Abstract
Continuous intraoperative optical coherence tomography (iOCT) integrated into the operating microscope is a new modification in the current operating microscope to aid in the surgical procedures involving both the anterior and the posterior segment. This helps in intraoperative planning, modification of the surgical steps if required and confirmation of the surgical endpoint in the operating room itself. iOCT was used for the successful management of descemet’s membrane detachment (DMD) following deep anterior lamellar keratoplasty (DALK) with intracameral injection of 20% Sulphur hexafluoride. The gas was injected under direct visualization through the microscope with continuous real time monitoring of the change in height of the detached Descemet’s membrane (DM). Additionally stab incisions were given through the anterior cornea due to the presence of residual fluid above the DM which was visible on continuous iOCT images. This led to the successful apposition of the DM which otherwise would have remained detached due to the residual fluid. This highlights the importance of continuous iOCT monitoring of the ophthalmic surgical procedures in order to produce a successful anatomical outcome of the surgery without disruption of the surgical procedure.
Keywords: Descemet’s membrane detachment, Continuous intraoperative OCT, RESCAN 700, Deep anterior lamellar keratoplasty
Introduction
Descemet’s membrane detachment (DMD) may occur following various intraocular procedures including deep anterior lamellar keratoplasty (DALK) which may result in the formation of a double chamber.1 Spontaneous resolution of DMD may occur if it is small2; however, in cases of large DMDs, intracameral air or isoexpansile gases are injected which tamponade the descemet’s membrane (DM).
In the presence of relatively clear cornea, DMD may be diagnosed on slit lamp examination. However, in cases of diffuse and severe corneal oedema, it may not be possible to visualize the DM. In such cases, anterior segment optical coherence tomography (ASOCT) is a useful tool to detect the presence, location and size of the DMD.
With the advent of an integrated ASOCT with the operating microscope, a real time imaging of the structures of the eye, both the anterior segment and the retinal layers is possible. It helps the surgeon to directly visualize the intraocular structures and monitor the intraoperative manoeuvres. Additionally, the surgical steps can be modified instantly and results thereof can be verified in the operating room itself.
We herein describe the successful management of DMD following DALK with the help of continuous intraoperative OCT (iOCT) guided intracameral injection of 20% sulphur hexafluoride (SF6).
Case report
The patient underwent manual descemetic DALK in the right eye for post chemical injury vascularized corneal opacity leaving behind the bare descemet’s membrane with donor cornea sutured to it. The vision in the right eye preoperatively was finger counting close to face with accurate projection of rays. Postoperatively on day one, a large DMD was present from limbus to limbus in the central cornea which was attributed to microperforations in the DM which might have occurred intraoperatively. In view of a large DMD, intracameral 20% SF6 injection was planned under the guidance of continuous intraoperative anterior segment OCT (OPMI LUMERA 700 and RESCAN 700, Carl Zeiss, Meditec, Germany).
The height and position of DMD were visualized on the continuous iOCT (Fig. 1). Under topical anaesthesia with proparacaine 0.5%, a 30 g needle attached to a syringe with 20% SF6 was then slowly advanced through the superior limbus at the 11 O’ clock position into the anterior chamber. The advancing edge of the needle as it traversed beneath the detached DM was clearly discernible on the real time OCT images. SF6 was slowly injected and the height of the detached DM was seen to decrease as the gas was injected (Fig. 2A and B); however, the OCT images revealed that there was still some fluid present between the posterior stroma and DM. Hence, stab incisions were given using the MVR blade (Alcon, Fort Worth, Texas) through the anterior part of the cornea at the 10 O’clock position. The tip of the MVR blade was visualized on the iOCT to occupy the supernumerary space above the DM (Fig. 2C and D). The fluid in the residual space decreased with the egress of the fluid and the attached DM was visualized on the continuous iOCT in the same surgical field (Fig. 3).
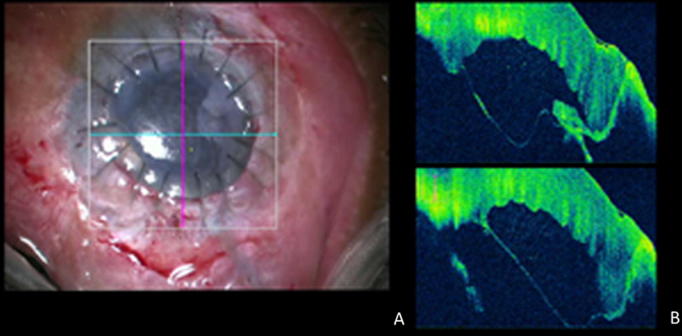
Figure 1.
Anterior segment intraoperative optical coherence tomography (iOCT). (A) Surgical view of a DMD in an operated case of DALK with the cross-hairs of the OCT scanner focussed on the centre of the cornea. (B) Continuous iOCT images of the cornea showing a large descemet’s membrane detachment in the centre of the cornea.
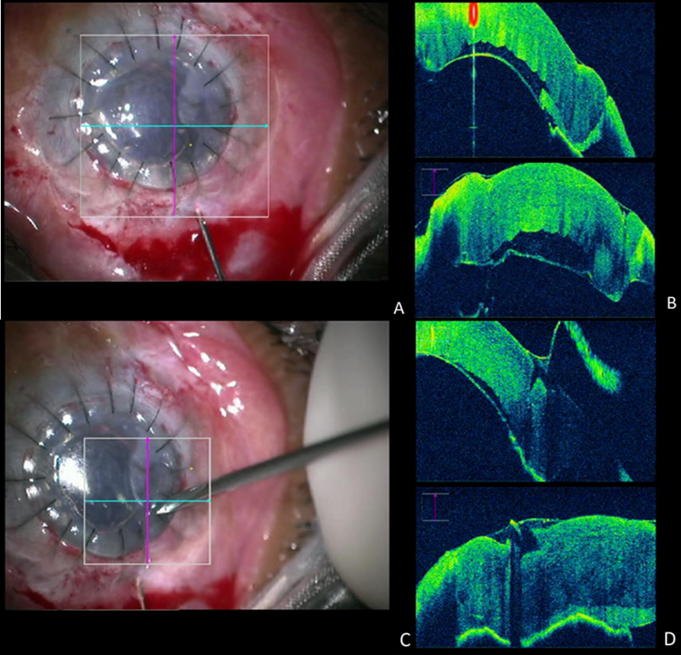
Figure 2.
Anterior segment intraoperative optical coherence tomography (iOCT). (A) Insertion of a 30 G needle with 20% SF6 injection into the anterior chamber at the 11 O’clock position. (B) iOCT images depicting a decrease in the height of descemet’s detachment following gas injection. (C) Insertion of the MVR blade through the anterior cornea at the 10 O’clock position to drain the residual fluid in the interface. (D) iOCT images showing a further decrease in the height of the descemet’s membrane detachment following the stab incision.
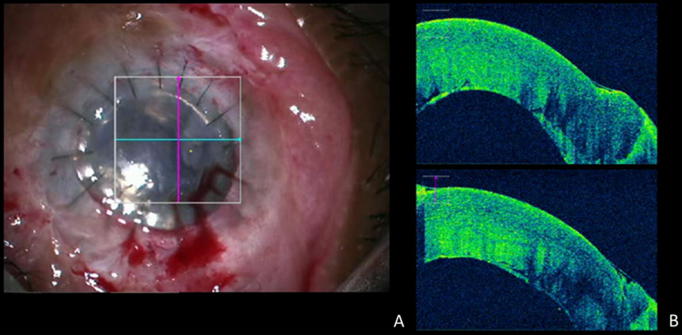
Figure 3.
Anterior segment intraoperative optical coherence tomography (iOCT). (A) Surgical view at the end of the procedure showing an attached descemet’s membrane. (B) The clinical finding is confirmed on iOCT with an attached descemet’s membrane.
Discussion
Spectral domain-OCT systems in a handheld or microscope-mounted fashion have been used both for anterior segment and for posterior segment surgery.3, 4, 5, 6 Till date, in all the microscope integrated iOCT systems, the OCT arm is mounted either in the optical path or on a side port and the OCT engine is placed outside the housing of the microscope. Hence, the microscope has to be moved away from the surgical field so that the OCT can capture the images leading to interruption of the surgical procedure causing surgical delay. In addition, an unscrubbed assistant is required to capture the OCT image.
The continuous iOCT system provides real time images by integration into the operating microscope which can be controlled with the foot pedal. Since the OCT is in-built into the microscope without being mounted on a separate arm, the anterior segment images are visualized adjacent to the surgical field through the surgeon’s eyepiece. This acts as a “third eye” for the surgeon and causes minimal disruption to the ongoing surgical procedure and does not require an assistant for capturing the images manually.
This prototype iOCT system has been used in two studies previously involving the calculation of intraocular lens power using the anterior chamber depth calculation intraoperatively.7, 8 The DISCOVER study was a preliminary study conducted with RESCAN 700 to determine the feasibility of the iOCT in the use of anterior segment and retinal surgeries.9
Till date, continuous iOCT systems have not been used in guiding lamellar corneal procedures. For the first time in the literature, we have used iOCT for real time monitoring of the reattachment of a detached DM with the help of intracameral SF6 injection. The presence of diffuse corneal oedema precludes the visibility of DM both on slit lamp biomicroscopy and on intraoperative visualization through the surgical microscope. It is difficult to discern conclusively while injecting intracameral air/gas whether the needle is beneath the DM or in the supra DM compartment. Many a times, the needle remains in the supernumerary space above the DM and air/gas injection leads to further enlargement of the DMD. In our case, with the continuous visualization of the anterior segment using iOCT the advancing edge of the needle was confirmed to lie beneath the DM. In our case, although the height of the DMD had decreased with SF6 injection residual fluid was still present above the DM. Stab incisions were then made through the anterior part of the corneal stroma to drain the fluid from the interface between the DM and posterior stroma, akin to the stab incisions in cases of Descemet’s stripping automated endothelial keratoplasty (DSAEK).
Continuous intraoperative real time OCT imaging is a new tool in the armamentarium of the lamellar corneal surgeons. This allows an accurate estimation of the depth of dissection with less chances of corneal perforation. As of now, the iOCT provides only qualitative details without providing the quantitative measurements of the anterior segment instantly. Further introduction of tools for quantitative analysis of the anterior and posterior segment will give a new dimension to this technology.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Hirano K., Kojima T., Nakamura M., Hotta Y. Triple anterior chamber after full-thickness lamellar keratoplasty for lattice corneal dystrophy. Cornea. 2001;20:530–533. doi: 10.1097/00003226-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Tu Kyaw Lin, Ibrahim M., Kaye S.B. Spontaneous resolution of Descemet membrane detachment after deep anterior lamellar keratoplasty. Cornea. 2006;25:104–106. doi: 10.1097/01.ico.0000167882.86137.fb. [DOI] [PubMed] [Google Scholar]
- 3.Ehlers J.P., Tam T., Kaiser P.K., Martin D.F., Smith G.M., Srivastava S.K. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014;34:1341–1346. doi: 10.1097/IAE.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlers J.P., Xu D., Kaiser P.K., Singh R.P., Srivastava S.K. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultrastructural alterations with intraoperative optical coherence tomography. Retina. 2014;34:213–221. doi: 10.1097/IAE.0b013e318297daf3. [DOI] [PubMed] [Google Scholar]
- 5.Scorcia V., Busin M., Lucisano A., Beltz J., Carta A., Scorcia G. Anterior segment optical coherence tomography-guided big-bubble technique. Ophthalmology. 2013;120:471–476. doi: 10.1016/j.ophtha.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 6.De Benito-Llopis L., Mehta J.S., Angunawela R.I., Ang M., Tan D.T. Intraoperative anterior segment optical coherence tomography: a novel assessment tool during deep anterior lamellar keratoplasty. Am J Ophthalmol. 2014;157:334–341. doi: 10.1016/j.ajo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Hirnschall N., Norrby S., Weber M., Maedel S., Amir-Asgari S., Findl O. Using continuous intraoperative optical coherence tomography measurements of the aphakic eye for intraocular lens power calculation. Br J Ophthalmol. 2015;99:7–10. doi: 10.1136/bjophthalmol-2013-304731. [DOI] [PubMed] [Google Scholar]
- 8.Hirnschall N., Amir-Asgari S., Maedel S., Findl O. Predicting the postoperative intraocular lens position using continuous intraoperative optical coherence tomography measurements. Invest Ophthalmol Vis Sci. 2013;54:5196–5203. doi: 10.1167/iovs.13-11991. [DOI] [PubMed] [Google Scholar]
- 9.Ehlers J.P., Kaiser P.K., Srivastava S.K. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014;98:1329–1332. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]