Abstract
Purpose
The aim of this study was to determine the frequency of clinically unsuspected ocular surface squamous neoplasia (OSSN) in cases of biopsied pterygium (PT).
Methods
We reviewed 15,016 cases presented at the Henry C. Witelson Ocular Pathology Laboratory during the period 1993–2013. All cases with a clinical diagnosis of PT were included. Histopathological diagnoses were reviewed and demographic data were retrieved from histopathological request forms. All cases associated with OSSN were re-evaluated independently by two ocular pathologists. The classification of OSSN in PT cases was made based on the Armed Forces Institute of Pathology (AFIP) recommendations.
Results
Two hundred and fifteen cases were diagnosed clinically as PT (1.43%) and 54% were from male patients. The average age at diagnosis was 53.4 ± 15.5 years. OSSN was identified in five cases (2.33%), and four of these cases were from female patients (80%). The average age of patients with PT and OSSN was similar to PT patients without OSSN (P > 0.05). Cases with OSSN were diagnosed as conjunctival intraepithelial neoplasia (CIN) I (60%), CIN II (20%), and CIN III (20%). There was complete agreement between the two pathologists (100%).
Conclusions
The relatively high rate of dysplasia in a low ultraviolet light index area challenges the main cause of this disease in our population, a hypothesis that should be evaluated in future studies. We suggest that all PT samples should be sent for histopathological evaluation even in areas with low ultraviolet light index.
Abbreviations: PT, pterygium; UV, ultraviolet; OSSN, ocular surface squamous neoplasia
Keywords: Pterygium, Squamous neoplasia, UV light
Introduction
Pterygium (PT) is a common conjunctival lesion of unknown etiology that can cause ocular irritation, visual disturbances, and most often cosmetic issues. Clinically, PT manifests as a wing-shaped fleshy growth, usually occurring on the nasal conjunctiva and cornea, growing toward the center of the cornea. Histologically, it shows elastotic degeneration of the substantia propria.1 The main risk factor for the development of this lesion has been found to be ultraviolet (UV) light exposure.2 This association is supported by the higher prevalence of PT in regions with high UV exposure, notably regions closer to the equator.3, 4
Ocular surface squamous neoplasia (OSSN) represents a broad spectrum of corneal and conjunctival epithelial neoplasias that range from dysplasia (conjunctival intraepithelial neoplasia [CIN] I, II, and III or mild, moderate or severe dysplasia respectively) to invasive squamous cell carcinoma (SCC).5 OSSN shares some risk factors with PT, in particular exposure to UV radiation. OSSN also varies worldwide with a prevalence of 1.9% estimated in Australia and age standardized incidence rates (cases/year/100,000 inhabitants) of 1.38 in Africa and an incidence of 0.05% in Europe.6 Because OSSN and pterygium share the same risk factors, they are believed to coexist and may even be related.7, 8
The relationship between both entities has been previously described in the literature. The prevalence of OSSN in PT is 9.8% in Brisbane, Australia8 and 5% in Sydney, Australia.7 In Florida, the prevalence is 1.7%,3 while it is 0% in Toronto, Canada.9 The different UV indices in various locations around the world are shown in Table 1. In addition, some case reports have shown that pterygium can masquerade as a wider array of epithelial neoplasia and even some hematological malignancies.10
Table 1.
Average UV index in different locations around the world. Modified from: [http://www.who.int/uv/intersunprogramme/activities/uv_index/en/index3.html].
| Country (City) | . | J | F | M | A | M | J | J | A | S | O | N | D |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia (Darwin) | 13°S | 12 | 13 | 12 | 10 | 8 | 8 | 8 | 10 | 11 | 13 | 12 | 12 |
| Canada (Vancouver) | 49°N | 1 | 1 | 3 | 4 | 6 | 7 | 7 | 6 | 4 | 2 | 1 | 1 |
| USA (Los Angeles) | 34°N | 3 | 4 | 6 | 8 | 9 | 10 | 10 | 9 | 7 | 5 | 3 | 2 |
The purpose of this study was to determine the frequency of OSSN in PT specimens in a low UV area, mainly Montreal, Quebec, Canada.
Methods
All specimens received between 1993 and 2013 at the Henry C. Witelson Ocular Pathology Laboratory (McGill University, Montreal, Canada) were reviewed. All cases with a clinical diagnosis of PT were included in the study. The final pathological diagnosis and clinical information, including age and gender, were obtained. Squamous lesions were classified pathologically according to the Armed Forces Institute of Pathology (AFIP) as PT, or CIN I, II, or III. All cases with dysplastic changes were reviewed independently by two pathologists. All data accumulation was in accordance with Canada and the Province of Quebec legislation and adhered to the tenets of the Declaration of Helsinki.
Data management and statistical analysis
Data are presented as percentages and means ± standard deviation (SD). The Student’s t-test was used to compare the average age at presentation of PT with and without OSSN. The association between gender and OSSN in PT was analyzed using Fisher exact test and odds ratio (OR) with 95% confidence intervals (Cis). Differences were considered significant when P < 0.05. Statistics were performed using Microsoft Excel (Microsoft, Redmond, WA, USA) and GraphPad Prism 5.0 software (San Diego, CA, USA).
Results
Of 15,016 cases presented to the Henry C. Witelson Ocular Pathology Laboratory during the period of 1993–2013, 215 (1.43%) PT cases were collected. Most of these cases (54%) were from male patients. The average age at diagnosis was 53.4 ± 15.5 years.
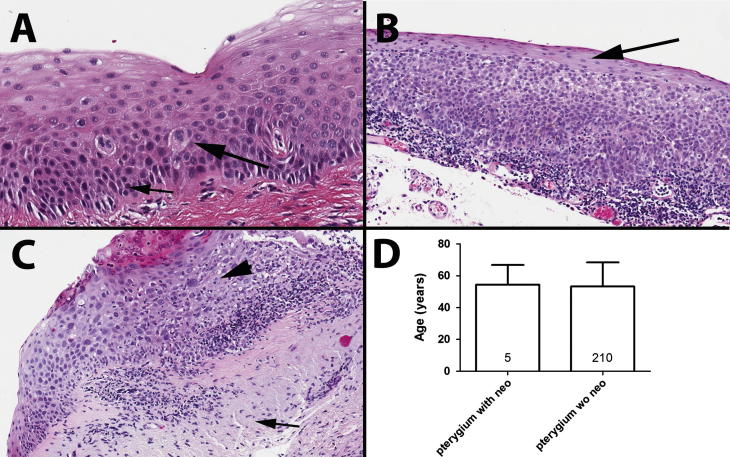
The frequency of epithelial neoplasia in PT cases was 2.33% (n = 5), of which four of the five cases were from female patients (80%). The average age at presentation of those cases was 59.2 ± 14.75. The average age of patients presenting with PT without neoplasia was 53.4 ± 15.5. Dysplastic changes in PT cases were CIN I (60%, Fig. 1A), CIN II (20%, Fig. 1B), and CIN III (20%, Fig. 1C). The average age of the patients presenting with PT with dysplasia did not significantly differ from patients with PT without dysplasia (P = 0.87). Similarly, there was no association between gender and OSSN in PT (P = 0.18).
Figure 1.
Representative examples of PT with OSSN. (a) Pterygium with CIN I (mild dysplasia). Large arrow shows large and irregular nuclei. Smaller arrow shows loss of polarity of the basal cells. (b) Pterygium with CIN II (moderate dysplasia). The dysplastic cells occupy two thirds of the epithelial thickness, showing maturation in the higher stratum (arrow). (c) Pterygium with CIN III (severe dysplasia). The dysplastic cells occupy the full thickness of the epithelium. Arrow head shows atypical mitotic figure in the upper part of the epithelium. Shorter arrow shows elastotic degeneration. (d) Average age of pterigyum with and without dysplasia.
Discussion
In this study, the frequency of OSSN within surgically excised PT was higher than expected (2.33%). This might be explained by the fact that PT is excised primarily when they cause visual problems by either being in close proximity to the visual axis or by inducing astigmatism, having a suspicious appearance and growth, or due to symptoms of irritation or cosmetic disturbances.11 As a result, cases with suspicious appearance will be sent for histological examination while benign looking cases will be simply discarded.
We strongly believe that the dysplastic findings (CIN) in PT cases emphasize the notion that every sample should be sent for microscopic analysis, because these patients need closer follow-up based on the fact that 10–24% of them will have OSSN recurrences within the next 15 years.12
Having a relatively high number of OSSN in cases of PT is unexpected in our area since Montreal has a relatively low yearly UV exposure (similar to the latitude of Vancouver) (Table 1). Our frequency rates were close to rates reported in Sydney and even higher than in Florida3, regions that are known to have higher yearly exposures to UV rays than Montreal. This could be partly explained by the fact that snow and snowfall persist late into spring in Montreal. Furthermore, it has been reported that UV rays reflected off snow plays a greater role in tissue damage13 with the addition that snowflakes have the highest UV reflectance. While these mechanisms have been postulated to cause corneal disease, such as photokeratitis and climatic droplet keratopathy,14 to the best of our knowledge, no strong evidence has demonstrated a relation with PT. Moreover, these results might also be explained by the high ethnic heterogeneity of the population in the Montreal region, including immigrants who come from countries near the equator. Unfortunately, however, this information is not available in medical charts. Also, it is important to highlight that although our institution is a referral center in ophthalmic pathology, none of the cases in this series was a referral, emphasizing the actual representation of our cases from our area.
Analysis of the demographic data showed that there was no significant difference in the average age of patients with PT associated with epithelial neoplasia and patients having PT without epithelial neoplasia (P = 0.87). Furthermore, the average age of dysplastic PT cases was even lower than those found in previous studies.15 Hence, age does not seem to be a significant demographic risk factor for the development of OSSN in PT cases in our study. Thus, PT samples in younger patients should also be sent for pathological evaluation. In addition, OSSN associated with PT was found to be more frequent in females than males (80% vs. 20%), which goes against a previous studies showing a higher prevalence of OSSN in men.15 These results are intriguing since most outer occupational activities are traditionally undertaken by males. This further brings into question the hypothesis that UV radiation is the main risk factor for OSSN development in our population, although our sample size is too small to come up with any definitive conclusions.
Most epithelial lesions associated with PT were CIN I (60%). This assumes progressive damage by the main risk factor of the region (UV or other) that has caused the progression to CIN I (mild dysplasia). To avoid overestimating CIN I lesions, all cases were reviewed by two pathologists and 100% concordance was found. All patients with OSSN should be followed on a regular basis to screen for the occurrence of new lesions in the conjunctiva because these patients already have enough risk factors for developing new OSSN in the currently uninvolved conjunctiva. In addition, we argue that all PT samples should be sent to pathology since PT and OSSN share similar risk factors, and therefore can co-exist.7, 8
Interestingly, most OSSN in the present study was found to be CIN I, as in Florida, while the most common neoplasia was CIN II in Australia. This might be explained by the strict and thorough evaluation of such lesions in North America where the lesions are removed before they develop into a CIN II or it might be explained in part by the higher UV exposure in Australia, which might lead to more rapid lesion progression.
The treatment options for cases of PT are controversial. One of the main problems encountered after surgical excision alone is the high recurrence rate which can reach up to 89%.16 All the cases of PT with OSSN in this cohort are primary cases from different patients and none of them is a recurrence. Table 2 shows the characteristics of each individual case with the follow-up.
Table 2.
Cases of pterygia associated with OSSN in our cohort.
| Case number | Gender | Age at biopsy | Degree of dysplasia | Follow-up |
|---|---|---|---|---|
| 1 | F | 58 | CIN I | No recurrence after 6 years f/u |
| 2 | F | 78 | CIN I | Lost f/u |
| 3 | F | 59 | CIN II | Lost f/u |
| 4 | F | 64 | CIN III | No recurrence after 2.5 years f/u |
| 5 | M | 37 | CIN I | Lost f/u |
OSSN: Ocular surface squamous neoplasia; M: Male; F: Female; CIN: Conjunctival intraepithelial neoplasia; f/u: follow-up.
Our study is not without limitations. For instance, it is important to highlight that our study is retrospective, and thus considers only biopsied lesions and may not represent the actual frequency of OSSN in PT in our region. However, our study shares this bias with similar studies from around the globe, and thus presents meaningful data. The relatively small number of cases in this series can be attributed to the lower incidence of PT in this area compared to other high risk areas6 and the possibility that some excised lesions are not sent to histopathological examination; however, the real incidence of OSSN in PT in this part of the world has not been well studied to date.
In conclusion, this study showed an unexpectedly relatively high frequency of OSSN in PT cases in a city where UV exposure is low. Moreover, age did not seem to be a significant risk factor to develop OSSN in cases of PT and most of these patients were female. We therefore highly suggest that all PT specimens that are surgically excised, regardless of the age and sex of the patient, should be sent for pathological evaluation. In addition, if an OSSN is found, the patient requires closer follow-up for screening of new lesions.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Jaros P.A., DeLuise V.P. Pingueculae and pterygia. Surv Ophthalmol. 1988;33(1):41–49. doi: 10.1016/0039-6257(88)90071-9. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie F.D., Hirst L.W., Battistutta D., Green A. Risk analysis in the development of pterygia. Ophthalmology. 1992;99(7):1056–1061. doi: 10.1016/s0161-6420(92)31850-0. [DOI] [PubMed] [Google Scholar]
- 3.Oellers P., Karp C.L., Sheth A. Prevalence, treatment, and outcomes of coexistent ocular surface squamous neoplasia and pterygium. Ophthalmology. 2013;120(3):445–450. doi: 10.1016/j.ophtha.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panchapakesan J., Hourihan F., Mitchell P. Prevalence of pterygium and pinguecula: the Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1998;26(Suppl. 1):S2–5. doi: 10.1111/j.1442-9071.1998.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 5.Basti S., Macsai M.S. Ocular surface squamous neoplasia: a review. Cornea. 2003;22(7):687–704. doi: 10.1097/00003226-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Gichuhi S., Sagoo M.S., Weiss H.A., Burton M.J. Epidemiology of ocular surface squamous neoplasia in Africa. Trop Med Int Health. 2013;18(12):1424–1443. doi: 10.1111/tmi.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chui J., Coroneo M.T., Tat L.T., Crouch R., Wakefield D., Di Girolamo N. Ophthalmic pterygium: a stem cell disorder with premalignant features. Am J Pathol. 2011;178(2):817–827. doi: 10.1016/j.ajpath.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirst L.W., Axelsen R.A., Schwab I. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol. 2009;127(1):31–32. doi: 10.1001/archophthalmol.2008.531. [DOI] [PubMed] [Google Scholar]
- 9.Yeung S.N., Kim P., Lichtinger A. Incidence of ocular surface squamous neoplasia in pterygium specimens: an 8-year survey. Br J Ophthalmol. 2011;95(4):592. doi: 10.1136/bjo.2010.197491. [DOI] [PubMed] [Google Scholar]
- 10.Subramaniam M.M., Tan M., Lee S.Y., Amrith S., Fredrik P., Ng S.B. Concomitant squamous cell carcinoma and myeloid sarcoma in pre-existing pterygium of the conjunctiva: diagnostic challenges. J Clin Oncol: Off J Am Soc Clin Oncol. 2012;30(10):e115–118. doi: 10.1200/JCO.2011.39.7844. [DOI] [PubMed] [Google Scholar]
- 11.Hirst L.W. The treatment of pterygium. Surv Ophthalmol. 2003;48(2):145–180. doi: 10.1016/s0039-6257(02)00463-0. [DOI] [PubMed] [Google Scholar]
- 12.Maudgil A., Patel T., Rundle P., Rennie I.G., Mudhar H.S. Ocular surface squamous neoplasia: analysis of 78 cases from a UK ocular oncology centre. Br J Ophthalmol. 2013;97(12):1520–1524. doi: 10.1136/bjophthalmol-2013-303338. [DOI] [PubMed] [Google Scholar]
- 13.Sliney D.H. Geometrical assessment of ocular exposure to environmental UV radiation–implications for ophthalmic epidemiology. J Epidemiol/Jpn Epidemiol Assoc. 1999;9(6 Supp. l):S22–32. doi: 10.2188/jea.9.6sup_22. [DOI] [PubMed] [Google Scholar]
- 14.Taylor H.R. Climatic droplet keratopathy and pterygium. Aust J Ophthalmol. 1981;9(3):199–206. doi: 10.1111/j.1442-9071.1981.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 15.Kao A.A., Galor A., Karp C.L., Abdelaziz A., Feuer W.J., Dubovy S.R. Clinicopathologic correlation of ocular surface squamous neoplasms at Bascom Palmer Eye Institute: 2001 to 2010. Ophthalmology. 2012;119(9):1773–1776. doi: 10.1016/j.ophtha.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 16.Singh G., Wilson M.R., Foster C.S. Mitomycin eye drops as treatment for pterygium. Ophthalmology. 1988;95(6):813–821. doi: 10.1016/s0161-6420(88)33104-0. [DOI] [PubMed] [Google Scholar]