Abstract
From 2007 to 2014 the New York State (NYS) Newborn Screening (NBS) program screened 2 million newborns for congenital adrenal hyperplasia (CAH). The data was analyzed to determine factors that affect 17α-hydroxyprogesterone levels and assist in developing algorithm changes that would improve the positive predictive value of the methodology being used. The concentration of 17-OHP in dried blood spots was measured using the AutoDELFIA Neonatal 17-OHP kit (Perkin Elmer, Turku, Finland). During the 8 year period of this study 2476 babies were referred, 105 babies were diagnosed with CAH (90 with the salt-wasting (SW), 8 with simple virilizing (SV), 5 with non-classical CAH, and 2 with another enzyme deficiency) and, 14 with possible CAH. Three false negative cases with SV-CAH were reported to the program. Of the total 108 known cases, 74 (69%) infants were detected by newborn screening in the absence of clinical information, or, known family history. The incidence of CAH in NYS is 1 in 18,170 with a ratio of SW to SV of 8.2:1. The incidence of CAH is lower in Black infants than in White, Hispanic and Asian infants. Despite a lower mean birth weight, female infants have a lower mean 17-OHP value than male infants and are under-represented in the referred category. As per other NBS programs the false positive rate is exacerbated by prematurity/low birth weight and by over-early specimen collection.
Keywords: Congenital adrenal hyperplasia, Newborn screening, 17α-Hydroxyprogesterone, 21-Hydroxylase deficiency
1. Introduction
Congenital adrenal hyperplasia (CAH; OMIM # 201910) is a group of autosomal recessive disorders that result from the deficiency of one of several enzymes involved in the steroidogenic pathway for cortisol biosynthesis. The most common cause of CAH, accounting for 90% of cases, is 21-hydroxylase deficiency resulting from mutations or deletions in the CYP21A gene (OMIM # 613815) [1]. Less common causes of CAH are deficiencies in the enzymes 11β-hydroxylase, 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase, steroidogenic acute regulatory protein, cholesterol side-chain cleavage enzyme and P450-oxidoreductase [2]. The symptoms of disease vary depending on the nature and severity of the enzyme deficiency as well as the sex of the individual. Approximately 75% of infants with classical CAH have the severe salt-wasting (SW) form of the disease with shock, dehydration, hyponatremia, and hyperkalemia leading to death [3]. Affected females have varying degrees of virilization of the external genitalia. The remaining 25% have the simple virilizing (SV) form of disease which is less severe. Non-classical (NC) CAH is generally late onset with symptoms such as hirsutism, infertility, acne and alopecia arising from androgen excess [4].
Untreated, babies with classic CAH could experience a life threatening adrenal crisis. However, treatment in the form of replacement hormone therapy (hydrocortisone or dexamethasone to replace cortisol and fludrocortisone to replace aldosterone) corrects the hormone deficiencies [3]. Life-long medication is required to prevent the return of symptoms in individuals with classic CAH. In addition, newborns with ambiguous genitalia should undergo an appropriate work-up by a medical team consisting of a pediatric endocrinologist, urologist, surgeon, geneticist, and psychologist who should assist parents in making fully informed decisions regarding gender assignment and treatment options such as genital reconstructive surgery [5].
Classic CAH has been considered an excellent candidate for newborn screening because it has a fairly high incidence, sensitive tests are available for its diagnosis, effective medications are available to treat the disease, and, early treatment reduces mortality. Therefore, many newborn screening programs throughout the world have screened newborns for the disease for many decades [6], [7], [8], [9], [10], [11], [12], [13]. To identify infants with the severe form of salt wasting CAH, most newborn screening tests measure the level of 17α-hydroxyprogesterone (17-OHP) which is elevated in affected infants. Some infants affected by non 21-hydroxylase forms of CAH will not be detected by these tests. In addition, non-SW-CAH with intermediate 17-OHP values are also occasionally missed.
The NYS NBS program initiated CAH screening in December 2002. Here we report on the results of the screening from 2007 to 2014 and include the algorithm changes that were made to reduce the number of false positive referrals. This time period includes at least 3 years of data before and after a significant change in the manufacturer's kit.
2. Materials and methods
The data presented here include results of CAH screening for 1,962,433 babies in NYS for an 8 year period from 2007 to 2014.
In NYS, ideally, a blood specimen is collected via a heel stick from all newborns on a Guthrie filter card 24–48 h after birth and sent to the NBS program with accompanying mother and infant demographic information. Throughout the time period of the study the program required that specimens be shipped overnight at ambient temperature. During accessioning, specimens were manually checked for suitability for testing. Unsuitable specimens such as those with serum rings, blood clots or specimens of insufficient quantity were not tested. Instead, a repeat specimen was requested. Since 2010, for any specimen that was collected when the infant was < 24 h old [henceforth referred to as a day of birth (DOB) specimen], even though it was tested for 17-OHP, a repeat specimen was nevertheless requested. There is a high rate of false positive and false negative results for specimens that are collected in the first 24 h of life [14]. In addition, with increasing prematurity, there is an increase in 17-OHP levels [15], therefore, without age adjusted cut-offs, premature infants would have a considerably higher false positive rate.
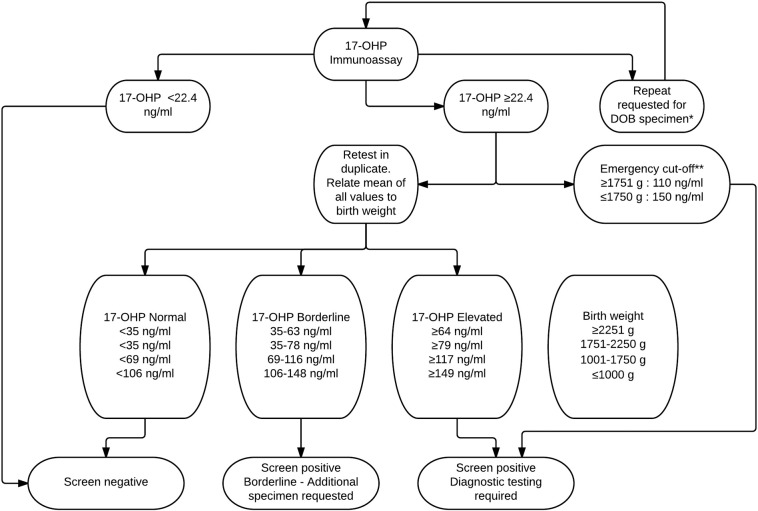
Three millimeter dried blot spots were punched into 96-well plates and the AutoDELFIA Neonatal 17α-hydroxyprogesterone (Perkin Elmer, Turku, Finland) kit was used to measure the 17-OHP level (see Fig. 1 for current CAH testing algorithm). The test is an FDA-approved time-resolved fluoroimmunoassay.
Fig. 1.
Current NYS screening algorithm for CAH.
*A repeat is requested when a specimen collected on day of birth (DOB) is received but the specimen is nevertheless tested. For DOB specimens: ≥ 1751 g infants are referred if the 17-OHP value is ≥ 110 ng/ml; 1001–1750 g infants are referred if the 17-OHP value is ≥ 117 ng/ml; ≤ 1000 g infants are referred if the 17-OHP value is ≥ 149 ng/ml. In addition, for specimens collected 14–40 days after birth: ≤ 1750 g babies are referred if the 17-OHP value is > 55 ng/ml and ≥ 1751 g babies are referred if the 17-OHP value is ≥ 35 ng/ml. For specimens collected > 40 days after birth, babies are referred if the 17-OHP value is ≥ 35 ng/ml. **Emergency level 17-OHP values are referred immediately and prior to repeat confirmatory screening. 17-OHP, 17-hydroxyprogesterone.
Unrelated to CAH screening, in July 2014, the program made a concerted effort to encourage hospitals to submit specimens from neonatal intensive care unit (NICU) babies prior to transfusion. This effort led to an increase in submission of DOB specimens.
2.1. Statistical analysis
Differences in 17-OHP mean values, ethnicities and infant weights were assessed using one-way ANOVA (analysis of variance), Kruskal-Wallis tests, Wilcoxon rank-sum tests, unpaired t tests or Chi-square tests. A p-value of < 0.05 was considered statistically significant.
3. Results
Approximately 2 million babies were screened for CAH over an 8 year period from 2007 to 2014 using a screen to detect elevated 17-OHP (Table 1). Race/ethnicity data was reported by health care providers who collect the blood specimens and is subjective, thus was suspended in 2012 (Table 2).
Table 1.
Yearly data for CAH referrals and confirmed cases in NYS from 2007 to 2014.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | Total 2007–14 | |
|---|---|---|---|---|---|---|---|---|---|
| Total infants screened | 255,044 | 252,632 | 249,255 | 245,072 | 241,938 | 241,420 | 238,250 | 238,822 | 1,962,433 |
| DOB specimen received | 3580 | 3727 | 4035 | 3944 | 4606 | 4421 | 4693 | 5587 | 34,593 |
| Total infants referred | 380 | 331 | 410 | 401 | 230 | 191 | 243 | 290 | 2476 |
| Borderline (repeat requested) | 1167 | 1062 | 1261 | 1090 | 1228 | 1306 | 1445 | 2015 | 10,574 |
| Confirmed cases | 14 | 7 | 15 | 13 | 13 | 16 | 10 | 17 | 105 |
| Possible cases | – | – | – | 4 | 2 | 1 | 5 | 2 | 14 |
| False negative cases reported | – | 1 | – | 2 | – | – | – | – | 3 |
DOB, day of birth.
Table 2.
Incidence of CAH and CAH subtype results in NYS from 2007 to 2014.
| CAH Incidence | Total Tested (%) | Referred (%) | Confirmed (%) | SW-CAH | SV-CAH | NC-CAH | Other Enzyme Deficiency | |
|---|---|---|---|---|---|---|---|---|
| Total | 1:18,170 | 1,962,433 | 2476 | 108a | 90 | 11a | 5 | 2 |
| Male | 1:18,280 | 1,005,444 (51.2) | 1,432 (57.8) | 55 (50.9) | 49 | 4 | 2 | 0 |
| Female | 1:18,050 | 956,856 (48.8) | 1,044 (42.2) | 53a (49.1) | 41 | 7a | 3 | 2 |
| White | 1:15,610 | 874,066 (44.5) | 767 (31.0) | 56a (51.9) | 46 | 8a | 1 | 1 |
| Hispanic | 1:17,450 | 331,589 (16.9) | 552 (22.3) | 19 (17.6) | 14 | 2 | 3 | 0 |
| Black | 1:24,840 | 298,057 (15.2) | 618 (25.0) | 12 (11.1) | 12 | 0 | 0 | 0 |
| Asian | 1:15,250 | 137,269 (7.0) | 104 (4.2) | 9 (8.3) | 7 | 1 | 0 | 1 |
| Native American | – | 3,009 (0.2) | 1 (0.04) | 0 | – | – | – | – |
| Other | 1:13,150 | 157,777 (8.0) | 225 (9.1) | 12 (11.1) | 11 | 0 | 1 | 0 |
| Not collectedb | – | 160,666 (8.2) | 209 (8.4) | – | – | – | – | – |
Includes false negative cases.
Racial/ethnic data was not collected on incoming specimens.
Multitiered thresholds were established based on the age and weight of the infant when specimens were collected. An emergency cut-off was established if the 17-OHP was extremely high (from July 2010 to 2014: 110 ng/ml for infants ≥ 1751 g and 150 ng/ml for infants ≤ 1750 g). In this case the program made the referral by phone prior to performing repeat confirmation testing. Elevated 17-OHP values triggered repeat testing in duplicate and the baby was referred based on the average value of three tests if the value remained above the cut-off level. If the average value of the three blood spots was borderline, then a repeat specimen was requested. In July 2010 the manufacturer changed the antibody used in the kit. Subsequent to the kit change, the cut-off for retesting for the kit was changed to a floating cut-off of 3% for a year after which new cut-off values were established. In order to decrease the number of false positives in normal weight infants whose specimens were collected on DOB, the referral cut-off for infants was raised in September 2014 to ≥ 110 ng/ml (Fig. 1). To reduce the false positive referrals additional changes were made to the algorithm that would create a borderline result and thus require an additional specimen be sent to the program: in October 2011 a borderline cut-off was established for low birth weight (LBW) infants ≥ 14 days of age, and, in September 2014 the cut-off level for the borderline category for ≥ 1751 g infants whose specimens were collected on DOB was raised. Additionally, borderline and referral levels for ≤ 1750 g babies whose specimens were collected between 14 and 40 days were also raised. Referrals were made by the NBS program to the pediatrician and appropriate specialty care center who then contacted parents to arrange diagnostic testing. The NBS program closed the case when they received a diagnosis and independent results. In some cases, a final diagnosis was not received because the infant was lost to follow up, parents refused further testing or the infant expired before diagnostic testing could be completed.
Repeat specimens were requested for 10,574 infants as their initial result was considered borderline (Table 1) and 2476 infants were referred for CAH. Of the total number of babies referred, 105 infants were diagnosed with CAH; 90 with SW-CAH, 8 with SV-CAH, 5 with NC-CAH, and 2 with “other enzyme deficiency” (Table 2). Of the 105 confirmed cases, 76 infants were referred based on the emergency cut-off for the assay; thus, the infants were referred based on preliminary data within 1 day of specimen receipt. Over the 8 year time period, 3 false negative cases were reported to the program although the expectation is that such cases are underreported. The annual rate of CAH in NYS was 13.5 cases per year. Of the 108 confirmed CAH cases, 55 were male and 53 were female, 3 of whom were not detected by newborn screening.
Our data show that there are race/ethnicity incidence differences with classical CAH more common in Asian, White and Hispanic infants and less common in Black infants (Table 2). In addition, statistically significant differences are observed in the average 17-OHP values of the different race/ethnicities with higher values in White infants and lower values in Asian infants (p < 0.0001)(Table 3).
Table 3.
Average 17-OHP (ng/ml) values for infants screened in NYS from 2007 to 2014.
| 2007 - June 2010 |
July 2010–2014 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. babies | Average 17-OHP | SD | Median | Range | No. babies | Average 17-OHP | SD | Median | Range | |
| Total babiesa | 863,902 | 15.0 | 13.4 | 12.3 | 0.1–1029.3 | 1,063,913 | 6.9 | 7.0 | 5.6 | 0.1–826.3 |
| Malea | 442,851 | 16.0 | 14.1 | 13.2 | 0.1–1029.3 | 544,092 | 7.4 | 7.4 | 6.1 | 0.1–716 |
| Femalea | 421,009 | 13.9 | 12.5 | 11.3 | 0.1–1004.3 | 519,739 | 6.3 | 6.6 | 5.2 | 0.1–826.3 |
| Whitea | 416,076 | 15.3 | 11.9 | 13.0 | 0.1–757.5 | 446,832 | 7.1 | 6.9 | 5.9 | 0.1–826.3 |
| Hispanica | 174,182 | 14.4 | 14.2 | 11.3 | 0.1–889.4 | 150,984 | 6.7 | 8.1 | 5.2 | 0.1–659 |
| Blacka | 144,224 | 15.1 | 16.3 | 11.6 | 0.1–1004.5 | 146,196 | 7.0 | 7.8 | 5.4 | 0.1–510 |
| Asiana | 61,254 | 14.2 | 12.2 | 11.8 | 0.1–657.2 | 74,418 | 6.1 | 5.7 | 5.0 | 0.1–479.3 |
| Native Americana | 1,395 | 13.9 | 10.3 | 11.7 | 1.9–133.4 | 1,581 | 6.6 | 6.0 | 5.4 | 0.9–123 |
| Othera | 64,820 | 14.8 | 13.9 | 12.2 | 0.1–1029.3 | 89,182 | 6.6 | 6.6 | 5.4 | 0.1–626.5 |
| Race/ethnicity not collecteda | 1,951 | 10.1 | 18.2 | 7.9 | 0.1–551.2 | 154,719 | 7.0 | 6.3 | 5.9 | 0.1–665.2 |
| Collected on DOB | 13,164 | 58.6 | 49.8 | 44.6 | 0.8–1140 | 21,429 | 26.3 | 24.3 | 20.2 | 0.1–610.3 |
| DOB for which repeat was received | 12,690 | 57.7 | 48.3 | 44.3 | 1–1140 | 20,776 | 26.4 | 24.3 | 20.2 | 0.4–610.3 |
| Repeat of the DOB | 12,690 | 25.2 | 30.8 | 16.9 | 0.1–926.6 | 20,776 | 12.9 | 19.2 | 7.4 | 0.1–755.5 |
| Borderline cases | 4,033 | 122.2 | 40.2 | 106.6 | 84.5–278.8 | 6,541 | 52.3 | 26.5 | 42.7 | 34.7–269 |
| Referrals | 1,332 | 195.5 | 121.4 | 156.6 | 85–1617.8 | 1,144 | 122.2 | 113.9 | 81.6 | 35–826.3 |
| Confirmed cases | 38b | 373.6 | 208.8 | 327.4 | 87.4–1029.3 | 66 | 303.6 | 181.4 | 274.7 | 37.8–659 |
Excluding day of birth (DOB) specimens.
Excluding one case that was closed based on results from an independent laboratory.
Our results also show that female infants have a lower average 17-OHP concentration than male infants (p < 0.0001 for data from July 2010 to 2014); a difference of 1.1 to 2.1 ng/ml depending on the kit used (Table 3). In addition, from 2007 to 2014, the mean weight (SD) of female infants was 3208 g (570.8), lower than male infants whose mean weight (SD) was 3325 g (596.1). This is a statistically significant difference (p < 0.0001). Furthermore, although 48.8% of the infants born in NYS are female, only 42.2% of referred babies are female but there is no significant difference between the numbers of males and females who are confirmed with disease. It should also be noted that we detected fewer cases of SV-CAH in male infants (4 vs 7) but a larger number of SW-CAH (49 vs 41), neither of which is statistically significant (Table 2).
Of the affected infants, 26 were reported to exhibit signs of abnormal virilization and 8 were reported as having a family history of CAH. This information is most probably under-reported to the NBS program. Due to the abnormal virilization, CAH was suspected in these infants prior to newborn screen results being available. Without reliable phenotypic diagnosis and/or family history, newborn screening detected an additional 74 (69%) CAH cases. Of these cases, 24 were female and 50 were male. Forty four of the confirmed cases were in the NICU when the NBS specimen was collected. Most (66%) of the babies in NICU were LBW and/or exhibited ambiguous genitalia.
From 2007 to 2014 the mean age of specimen collection was 2.6 days, the median was 2.0 days, and range was 0 to 783 days. Although specimen collection after 30 days of age is not encouraged by the NBS program, rare cases do occur. The program received 34,593 (1.8%) specimens that were collected on the DOB. A second specimen was requested and in response 33,466 (97%) specimens were received. As expected, specimens that had been collected on DOB had a higher mean 17-OHP value than specimens that were collected ≥ 24 h after birth (Table 3). The mean 17-OHP value for the repeat specimens was reduced by approximately 50% indicating a significant drop in 17-OHP values after the first day of life (Table 3).
From July 2010 to 2014, excluding DOB specimens, the program received 14,769 specimens for extremely LBW infants (≤ 1000 g), 29,321 specimens from very LBW infants (1001–1750 g), 41,142 specimens from LBW infants (1,751–2250 g) and 1.1 million specimens from normal birth weight (NBW) infants (≥ 2251 g). The mean (median) 17-OHP values for each of these groups were 21.0 (11.2), 15.0 (9.6), 11.8 (8.3) and 6.5 (5.5) ng/ml. From 2007 to June 2010 a similar decline in 17-OHP was observed with increasing weight of the newborns (data not shown).
Repeat specimens were requested for all borderline cases (Fig. 1). Of the 10,574 infants with borderline results a repeat specimen was received for 9402 infants, independent results from serum testing were obtained for 863 infants and despite further follow up, no specimens were received for the remainder. Results from independent testing, as well as, elevated 17-OHP results from repeat testing led to the referral of 103 cases. Seven of these cases were subsequently confirmed with CAH.
During the time period 2007–2014, three cases of confirmed SV-CAH that had normal newborn screens were reported to the program (Table 2). All three infants were White females of NBW with ambiguous genitalia. A case in 2008 had a newborn screen result of 59.9 ng/ml (cut-off ≥ 85 ng/ml) for 17-OHP. The other 2 cases were born in 2010 and had an initial 17-OHP concentration of 79.5 ng/ml (cut-off ≥ 85 ng/ml) and 10 ng/ml (cut-off ≥ 35 ng/ml). Due to the abnormal genitalia (and family history in one case), and, despite the normal NBS, diagnostic testing was performed and confirmed SV-CAH.
Fourteen cases ranging in date from 2010 to 2014 were closed as possible disease (Table 1). One case was a 1,010 g deceased infant, 11 others were premature infants in the NICU, one NBW infant was reported to have transient elevated 17-OHP, and, one NBW infant was a homebirth whose mother could not be contacted by the pediatrician and did not visit the specialty care center for follow-up. In general, when cases are closed as possible disease, the protocol is for the pediatric endocrinologist at the specialty care center to continue to follow the baby.
From 2007 to June 2010, using the testing methodology and algorithm of the time period, the incidence of CAH in NYS was 1 in 21,390, the sensitivity of the method was 95%, the specificity was 99.9%, the positive predictive value (PPV) was 2.9% and the negative predictive value (NPV) was 100%. From July 2010 to 2014, the specificity and NPV were unchanged but the sensitivity increased to 98.5% and the PPV doubled (5.8%). The incidence of CAH was 1 in 16,200. The overall incidence from 2007 to 2014 was 1 in 18,170.
The PPV was higher (12.6%) for full-term infants than for pre-term infants (1%) as determined by weight rather than gestational age. Of the 2,357 cases that were referred but were closed as “no disease”, 1467 (62%) were from LBW (< 2251 g) infants and 486 (21%) were from infants whose specimen was collected on DOB. Many of the LBW infants were referred after a second or third specimen was received and tested. This is because the cut-off levels for the ≥ 14 day old infants is lower than the < 14 day old infants and this lower cut-off leads to additional referrals. In September 2014 the cut-off level was raised and the effect on false positive screens is being monitored. Five hundred and sixty nine (24%) of the false positive cases were from > 2250 g babies whose specimens were collected at least 24 h after birth. Compared to this category, LBW and specimen collection on DOB remain significant causes of false positive results.
4. Discussion
Newborn screening for CAH reduces the time to diagnosis of the disease and is therefore crucial for infants who are not diagnosed clinically in a timely manner. In populations that are not screened, salt wasting is associated with learning disabilities and behavioral problems and adrenal crisis can lead to death.
From 2007 to 2014 screening of 2 million infants in NYS led to a repeat request for specimens from 10,574 infants, referral of 2476 infants, and, in the detection of 105 cases of confirmed CAH. Three false negative cases were also reported to the program. In 2010 due to the kit antibody change and the change in cut-off levels, the referral numbers were reduced but the number of borderline cases increased.
Our results indicate sex differences in mean 17-OHP concentrations as has previously been reported in Saudi Arabia, Cuba, Wisconsin and Iowa with mean levels higher in males than females [16], [17], [18], [19]. In contrast, in Colorado female infants were reported to have higher 17-OHP values which was explained by their lower birth weights [20]. In our population female infants also have a lower mean birth weight than male infants which would suggest that their 17-OHP value should be higher if there were no sex differences. Furthermore, female infants are underrepresented in the referred infant category (p < 0.0001) which may be explained by the lower mean 17-OHP values observed in females. In addition, in Wisconsin sensitivity of screening was found to be 83% in male infants but only 60% in female infants [18]. During the 3.5 year period of their study, of the 8 false negative cases reported, 7 were female [18]. It would be difficult to determine the true number of false negative males as the clinical symptoms are less clear. In NYS, during an 8 year period, all three false negative cases were females with SV-CAH. The 17-OHP values of 2 of the cases were not near the cut-off values, therefore, if the cut-off was reduced to a level that would make these cases positive, it would give rise to a very large number of additional false positives. All three infants exhibited abnormal genitalia and were therefore diagnosed despite the negative screens. The infant born in 2008 had two siblings who had been born prior to 2007 and who had CAH but whose newborn screens had also been in the normal range. Mutation analysis in the infant born in 2010 resulted in the detection of I172N and a 30 kb deletion. The 30 kb deletion in the HLA class III region of chromosome 6 is a common mutation that in homozygous form causes SW-CAH [21]. The I172N mutation yields different CAH phenotypes although it is most frequently associated with the SV form of the disease [22], [23]. Screening for CAH is primarily designed to detect the severe life threatening form of SW and SV-CAH and therefore the expectation is that less severe SV and NC forms of CAH may be missed.
The average 17-OHP levels, as well as, incidence of CAH was different among the different race/ethnicities (Table 2, Table 3). The highest incidence was observed in Asian (1 in 15,250) and White infants (1 in 15,610) and the lowest incidence in Black infants (1 in 24,840). Black infants constituted 25% of the referred infants even though only 15.2% of babies born in NYS were Black and only 11.1% of confirmed cases were Black (Table 2). This indicates that Black infants are over-represented in the false positive group. In Texas and some other states in the U.S. a low incidence of classic CAH has also been reported for African-American infants compared to White and Hispanic infants [8], [24]. No CAH cases were observed in Native Americans in NYS, however, the numbers screened in this group were small. Our program discontinued the collection of race/ethnicity data on the Guthrie form in 2012.
Newborn screening for CAH has a high rate of false positive results especially among premature/LBW infants. In these infants transient elevated 17-OHP levels is due to adrenal immaturity, stress at birth, and, early specimen collection [25], [26]. As a result, most NBS programs use weight adjusted criteria to reduce the number of false positive results [8], [27], [28], [29]. In Bavaria, adjustments are made to cut-offs depending on age at sampling, in addition to birth weight [29]. The NYS NBS program has multitiered cut-off levels for premature infants (based on various weight categories) and automatically requests a repeat specimen when a DOB specimen is received. Despite this algorithm, LBW infants and infants whose specimens were collected on DOB were over-represented in the referred category. Sixty two percent of referred infants weighed ≤ 2250 g and 21% of specimens from referred infants were collected on DOB, while 24% of referred infants weighed > 2250 g infants and had their specimens collected at least 24 h after birth. These data show that the majority of false positives in NYS are generated by screening LBW infants and by early specimen collection. Throughout the time period of this study, there was an increase in submission of DOB specimens because unrelated to CAH screening, the NBS program encouraged submitters to collect a specimen prior to transfusions (Table 1). This had the unintentional consequence of increasing CAH referrals, especially during 2014.
In total there are only 9 confirmed CAH cases in the 3 established LBW categories during the 8 year time period of this study making it difficult to re-assess cut-offs for each category. Having evaluated epidemiological, clinical and biological data from premature infants in France, Huet and colleagues have proposed that screening in children born before 32 weeks of gestation be discontinued as the 17-OHP results are irrelevant [30]. Results from Minnesota show that performing 3 screens within the first month of life on LBW infants in NICU reduces the false positive rate [31]. NYS also requests multiple specimens from NICU babies including a specimen at discharge. Elevated 17-OHP results in the absence of clinical symptoms and other laboratory results in premature/LBW infants are unlikely to give rise to true positive cases. It is therefore fortunate that premature, LBW infants are often in NICU where they are monitored frequently and are therefore at low risk of suffering from a salt wasting crisis. Infants with transiently elevated 17-OHP, as opposed to CAH, exhibit normal biochemistry such as acid-base equilibrium, glycemia and electrolytes, normal physical examination, and higher perinatal stress factors [32]. Therefore, when making a diagnosis these characteristics should be taken into consideration in cases of elevated 17-OHP in premature infants.
Data from the NBS programs in Switzerland and the Netherlands suggest that the efficacy of 17-OHP screening can be improved by adjusting cut-offs to gestational age rather than on birth weight [33], [34]. Unfortunately reported gestational age is not as reliable and accurate as birth weight data and therefore many programs, including NYS, use birthweight categorization to classify different degrees of prematurity. Linder et al. [35] report that gestational age has a greater effect on 17-OHP concentration than birth weight and that for very young and LBW infants, 17-OHP concentration decline sharply in the first 2 weeks of life and thereafter stabilize to full-term values within the first 3 months of life.
Some NBS programs perform a second tier test such as Liquid Chromatography Tandem Mass Spectrometry (HPLC-TMS) that measures the levels of adrenal steroids in dried blood spots. However, in Minnesota despite using second tier steroid profiling, the PPV for CAH testing remains relatively low [36]. Alternatively, second tier molecular genetic analysis has been proposed [37]. A definitive diagnosis is made by the cosyntropin (a short-acting adrenocorticotropic hormone) stimulation test [14].
Some states in the U.S. routinely test 2 specimens. Colorado, for example, collects a specimen within the first 3 days of life and the second screen is obtained 8–14 days after birth. That state has reported that the second screen identified 28% of classical-CAH cases that would have been missed by the initial screen alone [20]. The Texas NBS program has also reported that had a second screen not been performed, 14% of classical CAH cases (all SV-CAH) would have been missed [8]. Some of the missed cases in the first screen were clinically diagnosed, several with identifiable genital abnormalities. In general, the first screen detects the SW form of CAH and is more prone to missing the SV form of disease and less severe CAH [23], [38], [39]. Most NBS programs do not perform a mandatory second screen as the cost effectiveness of performing the mandatory second screen for all newborns is controversial. Some NBS programs have reported missed cases of SV-CAH and low or no cases of false negative SW-CAH [18], [40], [41]. Other programs however, have reported that both SW and SV cases of CAH are missed [36], [42]. In a study in Minnesota, 22% of diagnosed patients with classic CAH were not identified by NBS [35]. This places infants, especially male infants, at risk of a potentially life-threatening adrenal or salt-wasting crisis. The expectation is that in the absence of NBS, female infants are more likely to be clinically diagnosed with severe CAH due to virilization of external genitalia whereas male infants are more likely to be clinically detected based on family history. In our experience 28 of 50 females with confirmed CAH detected by NBS were reported as having ambiguous genitalia or family history, therefore in the absence of NBS, 42% of females would not have been detected. In addition, 3 male infants were reported as having family history. Therefore, 95% of male infants would not have been detected based on family history. Using the NYS algorithm the sensitivity of screening was 94% in females and 100% in males. NBS detected both male and female cases of CAH prior to the establishment of a clinical diagnosis and was particularly helpful in detecting SW-CAH in male infants. It should be noted that clinical information is under-reported to our program so the advantage of NBS is exaggerated, but nevertheless significant.
The mean age at which NBS specimens were collected in NYS infants was 2.6 days and in 76 of 105 (72%) confirmed cases the turn-around time for referring the infants was one working day. This turnaround time is very beneficial in initiating timely treatment since an adrenal crisis can occur as early as at the end of the first week of life [43].
In NYS, as per most other NBS programs, the main reasons for the high repeat requests and referral rates are elevated 17-OHP levels due to prematurity and early collection of specimens (i.e. collection on the DOB). Algorithm improvements throughout the years and the antibody change in the kit in July 2010 led to an increase in the sensitivity and PPV of the assay. The new 17-OHP assay is less sensitive to cross-reacting steroids related to 17-OHP that tend to circulate at particularly high levels in premature infants [43]. In order to improve the current PPV of 5.8% for CAH screening even further the NYS NBS program plans to make additional changes to the algorithm. The cut-off values for LBW infants whose specimens are collected 14–40 days and > 40 days after birth, will be based on weight cut-offs rather than separate categories based on age of infant. In addition, the referral cut-off for specimens collected on DOB will be raised. An additional specimen will be requested in these cases, many of which are NICU cases who are being monitored. This will require that the program also educate submitters to ensure collection of a specimen when infants are discharged from the NICU. These changes will make a significant reduction in the number of referrals of infants whose specimens are collected on DOB and LBW infants whose specimens were collected 14–40 days after referral. From July 2010 to 2014 referrals in the former category would have been reduced by 70% with no false negatives, while the referral in the latter category would have been reduced by 90% and an additional specimen would have been requested for the three NC and one SV confirmed CAH cases that would no longer fall within the referral category. The large majority of specimens collected at 14–40 days are second or third specimens from LBW babies whose 17-OHP value has decreased since the first specimen but still fall within the current referral level. By eliminating many of these unnecessary referrals, costs and additional work to the healthcare system will be decreased and more importantly psychological stress for many families will be reduced.
Funding source
Newborn screening in NYS is funded by the State of NY.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors thank Amy McGeoch for invaluable help in performing follow up of CAH cases, Dr. Denise Kay for assistance with newborn screening data and Erin Dauerer for assistance with statistical analysis.
References
- 1.White P.C. Neonatal screening for congenital adrenal hyperplasia. Nat. Rev. Endocrinol. 2009;5:490–498. doi: 10.1038/nrendo.2009.148. [DOI] [PubMed] [Google Scholar]
- 2.Turcu A.F., Auchus R.J. The next 150 years of congenital adrenal hyperplasia. J. Steroid Biochem. Mol. Biol. 2015;153:63–71. doi: 10.1016/j.jsbmb.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speiser P.W., Azziz R., Baskin L.S., Ghizzoni L., Hensle T.W., Merke D.P., Meyer-Bahlburg H.F., Miller W.L., Montori V.M., Oberfield S.E., Ritzen M., White P.C., Society Endocrine. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witchel S.F., Azziz R. Nonclassic congenital adrenal hyperplasia. Int. J. Pediatr. Endocrinol. 2010;2010:625105. doi: 10.1155/2010/625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee P.A., Nordenström A., Houk C.P., Ahmed S.F., Auchus R., Baratz A., Baratz Dalke K., Liao L.M., Lin-Su K., Looijenga L.H., 3rd, Mazur T., Meyer-Bahlburg H.F., Mouriquand P., Quigley C.A., Sandberg D.E., Vilain E., Witchel S., the Global DSD Update Consortium. Global Disorders of Sex Development Update since 2006 Perceptions, approach and care. Horm. Res. Paediatr. 2016 doi: 10.1159/000442975. [DOI] [PubMed] [Google Scholar]
- 6.Pang S.Y., Wallace M.A., Hofman L., Thuline H.C., Dorche C., Lyon I.C., Dobbins R.H., Kling S., Fujieda K., Suwa S. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 1988;81:866–874. [PubMed] [Google Scholar]
- 7.Gidlöf S., Wedell A., Guthenberg C., von Döbeln U., Nordenström A. Nationwide neonatal screening for congenital adrenal hyperplasia in Sweden: a 26-year longitudinal prospective population-based study. J. Am. Med. Assoc. Pediatr. 2014;168:567–574. doi: 10.1001/jamapediatrics.2013.5321. [DOI] [PubMed] [Google Scholar]
- 8.Therrell B.L., Berenbaum S.A., Manter-Kapanke V., Simmank J., Korman K., Prentice L., Gonzalez J., Gunn S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–590. doi: 10.1542/peds.101.4.583. [DOI] [PubMed] [Google Scholar]
- 9.Nascimento M.L., Cristiano A.N., Campos Td, Ohira M., Cechinel E., Simoni G., Lee Jv, Linhares R.M., Silva P.C. Ten-year evaluation of a neonatal screening program for congenital adrenal hyperplasia. Arq. Bras. Endocrinol. Metabol. 2014;58:765–771. doi: 10.1590/0004-2730000003310. [DOI] [PubMed] [Google Scholar]
- 10.Shetty V.B., Bower C., Jones T.W., Lewis B.D., Davis E.A. Ethnic and gender differences in rates of congenital adrenal hyperplasia in Western Australia over a 21 year period. J. Paediatr. Child Health. 2012;48:1029–1032. doi: 10.1111/j.1440-1754.2012.02584.x. [DOI] [PubMed] [Google Scholar]
- 11.Morikawa S., Nakamura A., Fujikura K., Fukushi M., Hotsubo T., Miyata J., Ishizu K., Tajima T. Results from 28 years of newborn screening for congenital adrenal hyperplasia in Sapporo. Clin. Pediatr. Endocrinol. 2014;23:35–43. doi: 10.1297/cpe.23.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odenwald B., Dörr H.G., Bonfig W., Schmidt H., Fingerhut R., Wildner M., Nennstiel-Ratzel U. Classic congenital adrenal hyperplasia due to 21-hydroxylase-deficiency: 13 years of neonatal screening and follow-up in Bavaria. Klin. Padiatr. 2015;227:278–283. doi: 10.1055/s-0035-1554639. [DOI] [PubMed] [Google Scholar]
- 13.Gruñieiro-Papendieck L., Chiesa A., Mendez V., Prieto L. Neonatal screening for congenital adrenal hyperplasia: experience and results in Argentina. J. Clin. Endocrinol. Metab. 2008;21:73–78. doi: 10.1515/jpem.2008.21.1.73. [DOI] [PubMed] [Google Scholar]
- 14.New M.I., Lorenzen F., Lerner A.J., Kohn B., Oberfield S.E., Pollack M.S., Dupont B., Stoner E., Levy D.J., Pang S., Levine L.S. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J. Clin. Endocrinol. Metab. 1983;57:320–326. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- 15.Wilson K., Hawken S., Ducharme R., Potter B.K., Little J., Thébaud B., Chakraborty P. Metabolomics of prematurity: analysis of patterns of amino acids, enzymes, and endocrine markers by categories of gestational age. Pediatr. Res. 2014;75:367–373. doi: 10.1038/pr.2013.212. [DOI] [PubMed] [Google Scholar]
- 16.al-Nuaim A.R., Abdullah M.A., Stevens B., Zain M. Effect of gender, birth weight and gestational age on serum 17-hydroxyprogesterone concentration and distribution among neonates in Saudi Arabia. Indian J. Pediatr. 1995;62:605–609. doi: 10.1007/BF02761890. [DOI] [PubMed] [Google Scholar]
- 17.González E.C., Carvajal F., Frómeta A., Arteaga A.L., Castells E.M., Espinosa T., Coto R., Pérez P.L., Tejeda Y., Del Río L., Segura M.T., Almenares P., Robaina R., Fernández J.L. Newborn screening for congenital adrenal hyperplasia in Cuba: six years of experience. Clin. Chim. Acta. 2013;421:73–78. doi: 10.1016/j.cca.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Varness T.S., Allen D.B., Hoffman G.L. Newborn screening for congenital adrenal hyperplasia has reduced sensitivity in girls. J. Pediatr. 2005;147:493–498. doi: 10.1016/j.jpeds.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Ryckman K.K., Berberich S.L., Shchelochkov O.A., Cook D.E., Murray J.C. Clinical and environmental influences on metabolic biomarkers collected for newborn screening. Clin. Biochem. 2013;46:133–138. doi: 10.1016/j.clinbiochem.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan C.L., McFann K., Taylor L., Wright D., Zeitler P.S., Barker J.M. Congenital adrenal hyperplasia and the second newborn screen. J. Pediatr. 2013;163:109–113. doi: 10.1016/j.jpeds.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 21.White P.C., Vitek A., Dupont B., New M.I. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc. Natl. Acad. Sci. U. S. A. 1988;85:4436–4440. doi: 10.1073/pnas.85.12.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.New M.I., Abraham M., Gonzalez B., Dumic M., Razzaghy-Azar M., Chitayat D., Sun L., Zaidi M., Wilson R.C., Yuen T. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc. Natl. Acad. Sci. U. S. A. 2013;110:2611–2616. doi: 10.1073/pnas.1300057110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkielstain G.P., Chen W., Mehta S.P., Fujimura F.K., Hanna R.M., Van Ryzin C., McDonnell N.B., Merke D.P. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2011;96:e161–e172. doi: 10.1210/jc.2010-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Held P.K., Shapira S.K., Hinton C.F., Jones E., Hannon W.H., Ojodu J. Congenital adrenal hyperplasia cases identified by newborn screening in one- and two-screen states. Mol. Genet. Metab. 2015;116:133–138. doi: 10.1016/j.ymgme.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korte C., Styne D., Merritt A.T., Mayes D., Wertz A., Helbock H.J. Adrenocortical function in the very low birth weight infant: improved testing sensitivity and association with neonatal outcome. J. Pediatr. 1996;128:257–263. doi: 10.1016/s0022-3476(96)70404-3. [DOI] [PubMed] [Google Scholar]
- 26.Ng P.C., Wong G.W.K., Lam C.W.K., Lee C.H., Wong M.Y., Fok T.F., Wong W., Chan D.C. Pituitary-adrenal response in preterm very low birth weight infants after treatment with antenatal corticosteroids. J. Clin. Endocrinol. Metab. 1997;82:3548–3552. doi: 10.1210/jcem.82.11.4392. [DOI] [PubMed] [Google Scholar]
- 27.Allen D.B., Hoffman G.L., Fitzpatrick P., Laessig R., Maby S., Slyper A. Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. J. Pediatr. 1997;130:128–133. doi: 10.1016/s0022-3476(97)70321-4. [DOI] [PubMed] [Google Scholar]
- 28.Barra C.B., Silva I.N., Pezzuti I.L., Januário J.N. Neonatal screening for congenital adrenal hyperplasia. Rev. Assoc. Med. Bras. 2012;58:459–464. [PubMed] [Google Scholar]
- 29.Olgemöller B., Roscher A.A., Liebl B., Fingerhut R. Screening for congenital adrenal hyperplasia: adjustment of 17-hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J. Clin. Endocrinol. Metab. 2003;88:5790–5794. doi: 10.1210/jc.2002-021732. [DOI] [PubMed] [Google Scholar]
- 30.Huet F., Godefroy A., Cheillan D., Somma C., Roussey M. [Do we need congenital adrenal hyperplasia screening for premature infants?] [Article in French] Arch. Pediatr. 2014;21:233–236. doi: 10.1016/j.arcped.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Sarafoglou K., Gaviglio A., Hietala A., Frogner G., Banks K., McCann M., Thomas W. Comparison of newborn screening protocols for congenital adrenal hyperplasia in preterm infants. J. Pediatr. 2014;164:1136–1140. doi: 10.1016/j.jpeds.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 32.Fernández B.H., Fernández M.E., Iñiguez E.D., Zubicaray B.E., Martín M.B.R., Arnao M.D.R., Sánchez A.R. Neonatal screening for congenital adrenal hyperplasia: transitory elevation of 17-hydroxyprogesterone. J. Clin. Endocrinol. Metab. 2011;24:155–162. [PubMed] [Google Scholar]
- 33.Steigert M., Schoenle E.J., Biason-Lauber A., Torresani T. High reliability of neonatal screening for congenital adrenal hyperplasia in Switzerland. J. Clin. Endocrinol. Metab. 2002;87:4106–4110. doi: 10.1210/jc.2002-012093. [DOI] [PubMed] [Google Scholar]
- 34.Van der Kamp H.J., Oudshoorn C.G., Elvers B.H., van Baarle M., Otten B.J., Wit J.M., Verkerk P.H. Cutoff levels of 17-alpha-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J. Clin. Endocrinol. Metab. 2005;90:3904–3907. doi: 10.1210/jc.2004-2136. [DOI] [PubMed] [Google Scholar]
- 35.Linder N., Davidovitch N., Kogan A., Barzilai A., Kuint J., Mazkeret R., Sack J. Longitudinal measurements of 17alpha-hydroxyprogesterone in premature infants during the first three months of life. Arch. Dis. Child Fetal Neonatal Ed. 1999;81:F175–F178. doi: 10.1136/fn.81.3.f175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarafoglou K., Banks K., Gaviglio A., Hietala A., McCann M., Thomas W. Comparison of one-tier and two-tier newborn screening metrics for congenital adrenal hyperplasia. Pediatrics. 2012;130:e1261–e1268. doi: 10.1542/peds.2012-1219. [DOI] [PubMed] [Google Scholar]
- 37.Kösel S., Burggraf S., Fingerhut R., Dörr H.G., Roscher A.A., Olgemöller B. Rapid second-tier molecular genetic analysis for congenital adrenal hyperplasia attributable to steroid 21-hydroxylase deficiency. Clin. Chem. 2005;51:298–304. doi: 10.1373/clinchem.2004.042416. [DOI] [PubMed] [Google Scholar]
- 38.Brosnan C.A., Brosnan P., Therrell B.L., Slater C.H., Swint J.M., Annegers J.F., Riley W.J. A comparative cost analysis of newborn screening for classic congenital adrenal hyperplasia in Texas. Public Health Rep. 1998;113:170–178. [PMC free article] [PubMed] [Google Scholar]
- 39.Votava F., Török D., Kovács J., Möslinger D., Baumgartner-Parzer S.M., Sólyom J., Pribilincová Z., Battelino T., Lebl J., Frisch H., Waldhauser F., the Middle European Society for Paediatric Endocrinology – Congenital Adrenal Hyperplasia (MESPE-CAH) Study Group Estimation of the false-negative rate in newborn screening for congenital adrenal hyperplasia. Eur. J. Endocrinol. 2005;152:869–874. doi: 10.1530/eje.1.01929. [DOI] [PubMed] [Google Scholar]
- 40.Heather N.L., Seneviratne S.N., Webster D., Derraik J.G., Jefferies C., Carll J., Jiang Y., Cutfield W.S., Hofman P.L. Newborn screening for congenital adrenal hyperplasia in New Zealand, 1994–2013. J. Clin. Endocrinol. Metab. 2015;100:1002–1008. doi: 10.1210/jc.2014-3168. [DOI] [PubMed] [Google Scholar]
- 41.Van der Kamp H.J., Noordam K., Elvers B., Van Baarle M., Otten B.J., Verkerk P.H. Newborn screening for congenital adrenal hyperplasia in the Netherlands. Pediatrics. 2001;108:1320–1324. doi: 10.1542/peds.108.6.1320. [DOI] [PubMed] [Google Scholar]
- 42.Thil'en A., Nordenström A., Hagenfeldt L., von Döbeln U., Guthenberg C., Larsson A. Benefits of neonatal screening for congenital adrenal hyperplasia (21-hydroxylase deficiency) in Sweden. Pediatrics. 1998;101 doi: 10.1542/peds.101.4.e11. [DOI] [PubMed] [Google Scholar]
- 43.Pang S., Clark A., Neto E.C., Giugliani R., Dean H., Winter J., Dhondt J.-L., Farriaux J.P., Graters A., Cacciari E., Balsamo A., Piazzi S., Suwa S., Kuroda Y., Wada Y., Naruse H., Kizaki T., Ichihara N., Arai O., Harada S., Fujieda K., Matsuura N., Suwa S., Kusuda S., Fukushi M., Mizushima Y., Kikuti Y., Yoyoura T., Saisho S., Shimozawa K., Matsumoto M., Webster D., Vilarinho L., Wallace A.M., Eguileor I., Marzana I., Dulin Iñiguez E., Fernandez Sanchez A., Gonzalez Gallego C., Hagenfeldt L., Guthenberg C., von Dobeln U., Thilen A., Larsson A., Torresani T., LeBlond C., Papadea C., Rumph F., Craft W., Kling S., Tsalikian E., Cook J., Getchell J., Susanin J., Mitchell M., Hofman L., Naylor E., Therrell B., Brown L., Prentice L., Glass M., Neier S. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: Newborn screening and its relationship to the diagnosis and treatment of the disorder. Screening. 1993;2:105–139. [Google Scholar]