Abstract
Importance: Basic essence of Pharmacovigilance is prevention of ADRs and its precise diagnosis is crucially a primary step, which still remains a challenge among clinicians. Objective: This study is undertaken with the objective to scrutinize and offer a notion of commonly used as well as recently developed methods of causality assessment tools for the diagnosis of adverse drug reactions and discuss their pros and cons. Evidence review: Overall 49 studies were recognized for all assessment methods with five major decisive factors of causality evaluation, all the information regarding reasons allocating causality, the advantages and limitations of the appraisal methods were extracted and scrutinized. Findings: From epidemiological information a past prospect is designed and subsequent possibility merged this background information with a clue in the individual case to crop up with an approximation of causation. Expert judgment is typically based on the decisive factor on which algorithms are based, nevertheless in imprecise manner. The probabilistic methods use the similar principle; however connect probabilities to each measure. Such approaches are quite skeptical and liable to generate cloudy causation results. Causation is quite intricate to ascertain than correlation in Pharmacovigilance due to numerous inherent shortcomings in causality assessment tools. Conclusions and relevance: We suggest that there is a need to develop a high quality assessment tool which can meticulously establish suitable diagnostic criteria for ADRs with universal acceptance to improvise the fundamental aspect of drug safety and evade the impending ADRs with the motive to convert Pharmacovigilance into a state of art.
Keywords: Adverse drug reactions, Causality assessment tools, Algorithms, Pharmacovigilance
1. Introduction
Almost all the drugs utilized for therapeutic benefits are associated with inevitable risks of adverse drug reactions, varying from very minor to exceedingly severe and infrequently lethal untoward effect (Curtin and Schulz, 2011). Furthermore, ADRs are undisputedly illustrated as one of the frequent causes of morbidity and mortality, in spite of wide-ranging and well-regulated registration practices for verifying drug efficacy and safety (Clavenna and Bonati, 2009, Nakamura, 2008), recent study from Sweden demonstrated ADRs as seventh most recurrent cause of death (Wester et al., 2008), and one in seven hospitalized patient develops an ADRs, asserting that ADRs as an important factor of morbidity and mortality (Davies et al., 2009).
The scientific method concerned with comprehension, recognition and prevention of adverse drug reactions is acknowledged as pharmacovigilance (Avery et al., 2011, Khan et al., 2012, Khan et al., 2013) and its basic essence is prevention of ADRs (Khan et al., 2013). It also needs to be emphasized that all ADRs are not preventable (Khan et al., 2013, Macedo et al., 2005), but precise diagnosis of ADRs is crucially a primary step to reduce those that are preventable, which still remains a challenge among clinicians (Macedo et al., 2005). Ability of adverse drug reactions to implicate any organ or system and its wide variety of clinical manifestations, often makes it difficult to diagnose (de Vries et al., 2008), and pharmacotherapy often makes the differentiation of patient’s sign and symptoms more complex, some of them might be due to disease process or due to one or more drugs. This demarcation requires a recognized and precise technique to attribute the causality of sign and symptom to a specific drug (Bates et al., 2003).
The causality appraisal basically comprises of evaluation of the probability that the detected untoward event is produced by a specific medication and hence it is recognized as an important tool of Pharmacovigilance (Macedo et al., 2005). The need of developing a standard presumption for the correlation, probability for a reported case of an alleged adverse drug reaction, was premeditated in an anticipation that in future this would guide toward a consistent and mimic able capability of the causality, which is essential to compute the risk–benefit relationship assessment of the medications (Du et al., 2012; WHO–UMC causality assessment (Accessed on 24 Feb, 2013); Arimone et al., 2010, Jones, 2005). Moreover, it also diminishes the incongruity between assessors, categorizes relationship likelihood and improves the scientific evaluation of ADRs (Du et al., 2012; WHO–UMC causality assessment (Accessed on 24 Feb, 2013); Arimone et al., 2010, Jones, 2005).
Paradoxically, almost all the causality assessment tools are intrinsically inconsistent, and additionally there is non-availability of analytical measures which can minimize their inter-rater and intra-rater inconsistency. This is overcome by utilization of some of their regular characteristics to facilitate in accomplishment of a conclusion on the alleged drug as well as certain queries which are utilized to determine the detail components of the ADRs. Subsequently, a variety of procedures are followed to interpret the responses from these queries to estimate the likelihood of an ADR (Macedo et al., 2005, Du et al., 2012, Arimone et al., 2010, Gallagher et al., 2011). Causality assessment tool is practically unworthy if reproducibility of the results is poor and may differ with background and experience of the evaluator (Davies et al., 2011).
In spite of the availability of large number of causality assessment tools, which vary from easy to intricate, still there is no unanimity on acceptance of any causality assessment tool as universal (Du et al., 2012, Gallagher et al., 2011, Davies et al., 2011). This study is undertaken with the objective to scrutinize and offer a notion of various commonly used as well as recently developed methods and discuss their pros and cons.
2. Methods
A systematic exploration was accomplished to get access of suitable articles/studies in the Pub med, Medline, Scopus, Cochrane database and Google scholar search engine from 1976 to July 2014. Additional search was also carried out from the Institute of Medicine, WHO and FDA. The searching procedure employed the keywords and/or the MeSH terms “algorithms”, “Bayesian scale”, “global introspection scale or assessment”, “causality assessment”, “causality assessment tools”, “diagnosis of ADRs”, “ADRs detection tools” and “assessment scale”, joined with any option of the following: “adverse drug reactions,” adverse drug event”, “pharmacovigilance”, “drug surveillance program”, “drugs side effects”, “drugs toxic effects”. The reference list of related articles was additionally explored to discern further potentially significant articles.
3. Included articles
Articles explored were original and review articles including both retrospective and prospective studies on ADRs detection tools or ADRs causality assessment tools in both adults and children as well as preventable ADRs on hospitalization and during hospitalized stay. The fundamental measures extracted from the primary studies were interrater reliability and reproducibility. Assessment of validity of included articles was done by observing the inclusion of “confounding variables” for instance simultaneous utilization of other drugs, absence of dechallenge, underlying disease and lack of authentic published illustrations of ADR.
4. Excluded articles
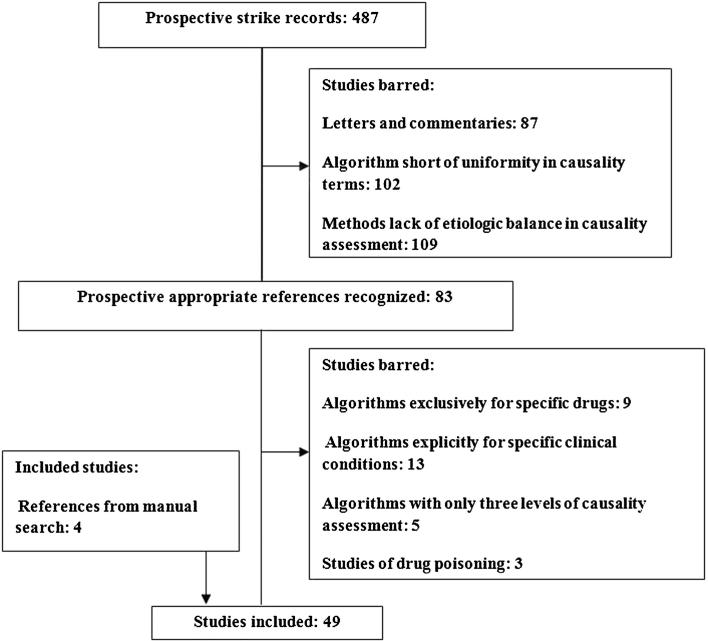
Algorithms short of uniformity in causality terms, methods lacking of etiologic balance in causality assessment, algorithms for specific drug only (e.g. Sodium Valproate or antimicrobials), algorithms for unambiguous clinical manifestation of ADRs studies (liver toxicity or cancers), algorithms which have only three levels for causality assessment and those related to therapeutic failures, drug induce poisoning were excluded. Duplicate references were also eliminated (see Fig. 1 for the flowchart on inclusion/exclusion studies)
Figure 1.
Flowchart of causality assessment tools.
Collaborator: Adults and children as characterized by the original study authors.
4.1. Data extraction and its analysis
Comprehensive information regarding study design, methods utilized for the diagnosis of adverse drug reactions with customary combination of five major decisive factors of causality evaluation explicitly, challenge, dechallenge, rechallenge, their past bibliographic account and etiologic substitutes were extracted and all the information regarding reasons allocating causality, the advantages and limitations of the appraisal methods was entered in data sheet. Furthermore, findings of the included studies were considered for generalizabilty, and all extracted articles were then scrutinized to determine the relationship between data collection period and aforementioned parameters of causality assessment of ADRs including unequivocal inter rater reliability and reproducibility.
4.2. Types of ADR tools and their salient features
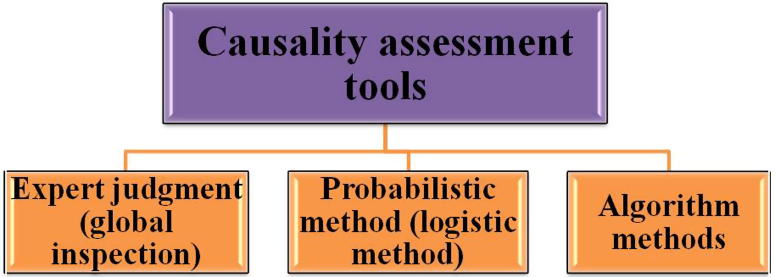
Several causality assessment methods are described to evaluate the causality linking pharmacotherapy with ADRs (Arimone et al., 2007, Macedo et al., 2003), during the past three decades dynamic endeavors are consistently going on, in order to materialize a universal causality assessment tool for the diagnosis of ADRs (Arimone et al., 2005, Théophile et al., 2010). Assessment of ADR probability necessitates a structure in order to abolish disagreements among the evaluators irrespective of their divergence in clinical specialization and experience (Macedo et al., 2005). These methods broadly utilize three approaches.
4.2.1. Expert judgment (global inspection)
This is most universally utilized method for causality evaluation of individual adverse drug reaction reports (Fig. 2). This is basically an individual assessment of ADR commonly executed by a clinical expert, employing his knowledge and past experience, nevertheless without use of any uniform tool in order to accomplish a conclusion concerning causality (Macedo et al., 2005, Arimone et al., 2007, Shapiro, 2004, Peter Ian Pillans, 2008).
Figure 2.
Types of causality assessment tools.
Advantage: It plays a major role in the identification and rating of potential ADRs (Arimone et al., 2007, Arimone et al., 2005, Théophile et al., 2010). Furthermore, it is often emphasized that expert or clinical judgment ought to play a leading role and remains to be par excellence in contrast to statistical methods (Shapiro, 2004, Peter Ian Pillans, 2008).
Limitation: Moreover, despite of its effectiveness clinical judgment is characterized by inter- and intra-rater contradiction, discernible prejudice, ambiguity and weak reproducibility (Koh et al., 2008, Karch and Lasagna, 1977, Kramer et al., 1979).
4.2.2. Probabilistic method (logistic method)
About all probabilistic methods are the derivatives of Bayes theorem (Macedo et al., 2005, Théophile et al., 2010) (Fig. 2). These methods require a probability for causality which are calculated from available knowledge, (previous estimation) in addition it also necessitates the specific findings in a case, which combined with the background information, determines the probability of drug causation for the case.(posterior estimate) (Macedo et al., 2005, Théophile et al., 2010).
Advantage: The logistic or probabilistic methods are apparently more sensitivity, they also have positive predictive value and in addition provide an outcome as incessant probabilities, and therefore appear to be quite creditable to utilize for a trustworthy assessment of adverse drug reactions in regular practice or automated evaluation of case reports of the suspected but still unknown ADRs (Du et al., 2012, Doherty, 2009).
Limitation: Nevertheless, the major drawbacks of these approaches are poor specificity and moreover practically complex as they require specifically calculated information data, such as specific ADR incidence, to reproduce the likelihood distribution (Du et al., 2012, Doherty, 2009).
4.2.3. Algorithm methods
The distinctive feature of algorithm method is that they comprise of sets of explicit queries with defined scores for computing the probability of a cause and effect correlation (Fig. 2). Fundamentally, it comprises of a questions in a sequence which can be responded by “Yes/No” with resultant allocation of plus or minus scores, finally a causality assessment is prepared by computing the number of points, relying on the point score, the strength of a causal relationship is subsequently judged as “definite, probable, possible or unlikely” (Théophile et al., 2013, Naranjo et al., 1981).
Advantage: Since last three decades numerous algorithms came with the assertion that estimated method of scoring has added discerning value of discrimination and more instantly recognizable (Arimone et al., 2007, Arimone et al., 2005, Macedo et al., 2005). Algorithms have attractive simplicity and therefore find comprehensive application for the assessment of ADRs, exclusively to eliminate or at least reduce inter-rater and intra-rater dissimilarity (Macedo et al., 2005, Théophile et al., 2013). In contrast to expert judgment and the Bayes’ approaches, algorithm had poor sensitivity but good specificity (Arimone et al., 2007, Doherty, 2009) (see Table 1). Furthermore, algorithm methods improvise the logical feature of causality assessment and they are frequently employed by the journals and various Pharmacovigilance centers to spot individual case reports (Doherty, 2009).
Table 1.
Common method used for ADR assessment tool and their important characteristics.
| Methods of causality assessment tools | Advantages | Limitations |
|---|---|---|
| Naranjo et al. (1981) | Simple and brief most extensively used | Dependability and validity not confirmed in children |
| Koh et al. (2008) | Higher sensitivity and specificity in comparison with other algorithm methods | Probability scores given by this algorithm are not assured to be the exact causality probabilities for the cases of ADRs |
| Karch and Lasagna (1977) | No specific advantage in comparison with any other methods | Reliability and validity not well established |
| Kramer et al. (1979) | No specific advantage in comparison with any other methods | Employ exhaustive flowcharts, excessively intricate and protracted for realistic application |
| Begaud (1984) | No specific advantage in comparison with any other methods | Its application requires 3-stage flow chart, not protracted but unable to employ all feature characteristically utilized in ADR appraisal |
| WHO – Uppsala monitoring center-causality assessment 1994 | Mainly planned as convenient tool for the assessment of individual case reports | It is a nonprobabilistic method and creates extensive unpredictability in evaluation |
Limitation: Conversely, algorithms methods are characterized by their inability to ascertain the causality consistently due to lack of regards to the “confounding variables” like underlying illness, concurrent use of other drugs and lack of available description of ADRs (Macedo et al., 2005). It is sturdily dependent on the weight of each sustaining or skeptical criterion that has been determined randomly by authors of each method (Arimone et al., 2007). They also suffer a major drawback to verify and invalidate the causality, besides inability to provide precise quantitative dimension of the probability of a relationship (Doherty, 2009). Nevertheless, none of the algorithms are unanimously acknowledged as a trustworthy or a recognized tool, this is exemplified by several studies that evaluation of same ADR reports by utilization of different algorithms, demonstrated significant variations of the results (Macedo et al., 2005, Arimone et al., 2007, Doherty, 2009).
4.3. Commonly used algorithms used and their tribulations
Algorithms are basically premeditated to increase inter-rater reliability and reduce the intra-rater agreement, and in comparison with clinical judgment, undoubtedly their score offers a fair degree of homogeneity or consensus (Doherty, 2009), this is exemplified by the use of Naranjo’s algorithm (Naranjo et al., 1981), which revealed that by using clinical judgment method, the intra rater agreement of a group of experts varies from 41% to 57%. In contrast when the same panel of expert utilized the Naranjo’s algorithm, there was statistically significant escalation of intra rater agreement from 80% to 97%. This highlights a higher degree of specificity for algorithms in detection of ADRs (Gallagher et al., 2011, Davies et al., 2011, Doherty, 2009, Naranjo et al., 1981, Koh et al., 2008) (see Table 1).
During the past three decade more than thirty algorithms of causality evaluation tools were developed, example of those commonly utilized includes the Naranjo’s algorithm (Naranjo et al., 1981), the Karch algorithm (Karch and Lasagna, 1977), the Kramer algorithm (Kramer et al., 1979), the Begaud algorithm (Begaud, 1984) and the WHO–UMC (WHO–UMC causality assessment (Accessed on 24 Feb, 2013)). All these algorithms are characterized by unambiguous similarities and dissimilarities.(see Table 1) Naranjo’s algorithm in comparison with the aforesaid methods has the advantage of being simple and brief, in addition to reduction in inter-rater disagreement and uncertainty in evaluation of potential ADRs and therefore utilized by Pharmacovigilance centers of several nations (Doherty, 2009). Conversely, reliability of Naranjo’s algorithm has been established in adults but not in children (Weiss et al., 2002). In a recent comprehensive review of ADRs in children (2012) which included 102 studies, nearly half of them utilized Naranjo’s algorithm for causality assessment and this may result in false estimation of ADRs in their studies (Smyth et al., 2012). Scrutiny of ADRs in a well organized approach necessitates an accurate and consistent causality evaluation tool with universal applicability for both adult and children and still needs improvisation (Khan, 2013, Du et al., 2012). The predicament of algorithms methods is that they frequently comprise of queries on dechallenge an rechallenge, and this is quite unrealistic and unethical due to involvement of safety concern for the patient, and at the same time exclusion of rechallenge restricts the gradation of causality only to a maximum “possible” (Doherty, 2009). Furthermore, inherent flaw of algorithms is depicted on its dependability to “Yes/No” response which seems to be characterized by recollect prejudice (Doherty, 2009). Furthermore, algorithms often suffer a major drawback to verify or invalidate the causality and at the same time, they have inability to provide precise quantitative dimension of the probability of an affiliation, however they are valuable in evaluation of causality in possible ADRs and improvement of scientific basis and learning of causality evaluation (Doherty, 2009).
4.4. Recent advances in causality assessment tools
The current approach for evaluation of suspected ADRs still incorporates the utilization of either clinical judgments or algorithms, notwithstanding their intrinsic limitation of inability to determine the probability of the ADR causality. It is exceedingly vital to have an algorithm which can determine the causality of ADR, as well as likelihood of causal relationship. During the past five years a few causality assessment tools are further added with the aspiration of enhancing the sensitivity, reproducibility and to provide quantitative estimation of a suspected ADR.
4.4.1. Genetic algorithm
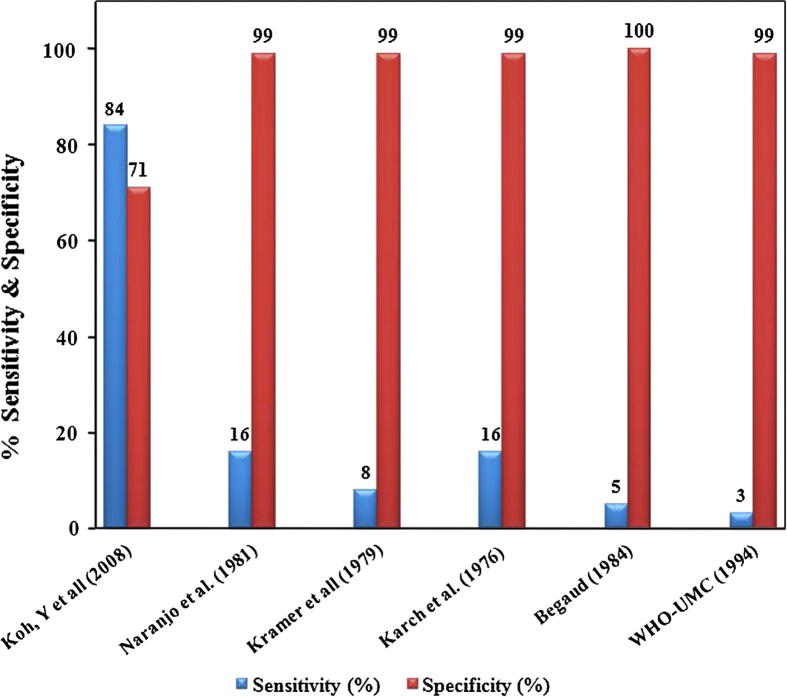
The prime objective of this method is to incorporate a probability score in the algorithm to facilitate the acquisition of a quantitative likelihood of suspected ADRs (Koh et al., 2008), this is reported to make possible the quantification of ADR signals and likely to have an beneficial impact of its utility in clinical practice as well as clinical trials of new drugs, in addition to have even more correct analysis of ADR data from spontaneous reporting in a big national Pharmacovigilance centers (Waller et al., 2005, Heckerling et al., 2007). This method used the concept of mutation and cross-over in order to find out new scoring system with reduction of optimization tribulations and calculating requirements. Such methods were employed effectively in prediction of urinary tract infection, diagnosis of multi-disorder and determination of therapeutic doses of radiation therapy (Heckerling et al., 2007, Cotrutz and Xing, 2003). This newer algorithm demonstrated higher degree of sensitivity and specificity in comparison with most of the prevalent algorithm methods (see Fig. 3) and its use can culminate into detection of higher numbers of cases correctly as definite ADRs (Koh et al., 2008). But, in view of the lack of definite methods to confirm the reliability of probabilities values of any of the prevailing algorithm, the probability scores given by this new algorithm are not a guarantee to be the exact causality probabilities for the cases of ADRs. Nevertheless, addition of an appendix to this algorithm claimed to enhance the reliability, sensitivity and reduces the variability with resultant consistent outcomes even when used by different users (Koh et al., 2008).
Figure 3.
Sensitivity and Specificity of CAT final.
4.4.2. Liverpool algorithm
There is no universal acceptance of even a single method of causality assessment tools as result of tribulations of reproducibility and legitimacy (Macedo et al., 2005, Agbabiaka et al., 2008, Avner et al., 2007, Garcia-Cortes et al., 2008), and moreover Naranjo’s tool finds wider application despite of its lack of sensitivity (Jones, 2005, Smyth et al., 2012). Development of this tool was aspired to overcome some of these limitations (Gallagher et al., 2012).
The basic design comprises of a flow diagram with classification approach based on robust binary decision ,where responses are further directed to precise queries, rather than scoring system and weighted answers as utilized in Naranjo’s tool which have a propensity to offer additional sway to a number of variables. This tool was developed by a clinical team which includes nurses, pharmacists and clinicians involving both adults and pediatric patients, and asserted to be user friendly, devoid of uncertainty and more suitable for causality evaluation (Gallagher et al., 2011). Furthermore, in comparison with Naranjo’s tool it was observed that inter-rater reliability was enhanced while intra-rater reliability remains unchanged. A significant enhancement of categorization of few definite cases was determined with this tool, which is never achieved with Naranjo’s tool (Gallagher et al., 2011, Théophile et al., 2013). This is substantiated in a recent study, that utilization of this tool in causality assessment, had remarkably demonstrated 37.8% of ADRs classified as ‘definite’ causality (Gallagher et al., 2012) Seemingly, the Liverpool algorithm demonstrates its utility for spontaneous reports and as well as clinical trials of new drugs (Gallagher et al., 2011). However, it is pertinent to observe that the validity of this tool was executed internally rather than independently by other investigators and claimed to be at par, nevertheless not better than several other tools (Gallagher et al., 2011, Gallagher et al., 2012, Naranjo et al., 1981, Koh and Li, 2005).
4.4.3. Pediatric algorithm
The propensity of children to develop ADRs is due to their intrinsic divergence in Pharmacokinetic and Pharmacodynamic of drugs, variation of their disease process from that of adults (Khan et al., 2013, Chien and Ho, 2011), and this is further substantiated by the lack of precise and logical causality evaluation tool unequivocally for this age group (Khan et al., 2013, Weiss et al., 2002, Smyth et al., 2012). This essentially inspired and premeditated this algorithm in order to afford a suitable, consistent, and comprehensible tool for ADR detection in neonates (Du et al., 2012). This specialized tool was developed by the experts of neonatal clinical pharmacology and it is based on actual patient data by using novel mathematical approach. The initial 24 item questionnaire with weighted scoring on Yes/No responses was reduced to 13 item questionnaire by regression analysis, validation of algorithm was done and inter-rater reliability was found to be greater than Naranjo’s algorithm (Du et al., 2012). This algorithm could find its relevance in newborn population, for the ADR detection for the neonatologist and pediatrician. Furthermore this innovative tool permits for the variables of underlying disease and simultaneous utilization of other drugs, while evaluating causality of the reaction (Du et al., 2012). These characteristics might enhance the ability of a clinician to disentangle the ADR from the clinical symptoms of the disease in neonates. Further authentication of this tool requires testing its validity and efficacy by random evaluation in a larger neonatal population with diverse ethnic and racial backgrounds (Du et al., 2012).
4.5. The current status of causality assessment tools
Causation is quite intricate to ascertain than correlation in Pharmacovigilance due to numerous inherent shortcomings in causality assessment tools, in clinical judgments widespread divergence is observed as result of clinician’s propensity to overrate or underestimate the probability of causality (Macedo et al., 2005, Arimone et al., 2005, Miremont et al., 1994, Talbot and Aronson, 2012), the execution of various algorithm is not simple and inter-rater agreement of different algorithms is quite large (Macedo et al., 2005, Arimone et al., 2007, Arimone et al., 2005, Théophile et al., 2013). Quite a few algorithms utilize flowcharts in which if there is a decisive factor in the early part of the procedure, then lack of its accomplishment due to deficiency of available information, often produces neither provable nor refutable data and therefore fails to be completely executed (Talbot and Aronson, 2012). Furthermore, rechallenge is frequently detrimental and impracticable (Macedo et al., 2005, Arimone et al., 2007, Arimone et al., 2005, Théophile et al., 2013, Doherty, 2009, Talbot and Aronson, 2012), and probabilistic methods is dependent on the preceding likelihood approximates that may perhaps not be there (Arimone et al., 2005, Doherty, 2009, Talbot and Aronson, 2012).
Since the adverse drug reactions are hardly ever unambiguous for the drugs with non-availability of specific diagnostic tests and rechallenging tests are unethical (Macedo et al., 2005, Arimone et al., 2007, Arimone et al., 2005, Théophile et al., 2013, Doherty, 2009, Talbot and Aronson, 2012), practicability to label ADRs as “certain” or “unlikely” are quite inconspicuous (WHO–UMC causality assessment (Accessed on 24 Feb, 2013, CDSCO, 2013, USFDA, 2013). However, in clinical trials, precision and transparency of reporting ADRs is essential with the aim of keeping a balance between individual safety and the scientific necessities of safety assessment, unfortunately this needs to be accomplished only by the present imprecise causality assessment tools.
The compensation of a clinical trial participant for serious adverse event as a result of investigational product entails three major aspects – ethics, causality and legitimacy (WHO–UMC causality assessment (Accessed on 24 Feb, 2013, Kulkarni and Bhatt, 2013). The predicament in causality assessment in presence of lack of morality often makes the process of legitimacy a futile effort (WHO–UMC causality assessment (Accessed on 24 Feb, 2013); Talbot and Aronson, 2012; CDSCO, 2013, USFDA, 2013). This induction of repercussions on compensatory rules by the causality assessment tools emphasizes the compelling need of their refinements.
The major rationale for the utilization of algorithms is to augment inter and interrater agreement, and it should be highlighted that, they are neither designed nor intended to replace medical diagnosis (Macedo et al., 2005, Gallagher et al., 2011). In algorithms, presence of some inappropriate questions leads to responses categorized as “unidentified”, which concludes in the lack of sensitivity with underestimation of probability of an ADR (Gallagher et al., 2011). Moreover, almost all the algorithms of causality assessment tools have shown lack of consistency and reproducibility of causality and therefore their reliability always remains uncertain (Gallagher et al., 2011).
The hallmark of algorithms is that, they have basic and intrinsic intricacies in establishing sensitivity and specificity of causality tools, and therefore an algorithm that works in one Pharmacovigilance dataset may not work in a different dataset. Consequently, in spite of rigorous attempts since last four decades across the globe, there is still non availability of universally accepted algorithm, as a gold standard. (Macedo et al., 2005, Théophile et al., 2010, Théophile et al., 2013, Doherty, 2009, Naranjo et al., 1981).
4.6. Recommendations for their future refinement
In view of revamping the monitoring of drug safety and improvisation of Pharmacovigilance, innovative techniques are required for the precise diagnosis of ADRs. Utilization of a DoTs classification of ADRs in causality assessment tools (Aronson and Ferner, 2003), afford an important insight, and give an impression to meticulously establish suitable diagnostic criteria for ADRs, we recommend that relentless positive efforts are required to move critically in this direction for refinement of existing tools by utilization of this classification.
Principally algorithms are usually designated as disproportionality analysis (DA), and several algorithms are proposed for causality assessment of ADRs, which utilizes the ratio of observed drug-event combinations and drug event combinations estimated by sheer likelihood (Hauben and Bate, 2009). Moreover, analytical methods based on DA are now being believed to be the most excellent quantitative screening methods for the recognition of unknown and uncommon ADRs, and also considered as valuable complement to signal detection approaches (Hauben and Bate, 2009). Furthermore, there is a worldwide surge of clinical information with escalating implementation of electronic medical records (EMRs). This leads to opening of new vistas to offer precise diagnosis, laboratory results, radiology results and algorithms for ADR detection signals (Strom and Kimmel, 2006). In addition EMR data also have several strong potentials which include adequate sample size, cost effectiveness and no prospect of recall prejudice (Park et al., 2011). We recommend the potential of EMRs could be utilized to create a novel perspective algorithm in the development of causality assessment tool, with the motive to convert Pharmacovigilance into a state of art.
The elementary core and prospect of causality assessment is to tag an ADR as “definite” or “unlikely” with the intention of transform the uncertainty to certainty, unfortunately contemporary methods of causality assessment fail to achieve this imperative aspect without a rechallenge procedure (Macedo et al., 2005, Arimone et al., 2007, Arimone et al., 2005, Doherty, 2009, Talbot and Aronson, 2012; CDSCO, 2013, USFDA, 2013). Conversely, we strongly assert that the current genomic research (Daly, 2010, Daly, 2012), coupled with innovations in comprehensive database informatics technology (Burns et al., 2013), could be utilized to deploy genetic biomarkers in the current and frequently used causality assessment tools in order to accomplish their basic objective.
The developing field of diagnostic testing for adverse drug reactions continues to create multifaceted issues that pharmaceuticals companies and healthcare providers must recognize. Because the value of precise and immaculate causality assessment tool is so intimately linked by many factors to the development process, a much higher level of coordination will be needed among all stakeholders to realize the full potential of innovative causality assessment tool. The dominance of algorithms in fact degrades clinical medicine, it is an essential part of such exploration, and yet it should be emphasized realistically that basically it is a tool. We believe that the excellent utilization of the attributes as well as collaboration of clinical medicine, clinical pharmacology and epidemiology with judicious observation, harmonized effectively with electronic medical records and genetic biomarkers could play a definitive and crucially important role in the diagnosis as well as documentation of adverse drug reactions and can produce striking successes instead of superimposing algorithms. Clinical judgment for this reason should occupy a driver’s seat in this imprecise discipline of determining causality of adverse drug reactions (Macedo et al., 2005, Shapiro, 2004, Karch and Lasagna, 1977, Kramer et al., 1979, Dennis and Randall, 2010).
5. Conclusion
-
•
Positive impact on quality of healthcare services can be significantly observed by reduction of ADRs in patients, and one of the most important key factors in reducing the incidence of ADRs is undoubtedly its precise diagnosis.
-
•
The current state of art in the diagnosis of ADRs is empirically based, expert judgment is typically based on the decisive factor on which algorithms are based, nevertheless in imprecise manner. The probabilistic methods use the similar principle; however connect probabilities to each measure. Such approaches are quite skeptical and liable to generate cloudy causation results.
-
•
With the objective of superb positive measures of Pharmacovigilance, there is a need to develop a high quality assessment tool which can meticulously establish suitable diagnostic criteria for ADRs with universal acceptance to improvise the fundamental aspect of drug safety and evades the impending ADRs.
Footnotes
What is already known about this subject. (1) Precise diagnosis of ADRs still remains a challenge among clinicians. (2) Demarcation requires a precise technique to attribute the causality of sign and symptom to a specific drug. (3) Diagnosis of ADRs still utilizes either clinical judgments or algorithms, despite their constraint of inability to resolve the probability of the ADR. What this study adds. (1) DoTs classification can establish suitable diagnostic criteria for ADRs. (2) Electronic medical record could be utilized to create a novel perspective algorithm. (3) Deployment of genetic biomarkers in the contemporary tools can escalate the likelihood to accomplish their basic objective.
Peer review under responsibility of King Saud University.
References
- World Health Organization causality assessment method. Available at <http://www.who-umc.org/defs.html> (accessed on 24.02.13).
- Agbabiaka T.B., Savović J., Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf. 2008;31(1):21–37. doi: 10.2165/00002018-200831010-00003. [DOI] [PubMed] [Google Scholar]
- Arimone Y., Begaud B., Miremont-Salame G., Fourrier-Reglat A., Moore N. Agreement of expert judgment in causality assessment of adverse drug reactions. Eur. J. Clin. Pharmacol. 2005;61:169–173. doi: 10.1007/s00228-004-0869-2. [DOI] [PubMed] [Google Scholar]
- Arimone Y., Miremont-Salamé G., Haramburu F., Molimard M., Moore N., Fourrier-Réglat A., Bégaud B. Inter-expert agreement of seven criteria in causality assessment of adverse drug reactions. Br. J. Clin. Pharmacol. 2007;64(4):482–488. doi: 10.1111/j.1365-2125.2007.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimone Y., Bidault I., Collignon A.E., Dutertre J.P., Gerardin M. Updating of the French causality assessment method. Fundament. Clin. Pharmacol. 2010;6:29. [Google Scholar]
- Aronson J.K., Ferner R.E. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ. 2003;327(7425):1222–1225. doi: 10.1136/bmj.327.7425.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery A.J., Anderson C., Bond C.M., Fortnum H., Gifford A., Hannaford P.C., Hazell L., Krska J., Lee A.J., McLernon D.J., Murphy E., Shakir S., Watson M.C. Evaluation of patient reporting of adverse drug reactions to the UK ‘Yellow Card Scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol. Assess. 2011;15:1–234. doi: 10.3310/hta15200. [DOI] [PubMed] [Google Scholar]
- Avner M., Finkelstein Y., Hackam D., Koren G. Establishing causality in pediatric adverse drug reactions: use of the Naranjo probability scale. Paediatr. Drugs. 2007;9:267–270. doi: 10.2165/00148581-200709040-00007. [DOI] [PubMed] [Google Scholar]
- Bates D.W., Evans R.S., Murff H., Stetson P.D., Pizziferri L., Hripcsak G. Detecting adverse events using information technology. J. Am. Med. Inform. Assoc. 2003;10(2):115–128. doi: 10.1197/jamia.M1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begaud B. Standardized assessment of adverse drug reactions: the method used in France Special workshop—Clinical. Drug Inf. J. 1984;18(3–4):275–281. doi: 10.1177/009286158401800314. [DOI] [PubMed] [Google Scholar]
- Burns L.C., Orsini L., L’italien G. Value-based assessment of Pharmacodiagnostic testing from early stage development to real-world use. Value Health. 2013;16(6 Suppl):S16–S19. doi: 10.1016/j.jval.2013.06.007. doi: http://dx.doi.org/10.1016/j.jval.2013.06.007. PubMed PMID: 24034307. [DOI] [PubMed] [Google Scholar]
- CDSCO Compensation in case of injury/death during a clinical trial. Available from: <http://www.cdsco.nic.in/GSR%2053(E)%20dated%2030.01.2013.pdf> (Last accessed on 2013 Feb 1).
- Chien J.Y., Ho R.J. Drug delivery trends in clinical trials and translational medicine: evaluation of pharmacokinetic properties in special populations. J. Pharm. Sci. 2011;100:53–58. doi: 10.1002/jps.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavenna A., Bonati M. Adverse drug reactions in childhood: a review of prospective studies and safety alerts. Arch. Dis. Child. 2009;94:724–728. doi: 10.1136/adc.2008.154377. [DOI] [PubMed] [Google Scholar]
- Cotrutz C., Xing L. Segment-based dose optimization using a genetic algorithm. Phys. Med. Biol. 2003;48:2987–2998. doi: 10.1088/0031-9155/48/18/303. [DOI] [PubMed] [Google Scholar]
- Curtin F., Schulz P. Assessing the benefit: risk ratio of a drug—randomized and naturalistic evidence. Dialog. Clin. Neurosci. 2011;13:183–190. doi: 10.31887/DCNS.2011.13.2/fcurtin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly A.K. Pharmacogenetics and human genetic polymorphisms. Biochem. J. 2010;429:435–449. doi: 10.1042/BJ20100522. [DOI] [PubMed] [Google Scholar]
- Daly A.K. Using genome-wide association studies to identify genes important in serious adverse drug reactions. Ann. Rev., Pharmacol. Toxicol. 2012;52(21):35. doi: 10.1146/annurev-pharmtox-010611-134743. [DOI] [PubMed] [Google Scholar]
- Davies E.C., Green C.F., Taylor S., Williamson P.R., Mottram D.R., Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS ONE. 2009;4(2):e4439. doi: 10.1371/journal.pone.0004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E.C., Rowe P.H., James S. An investigation of disagreement in causality assessment of adverse drug reactions. Pharmaceut. Med. 2011;25(1):17–24. [Google Scholar]
- de Vries E.N., Ramrattan M.A., Smorenburg S.M., Gouma D.J., Boermeester M.A. The incidence and nature of in-hospital adverse events: a systematic review. Qual. Saf. Health Care. 2008;17(3):216–223. doi: 10.1136/qshc.2007.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis F.T., Randall P.S. Identification and reduction of adverse. J. Healthcare Leadership. 2010;2:43–48. http://dx.doi.org/10.2147/JHL.S8022. [Google Scholar]
- Doherty M.J. Algorithms for assessing the probability of an adverse drug reaction. Respirat. Med. CME. 2009;2:63–67. [Google Scholar]
- Du W., Tutag Lehr V., Lieh-Lai M., Koo W., Ward R.M., Rieder M.J. An algorithm to detect adverse drug reactions in the neonatal intensive care unit: a new approach. J. Clin. Pharmacol. 2012 doi: 10.1177/0091270011433327. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Gallagher R.M., Kirkham J.J., Mason J.R. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS ONE. 2011;6(12):e28096. doi: 10.1371/journal.pone.0028096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R.M., Mason J.R., Bird K.A., Kirkham J.J., Peak M., Williamson P.R., Nunn A.J., Turner M.A., Pirmohamed M., Smyth R.L. Adverse drug reactions causing admission to a pediatric hospital. PLoS ONE. 2012;7(12):e50127. doi: 10.1371/journal.pone.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cortes M., Lucena M.I., Pachkoria K., Borraz Y., Hidalgo R. Evaluation of Naranjo Adverse Drug Reactions Probability Scale in causality assessment of drug-induced liver injury. Aliment. Pharmacol. Ther. 2008;27:780–789. doi: 10.1111/j.1365-2036.2008.03655.x. [DOI] [PubMed] [Google Scholar]
- Hauben M., Bate A. Decision support methods for the detection of adverse events in post-marketing data. Drug Discov Today. 2009;14(7–8):343–357. doi: 10.1016/j.drudis.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Heckerling P., Canaris G., Flach S., Tape T., Wigton R., Gerber B. Predictors of urinary tract infection based on artificial neural networks and genetic algorithms. Int. J. Med. Inf. 2007;76:289–296. doi: 10.1016/j.ijmedinf.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Jones, J.K., 2005. Determining causation from case reports. Pharmacoepidemiology. In: Strom, B.L. (Ed.), J. Wiley: Chichester, England, 2005.
- Karch F.E., Lasagna L. Toward the operational identification of adverse drug reactions. Clin. Pharmacol. Ther. 1977;21(3):247–254. doi: 10.1002/cpt1977213247. [DOI] [PubMed] [Google Scholar]
- Khan L.M. Comparative epidemiology of hospital-acquired adverse drug reactions in adults and children and their impact on cost and hospital stay – a systematic review. Eur. J. Clin. Pharmacol. 2013;69:1985–1996. doi: 10.1007/s00228-013-1563-z. [DOI] [PubMed] [Google Scholar]
- Khan L.M., Al-Harthi S.E., Saadah O.I. Impact of Pharmacovigilance on adverse drug reactions reporting in hospitalized internal medicine patients at Saudi Arabian teaching hospital. Saudi Med. J. 2012;33(8):863–868. [PubMed] [Google Scholar]
- Khan L.M., Al-Harthi S.E., Saadah O.I. Adverse drug reactions in hospitalized pediatric patients of Saudi Arabian University Hospital and impact of Pharmacovigilance in reporting ADR. Saudi Pharmac. J. 2013;21:261–266. doi: 10.1016/j.jsps.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh Y., Li S.C. A new algorithm to identify the causality of adverse drug reactions. Drug Saf. 2005;28:1159–1161. doi: 10.2165/00002018-200528120-00010. [DOI] [PubMed] [Google Scholar]
- Koh Y., Yap C.W., Li S.C. A quantitative approach of using genetic algorithm in designing a probability scoring system of an adverse drug reaction assessment system. Int. J. Med. Informatics. 2008;77(6):421–430. doi: 10.1016/j.ijmedinf.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Leventhal J.M., Hutchinson T.A., Feinstein A.R. Algorithm for the operational assessment of adverse drug reactions, I: background, description, and instructions for use. JAMA. 1979;242(7):623–632. [PubMed] [Google Scholar]
- Kulkarni A., Bhatt A. Causality assessment: a casualty of compensation? Perspect. Clin. Res. 2013 Oct;4(4):196–198. doi: 10.4103/2229-3485.120166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo A.F., Marques F.B., Ribeiro C.F., Teixeira F. Causality assessment of adverse drug reactions: comparison of the results obtained from published decisional algorithms and from the evaluations of an expert panel, according to different levels of imputability. J. Clin. Pharm. Ther. 2003;28:137–143. doi: 10.1046/j.1365-2710.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- Macedo A.F., Marques F.B., Ribeiro C.F., Teixeira F. Causality assessment of adverse drug reactions: comparison of the results obtained from published decisional algorithms and from the evaluations of an expert panel. Pharmacoepidemiol. Drug Saf. 2005;14(12):885–890. doi: 10.1002/pds.1138. [DOI] [PubMed] [Google Scholar]
- Miremont G., Haramburu F., B́egaud B., Ṕeŕe J.C., Dangoumau J. Adverse drug reactions: physicians’ opinions versus a causality assessment method. Eur. J. Clin. Pharmacol. 1994;46(4):285–289. doi: 10.1007/BF00194392. [DOI] [PubMed] [Google Scholar]
- Nakamura Y. Pharmacogenomics and drug toxicity. N. Engl. J. Med. 2008;359:856–858. doi: 10.1056/NEJMe0805136. [DOI] [PubMed] [Google Scholar]
- Naranjo C.A., Busto U., Sellers E.M., Sandor P., Ruiz I., Roberts E.A. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- Park M.Y., Yoon D., Lee K., Kang S.Y., Park I., Lee S.H. A novel algorithm for detection of adverse drug reaction signals using a hospital electronic medical record database. Pharmacoepidemiol. Drug Saf. 2011;20(6):598–607. doi: 10.1002/pds.2139. doi: 10.1002/pds.2139. [DOI] [PubMed] [Google Scholar]
- Peter Ian Pillans Clinical perspectives in drug safety and adverse drug reactions. Expert Rev. Clin. Pharmacol. 2008;1(5):695–705. doi: 10.1586/17512433.1.5.695. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Clinical judgment, common sense and adverse reaction reporting. Pharmacoepidemiol. Drug Saf. 2004;13:511–513. doi: 10.1002/pds.999. [DOI] [PubMed] [Google Scholar]
- Smyth R.M., Gargon E., Kirkham J., Cresswell L. Adverse drug reactions in children—a systematic review. PLoS ONE. 2012;3:e24061. doi: 10.1371/journal.pone.0024061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom B.L., Kimmel S.E. John Wiley & Sons; Chichester, West Sussex; Hoboken, NJ: 2006. Textbook of Pharmacoepidemiology. pp. 168–169. [Google Scholar]
- Talbot, J., Aronson, J.K., 2012. Causality algorithms. Stephens’ detection and evaluation of adverse drug reactions: principles and practice, sixth ed., John Wiley & Sons Ltd., UK, 2012, pp. 65–68.
- Théophile H., Arimone Y., Miremont-Salamé G., Moore N., Fourrier-Réglat A., Haramburu F., Bégaud B. Comparison of three methods (consensual expert judgment, algorithmic and probabilistic approaches) of causality assessment of adverse drug reactions: an assessment using reports made to a French Pharmacovigilance center. Drug Saf. 2010;33(11):1045–1054. doi: 10.2165/11537780-000000000-00000. Pub Med PMID: 20925441. [DOI] [PubMed] [Google Scholar]
- Théophile H., André M., Miremont-Salamé G., Arimone Y., Bégaud B. Comparison of three methods (an updated logistic probabilistic method, the Naranjo and Liverpool algorithms) for the evaluation of routine Pharmacovigilance case reports using consensual expert judgment as reference. Drug Saf. 2013;36(10):1033–1044. doi: 10.1007/s40264-013-0083-1. Pub Med PMID: 23828659. [DOI] [PubMed] [Google Scholar]
- USFDA Guidance for Industry and Investigators Safety Reporting Requirements for INDs and BA/BE Studies. <http://www.fda.gov/downloads/Drugs/.../Guidances/UCM227351.pdf> (last accessed on 11.12.13).
- Waller P., Heeley E., Moseley J. Impact analysis of signals detected from spontaneous adverse drug reaction reporting data. Drug Saf. 2005;28:843–850. doi: 10.2165/00002018-200528100-00002. [DOI] [PubMed] [Google Scholar]
- Weiss J., Krebs S., Hoffmann C. Survey of adverse drug reactions on a pediatric ward: a strategy for early and detailed detection. Pediatrics. 2002;110:254–257. doi: 10.1542/peds.110.2.254. [DOI] [PubMed] [Google Scholar]
- Wester K., Jonnson A.K., Sigset O., Druid H., Hagg S. Incidence of fatal adverse drug reactions: a population based study. Br. J. Clin. Pharmacol. 2008;65:573–579. doi: 10.1111/j.1365-2125.2007.03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]