Abstract
As compared to gel and other topical preparations microemulgel has been prepared by screening of oils, emulsifier, and co-emulsifier on bases of solubility of an API in it. An API has high solubility and oil may also have more or less pharmacological property, so it may assist the therapeutic action of API. Due to presence of oil portion, it leads to more penetration of API in the skin. Oil Micelle Size was less than 500 nm which provides more area for absorption of API in the skin so more penetration and more effective than macro-emulsion. Microemulgel has an advantage of emulgel that has dual benefits of micro-emulsion and gel and several other desirable properties like good consistency, thyrotrophic, greaseless, easily spreadable as well as removable, emollient, non-staining, water soluble, longer shelf-life, bio-friendly, transparent, pleasant appearance, ability of patients for self-medication, termination of medications will be easy, etc.
Keywords: Microemulgel, Micro-emulsion based gel
1. Introduction
1.1. Introduction to micro-emulsion
The concept of micro-emulsion was first introduced in 1940 by Hoar and Schulman (Lowrance and Gareeth, 2012). Micro-emulsion is a clear, stable, isotropic mixture of oil, water and surfactant frequently used in combination with a co-surfactant. Micro-emulsion based drug delivery consists of delivering a drug dissolved in a mixture of one or more excipients which may be a mono, di and tri-glycerides, lipophilic and hydrophilic surfactants and a co-surfactant (Kalhapure and Akamanchi, 2012). When the drug is delivered through lipid formulation, it remains in the dissolved state throughout its transit. The absorption of drug presented in solubilized form within a colloidal dispersion is enhanced since the drug dissolution step is partially divided.
In the year 2000, Pouton classified lipid based formulations into three categories based on their composition and properties as in Table 1.
Table 1.
Typical properties of Type I, II, IIIA, IIIB, and IV lipid formulations Pouton (1985).
| Sr. no. | Composition (%) | Type I | Type II | Type IIIA | Type IIIB | Type IV |
|---|---|---|---|---|---|---|
| 1 | Triglycerides or mixed glycerides | 100 | 40–80 | 40–80 | <20 | – |
| 2 | Surfactant | – | 20–60 (HLB<12) | 20–40 (HLB > 11) | 20–50 (HLB > 11) | 40–80 |
| 3 | Co-surfactant Hydrophilic co-solvents | – | – | 0–40 | 20–50 | 0–50 |
| 4 | Particle size of dispersion (nm) | Coarse | 100–250 | 100–250 | 50–100 | – |
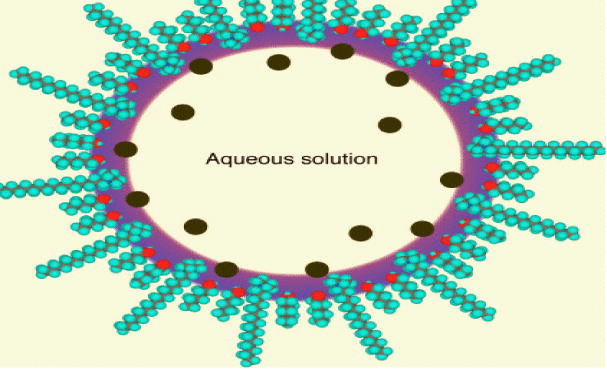
Micro-emulsions are isotropic mixtures of oil, surfactant, co-surfactant (solubilizer) and drug falling under class III B in Table 1. The basic principle of this system is to form o/w type of micro-emulsion under gentle agitation following dilution by aqueous phase. This micro-emulsion keeps the drug in a solubilized form and small sized formed droplets provide large interfacial area for drug absorption. Apart from solubilization, the presence of lipid in a formulation further helps to improve bioavailability by enhancing the permeability of the drug (Pouton, 2000) Table 2.
Table 2.
Advantages of micro-emulsion over emulsion (Holmberg et al. (2010).
| Sr. no. | Conventional (macro) emulsion | Micro-emulsion |
|---|---|---|
| 1 | 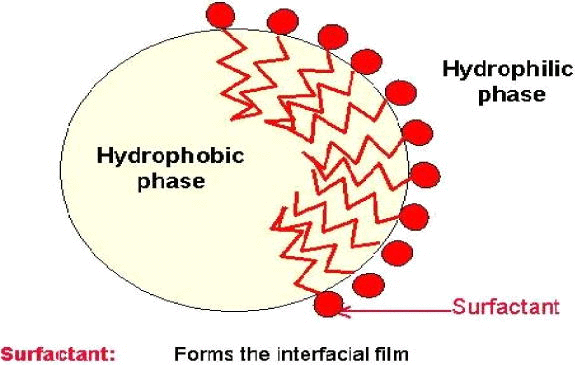 |
 |
| 2 | Two phase system renders it thermodynamically unstable | One phase system renders it thermodynamically stable |
| 3 | Poorer long term storage stability and often tend to coalesce, creaming/sedimentation or phase separation | Better long term storage stability |
| 4 | Comparatively less bioavailability | Reduction in dose by the enhancement in bioavailability |
| 5 | Comparatively less lipophilic transport | Enhanced lymphatic transport due to lipids Holm et al. (2003) |
| 6 | High intra and inter subject variability | Reduced intra and inter subject variability Gattefosse-self emulsifying formulation (2013) |
| 7 | Difficult manufacturing and scale up | Easy manufacture and scale up |
| 8 | Thermodynamically unstable | Excellent kinetic stability |
| 9 | Cloudy white | Clear |
| 10 | Large input of energy for method of preparation | No large input of energy for method of preparation |
| 11 | Globule size more than 500 nm | Micelle size are 5–500 nm |
Micro-emulsion is prepared when entropy changes that fevers dispersion is greater than the free energy required to increase the surface area between the oil and aqueous phase of the dispersion. The change in free energy (ΔG) associated with the process of emulsification, ignoring the free energy of mixing, can be expressed by (McClements, 2012)
where Ni = the number of droplets of radius ri, σ = the interfacial energy.
Micro-emulsification will occur only when the interfacial energy is low. However, emulsions are not thermodynamically stable as the oil phase and the aqueous phase will tend to separate with time to reduce the interfacial area and also free energy of the system. Therefore, the presence of surfactants will help to reduce interfacial tensions by forming a barrier around the oil droplets and hence the free energy of the systems. On the other hand, related emulsification with the formation of liquid crystalline phase. Liquid crystalline phase is the phase between liquid and crystal phases. A liquid crystal has both the properties of a crystal as well as a liquid. When additional energy is exerted onto the liquid crystalline phase, it will turn into liquid phase. For micro-emulsifying systems, when the oil phase is introduced into the aqueous phase with gentle agitation, the aqueous phase will penetrate through the interface into the oil phase until the interface of the two phases is disrupted. Consequently, oil droplets are formed resulting in emulsification. Thus, the ease of emulsification is governed by the ease of water penetration into the various liquid crystals or gel phases formed on the surface of droplets. The liquid crystal formation surrounding the oil droplets will increase the stability of the emulsion. Nevertheless, the relationship between liquid crystal formation and emulsion formation could be more complicated as it appeared to be. Various factors can affect the process of emulsification, such as the nature of oil/surfactant pair, the surfactant concentration used as well as the temperature. Moreover, the presence of drug compound will alter the emulsion characteristics, probably by interacting with the liquid crystalline phase (Lowrance and Gareeth, 2012) Table 3.
Table 3.
Different types of dispersions.
| Appearance | Particle size range | Type |
|---|---|---|
| Transparent | 10–140 nm | Micro-emulsion |
| Translucent | 140–200 nm | |
| Turbid | 200 nm-few microns | Emulsion |
| Presence of particle | Depended on API | Dispersion |
1.2. Introduction to emulgel
When both emulsion and gel are used in a combined form the dosage form prepared is named as Emulgel (Mohamed, 2004). As the name suggests it is the combination of emulsion and gel (Abd El-Bary et al., 2001). Therefore, it has been recently used as vehicle to deliver various drugs to the skin for topical as well as systemic actions (Ajazuddin et al., 2013). In fact, the presence of a gelling agent in water phase converts an ancient emulsion into emulgel (Vijya et al., 2011). The direct (oil-in-water) system is used to entrap lipophilic drugs, while hydrophilic drugs are encapsulated in the reverse (water-in-oil) system (Khullar et al., 2011, Vincent). Emulsions have a certain degree of elegance and are easily washable whenever required (Neishboor et al., 2013). They also have a high ability to penetrate the skin (Mohamed, 2004). Topically used emulgels have several desirable properties like being thixotropic, greaseless, easily spreadable as well as removable, emollient, non-staining, water soluble, longer shelf-life, bio-friendly, transparent, pleasant appearance etc (Jain et al., 2011, Setty et al., 2010).
1.3. Introduction to microemulgel
When both micro-emulsion and gel are used in combination dosage forms the prepared formulations are called as microemulgel, having the advantages of both emulgel as well as micro-emulsion. Both hydrophilic and hydrophobic types of drugs are incorporated into dosage forms. They provide a large surface area for drug absorption and oil portion increases the bioavailability by improving permeability of drugs. Also the stability of micro-emulsion is increased when it is incorporated in gel. Over to micro-emulsions, microemulgels have a certain degree of elegance and easily washable whenever required.
1.4. Ideal characteristics of drug and excipients
There are some necessities of drug candidates and excipients used for topical or ophthalmic drug delivery system as listed in Table 4.
Table 4.
Ideal properties of drug candidate Shingade et al. (2012).
| Parameter | Properties |
|---|---|
| Dose | Should be low (less than 10 mg) |
| Half-life | 10 hr or less |
| Molecular weight | 400 Dalton or less |
| Partition coefficient | Log p (octanol–water) between −0.8 and 4 |
| Skin permeability coefficient | More than 0.5 × 10−3 cm/hr |
| Skin reaction | Non irritating and non-sensitizer |
| Oral bioavailability | Low |
| Therapeutic index | Low |
| Polarity | Less |
| Molecular size | Small |
Same way the excipients utilized should be IIG listed, GRAS listed or biologically safe, non-irritant, non-allergic, concentration under guideline, should have little or no deleterious effect on activity and stability of final product, compatible with API and other excipients etc.
2. Study protocol
2.1. Aim
Formulation, Development and Evaluation of Microemulgel.
2.2. Objective
-
•
Prepared and optimized micro-emulsion from selected oil with emulsifier and co-emulsifier in which an API has the maximum solubility.
-
•
Optimized formula of microemulgel with different grades and different concentrations of the gelling agent by applying suitable statistical design.
-
•
To evaluate the combined effect of oil and API and compare it to API alone and available marketed preparation.
2.3. Rationale of study
-
•
Microemulgel could bring about dual control release system i.e. gel and micro-emulsion with an increase in the pharmacological activity at the site of action and reduction in side effects with advantages of emulgel.
-
•
Oil probably is having more or less pharmacological property, surfactant and co-surfactant one from each category will be screened on the basis of solubility studies of API(s) in it, so the problem of solubility of API will be overcome.
-
•
Microemulgel formulation enhances the skin deposition of API, thereby presumably enhancing its therapeutic activity.
3. Methodology
-
−
Identification of API: As per pharmacopoeia procedure.
-
−
Scanning and Calibration curve of API in solvent and in Phosphate buffer at specific pH: As per pharmacopoeia procedure.
-
−
Identification of Excipients: As per pharmacopoeia procedure.
-
−
Short listing of oils that have no interference of absorbance of API (generally between 200 and 400 nm.)
-
−
Screening of oil among the shortlisted oils, emulsifier and co-emulsifier on the basis of solubility study.
-
−
Selection of emulsifier, co-emulsifier, its ratio and oil.
-
−
API-Excipients compatibility study.
-
−
Preparation of Pseudo ternary phase diagram.
-
−
Application of Mixture design.
-
−
Preparation of API loaded micro-emulsion.
-
−
Optimization of micro-emulsion: By design expert 9.0.3.1 software or Minitab 7.0.
-
−
Formulation and Development of API loaded microemulgel using suitable design of experiments.
-
−
EVALUATION PARAMETERS.
3.1. Preparation of micro-emulsion and microemulgel
-
•
Viscosity: Brookfield Rotational viscometer will be used to measure viscosity (Djordjevic et al., 2004).
-
•
pH: pH will be measured by digital pH meter.
-
•
Drug Content: API based micro-emulsion will be subjected to extract API from micro-emulsion in appropriate solvent. Suitable dilution will be made with solvent and concentration will be measured by UV visible spectroscopic method at λmax nm by keeping solvent as reagent blank (Surjyanarayan et al., 2010).
-
•
Centrifugation: This parameter will be measured to evaluate physical stability. Micro-emulsion will be centrifuged at ambient temperature and 5000 RPM for 10 min to evaluate the system for creaming or phase separation. System will be observed visually for appearance (Karla et al., 2010).
-
•
Conductivity: Electric conductivity of micro-emulsion will be measured at ambient temperature with digital conductometer.
-
•
Dilution Test: If continuous phase is added into micro-emulsion, it will not be separated into phases. 50–100 times continuous phase dilution of micro-emulsion will be carried out and visually checked for phase separation and clarity (Mandal and Mandal, 2011).
-
•
% Transmittance Measurement: Micro-emulsion will be diluted to 50–100 times with continuous phase. The % transmittance of formulation was measured using UV Visible spectrophotometer at a specific wavelength using UV–Visible spectrophotometer against continuous phase as blank (Patel et al., 2013, Spectrometry Skill).
-
•
Zeta potential and Micelle Size analysis: Micelle size, Size distribution and zeta potential of micro-emulsion will be determined using particle size analyzer (Shinde et al., 2007).
-
•
In-vitro-Release Study: By Franz diffusion cell.
3.2. Evaluation of micro-emulsion and microemulgel
-
•
Physical Examinations: Microemulgel was inspected for their color, homogeneity, consistency, texture, etc.
-
•
pH: pH of the 1% aqueous solution of the prepared microemulgels will be measured by digital pH meter.
-
•
Spreadability Measurement: To determine the spreadability of microemulgel, 0.5 gm of microemulgel will be placed within a circle of 1 cm diameter pre-marked on a glass plate, over which second plate will be placed. A weight of 5 gm will be allowed to rest on the upper glass plate for 5 min. The increase in diameter due to microemulgel, the spreading will be noted, which was cm/gm-sec (Patel et al., 2013).
-
•
Syneresis measurement test: Upon standing sometimes gel system shrinks a bit and little liquid is pressed out. This phenomenon is known as Syneresis. In this test, microemulgel will be put in a cylindrical plastic tube with a perforated bottom which will be covered with filter paper (Whatman No. 41). These tubes will then placed in centrifuge tubes and centrifuged for 15 min. The cylindrical plastic tube and liquid which separated from microemulgel will be weighed. The percentage of Syneresis will be then calculated as (Charoenrein et al., 2011).
-
•
Rheological study: Mainly viscosity will be determined at 37 °C by means of Brookfield Viscometer.
-
•
Drug content determination: Drug content in microemulgel will be measured by dissolving 1gm of microemulgel in solvent by sonication. Absorbance will be measured after suitable dilution at λmax nm using UV spectrophotometer.
-
•
Tube Test (Extrudability Test): Force required to measure to extrude material from tube to evaluate microemulgel formulation for extrudability (Singla et al., 2012).
-
•
In-vitro-Release Study: By Franz diffusion cell at 37 °C.
-
•Drug release kinetics study: Results of in vitro release profile obtained for all batches will be plotted in models of data treatment as
-
•Zero – order kinetic model – % CPR Vs time.
-
•First – order kinetic model – log cumulative percent drug remaining Vs time.
-
•Higuchi’s model – cumulative percent drug released vs. square root of time.
-
•Korsmeyer/Peppa’s model – log cumulative percent drug released Vs log time.
-
•Hixson crowell model – Cube root of % drug to be remaining Vs Time.
-
•
-
•
Skin Irritation: By Draize-patch test in Rabbit.
-
•
In-vivo study: In animal study.
-
−
Optimization of Microemulgel.
-
−
Microbial Assay of optimized microemulgel: If an API has bacteriostatic or fungistatic activity then this test is required to be carried out. Ditch plate technique will be used for evaluation of bacteriostatic or fungistatic activity of an API (Berry et al., 2004).
where L1 = Total length of the streaked culture, and L2 = length of inhibition
-
−
Accelerated Stability study of optimized microemulgel: Sample of API loaded microemulgel will be sealed in ampoule and then placed in an accelerated stability chamber at 40 °C ± 5 °C temperature and 70% ± 5% RH. Duplicate sample will be withdrawn at 1, 2 and 3 months to evaluate their physicochemical parameters. The physical stability will be evaluated by visual inspection for physical changes such as phase separation and drug precipitation. Chemical stability will be expressed as the content of drug determined by UV visible spectroscopic method at λmax nm (ICH, 2013).
4. Conclusion
Mostly drugs are very much effective by oral and/or parenteral routes but have drawbacks of unwanted side effects, so the requirement of alternate routes of administration like topical, ophthalmic, vaginal etc. Mostly drugs are poorly water soluble, so have the problem of penetrating through the skin. Selection of oil, emulsifiers and co-emulsifiers for preparation are based on solubility of it in them, so the problem of solubility would be overcome. Oil portion has more or less pharmacological action and itself enhances penetration. Microemulgel enhances deposition of drug moieties at the site, so therapeutic activity is also increased. Stability is more as compared to micro-emulsion.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Bary A., Shalaby S., Abd El-Aal S. Formulation and stability of chloramphenicol gel and emulgel. Bull. Fac. Pharm. 2001;39:89–99. [Google Scholar]
- Ajazuddin, Alexander A., Khichariya A., Gupta S., Patel R.J. Recent expansions in an emergent novel drug delivery technology: emulgel. J. Control Release. 2013;171(2):122–132. doi: 10.1016/j.jconrel.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Berry H.W., Russell A.D., Denyer S., Hodges N.A., Gorman S.P. 7th ed. Blackwell Scientific Publications; Oxford, UK: 2004. Pharmaceutical Microbiology; pp. 197–198. [Google Scholar]
- Charoenrein S., Tatirat O., Rengsutthi K., Thongngam M. Effect of konjac glucomannan on syneresis, textural properties and the microstructure of frozen rice starch gels. Carbohydr. Polymers. 2011;83:291–296. [Google Scholar]
- Djordjevic L., Primorac M., Stupar M., Krajisnik D. Characterization of caprylocaproyl macrogolglycerides based micro-emulsion drug delivery vehicles for an amphiphilic drug. Int. J. Pharm. 2004;271(1):11–19. doi: 10.1016/j.ijpharm.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Gattefosse-self emulsifying formulation, 2013. <http://www. gattefosse.com/en/self-emulsifying-lipid-formulation/liquid-self kgx.html>
- Holm R., Porter J.C., Edwards G.A., Mullertz A., Kristensen H.G. Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in SMEDDS containing structured triglycerides. Int. J. Pharm. 2003;20:91–97. doi: 10.1016/s0928-0987(03)00174-x. [DOI] [PubMed] [Google Scholar]
- Holmberg et al. Self emulsifying drug delivery system, US Patent 7736666; 2010.
- ICH guideline for accelerated stability study, 2013. <http://www.ich.org/fileadmin/public_website/ICH_products/guidelines/Quality/Q1F/Stability_Guide_WHO.pdf>.
- Jain A., Deveda P., Vyas N., Chauhan J., Khambete H. Development of antifungal emulsion based gel for topical fungal infection(s) Int. J. Pharm. Dev. Res. 2011;2(12):18–25. [Google Scholar]
- Kalhapure R.S., Akamanchi K.G. Oleic acid based heterolipid synthesis, characterization and application in self-microemulsifying drug delivery system. Int. J. Pharm. 2012;425(2):9–18. doi: 10.1016/j.ijpharm.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Karla R., Maulik R.S., Badgujar L., Paradkar A.R., Mahadik K.R. Development and characterization of micro-emulsion Formulations for transdermal delivery of aceclofenac: a research. Int. J. Drug Form. Res. 2010;1(1):359–386. [Google Scholar]
- Khullar R., Saini S., Seth N., Rana A.C. Emulgels: a surrogate approach for topically used hydrophobic drugs. Int. J. Pharm. Biol. Sci. 2011;1(3):117–128. [Google Scholar]
- Lowrance M.J., Gareeth D.R. Micro-emulsion based media a Novel drug delivery systems. Adv. Drug Dev. Rev. 2012;64:175–193. [Google Scholar]
- Mandal S., Mandal S.S. Micro-emulsion drug delivery system: a platform form improving dissolution rate of poorly water soluble drug. Int. J. Pharm. Sci. Nanotech. 2011;3(4):1214–1219. [Google Scholar]
- McClements D.J. Nanoemulsions versus micro emulsions: terminology, differences, and similarities. R. Soc. Chem. Soft Matter. 2012;8:1719–1729. [Google Scholar]
- Mohamed M.I. Topical emulsion gel composition comprising diclofenac sodium. AAPS J. 2004;6(3):1–7. [Google Scholar]
- Neishboor E.R., Jallili R., Hartwell R., Leung V., Carr N. Topical application of a film-forming emulgel dressing that controls the release of stratifin and acetylsalicylic acid and improves/prevents hypertrophic scarring. Wound Repair Regen. 2013;21(1):55–65. doi: 10.1111/j.1524-475X.2012.00857.x. [DOI] [PubMed] [Google Scholar]
- Patel R.B., Patel M.R., Bhatt K.K., Patel B.G. Formulation and evaluation of micro-emulsion based drug delivery system for intranasal administration of olanzapine. Int. J. Biomed. Pharm. Sci. 2013;1(7):20–27. [Google Scholar]
- Patel P., Monapara M.A., Mandal S.N., Patel N., Rajesh K.S. Formulation and evaluation of Micro-emulsion based gel of Itraconazole. Pharmagene. 2013;1(2):32–36. [Google Scholar]
- Pouton W.C. Self-emulsifying drug delivery systems: assessment of the efficiency of emulsification. Int. J. Pharm. 1985;27:335–348. [Google Scholar]
- Pouton W.C. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and self-microemulsifying drug delivery systems. Eur. J. Pharm. Sci. 2000;11(2):593–598. doi: 10.1016/s0928-0987(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Setty C.M., Rupal S.B., Pathan I.B. Development of Valdecoxib topical gels: effects of formulation variables on the release of Valdecoxib. Int. J. Pharm. Pharm. Sci. 2010;2(1):70–73. [Google Scholar]
- Shinde A.J., Patil R.R., Devranjan P.V. Micro-emulsion of lamotrigine for intranasal delivery. Ind. J. Pharm. Sci. 2007;69(5):721–722. [Google Scholar]
- Shingade G.M., Aamer Q., Sabale P.M., Grampurohit N.D., Gadhave M.V. Review on: recent trend on transdermal drug delivery system. J. Drug Dev. Ther. 2012;2(1):66–75. [Google Scholar]
- Singla V., Sanini S., Rana A.C., Singh G. Development and evaluation of topical emulgel of lornoxicam using different polymer bases. Int. Pharm. Sci. 2012;2(3):36–44. [Google Scholar]
- Spectrometry Skill, 2014. <www.austincc.edu/mlt/chem/Spec%20Lab_2014.do>.
- Surjyanarayan M., Mandal S.S., Sawant K.K. Design and development of micro-emulsion drug delivery system of atorvastatin and study its intestinal permeability in rats. Int. J. Drug Dev. Tech. 2010;2:69–75. [Google Scholar]
- Vijya P.B., Shanmugam V., Laxmi P.K. Development and optimization of novel diclofenac emulgel for topical drug delivery. Pharm. Glob. Int. J. Compr. Pharm. 2011;9(10):1–4. [Google Scholar]
- Vincent C.H., Principle of skin therapy, 13. <http://www.dermweb.com/therapy>.


