Abstract
In the modern world, a number of therapeutic proteins such as vaccines, antigens, and hormones are being developed utilizing different sophisticated biotechnological techniques like recombinant DNA technology and protein purification. However, the major glitches in the optimal utilization of therapeutic proteins and peptides by the oral route are their extensive hepatic first-pass metabolism, degradation in the gastrointestinal tract (presence of enzymes and pH-dependent factors), large molecular size and poor permeation. These problems can be overcome by adopting techniques such as chemical transformation of protein structures, enzyme inhibitors, mucoadhesive polymers and permeation enhancers. Being invasive, parenteral route is inconvenient for the administration of protein and peptides, several research endeavors have been undertaken to formulate a better delivery system for proteins and peptides with major emphasis on non-invasive routes such as oral, transdermal, vaginal, rectal, pulmonary and intrauterine. This review article emphasizes on the recent advancements made in the delivery of protein and peptides by a non-invasive (peroral) route into the body.
Keywords: Proteins, Peptides, Insulin, Permeability, Enzyme inhibitor, Peroral
1. Introduction
Proteins and peptides are the building blocks of life and are now evolving as a very promising brand of therapeutic entities. Once a rarely used subset of medical treatments, therapeutic proteins have increased dramatically in number and frequency of use since the introduction of first recombinant protein therapeutic viz. human insulin, 25 years ago. Therapeutic proteins and peptides hold a significant role in almost every field of medicine, but this role is still only in its infancy. The foundation for the popularity of protein therapeutics was laid down with the regulatory approval of recombinant insulin by the US Food and Drug Administration (FDA) in 1982, which became the first commercially-available recombinant protein and a source of major therapy for patients suffering from diabetes mellitus (Leader et al., 2008). Three decades have passed since the inauguration of approval of first recombinant protein i.e. insulin by the FDA, and its clinical success has inspired the field of therapeutic proteins into a wider horizon ever since, with more than 130 different proteins or peptides already approved for clinical use by the FDA till 2008 alone, and many more in the development pipeline.
A better understanding of molecular biology and biochemistry behind the macromolecular endogenous proteins, peptides and peptidergic molecules, and their role in various body functions and pathological conditions has led to the realization of the enormous therapeutic potential of proteins and peptides in the last few decades. Consequently, a variety of new therapeutic proteins have been developed showing therapeutic benefits in the treatment of ailments like diabetes, cancer which offer several advantages over the conventional small-molecule drugs. Firstly, proteins often serve a highly specific and complex set of functions in the body that cannot be mimicked by simple chemical compounds. Secondly, since the action of proteins is highly specific, there is often less potential for therapeutic protein to interfere with normal biological processes and cause adverse effects. Thirdly, because the body naturally produces many of the proteins that are used for therapeutic purpose, these agents are often well-tolerated and are less likely to elicit immune responses. Fourthly, for diseases in which a gene is mutated or deleted, protein therapeutics can provide an effective replacement for the treatment without the need for gene therapy, which is not currently available for most genetic disorders. Fifthly, the clinical development and FDA approval time of protein therapeutics may be faster than that of small-molecule drugs. A study published in 2003 showed that the average clinical development and approval time was more than one year faster for 33 protein therapeutics approved between 1980 and 2002 than for 294 small-molecule drugs approved during the same time period. Lastly, because proteins are unique in form and function, companies are able to obtain far-reaching patent protection for protein therapeutics. The last two advantages make proteins an attractive alternative from a financial perspective compared with small-molecule drugs (Leader et al., 2008).
As a result of intensive research efforts in both academic and industrial laboratories, recombinant DNA, protein and peptide engineering and tissue culture techniques can now be used to obtain proteins and peptides for therapeutic use on a commercial scale which resemble an endogenous molecule and thus provoke fewer or minimal immunological responses. Though the initial problems related to obtaining non-immunogenic protein therapeutics in purer form at commercial scales have been overcome to quite some extent, their formulation and optimum delivery still remains the biggest challenge to pharmaceutical scientists. There are now many examples (Octreolin®, Sandimmune®, AI-401, HDV-I, Capsulin™, Oraldel™, IN-105, Oral-Lyn™, CLEC®, ORMD-0801, Eligen® etc.) in which proteins have been used successfully for therapeutic purposes (mentioned in detail later in this review under clinical applications). Nonetheless, potential protein therapies that have failed so far outnumber the successes, in part owing to a number of challenges that are faced in the development and use of protein therapeutics.
Route of administration is a critical factor in any therapeutic intervention which governs both the pharmacokinetics and efficacy of the drug. For protein and peptide therapeutics, an interplay of poor permeability characteristics, luminal, brush border, and cytosolic metabolism, and hepatic clearance mechanisms result in their poor bioavailability from oral and non-oral mucosal routes. Hence, at present these drugs are usually administered by parenteral route. However, inherent short half-lives of penetrating peptides (PP) and almost warranted chronic therapy requirements in a majority of cases make their repetitive dosing a necessity. Frequent injections, oscillating blood drug concentrations and low patient acceptability make even the simple parenteral administration of these drugs problematic. This has prompted researchers to develop new delivery systems capable of delivering such a class of drugs in a more effective manner. Although there have been reports of successful delivery of various PP therapeutics across non-peroral mucosal routes, peroral route continues to be the most intensively investigated route for PP administration. This interest in the peroral route, despite enormous barriers to drug delivery that exist in the gastrointestinal tract (GIT), can be very well appreciated from obvious advantages such as ease of administration, large patient acceptability, etc. Potential cost savings to the health care industry further augment the advantages of peroral systems in terms of patient compliance and acceptability, since peroral formulations do not require sophisticated sterile manufacturing facilities or the direct involvement of health care professionals.
There is a need to design an approach which not only protects the protein/peptide from enzymatic degradation but also aids in enhancing its absorption without altering its biological activity (Gupta et al., 2013). Although the oral delivery of proteins and peptides remains an attractive option, but to reach its true potential the challenges must be met. Oral delivery of proteins and peptides has long been hailed as the ‘Holy Grail’ of drug delivery by showing great potential but also presenting problems in their development (Shen, 2003).
The current article deals with the possibilities being explored in the oral delivery of protein and peptide therapeutics, the challenges in their development and the current and future prospects, with focus on technology trends in the market to improve the bioavailability of proteins and peptides and effect of different forms of therapeutic proteins by oral routes.
2. Peroral route: promises and pitfalls
Oral delivery is the most sought after route of administration for most of the drugs and pharmaceutical products, which depends on the drug’s molecular structure or weight (Elsayed et al., 2009). Bioavailability is dependent upon the molecular mass of drugs if molecular mass increases above 500–700 Da, bioavailability of drugs decreases sharply whereas bioavailability is essentially independent of molecular mass for drugs of less than 500–700 Da (Donovan et al., 1990). Proteins have important therapeutic roles, such as insulin which is a major therapeutic agent for the management of insulin-dependent diabetes mellitus (Type 1) and for many patients with non-insulin dependent diabetes mellitus (Type 2) (El-Sayed et al., 2007, Khan, 2003). Intestinal mucosa is considered as a very complex structure. On the basis of adhesion in gastrointestinal tract, there are two main targeting areas, i.e. mucosal tissue and mucus gel layer. It may be due to adhesive interaction with mucoadhesive polymers either through non-specific (Van der waal and hydrophobic interaction) or specific interaction between complementary structures. On the other hand, regular renewal of mucosal surface by a turnover process restricts muco-adhesive drug delivery system (Ponchel and Irache, 1998). Currently, pharmaceutical strategies aim to increase the bioavailability, overcome the enzymatic degradation, enhance the permeability and develop safe, efficacious and highly-potent proteinous drugs (Hamman et al., 2005, Shah et al., 2002) Proteins have been transported (actively) through the epithelial lining of the small intestine in membrane-bound vesicles after binding to the cell-surface receptor. Very few portions are released at the baso-lateral membranes and then secreted in the intact form in the intestinal space. (Strous and Dekker, 1992). Drug absorption depends upon the age, diet and disease state (Morishita and Peppas, 2006). Mucus covers the epithelial cell surface, hence hampering the diffusion of peptide drugs. The goblet cells continuously secrete highly viscous gel whose viscosity enhances strongly towards the cell surface (Camenisch et al., 1998). Protein and peptides most commonly follow the paracellular route as compared to transport through the lipophilic cell membrane. Metabolic barriers consist of brush border peptidases and luminal proteases such as trypsin, α-chymotrypsin, elastase and carboxypeptidase. These enzymes easily degrade the therapeutic proteins and peptides administered through oral routes. Recently, there are only two oral proteins and peptides, e.g. Interferon-α and Human growth hormone (HGH) in clinical developmental stage (Orive et al., 2004) FDA has approved three drugs which augment glucagon like peptide-1 (GLP-1) production, on the basis of incretin based therapy for potential treatment in Type 2 Diabetes mellitus (Peters, 2010). It was reported that the intestinal uptake of therapeutic protein through biodegradable nanoparticles was enhanced by particle size reduction (enhanced dissolution) (Bakhru et al., 2013).
Insulin releases from pancreatic β-cells into the hepatic portal vein and then into the liver which is the primary site of action, whereas, parental route and other delivery systems (buccal, pulmonary, and nasal) deliver the drug directly into the systemic circulation. In this delivery system, the drug reaches the systemic circulation bypassing the first-pass metabolism, but in case of oral delivery, insulin first reaches the liver (20% of drug dose is available in liver) and then to the peripheral tissue. Oral route of administration is closer to the natural physiological route of insulin (Rekha and Sharma, 2013).
2.1. Transport mechanism of macromolecules
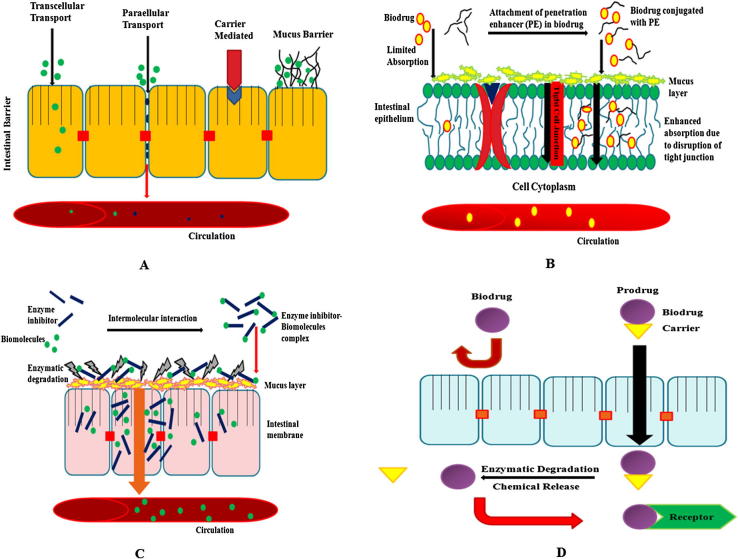
Large numbers of mechanisms are responsible for penetration such as simple diffusion (paracellular and transcellular), carrier-mediated transport, active transport and pinocytosis or endocytosis (Salamat-Miller et al., 2005). Proteins and peptides have very low log P (<0) value. Those drugs have lack of lipophilicity, no passive absorption can take place and are absorbed through paracellular pathways (restricted to small molecules, less than 100–200 Da) (Camenisch et al., 1998). The paracellular space lies between 10 and 30–50Å, therefore the paracellular route is not feasible for large macromolecules. But in the case of insulin, it is adsorbed on the apical membrane and is internalized by specific types of endocytosis processes (Agarwal and Khan, 2001). Few numbers of protein and peptides show practically active transport by binding to the cell surface receptor or binding sites in the epithelial lining of the small intestine (membrane bound vesicles) (Bastian et al., 1999). The most commonly used transport mechanism is passive diffusion with two ways of transport: first, paracellular (transport of drug through the intercellular space between the cells) and second, transcellular (involves passage into or across the cells), and is shown in Figure 1A. Transportation of drugs depends on overall molecular geometry, lipophilicity and charge of the transport pathway across the oral mucosa (Brayden and Mrsny, 2011). A minimum level of lipophilicity is essential in drugs to partition into the epithelial membrane and absorbed through transcellular passive diffusion (Camenisch et al., 1998). Transport of therapeutic molecules from the gastrointestinal tract into the systemic circulation is through the mucosal layer then through the areolar layer. Other two intestinal layers (areolar or submucosal) connect together the mucus and muscular layers (Blanchette et al., 2004). Muscular and mucus layers are the strongest layers of the intestine which consists of the loose, filamentous areolar tissue containing lymphatics, nerves and blood vessels (Rekha and Sharma, 2013).
Figure 1.
(A) Transport mechanism of biodrug through the intestinal epithelium membrane, (B) Probable mechanism of penetration enhancer, and (C) enzyme inhibitors, (D) Representative mechanism of prodrug absorption and its activation.
Membrane perturbing in order to increase transcellular permeation, was shown on human Caco-2 epithelial cell monolayers when exposed at maximum concentration and demonstrated tolerance in vitro, but the best way is to attach any ligand on molecules that opens the tight junctions (Brayden and Mrsny, 2011, Aungst, 2000).
2.2. Challenges associated with oral protein delivery
The unfriendly physiochemical properties of proteins and peptides have created great challenges for the formulation scientists, and have therefore resulted in a need to develop other routes of administration, such as oral, nasal, buccal, pulmonary, transdermal, rectal and ocular (Park et al., 2011). Use of proteins and peptides as therapeutic agents is limited due to lack of an effective route and method of delivery. Various critical issues associated with therapeutic protein and peptide delivery, that have drawn the attention of formulation scientists include the following:
-
(i)
Proteins and peptides are high molecular weight biopolymers which serve various functions, such as enzymes, structural elements, hormones or immunoglobulins, and are involved in several biological activities. However, large molecular weight, size and presence of both hydrophilic and hydrophobic appendages in their structure, render proteins difficult to enter into cells and other body compartments, and thus impart poor permeability characteristics through various mucosal surfaces and biological membranes. Commonly, therapeutic proteins and peptides are hydrophilic with a log P < 0 (Camenisch et al., 1998).
-
(ii)
Many therapeutic proteins and peptides are efficacious in large part because of their tertiary structure, which can be lost under various physical and chemical environments, resulting in their denaturation or degradation with a consequent loss of biological activity, thereby making these molecules inherently unstable.
-
(iii)
Many proteins and peptides have very short biological half-lives in vivo due to their rapid clearance in liver and other body tissues by proteolytic enzymes, protein-modifying chemicals or through other clearance mechanisms.
-
(iv)
Protein and peptide degradation is highest in the stomach and duodenum and is significantly decreased in the ileum and colon. Various delivery systems have been developed to target absorption from the colon and ileum as a result, and minimize exposure of drug to proteolytic enzymes. Thick enteric coating formulation has been used to target both the ileum and colon due to delay in the release of drug for a sufficient period of time. However there is an additional drawback such as potential changes in colon microflora, delay drug absorption and risk of absorption, along with drugs with endotoxins and other potentially harmful compounds residing in this intestinal region (Rubinstein, 1995, Van den and Kinget, 1995).
-
(v)
As proteins and peptides deliver specific actions and are highly potent, a precise clinical dosing is of utmost importance.
-
(vi)
The body may mount an immune response against the therapeutic protein and peptide. In some cases, this immune response may neutralize the protein and even cause a harmful reaction in the recipient. Recombinant technology and other advances have allowed the development of various antibody products that are less likely to provoke an immune response than unmodified murine antibodies, because in humanized antibodies, portions of the antibody that are not critical for antigen-binding specificity are replaced with human Ig sequences that confer stability and biological activity on the protein, but do not provoke an anti-antibody response. Exclusive human antibodies can be produced using transgenic animals or phage display technologies.
-
(vii)
For a protein to be physiologically active there is a need for some post-translational modifications, such as glycosylation, phosphorylation and proteolytic cleavage. These requirements may dictate the use of specific cell types that are capable of expressing and modifying the proteins appropriately. Thus, recombinant proteins can be synthesized in a genetically-engineered cell type for large-scale production.
-
(viii)
The costs involved in developing therapeutic proteins and peptides are high due to the expensive intermediate technologies involved in their designing (Leader et al., 2008, Mahato et al., 2003).
Penetration of drug through oral mucosa into systemic circulation is a major hindrance in their absorption. A hydrophilic large molecular weight drug such as protein and peptides are easily degraded by oral route, as a result they are not or very less available in the systemic circulation (Mahato et al., 2003, Antunes et al., 2013). Aoki et al. (2005) demonstrated through his in vitro studies that the mucus layer plays a critical role in the absorption of insulin across the small intestinal. In these studies mucus layers are removed from the intestinal segments using hyaluronidase without affecting the integrity of the epithelial part of the intestine. The transportation of therapeutic protein through hyaluronidase-treated small intestine was found to be significantly higher in comparison to the control group treated with phosphate buffered saline, PBS (Aoki et al., 2005).
3. Formulation approaches for oral delivery of proteins and peptides
The two important approaches for formulation of protein and peptides by oral route include: use of absorption enhancers and enzymatic inhibitor. Being charged, large in size and hydrophilic, proteins and peptides are notoriously poor permeators (and thus exhibit poor oral bioavailability per se). The former approach offers an opportunity to counter balance this permeation problem of therapeutic proteins. The latter approach is an answer to the instability exhibited by proteins on account of a plethora of proteolytic enzymes present in the GIT which have inherent dietary protein-digesting functions. Various strategies for the development of oral protein and peptides are given below.
3.1. Enzyme inhibitors (protease inhibitors)
Macromolecules, such as proteins and carbohydrates, are broken down in the digestive system into simpler molecules, viz. amino acids and sugars, respectively, which are easily absorbed because intact protein absorption is typically minimal (<1%) (Iyer et al., 2010). Various types of enzymes (endopeptidases and exopeptidases) are responsible for the cleavage of amino acid chains, (e.g. trypsin, chymotrypsin, elastase, pepsin and carboxypeptidases etc.). Each type of enzyme is specific for the cleavage of particular links of amino acids and different targeted inhibitors (Lueben et al., 1996, Bernkop-Schnurch et al., 1997, Gamboa and Leong, 2013). First approach is the use of enzyme inhibitors such as aprotinin and soybean trypsin inhibitors, camostat mesilate and chromostatin, but administration of such types of protease inhibitors for long duration results in the deficiency of these enzymes in humans (Figure 1C) (Yamamoto et al., 1994, Tozaki et al., 1997). A novel class of enzyme inhibitor, chicken and duck ovomucoids has been recently reported, and a formulation has been developed wherein the insulin and duck ovomucoids offered 100% protection against the action of trypsin and α-chymotrypsin (Agarwal and Khan, 2001). In another case study, polymer inhibitor conjugates such as carboxymethyl cellulose-Elastinal (CMC-Ela) have shown in vitro protection against enzymes trypsin, α-chymotrypsin and Elastase. After 4 h of incubation, nearly 33% of the therapeutic protein was found to be active against the elastase (Park et al., 2011, Marschutz and Bernkop-Schnurch, 2000).
Serpin (Serine protease inhibitor) forms covalent complexes with the target protease and in such a way, the protein is protected from the protease enzymes. On the basis of structural studies, it has been demonstrated that inhibitory members of the group undergo conformational changes, known as stressed and relaxed transition, and conformational change which is the critical step in the mechanism of inhibition of a targeted protease (Egelund et al., 1998).
3.2. Absorption enhancers (permeation enhancers)
Penetration enhancers (PEs) directly transport protein molecules through the epithelium without major effects on their solubility (Brayden and Mrsny, 2011). PEs are commonly classified as either tight junction (TJ) selective, in order to increase paracellular permeability through slight modification of TJ functional properties or in order to increase transcellular permeation (membrane perturbing). These mechanisms ascertained using human Caco-2 epithelial cell monolayer at the maximum concentration in which the systems can tolerate in vitro conditions. In early 1990s, there was some consensus that the smarter strategies for poorly permeable drugs were to opt for specific agents, that opened the tight junction of the epithelial cell membrane, but the latter strategies suggested that membrane perturbation was considered potentially toxic (Maher et al., 2009). Enhancers have been studied for oral insulin delivery, such as fatty acids and bile salts, which enhance the permeability across the mucosal walls (Obata et al., 2000). They open up the tight junctions reversibly and improve the permeability of insulin and several other proteins (Figure 1B). A novel absorption enhancer, viz. Zonula occludens toxin (ZOT) (Salama et al., 2006), chitosan (Prego et al., 2005), thiolated polymers (Bernkop-Schnurch, 2005) and Pz-peptide have all been studied as penetration enhancers for oral insulin delivery, and have resulted in effective reduction of glucose levels in the body (Fasano and Uzzau, 1997). Sachdeva et al. (1997) reported that proteases (pancreatic enzymes) are less active against small peptides, such as cyclosporine and vasopressin analogues (Sachdeva et al., 1997). Leone-bay et al. (2001) described a new class of molecules that alter the conformation of proteins reversibly and provide facility for their transport across mucosa (Leone-Bay et al., 2001). The most common drawback of penetration enhancers in the case of long-term usage is that they may damage or even dissolve the biomembrane, leading to local inflammation (Iyer et al., 2010).
Surfactants also enhance the transcellular transport by disrupting the lipid bilayer and make it more permeable to drugs (Lecluyse and Sutton, 1997), a mechanism very similar to that of chelating agents which form a complex with calcium ions and rupture the tight junctions and facilitate the transport of proteins (Aungst, 2000, Park et al., 2011). When proteins and peptides are given with lipophilic carriers, they enhance their absorption (Sood and Panchagnula, 2001) such as insulin, human growth hormone (HGH), calcitonin and recombinant parathyroid hormone (Lee et al., 2005, Kidron et al., 2004). The carrier alters the lipid solubility and then makes access to pore of the integral membrane (Leone-Bay et al., 2001). Merrion Pharmaceuticals (Dublin, Ireland) produced a novel formulation of alendronate with paracellular penetration enhancer known as Almerol™ formally known as MER-103. Almerol™ was found to have better bioavailability and fewer side effects as compared to alendronate for the treatment of osteoporosis (Walsh et al., 2011, Frost, 2008).
3.3. Mucoadhesive polymeric systems
They have a changing swelling behavior in response to the environmental factors, such as ionic strength, electric field, light, temperature and pH (Park et al., 2011). The most common approach for the encapsulation of oral insulin is using mucoadhesive polymers, such as chitosan (Mathiowitz et al., 1997), poly [lactic-co-glycolic acid] (PLGA) (Damge et al., 1988), thiolated polymer and alginate, which have been studied extensively (Takka and Acarturk, 1999). Chitosan is a natural non-toxic, biocompatible and biodegradable polymer (Hejazi and Amiji, 2003). When a peptide (transforming growth factor [TGF-β]) was delivered with chitosan, as a result, a 6–7-fold enhancement of permeability of TGF-β with chitosan was attained. This resulted in the healing of the oral mucosa by arresting epithelial cell division and thus destruction of the cells from the effects of anticancer therapy (Senel et al., 2000). Mucoadhesive polymer adheres to the mucus and increases the drug concentration gradient. When insulin was encapsulated with Poly (methacrylic acid-g-ethylene glycol)[P(MAA-g-EG)], [P(MAA-g-EG)] being a pH sensitive mucoadhesive polymer, showed pH-dependent swelling behavior, as a result of formation or dissociation of inter-polymer complex [MAA-g-EG] polymer and it showed ∼10% bioavailability of orally-administered insulin encapsulated with pH sensitive mucoadhesive polymer as compared to insulin (Lowman and Peppas, 1997, Peppas and Klier, 1991). Thiolated polymers (thiols side chains) have strong mucoadhesive properties due to covalent bonding with cysteine-rich subdomains of the mucus glycoprotein (Leitner et al., 2003). Alone, protein encapsulated in polymer did not show efficient absorption as compared to polymer with enzyme inhibitor or protease inhibitor. Encapsulation leads to successful protection of the protein formulations from enzymatic degradation and also gets successful results. Currently, only two peptide- and protein-based drugs (Interferon-α and human growth hormone (hGH)) that can be given orally are known to be in clinical development (Renukuntla et al., 2013).
3.4. Novel carrier systems
A large number of carriers for proteins and peptides delivery, such as emulsions, nanoparticles, microspheres and liposomes, have been used to protect the protein formulation against the harsh environment of the GI tract (acidic medium and enzymes). Emulsion developed by using lipophilic surfactant-coated insulin decreased its degradation and increased its permeation. The critical drawback of emulsions is its physiochemical stability (Toorisaka et al., 2003). Stability problem of emulsions may be overcome by dry emulsion formulations, which are prepared by spray drying, lyophilisation or evaporation (Dollo et al., 2003). Liposomes have also been exploited to improve the bioavailability of proteins from the intestinal tract (Park et al., 2011). Liposomal system containing insulin and sodium taurocholate markedly reduced the blood glucose levels after oral administration and showed a high in vitro/in vivo correlation in the Caco-2 cell model (Degim et al., 2004). Langer and his colleagues developed polymerized liposomes with covalent double bonds to improve the stability of biomolecules against the harsh environments (Langer, 1998).
Carrier nanoparticles consisting of lipophilic polystyrene, mucoadhesive chitosan and PLA-PEG were detected in both epithelial and Peyer’s patches after inter-duodenal administration of drug molecules (Sakuma et al., 2001). Peyer’s patches are the follicles of lymphoid tissue which contain M-cells. M-cells have an important role in particle uptake. Particle size and surface charge are important factors related to the uptake of particulates by M-cells (Shakweh et al., 2005, Brayden et al., 2005). Polymeric nanoparticles can be used to easily entrap and encapsulate therapeutic proteins and peptides and lead to the targeted area. It can be smoothly functionalized for off opsonisation, and therefore has shown reduced toxicity towards the non-target areas (peripheral tissues) (Chan et al., 2010). Kafka et al. (2011) investigated the in vitro and in vivo studies of gonadotropin releasing hormone-loaded nanoparticles. Different in vitro conditions (artificial gastric juice, simulated intestinal fluid and brushtail possum plasma) were studied, and it was found that less than 5% of the hormone was released over 6 h in artificial gastric juice and simulated intestinal fluid, and 60% of it was released in brushtail possum tail plasma over 1 h. In vivo study showed that sufficient therapeutic levels of these proteins were achieved from drug-loaded nanoparticles in the systemic circulation.
It was investigated that mucoadhesive nanoparticles increased the residence time of the drug moiety because it allows the attachment of drug molecules into the mucous membrane of GIT. The concepts behind these nanocarriers can reduce clearance through the alimentary canal and lead to increased bioavailability of therapeutic protein (Carvalho et al., 2010). Makhlof et al. (2010) revealed the permeation-enhancing properties of the mucoadhesive nanoparticles. Fluorescein isothiocyanate dextran (FITC dextran) -loaded polyelectrolyte complexes were prepared by interaction of spermine, polyacrylic acid and FITC dextran. Confocal microscopy has been investigated for prolonged penetration using fluorescein isothiocyanate dextran for in vitro and in vivo conditions. It was concluded that the drug loaded mucoadhesive nanoparticles showed prolonged penetration (5–5.56-fold) as compared to free FITC dextran through confocal microscopy.
3.5. Derivatization or chemical modification of proteins and peptides
Another approach is the derivatization of proteins and peptides by using polyethylene glycol in order to protect the protein from enzymatic degradation and also to improve the solubility (Clement et al., 2002). Lipidization, which is the covalent interaction of hydrophobic moiety or non-covalent conjugation with the hydrophobic moiety, results in the increase in the hydrophobicity of proteins and peptides (Goldberg and Gomez-Orellana, 2003). This approach has been used in the clinic and has provided multiple drug candidates. Some others are the formation of an inclusion complex with leucine encephalin, protection of the peptides against enzymatic degradation and also enhancement of absorption (Basu et al., 2006). Chemical modification can be done by exploiting the carbohydrate moiety (glycoproteins) attached to protein or side chain of protein (Calceti et al., 2004). The deamination of first amino acid and substitution of last l-Arginine with d-Arginine along with simultaneous substitution of fourth amino acid with valine forms 1-Deamino-8-D-ArginineVasopressin (DDAVP). Such derivative forms of vasopressin are two-times more potent than simple vasopressin (Shaji and Patole, 2008). Transport of proteins and peptides have been studied with and without absorption enhancers (Morishita and Peppas, 2006) through buccal epithelia, for example, TRH (Thyrotropin-releasing hormone) and the LHRH (luteinizing hormone-releasing hormone) analogue buserelin, a lauroyl tripeptide, the vasopressin fragment DGAVP, and insulin resulting in increased bioavailability of protein molecules (Jana et al., 2010).
3.6. Prodrug strategies
The prodrug is actually an active pharmacological moiety which has been converted into inactive form through chemical modification, and when administrated changes into the active form by enzymatic or non-enzymatic reactions (Figure 1D). It is complete bioreversible cyclization (Jana et al., 2010). These approaches enhance the solubility, permeability and targeting of small molecules but it faces challenges, such as limitation in methodology, stability of proteins and structural complexity (Hsieh et al., 2009).
Drug + Carrier = “Prodrug” which after enzymatic degradation gives free drug and carrier
A recent approach has enhanced the hydrophobicity and targeting through a lipid raft which has been conjugated with the protein moiety, as well as attached a specific transporter in the parent drug (Renukuntla et al., 2013). Prodrug approach may help in the absorption of various biomolecules such as RNA, DNA, oligonucleotides and proteins (enzymes, proteinous drugs and hormones) (Vadlapudi et al., 2012). (Lue5)-enkephaline was chemically-modified by phenyl propionic acid into a prodrug, which was found to improve their permeability across the Caco-2 1680-fold than the parent moiety (Cronauer et al., 2003).
3.7. Novel approaches
Novel vesicular delivery systems containing bile salts are known as “bilosome”, which act as penetration enhancers and improve bioavailability (Sizer, 1997). Sadeghi et al. (2009) developed a gas-empowered delivery system for carbon dioxide-forced transport of the protein to the surface of the small intestine. Insulin, together with a mucoadhesive polymer, trimethyl chitosan (a permeation enhancer) and polyethylene oxide, was delivered with carbon dioxide gas to the surface of the small intestine. This model enhanced the bioavailability of insulin up to seven-fold (Sadeghi et al., 2009).
A novel conjugation of iron and polysaccharide multi-layered microcapsules was developed for the continuous release of insulin (known as controlled delivery system). Multi-layered insulin-loaded microcapsules were prepared through layer-by-layer deposition of dextran sulfate and oppositely-charged Fe+3(ferric ion) onto the surface of insulin microcapsules. In this model, two oppositely-charged substances (dextran acts as negatively-charged moiety and ferric ions act as the positive moiety) adhere on the insulin and result in the formulation of multi-layered insulin microcapsules (Zheng et al., 2009).
3.8. Novel functionality to macromolecules
3.8.1. Endogenous cell carrier systems
The endogenous carrier mechanisms are receptor-mediated endocytosis and membrane transporters. In some cases, when a drug is conjugated to a dipeptide, it gets detected by a peptide influx transporter, which in turn enhances its oral absorption (Morishita and Peppas, 2006). Efflux transport systems such as P-glycoproteins lead to inefficient bioavailability of proteins and peptides, and therefore, certain P-gp inhibitors are used with proteins and peptides to increase the bioavailability (Varma et al., 2003). The membrane transport is possible for small drug molecules; whereas receptor-mediated endocytic system does not have any limitation regarding the size of the drugs (Morishita and Peppas, 2006). Receptor-detectable ligands, such as vitamin B12, transferrin, invasins, viral haemoaggulitinin, toxin and lectin, can be bound to the protein molecules to enhance the intercellular delivery to target cells (Russell-Jones, 2004, Lim and Shen, 2005). In cases of oral delivery system of proteins and peptides such as insulin and granulocyte colony-stimulating factors (G-CSF), they are conjugated with transferrin carrier to improve the bioavailability (Bai et al., 2005). There is a broad scope of use of recombinant fusion protein technology, and it may be useful for the future development of oral and buccal delivery systems for proteins and peptides (Table 1 and Table 2).
Table 1.
Various approaches for oral delivery of therapeutic proteins.
| Approach | Examples | Effects on bioavailability | Drawbacks | References |
|---|---|---|---|---|
| Absorption enhancers | Bile salts, fatty acids, Surfactants (anionic, cationic, and nonanionic) chelators, Zonular OT, esters, cyclodextrin, dextran sulfate, azone, crown ethers, EDTA, sucrose esters, and phosphotidyl choline | Enhanced bioavailability by increased membrane permeation | Available transport systems of both proteins/peptides and undesirable molecules in GIT | Brayden and Mrsny (2011) |
| Enzyme inhibitors (protection against enzymes) | Sodium glycocholate, camostate mesilate, bacitracin soyabean, trypsin inhibitor, CROVM, DKOVM, polymer inhibitor conjugates, carbomers, polycarbophil, bestatin, aprotinin, and streptozocin | Resisted enzymes degradation in stomach and intestines | Produced severe side effects in the treatment of chronic diseases such as diabetes, etc. | Park et al., 2011, Iyer et al., 2010 |
| Mucoadhesive polymers | P(MAA-g-EG) hydrogel microparticles, lectin–conjugated alginate microparticles, thiolated polymer, natural oligosaccharides gum, drum dried waxy maize starch, carbopol 974P, chitosan derivatives, sea curve 240, scleroglucan, HE-starch, hydroxyl propyl cellulose, celloulose derivatives, pectin, xanthan gum, polycarbophil, amino dextran, DEAE-dextran | Site–specific delivery and improved membrane permeation | Limitation due to the mucus turnover in absorption sites (intestine) | Senel et al. (2000) |
| Formulation vehicles | -Emulsion
|
Protection against acids and enzymes | Physiochemical instability in case of long term storage | Park et al., 2011, Toorisaka et al., 2003 |
-Liposomes
|
Improve physical stability | Low loading efficiency of hydrophobic drugs | ||
-Microsphere
|
Restrict release of protein to favorable area of GIT | Difficulty of precise control-Avoidance of particle aggregation | ||
-Nanoparticle
|
Increase membrane permeation Increase intestinal epithelial absorption |
|||
| Derivatization of proteins | Polyethylene glycol | Protected against enzymatic degradation as well as enhanced the solubility | Non-specific pegylation | Clement et al. (2002) |
| Endogenous cell carrier system | Vitamin B12, transferrin, invasins, viral haemoaggulitinin, toxin, and lectin | To enhance the intercellular delivery system to target cells, enhanced oral absorption | Limited to transporting of small drugs. | Bai et al., 2005, Morishita and Peppas, 2006 |
| Cell penetrating peptides | Proteins were enabled to be delivered into cells or tissues by hybridizing with target molecules | Enhanced bioavailability and targeting of proteins | Toxic effect | Morishita and Peppas (2006) |
| Prodrug approach | Phenyl propionic acid | Prodrug permeability improved 1608fold than parent drug | Lack of methodology, structural complexity, stability problem of protein | Renukuntla et al., 2013, Hsieh et al., 2009 |
Abbreviations: CROVM, Chicken ovomucoid; DEAE, Diethylaminoethyl cellulose; DKOVM, Duck ovomucoid; EDTA, Ethylenediaminetetraacetic acid; PLGA-PEG, Poly(lactic-co-glycolic) acid-Polyethylene glycol; PMAA, Poly(methyl methacrylate); P(MAA-g-EG), Poly(methacrylic acid-g-ethylene glycol; S/O/W, Solid-in-oil-in-water.
Table 2.
Different nanocarrier systems and models for oral delivery of proteins.
| Proteins | Carrier system | Models | Reference |
|---|---|---|---|
| Insulin | Nano-cubicles | STZ-induced diabetic Rat | Chung et al. (2002) |
| Insulin, calcitonin, HGF (Human granulocyte colony stimulating factors) | Nanocapsules | – | Oppenheim et al. (1982) |
| Salmon calcitonin | PLGA-nanoparticle | Rat in vivo | Sang and Park (2004) |
| Insulin | Acrylic-based co-polymer nanoparticles | STZ-induced diabetes in rat | Foss et al. (2004) |
| Cyclosporine | Lipid microemulsions | Rat in vivo | Constantinides (1995) |
| Leucine encephalin | Sugar coupling with cellobiose and gentiobiose | _ | Mizuma et al. (1996) |
| Insulin | Chitosan nanoparticles | Alloxan–induced diabetic rat | Pan et al. (2002) |
| HIV Protease (CGP57813) | pH sensitive nanoparticles | Rat in vivo | Leroux et al. (1996) |
| DGAVP | Niosomes | – | Yoshida et al., 1992 |
Abbreviations: DGAVP, desglycinamide-(Arg8)-vasopressin; HIV Protease (CGP 57813), is a peptidomimetic inhibitor of human immunodeficiency virus type 1 (HIV-1) protease; STZ, streptozocin.
3.8.2. Cell-penetrating peptides (CPPs)
Cell-penetrating peptides (CPPs), also known as protein transduction domains (PTD), are made up of 3–30 protein residues (Munyendo et al., 2012). CPPs consist of two groups, one is HIV-1 Tat peptide (cationic peptide) and artificial oligoarginine, and the other group is penetratin derived from Drosophila antennapedia homeoprotein (amhiphilic peptides) (Nakase et al., 2008, Derossi et al., 1996). They are employed to enhance the internalization of various biomolecules such as DNA, RNA, oligonucleotides, proteins and peptides (De Coupade et al., 2005). A group of small peptides such as TAT, oligoarginine and penetratin have been used to internalize different protein and peptide formulations into cells. The peptide enabled the delivery of the macromolecules, microparticles, liposomes and nanoparticles into cells or tissues by hybridizing with the target molecules. With regard to the harmful effects of the peptides, TAT has been shown to cause practically no toxic effects to membranes and in most of the in vivo applications, no undesirable effect has been detected (Zorko and Langel, 2005). It has been identified that penetration occurred in the cell membrane and they can cause a small disturbance in the membrane leading to enhanced absorption of proteins and peptides through the oral route. Peptide strategy is based on a non-specific delivery system, whereas it is proposed for the enhanced bioavailability and targeting of proteins and peptides through the oral route (Morishita and Peppas, 2006). Enhancement of safety and efficacy, and reduction in toxic effects are mandatory for the development of this delivery system for proteins and peptides. By co-administering the typical CPP with the insulin, enhanced intestinal bioavailability of insulin up to 30% was observed (Noriyasu et al., 2013).
4. Clinical application of oral proteins and peptides
Oral delivery systems for proteins and peptides are still in development stages. Oral delivery, being non-invasive, is the most favored route of drug administration. This is illustrated by the fact that oral delivery represents approximately US$ 25 billion worldwide (Werle et al., 2007). Various techniques for proteins and peptides delivery used by industries, are highlighted in this section (Table 3).
Table 3.
Technologies for oral delivery of proteins under clinical development by companies.
| Company | Product name | Technology | Formulation | Development phase | Product | References |
|---|---|---|---|---|---|---|
| Apollo Life Science | Oraldel™ | Nanoparticles | Tablet | Clinical phase I b | Insulin, TNF-blocker | http://apollolifesciences.com |
| Emisphere | Eligen | Penetration enhancers-Salcaprozate sodium | Tablet | Phase II | Calcitonin, insulin, PTH, heparin, calcitonin, enzymes (lipases, esterases, proteases) | http://emisphere.com |
| Nobex/Biocon | HIM2 | Pegylation + PE | Liquid | Abandoned | Insulin, enkephalin, calcitonin, PTH | Wajberg et al., 2004 |
| Oramed | ORMD-0801 ORMD-0901 |
Salts of EDTA (enteric coated + PE) | Capsule | Phase I | Insulin/Exenatide | Kidron et al., 2004 |
| Diasome pharmaceuticals | Hepatic-directed vesicles-insulin (HDV-1) | Liposomal insulin | Tablet | Phase II/III | Insulin | Schwartz et al., 2008 |
| Diabetology | Capsulin | PE | Capsule | Phase II | Insulin | Whitelaw et al., 2005 |
| Coremed | Intesulin | Nanoparticle encapsulation | Capsule | Preclinical | Insulin | Carino et al., 2000 |
| Merrion pharma (Ireland) with Novo-Nordisk (Denmark) | Vetsulin | PE (sodium caprate {C10}) | Matrix tablet | Phase I | Insulin and GLP-1 analogues | Walsh et al., 2011 |
| Chiasma (Israel) | Octreolin | PE (sodium caprylate{C8}) | Suspension | Phase I (phase I completed, phase III enrolling | Octreotide | http://chiasmapharma.com |
| Unigene/Tarsa (USA) | PeptelligenceTM | PE (Citric acid + acyl carnitine) | pH-dependent coated dosage form | 2011, Phase III completed | Salmon calcitonin | http://tarsatherapeutics.com |
| Altus | CLEC® | Protein crystallization | Tablet | Trial and error approach | Calcitonin and other polypeptides | Margolin (1996) |
| Generex | Oral–Lyn ™ | PE | Spray devices and aerosol particles | Phase IV | Insulin, Macrotonin | http://www.generex.com |
| Endorex | Orasome TM | Polymerized liposome | – | Phase II | Insulin, growth hormones, vaccines | Okada et al., 1998 |
| Provalis PLC | MacrulinTM | Lipid based microemulsion | Emulsion | Phase II | Insulin, Salmon calcitonin | Cilek et al., 2006 |
| Eli–lily | AI-401 | Enzyme inhibitor | Oral formulation | Phase II | Insulin | http://autoimmuneinc.com |
Abbreviations: EDTA, Ethylene diaminetetraacetic acid; PE, Penetration enhancers; PTH, Parathyroid hormone; TNF, Tumor necrosis factor.
4.1. Eligen®: Emisphere Technologies (USA)
This technology improves the transport of drugs through the intestinal epithelium when a small carrier, (N-(8-(2-hydroxybenzoyl) amino) caprylic acid), is attached non-covalently with biomolecules, but the complex formation does not affect the chemical properties of biomolecules and the interaction is reversible. The drug-carrier complex is able to cross the epithelial membrane and break the non-covalent bond between the drug and carrier, because it occurs spontaneously by simple diffusion on entering the blood circulation (Grosz et al., 2000, Wu and Robinson, 1999). These techniques play an important role in protection from digestive enzymes, as well as impart enhanced hydrophobic character to the macromolecules. Mostly, the molecular size is in the range from 500 to 1500 Da (Walsh et al., 2011). In pharmacokinetic studies it was found that Cmax for insulin was reached after ∼20 min from the time of administration, and the insulin level returned to the baseline within 80–120 min. Two most recently developed acylated entities are N-(8-(2-hydroxybenzoyl) amino) caprylic acid (SNAC) and N-(5-cholorosalicyloyl)-8-aminocaprylic acid (5-CNAC).SNAC was found to decrease transepithelial electrical resistance in Caco-2 monolayers, as well as improve the release of lactate dehydrogenase (LDH), suggesting that transcellular transport enhancement can also be a part of its mechanism (Hess et al., 2005). In vitro studies represented cytotoxicity in cell lines, but in animal models did not show pathological changes. An oral enteric-coated formulation for sCT (salmon calcitonin) has been found to possess higher efficacy than the nasal route of drug. In 2011, oral 5-CNAC/sCT failed in the phase III of clinical trials (Karsdal et al., 2011). If higher doses of insulin are given to volunteers then they showed a meaningful drop in HbA1c only after 3 months of studies. The high dose makes the therapy cost-effective and ensures the commercial viability of oral proteins and peptides in the marketplace. At present, no clinical efficacy of such a system has been represented till date (Emisphere Technologies, Inc., 2006).
4.2. ORMD-0801: Oramed Company (Jerusalem, Israel)
The technology came with enteric-coated oral capsules wherein the protein part is released in the intestine with the help of penetration enhancers (Craik et al., 2013). Effect of oral insulin was determined by studies in eight volunteers in the fasted condition and demonstrated reduced glucose levels (7–35%) and also a decline in the C-peptide level (13–87%) in all formulations. When the studies were conducted on fed volunteers, release of insulin was found to be adversely affected by meal and GIT motility. The onset and duration of action from time of administration was found to be 2 h and 5–6 h, respectively (Walsh et al., 2011).
4.3. CLEC®: Altus (USA)
Cross-linked enzyme crystal (CLEC) method mostly comprises of two steps including, first, batch crystallization of enzymes and second, crosslinking of enzyme microparticle (1–100 μm) with cross-linking agents, such as glutaraldehyde. These above two steps must be optimized in order to ensure efficacy and safety (Judge et al., 1998). Altus has produced different CLEC® enzyme products, such as lipases, esterase and protease, but they have certain risks. Crystallization of proteins is not an easy step, therefore sometimes the crystalline state may be inactive. Crystallization of biomolecules has several advantages, viz. higher solubility of the crystalline form over the amorphous form, easy purification of protein and concentrated protein crystals being beneficial for certain cases which require high doses at the site of action (Margolin, 1996).
4.4. Oral-Lyn™: Generex Biotechnology Corp. (Canada)
Oral-Lyn™ is delivered to the oral cavity through the Rapidmist™ device (aerosol-type device containing non-chloroflurocarbon propellant, penetration enhancers and stabilizers) to the oral cavity which permeates across the buccal epithelium and reaches the blood circulation (Bernstein, 2006). Oral-Lyn™ delivery system has a sufficiently large micellar size (larger than 7 μm), therefore, it does not enter the respiratory system. A study was carried out to claim that Oral-Lyn is a safe formulation in which Oral-Lyn™ without insulin formulation was administrated to 40 dogs or nearly 1000 patients and did not show any abnormalities in the buccal mucosa. These formulations were found to be also effective in type-2 diabetes, whose patients were resistant to diet, exercise, metformin, sulphonylureas and thiazolidenes. After the approval of Oral Lyn™ in India for the purpose of import, commercialization, marketing and sales for both types of diabetes, it has been issued the license, where the product has been renamed as Oral Recosulin (Shreya Life Sciences Pvt. Limited). Generex Biotechnology has claimed that it is close to completing the Indian clinical study needed to secure commercialization approval from the Central Drugs Standard Control Organization (CDSCO), Directorate General of Health Services, Ministry of Health and Family Welfare, and is awaiting advice from Shreya Life Sciences as to the anticipated timing of these initiatives. Generex Biotechnology Corp. has recently launched the Oral Recosulin for the treatment of Type-1 and 2 diabetes since 2009 (Generex biotechnology corporation, 2009).
4.5. IN-105: Nobex and Biocon (India)
Nobex technology (HIM2) is used in an oral delivery system which has been developed by Biocon. In this technique, enhancement of the hydrophobic character of proteins is achieved by chemical modification of insulin with a small PEG and penetration enhancers. New modified analogue called IN-105, which is an advanced new generation molecule to HIM2 (hexyl-insulin mono-conjugate 2) was prepared (Wajberg et al., 2004). Introducing hydrophobicity to proteins by simple chemical linkage of the primary amine group of the Lys-29 residue in the beta chain of insulin and amphiphilic oligomer resulted in enhanced transcellular transportation, increased protein stability and resistance to enzymatic degradation when administrated as oral semisolid hard gelatin capsules (Clement et al., 2002, Kipnes et al., 2003). A study was conducted on 20 patients with T2DM (Type-2 diabetes mellitus) poorly-controlled on metformin. The doses given were as follows: 10, 15, 20, 30 mg of IN-105 and were compared with the placebo control arm. The study concluded that the onset of action occurred 10 min after administration of IN-105 and duration of action was near about 1.5–2 h. Biocon did phase IV trials for IN-105 and marketed it as Insugen in India (Kumar, 2009).
4.6. Oraldel™: Apollo Life Sciences (Australia)
Studies on Oraldel™ delivery system showed that it protects and transports biomolecules (insulin), which are encapsulated inside them. The nanoparticles are composed of carbohydrate-based sugar (Rieux et al., 2005), protected polymer coated with cyanocobalamin (Vitamin B12) (Petrus et al., 2007). These formulations have the ability to entrap 100% protein with vitamin B12, and as a result they protect proteins from enzymatic degradation, as well as enhance the transportation of proteins (Park et al., 2011). Various sizes of insulin nanoparticles are delivered by Apollo Life Sciences. Other categories of drugs, such as TNF blockers for the treatment of rheumatoid arthritis, are under development stages. The global market of anti-TNF was almost US $ one trillion in 2006, growing at over 30% per year (Craik et al., 2013, Source Apollo life sciences, 2010).
4.7. Capsulin™: Diabetology (Jersey, UK)
In UK, Capsulin™ is under clinical trials by Diabetology, which shows the onset of action within 30 min and duration of action up to 4–6 h. During the fasting condition, higher doses (300 I.U.), given to healthy volunteer with T1DM (Type-1 diabetes mellitus), showed a sudden fall in blood glucose level (1.6 mmol/l) and minimum doses (150 I.U.) which represented lowering of blood glucose levels (0.02 mmol/l). On the basis of clinical trial data, it was found that Capsulin™ has the ability to control the progression of diabetic conditions (Schwartz et al., 2008).
4.8. HDV-1: Diasome Pharmaceuticals (USA)
The concept of liposomal (vesicular) delivery system is growing by Diasome Pharmaceuticals. It is available in non-invasive (oral) and invasive (subcutaneous) forms. The study of 6 volunteers (with T2DM- Type-2 diabetes mellitus), which was based on comparison between placebo and doses in the ranging trial of oral HDV-1, represented significantly lowered mean and increased PPG area curve as determined over a period of 14 h as compared with placebo, which demonstrated non-linearity. The position of this drug is not clear due to insufficient data of pharmacokinetics. If HDV-1 is used for a long duration, it becomes tough to control over-glycemic levels due to the development of resistance (Skyler et al., 2005).
4.9. AI-401: Eli-Lily (USA)
Eli-Lily is still developing AI-401 for the oral delivery of proteins (recombinant product of human insulin). Besides Oral-Lyn and HIM2, AI-401 is used for the prevention and treatment of Type-1 diabetes. This technique uses the concept of oral-tolerance therapy. The data of Type-1 diabetes are organized by the oral insulin arm of NIH-sponsored diabetes prevention and is advantageous for type-1 diabetes patients (Source FDA, 2003, http://www.autoimmuneinc.com).
4.10. Sandimmune®: Novartis Pharmaceuticals (USA)
Sandimmune® is a brand of Novartis, which consists of a small hydrophobic cyclic polypeptide of 11 amino acids called cyclosporine, and is available in the form of a capsule. It is used as an immunosuppressant for organ transplant rejection in kidney, liver and heart, as well as for the treatment of auto-immune diseases (psoriasis and rheumatoid arthritis) (Holt et al., 1995). It has a specific chemical structure of cyclosporine, therefore absolute bioavailability is about 30%. The uptake of cyclosporine is easy from intestine, and they are protected from enzymatic action due to its lipophilicity and unique structure of the molecules. When cyclosporine contacts with the aqueous environment it immediately forms a micro-emulsion (Salama et al., 2010).
4.11. Octreolin®: Chiasma (Israel)
Transient permeability enhancer (TPE) system is an enteric-coated formulation which facilitates intestinal absorbance of drug molecules with limited intestinal bioavailability. It is formulated from sodium caprylate (C8) in hydrophobic microparticles and is agitated with castor oils or medium-chain glycerides, yielding emulsions (oily suspension) (www.chiasmapharma.com). The FDA has approved the orphan status for the Octreotide formulation, Octreolin®. During the phase III trials it (Octreolin®) showed no side effects in all the 12 individuals. Most effective molecular weight of biomolecules that enhanced the permeation of (TPE) is 4–10 KDa (Carino et al., 2000). C10 and C12 have more promoting action than C8, in emulsion as an additive and its combination, to give TPE (www.chiasmapharma.com).
5. Conclusion and future prospects
Oral delivery of proteins and peptides is the most efficient way to replace the invasive route as well as a very interesting and promising area for research. The strategy for development of oral biomolecules has always been challenged for the researchers due to their high molecular weight, chemical or enzymatic degradation, and impermeability through the intestinal mucosa. The growing field of biotechnology has allowed cost-effective and pilot-scale production of proteins and peptides and it is used for oral delivery. In recent times, large numbers of proteins are invented through the oral route such as Oral Recosulin, Octreolin® and Sandimmune® etc., in which a few are in clinical stage of development. As discussed in the review, nanotechnology offers various efficient carriers for the delivery of proteins such as solid lipid nanoparticles, nanostructured lipid carrier, liposomes, niosomes, cubosomes and nanoparticles, etc. Various efficient approaches were discussed for formulation development of oral delivery of therapeutic proteins and it can be implemented in large-scale production. Protein stability during formulation, and the product development costs remain major challenges in pilot scale-up of these novel products which need to be addressed at all levels of research and development for this novel technology to be successfully transferred from the bench to the bedside.
Acknowledgement
The authors are thankful to Ms Sobiya Zafar, Department of Pharmaceutics, Jamia Hamdard, New Delhi for her linguistic support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Musarrat Husain Warsi, Email: mhwarsi@gmail.com.
Farhan Jalees Ahmad, Email: farhanja_2000@yahoo.com.
References
- Agarwal V., Khan M.A. Current status of the oral delivery of insulin. Pharm. Tech. 2001;25(10):76–90. [Google Scholar]
- Antunes F., Andrade F., Ferreira D., Morck N.H., Sarmento B. Models to predict intestinal absorption of therapeutic peptides and proteins. Curr. Drug Metab. 2013;14(1):4–20. [PubMed] [Google Scholar]
- Aoki Y., Morishita M., Asai K., Akikusa B., Hosoda S., Takayama K. Region dependent role of the mucous/glycocalyx layers in insulin permeation across rat small intestinal membrane. Pharm. Res. 2005;22(11):1854–1862. doi: 10.1007/s11095-005-6137-z. [DOI] [PubMed] [Google Scholar]
- Aungst B.J. Intestinal permeation enhancers. J. Pharm. Sci. 2000;89(4):429–442. doi: 10.1002/(SICI)1520-6017(200004)89:4<429::AID-JPS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bai S., Thummel R., Godwin A.R., Nagase H., Itoh Y., Li L. Matrix metalloproteinase expression and function during fin regeneration in zebrafish: analysis of MT1-MMP, MMP2 and TIMP2. Matrix Bio. 2005;24(4):247–260. doi: 10.1016/j.matbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Bakhru S.H., Furtado S., Morello A.P., Mathiowitz E. Oral delivery of proteins by biodegradable nanoparticles. Adv. Drug Del. Rev. 2013;65(6):1–11. doi: 10.1016/j.addr.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Bastian S.E.P., Walton P.E., Ballard F.J., Belford D.A. Transport of IGF-I across epithelial cell monolayers. J. Endo. 1999;162(3):361–369. doi: 10.1677/joe.0.1620361. [DOI] [PubMed] [Google Scholar]
- Basu A., Yang K., Wang M., Liu S., Chintala R., Palm T. Structure-Function Engineering Of Interferon-Beta-1b For Improving Stability, Solubility, Potency, Immunogenicity, And Pharmacokinetic Properties By Site-Selective Mono-Pegylation. Bioconjugate Chem. 2006;17(3):618–630. doi: 10.1021/bc050322y. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A. Thiomers: a new generation of mucoadhesive polymers. Adv. Drug Del. Rev. 2005;57(11):1569–1582. doi: 10.1016/j.addr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A., Paikl C., Valenta C. Novel bioadhesive chitosan-EDTA conjugate protects leucine enkephalin from degradation by aminopeptidase N. Pharm. Res. 1997;14(7):917–922. doi: 10.1023/a:1012108118670. [DOI] [PubMed] [Google Scholar]
- Bernstein G. Drug delivery in diabetes: oral-lyn needle-free buccal delivery of insulin. Drug Delivery Ltd. 2006:15–18. [Google Scholar]
- Blanchette J., Kavimandan N., Peppas N.A. Principles of transmucosal delivery of therapeutic agents. Biomed. Pharmacol. 2004;58(3):142–151. doi: 10.1016/j.biopha.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Brayden D.J., Mrsny R.J. Oral peptide delivery: prioritizing the leading technologies. Ther. Delivery. 2011;2(12):1567–1573. doi: 10.4155/tde.11.114. [DOI] [PubMed] [Google Scholar]
- Brayden D.J., Jepson M.A., Baird A.W. Keynote review: intestinal Peyer’s patch M cells and oral vaccine targeting. Drug Discovery Today. 2005;10(11):1145–1157. doi: 10.1016/S1359-6446(05)03536-1. [DOI] [PubMed] [Google Scholar]
- Calceti P., Salmaso S., Walker G., Bernkop-Schnürch A. Development and in vivo evaluation of an oral insulin-PEG delivery system. Eur. J. Pharm. Sci. 2004;22(4):315–323. doi: 10.1016/j.ejps.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Camenisch G., Alsenz J., Waterbeemd H.V., Folkers G. Estimation of permeability by passive diffusion through caco-cell monolayers using the drugs’ lipophilicity and molecular weight. Eur. J. Pharm. Sci. 1998;6(4):317–324. [PubMed] [Google Scholar]
- Carino G.P., Jacob J.S., Mathiowitz E. Nanosphere based oral insulin delivery. J. Controlled Release. 2000;65(1–2):261–269. doi: 10.1016/s0168-3659(99)00247-3. [DOI] [PubMed] [Google Scholar]
- Carvalho F.C., Bruschi M.L., Evangelista R.C., Gremia M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010;46(1):1–17. [Google Scholar]
- Chan J.M., Valencia P.M., Zhang L. Polymeric nanoparticles for drug delivery. Methods Mol. Bio. 2010;624:163–175. doi: 10.1007/978-1-60761-609-2_11. [DOI] [PubMed] [Google Scholar]
- Chung H., Kim J., Um J.Y., Kwon I.C., Jeong S.Y. Self-Assembled ‘Nanocubicle’ As a Carrier for Peroral Insulin Delivery. Diabetologia. 2002;45(3):448–451. doi: 10.1007/s00125-001-0751-z. [DOI] [PubMed] [Google Scholar]
- Cilek A., Celebi N., Tirnaksiz F. Lecithin-based microemulsion of a peptide for oral administration: preparation, characterization, and physical stability of the formulation. Drug Del. 2006;13(1):19–24. doi: 10.1080/10717540500313109. [DOI] [PubMed] [Google Scholar]
- Clement S., Still J.G., Kosutic G., McAllister R.G. Oral Insulin product hexyl-insulin monoconjugate 2 (HIM2) in type 1 diabetes mellitus: the glucose stabilization effects of HIM2. Diabetes Tech. Ther. 2002;4(4):459–466. doi: 10.1089/152091502760306544. [DOI] [PubMed] [Google Scholar]
- Constantinides P.P. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm. Res. 1995;12(11):1561–1572. doi: 10.1023/a:1016268311867. [DOI] [PubMed] [Google Scholar]
- Craik J.D., Fairlie D.P., Liras S., Price D. The future of peptide-based drugs. Chem. Bio. Drug Design. 2013;81(1):136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- Cronauer M.V., Schulz W.A., Burchardt T., Anastasiadis A.G., De la Taille A., Ackermann R. The androgen receptor in hormone-refractory prostate cancer: relevance of different mechanisms of androgen receptor signalling. Int. J. Onco. 2003;23(4):1095–1102. doi: 10.3892/ijo.23.4.1095. [DOI] [PubMed] [Google Scholar]
- Damge C., Michael C., Aprahamian M., Couvreur P. New approach for oral administration of insulin with polyalkylcyanoacrylate nanocapsules as oral carrier. Diabetes. 1988;37(2):247–251. doi: 10.2337/diab.37.2.246. [DOI] [PubMed] [Google Scholar]
- De Coupade C., Fittipaldi A., Chagnas V., Michel M., Carlier S., Tasciotti E. Novel human-derived cell-penetrating peptides for specific subcellular delivery of therapeutic biomolecules. Biochem. J. 2005;390(2):407–418. doi: 10.1042/BJ20050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degim Z., Unal N., Eşsiz D., Abbasoglu U. The effect of various liposome formulations on insulin penetration across Caco-2 cell monolayer. Life Sci. 2004;75(23):2819–2827. doi: 10.1016/j.lfs.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Derossi D., Calvet S., Trembleau A., Brunissen A., Chassaing G., Prochiantz A. Cell internalization of the third helix of the Antennapediahomeodomain is receptor-independent. J. Bio. Chem. 1996;271(1):18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- Dollo G., LeCorre P., Guérin A., Chevanne F., Burgot J.L., Leverge R. Spray-dried redispersible oil-in-water emulsion to improve oral bioavailability of poorly soluble drugs. Eur. J Pharm. Sci. 2003;19(4):273–280. doi: 10.1016/s0928-0987(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Donovan M.D., Flynn G.L., Amidon G.L. Absorption of polyethylene glycols 600 through 2000: the molecular weight dependence of gastrointestinal and nasal absorption. Pharm. Res. 1990;8:863–868. doi: 10.1023/a:1015921101465. [DOI] [PubMed] [Google Scholar]
- Egelund R., Rodenburg K., Andreasen P., Rasmussen M., Guldberg R., Petersen T. An ester bond linking a fragment of a serine proteinase to its serpin inhibitor. Biochemistry. 1998;37(18):6375–6379. doi: 10.1021/bi973043+. [DOI] [PubMed] [Google Scholar]
- El-Sayed K., Morishita M., Onuki Y., Takayama K. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv. Drug Delivery Rev. 2007;59(15):1521–1546. doi: 10.1016/j.addr.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Elsayed A., Remawi M.A., Qinna N., Farouk A., Badwan A. Formulation and characterization of an oily-based system for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2009;73(2):269–279. doi: 10.1016/j.ejpb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Fasano A., Uzzau S. Modulation Of intestinal tight junctions zona occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J. Clin. Invest. 1997;99(6):1158–1164. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss A.C., Goto T., Morishita M., Peppas N.A. Development of acrylic-based copolymers for oral insulin delivery. Eur. J. Pharm. Biopharm. 2004;57(10):163–169. doi: 10.1016/S0939-6411(03)00145-0. [DOI] [PubMed] [Google Scholar]
- Frost, S., Merrion pharmaceuticals (2008). http://www.merrionpharma.com/archive/FandS-MP-051108.pdf (Accessed on 8 January 2013.
- Gamboa J.M., Leong K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Delivery Rev. 2013;65(6):800–810. doi: 10.1016/j.addr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M., Gomez-Orellana I. Challenge for the oral delivery of macromolecules. Nat. Rev. Drug Dis. 2003;2(4):289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- Grosz A., Heintz A., Mlynek G.M., Calvo L.J., Robinson J.R. Carrier-enhanced human growth hormone absorption across isolated rabbit intestinal tissue. Int. J. Pharm. 2000;197(1):113–121. doi: 10.1016/s0378-5173(99)00322-1. [DOI] [PubMed] [Google Scholar]
- Gupta S., Jain A., Chakraborty M., Sahni J.K., Ali J., Dang S. Oral delivery of therapeutic proteins and peptides: a review on recent developments. Drug Delivery. 2013;20(6):237–246. doi: 10.3109/10717544.2013.819611. [DOI] [PubMed] [Google Scholar]
- Hamman J.H., Enslin G.M., Kotze A.F. Oral delivery of peptide drugs. BioDrugs. 2005;19(3):165–177. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- Hejazi R., Amiji M. Chitosan-based gastrointestinal delivery system. J. Controlled Release. 2003;89(2):151–165. doi: 10.1016/s0168-3659(03)00126-3. [DOI] [PubMed] [Google Scholar]
- Hess S., Rotshild V., Hoffman A. Investigation Of the enhancing mechanism of sodium N-[8-(2-hydroxybenzoyl)amino)caprylate effect on the intestinal permeability of polar molecules utilizing a voltage clamp method. Eur. J. Pharm. Sci. 2005;25(2–3):307–312. doi: 10.1016/j.ejps.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Holt D.W., Mueller E.A., Kovarik J.M., Van Bree J.B., Richard F., Kutz K. Sandimmune nerol pharmacokinetics: impact of new oral formulation. Trans. Proc. 1995;27(1):1434–1447. [PubMed] [Google Scholar]
- Hsieh P.W., Hung C.F., Fang J.Y. Current prodrug design for drug discovery. Curr. Pharm. Design. 2009;15(19):2236–2250. doi: 10.2174/138161209788682523. [DOI] [PubMed] [Google Scholar]
- Source AutoImmune Inc., additional information related data available on http://www.autoimmuneinc.com.
- Iyer H., Anand K., Verma M. Oral insulin – a review of current status, diabetes. Obes. Met. 2010;12(3):179–185. doi: 10.1111/j.1463-1326.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- Jana S., Mandlekar S., Marathe P. Prodrug design to improve pharmacokinetic and drug delivery properties: challenges to the discovery scientists. Curr. Med. Chem. 2010;17(32):3874–3908. doi: 10.2174/092986710793205426. [DOI] [PubMed] [Google Scholar]
- Judge R.A., Forsythe E.L., Pusey M.L. The effect of protein impurities on lysozyme crystal growth. Biotech. Bioeng. 1998;59(6):776–785. doi: 10.1002/(sici)1097-0290(19980920)59:6<776::aid-bit14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kafka A.P., McLeod B.J., Radesa T., McDowell A. Release and bioactivity of PACA nanoparticles containing D-Lys6-GnRH for brushtail possum fertility control. J. Controlled Release. 2011;149(3):307–313. doi: 10.1016/j.jconrel.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Karsdal M.A., Henriksen K., Bay-Jensen A.C., Molloy B., Arnold M., John M.R. Lessons learned from the development of oral calcitonin: the first tablet formulation of a protein in phase III clinical trials. J. Clin. Pharmacol. 2011;51(4):460–471. doi: 10.1177/0091270010372625. [DOI] [PubMed] [Google Scholar]
- Khan N.M.G. New development in insulin delivery. Drug Dev. Ind. Pharm. 2003;29(3):253–265. doi: 10.1081/ddc-120018199. [DOI] [PubMed] [Google Scholar]
- Kidron M., Dinh S., Menachem Y., Abbas R., Variano B., Goldberg M. A novel per-oral insulin formulation: proof of concept study in non-diabetic subjects. Diabetes Med. 2004;21(4):354–357. doi: 10.1111/j.1464-5491.2004.01160.x. [DOI] [PubMed] [Google Scholar]
- Kipnes M., Dandona P., Tripathy D., Still J.G., Kosutic G. Control of postprandial plasma glucose by an oral insulin product (HIM2) in patients with type 2 diabetes. Diabetes Care. 2003;26(2):421–426. doi: 10.2337/diacare.26.2.421. [DOI] [PubMed] [Google Scholar]
- Kumar, P., 2009. A Five Period, Open Label, Placebo Controlled Study to evaluate the Safety, Tolerability, Pharmacodynamics and Pharmacokinetics of a Single Ascending Dose of IN-105 under Fed Conditions in T2DM subjects. CTRI/2009/091/000479.
- Langer R. Drug delivery and targeting. Nature. 1998;392(6679 suppl.):5–10. [PubMed] [Google Scholar]
- Leader B., Baca Q.J., Golan D.E. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Dis. 2008;7(1):21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- Lecluyse E.L., Sutton S.C. In vitro models for selection of development candidates. Permeability studies to define mechanisms of absorption enhancement. Adv. Drug Delivery Rev. 1997;23(1–3):163–183. [Google Scholar]
- Lee S.J., Lee D.Y., Lee S.K., Kim Y., Lee Y., Byun Y. A new drug carrier, N α -deoxycholyl-l-lysyl-methylester, for enhancing insulin absorption in the intestine. Diabetes. 2005;48(3):405–411. doi: 10.1007/s00125-004-1658-2. [DOI] [PubMed] [Google Scholar]
- Leitner V.M., Walker G.F., Bernkop-Schnurch A. Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins. Eur. J Pharm. Biopharm. 2003;56(2):207–214. doi: 10.1016/s0939-6411(03)00061-4. [DOI] [PubMed] [Google Scholar]
- Leone-Bay A., Sato M., Paton D., Hunt A.H., Sarubbi D., Carozza M. Oral delivery of biologically active parathyroid hormone. Pharm. Res. 2001;18(7):964–970. doi: 10.1023/a:1010936227570. [DOI] [PubMed] [Google Scholar]
- Leroux J.C., Cozens R., Roesel J.L., Galli B., Doelker E., Gurny R. PH-Sensitive nanoparticles an effective means to improve the oral delivery of HIV-1 protease inhibitors in dogs. Pharm. Res. 1996;13(3):485–487. doi: 10.1023/a:1016073416332. [DOI] [PubMed] [Google Scholar]
- Lim C.J., Shen W.C. Comparison of monomeric and oligomeric transferrin as potential carrier in oral delivery of protein drugs. J. Controlled Release. 2005;106(3):273–286. doi: 10.1016/j.jconrel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Lowman A.M., Peppas N.A. Analysis of complexation/decomplexation phenomena in graft copolymer networks. Macromolecules. 1997;30(10):4959–4965. [Google Scholar]
- Lueben H.L., De Leeuw B.J., Perard D., Lehr C.M., De Boer A.G., Verhoef J.C. Mucoadhesive polymers in peroral peptide drug delivery: I, influence of mucoadhesive excipients on the proteolytic activity of intestinal enzymes. Eur. J. Pharm. Sci. 1996;4(2):117–128. [Google Scholar]
- Mahato R.I., Narang A.S., Thoma L., Miller D.D. Emerging trends in oral delivery of peptide and proteins. Crit. Rev. Ther. Drug Carr. Sys. 2003;20(2–3):153–214. doi: 10.1615/critrevtherdrugcarriersyst.v20.i23.30. [DOI] [PubMed] [Google Scholar]
- Maher S., Leonard T.W., Jacobsen J., Brayden D.J. Safety and efficacy of sodium caprate in promoting oral drug absorption: from in vitro to the clinic. Adv. Drug Delivery Rev. 2009;61:1427–1449. doi: 10.1016/j.addr.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Makhlof A., Werle M., Tozuka Y., Takeuchi H. A mucoadhesive nanoparticulate system for the simultaneous delivery of macromolecules and permeation enhancers to the intestinal mucosa. J. Controlled Release. 2010;149(1):81–88. doi: 10.1016/j.jconrel.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Margolin A.L. Novel crystalline catalysts. Tibtech. 1996;14(2):223–230. [Google Scholar]
- Marschutz M.K., Bernkop-Schnurch A. Oral peptide drug delivery: polymer-inhibitor conjugates protecting insulin from enzymatic degradation in vitro. Biomaterials. 2000;21(14):1499–1507. doi: 10.1016/s0142-9612(00)00039-9. [DOI] [PubMed] [Google Scholar]
- Mathiowitz E., Jacob J.S., Jong Y.S., Carino G.P., Chickering D.E., Chaturvedi P. Biologically erodible microspheres as potential oral drug delivery systems. Nature. 1997;386(6623):410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- Mizuma T., Ohta K., Koyanagi A., Awazu S. Improvement of intestinal absorption of leucine enkephalin by sugar coupling and peptidase inhibitors. J. Pharm. Sci. 1996;85(8):854–857. doi: 10.1021/js950507l. [DOI] [PubMed] [Google Scholar]
- Morishita M., Peppas N.A. Is the oral route possible for peptide and protein drug delivery? Drug Discovery Today. 2006;11(19–20):905–910. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Munyendo W.L.L., Huixia Lv., Benza-Ingoula H., Baraza L.D., Zhou J. Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules. 2012;2(2):187–202. doi: 10.3390/biom2020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase I., Takeuchi T., Tanaka G., Futaki S. Methodological and cellular aspects that govern the internalization mechanisms of arginine-rich cell-penetrating peptides. Adv. Drug Delivery Rev. 2008;60(4–5):598–607. doi: 10.1016/j.addr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Noriyasu K., Nielsen E.J.B., El-Sayed K., Takeda-Morishita M. Noninvasive insulin delivery: the great potential of cell-penetrating peptides. Ther. Delivery. 2013;4(3):315–326. doi: 10.4155/tde.12.164. [DOI] [PubMed] [Google Scholar]
- Obata Y., Sesumi T., Takayama K., Isowa K., Grosh S., Wick S. Evaluation of skin damage caused by percutaneous absorption enhancers using fractal analysis. J. Pharm. Sci. 2000;89(4):556–561. doi: 10.1002/(SICI)1520-6017(200004)89:4<556::AID-JPS13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Okada, J., Cohen, S., Langer, R., 1998. Oral delivery of vaccines using polymerized liposomes. In US patent 5762, 904.
- Oppenheim R.C., Stewart N.F., Gordon L., Patel H.M. The production and evaluation of orally administered insulin nanoparticles. Drug Dev. Ind. Pharm. 1982;8(4):531–546. [Google Scholar]
- Orive G., Gascon A., Hernandez R., Dominiguez-Gil A., Pedraz J. Techniques: new approaches to the delivery of biopharmaceuticals. Trends Pharmacol. Sci. 2004;25(7):382–387. doi: 10.1016/j.tips.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pan Y., Li Y.J., Zhao H.Y., Zheng J.M., Xu H., Wei G. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002;249(1–2):139–147. doi: 10.1016/s0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- Park K., Kwon I.C., Park K. Oral protein delivery: current status and future prospect. React. Funct. Polym. 2011;71(3):280–287. [Google Scholar]
- Peppas N.A., Klier J. Controlled release by using poly(methacrylic acid-g-ethylene glycol) hydrogels. J. Controlled Release. 1991;16(1–2):203–214. [Google Scholar]
- Peters A. Incretin-based therapies: review of current clinical trial data. Am. J. Med. 2010;123(3 suppl.):S28–S37. doi: 10.1016/j.amjmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Petrus A.K., Vorthermsf A.R., Fairchild T.J., Doyle R.P. Vitamin B12 as a carrier for the oral delivery of insulin. Chemmedchem. 2007;2(12):1717–1721. doi: 10.1002/cmdc.200700239. [DOI] [PubMed] [Google Scholar]
- Ponchel G., Irache J.M. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv. Drug Delivery Rev. 1998;34(23):191–219. doi: 10.1016/s0169-409x(98)00040-4. [DOI] [PubMed] [Google Scholar]
- Prego C., Garcia M., Torres D., Alonso M.J. Transmucosal Macromolecular Drug Delivery. J. Controlled Release. 2005;101(1–3):151–162. doi: 10.1016/j.jconrel.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Rekha M.R., Sharma C.P. Oral delivery of therapeutic protein/peptide for diabetes – Future Perspectives. Int. J. Pharm. 2013;440(1):48–62. doi: 10.1016/j.ijpharm.2012.03.056. [DOI] [PubMed] [Google Scholar]
- Renukuntla J., Vadlapudi A.D., Patel A., Boddu S.H.S., Mitra A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013;447(1–2):75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieux D., Ragnarsson E.G., Gullberg E., Préat V., Schneider Y.J., Artursson P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur. J. Pharm. Sci. 2005;25(4–5):455–465. doi: 10.1016/j.ejps.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Rubinstein A. Approaches and opportunities in colon specific drug delivery. Crit. Rev. Ther. Drug Car. Sys. 1995;12:101–149. doi: 10.1615/critrevtherdrugcarriersyst.v12.i2-3.10. [DOI] [PubMed] [Google Scholar]
- Russell-Jones G.J. Use of targeting agents to increase uptake and localization of drugs to the intestinal epithelium. J. Drug Target. 2004;12(2):113–123. doi: 10.1080/10611860410001693760. [DOI] [PubMed] [Google Scholar]
- Sachdeva A., Gulati R., Gupta U., Bapna J.S. Comparative bioavailability of two brands of cyclosporine. Ind. J. Pharmacol. 1997;29(2):135–137. [Google Scholar]
- Sadeghi M., Avadi M.R., Ejtemaimehr S., Abashzadeh S., Partoazar A., Dorkoosh F. Development of a gas empowered drug delivery system for peptide delivery in the small intestine. J. Controlled Release. 2009;134(1):11–17. doi: 10.1016/j.jconrel.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Sakuma S., Hayashi M., Akashi M. Design of nanoparticles composed of graft copolymers for oral peptide delivery. Adv. Drug Delivery Rev. 2001;47(1):21–37. doi: 10.1016/s0169-409x(00)00119-8. [DOI] [PubMed] [Google Scholar]
- Salama N.N., Eddington N.D., Fasano A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Delivery Rev. 2006;58(2):15–28. doi: 10.1016/j.addr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Salama, P., Mamluk, R., Marom, K., Weinstein, I., Tzabari, M., 2010. Pharmaceutical Combinations And Related Methods Of Delivery. (LandoAndAnastasi), USP Application 01056275A1.
- Salamat-Miller N., Chittchang M., Johnston T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Delivery Rev. 2005;57(11):1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Sang H.Y., Park T.G. Biodegradable nanoparticles containing protein, fatty acid complexes for oral delivery of salmon calcitonin. J. Pharm. Sci. 2004;93(2):488–495. doi: 10.1002/jps.10573. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Geho B., Rosenberg L., Lau J. A 2-week randomized, active comparator study of two HDV-Insulin routes (SC and oral) and SC human insulin in patients with type 1 diabetes mellitus. Diabetes. 2008;57(1 suppl.):A124. [Google Scholar]
- Senel S., Kremer M.J., Kas S., Wertz P.W., Hincal A.A., Squier C.A. Enhancing effect of chitosan on peptide drug delivery across buccal mucosa. Biomaterials. 2000;21(20):2067–2071. doi: 10.1016/s0142-9612(00)00134-4. [DOI] [PubMed] [Google Scholar]
- Shah R.B., Ahsan F., Khan M.A. Oral delivery of proteins: progress and prognostication. Crit. Rev. Ther. Drug Car. Sys. 2002;19(2):135–169. doi: 10.1615/critrevtherdrugcarriersyst.v19.i2.20. [DOI] [PubMed] [Google Scholar]
- Shaji J., Patole V. Protein and peptide drug delivery: oral approaches. Ind. J Pharm. Sci. 2008;70(3):269–277. doi: 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakweh M., Besnard M., Nicolas V., Fattal E. Poly (lactide-co-glycolide) particles of different physicochemical properties and their uptake by peyer’s patches in mice. Eur. J. Pharm. Biopharm. 2005;61(1–2):1–13. doi: 10.1016/j.ejpb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Shen W.C. Oral peptide and protein delivery: unfulfilled promises? Drug Discovery Today. 2003;8(14):607–608. doi: 10.1016/s1359-6446(03)02692-8. [DOI] [PubMed] [Google Scholar]
- Sizer P.J.H. Towards an oral influenza vaccine. Trends Biotech. 1997;15(8):282–285. doi: 10.1016/S0167-7799(97)01070-6. [DOI] [PubMed] [Google Scholar]
- Skyler J.S., Krischer J.P., Wolfsdorf J., Cowie C., Palmer J.P., Greenbaum C. Effects of oral insulin in relatives of patients with type 1 diabetes: the diabetes prevention trial-type 1. Diabetes Care. 2005;28(5):1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- Sood A., Panchagnula R. Peroral route: an opportunity for protein and peptide drug delivery. Chem. Rev. 2001;101(11):3275–3303. doi: 10.1021/cr000700m. [DOI] [PubMed] [Google Scholar]
- Source Apollo life sciences, Apollo life science press release (2010) on additional clinical data from http://www.apollolifesciences.com.
- Source Chiasma Pharma, Chiasma press release (2011) on additional clinical data from phase III oral TPE available from URL: http://www.chiasmapharma.com/page.asp?id=20.
- Source Emisphere Technologies, Inc. Emisphere’s press release (October 6, 2006) on additional clinical data from phase 2 oral insulin trial. Available from URL: http://www.emisphere.com.
- Source FDA. Gov, (November 2003), on additional clinical data from oral cyclosporine trial (Novartis). Available from URL: http://www.accessdata.fda.gov/scripts/cderfob/docsltempai.cfm.
- Source Generex biotechnology corporation, Press release (2009) on additional clinical data from http://www.generex.com.
- Source Tarsa Therapeutics, http://www.tarsatherapeutics.com/News/091811.Html (Accessed November 10th, 2013).
- Strous G.J., Dekker J. Mucin-type glycoproteins. Crit. Rev. Biochem. Mol. Bio. 1992;27(1–2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Takka S., Acarturk F. Calcium alginate microparticles for oral administration. I: effect of sodium alginate type on drug release and drug entrapment efficiency. J. Microencap. 1999;16(3):275–290. doi: 10.1080/026520499289013. [DOI] [PubMed] [Google Scholar]
- Toorisaka E., Ono H., Arimori K., Kamiya N., Goto M. Hypoglycemic effect of surfactant-coated insulin solubilized in a novel solid-in-oil-in-water (S/O/W) emulsion. Int. J. Pharm. 2003;252(1–2):271–274. doi: 10.1016/s0378-5173(02)00674-9. [DOI] [PubMed] [Google Scholar]
- Tozaki H., Emi Y., Horisaka E., Fujita T., Yamamoto A., Muranishi S. Degradation of insulin and calcitonin and their protection by various protease inhibitors in rat caecal contents: implications in peptide delivery to the colon”. J. Pharm. Pharmacol. 1997;49(2):164–168. doi: 10.1111/j.2042-7158.1997.tb06773.x. [DOI] [PubMed] [Google Scholar]
- Vadlapudi A.D., Vadlapatla R.K., Kwatra D., Earla R., Samanta S.K., Pal D. Targeted lipid based drug conjugates: a novel strategy for drug delivery. Int. J. Pharm. 2012;434(1–2):315–324. doi: 10.1016/j.ijpharm.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den M.G., Kinget R. Oral colon-specific drug delivery: a review. Drug Delivery. 1995;2:81–93. [Google Scholar]
- Varma M.V.S., Ashokraj Y., Dey C.S., Panchagnula R. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol. Res. 2003;48(4):347–359. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- Wajberg E., Miyazaki Y., Triplitt C., Cersosimo E., Defronzo R.A. Dose-response effect of a single administration of oral hexyl-insulin monoconjugate 2 in healthy non-diabetic subjects. Diabetes Care. 2004;27(12):2868–2874. doi: 10.2337/diacare.27.12.2868. [DOI] [PubMed] [Google Scholar]
- Walsh E.G., Adamczyk B.E., Chalasani K.B., Maher S., Toole E.B.O., Fox J.S. Oral Delivery of Macromolecules: Rationale Underpinning Gastrointestinal Permeation Enhancement Technology (GIPET1) Ther. Delivery. 2011;2(12):1595–1610. doi: 10.4155/tde.11.132. [DOI] [PubMed] [Google Scholar]
- Werle V., Brigitta L., Daniel E., Florian F. Design and evaluation of chitosan-aprotinin conjugate for the peroral delivery of therapeutic peptides and protein susceptible to enzymatic degradation. J. Drug Target. 2007;15(5) doi: 10.1080/10611860701349141. 327-323. [DOI] [PubMed] [Google Scholar]
- Whitelaw, C. D., Kelly, C. A., Ironmonger, W., Cunliffe, C., New, R., Phillips, J. N., 2005. Absorption Of Orally Ingested Insulin In Human Type 1 Diabetic Subjects: Proof Of Concept Study. San Diego: American Diabetes Ass. 65th Scientific Sessions.
- Wu S.J., Robinson J.R. Transport of human growth hormone across Caco-2 cells with novel delivery agents: evidence for P-glycoprotein involvement. J. Controlled Release. 1999;62(1–2):171–177. doi: 10.1016/s0168-3659(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.T., Rikyuu K., Tsuji T., Fujita T., Murakami M. Effects of various protease inhibitors on the intestinal absorption and degradation of insulin in rats. Pharm. Res. 1994;11(10):1496–1500. doi: 10.1023/a:1018968611962. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Lehr C.M., Kok W., Junginger H.E., Verhoef J.C., Bouwistra J.A. Niosomes for oral delivery of peptide drugs. J. Controlled Release. 1992;21(1–3):145–153. [Google Scholar]
- Zheng J., Yue X., Dai Z., Wang Y., Liu S., Yan X. Novel iron polysaccharide multilayered microcapsules for controlled insulin release. Actabiomaterialia. 2009;5(5):1499–1507. doi: 10.1016/j.actbio.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Zorko M., Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv. Drug Delivery Rev. 2005;57(4):529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]