Abstract
Ulcerative colitis is the chronic relapsing multifactorial gastrointestinal inflammatory bowel disease, which is characterized by bloody or mucus diarrhea, tenesmus, bowel dystension, anemia. The annual incidence of ulcerative colitis in Asia, North America and Europe was found to be 6.3, 19.2 and 24.3 per 100,000 person-years. The major challenge in the treatment of ulcerative colitis is appropriate local targeting and drug related side-effects. To overcome these challenges, microparticulate systems seem to be a promising approach for controlled and sustained drug release after oral administration. The main goal of this article is to explore the role of microparticles in ulcerative colitis for the appropriate targeting of drugs to colon. There are different approaches which have been studied over the last decade, including prodrugs, polymeric approach, time released system, pH sensitive system, which show the site specific drug delivery to colon. Among these approaches, microparticulate drug delivery system has been gaining an immense importance for local targeting of drug to colon at a controlled and sustained rate. Combined approaches such as pH dependent and time dependent system provide the maximum release of drug into colon via oral route. This article embraces briefly about pathophysiology, challenges and polymeric approaches mainly multiparticulate systems for site specific drug delivery to colon in sustained and controlled manner so that drug related side-effects by reducing dosage frequency can be minimized.
Keywords: Ulcerative colitis, Inflammatory bowel disease, Multiparticulate carrier, pH sensitive polymer, Colon
1. Introduction
Inflammatory bowel diseases are the idiopathic chronic multifactorial inflammatory diseases of gastrointestinal tract, which mainly include ulcerative colitis and Crohn’s disease (Talaei et al., 2013). Small intestine and large intestine or colon are the main regions involved in inflammatory bowel diseases, which are marked by the chronic inflammation in specific mucosal or transmural locations (Friend, 2005). Appropriate local targeting is the main challenge in the treatment of ulcerative colitis. For improved localization, a well designed drug delivery system is beneficial to enhance therapeutic efficacy. There are many approaches used for appropriate targeting like pro drug approach, conjugate approach, and time released system, pH dependent system, multiparticulate system, nanoparticulate system, probiotic approach. Among all, microparticulate system is one of the best approaches for controlled drug delivery in specific site of inflammation. Microparticles are small free flowing particles consisting of natural or synthetic polymers having particle diameter ranging from 1 to 1000 μm. With the advancement in biotechnology, genomics, lots of potent and specific therapeutics have been formed. Due to various problems such as low solubility, poor stability, narrow therapeutic index of many new drugs, there is a corresponding need for safer drug delivery. The delivery should be designed in such a way to provide active agent in right amount, at right time, to proper location in the body to increase patient compliance and minimize side-effects. There is wide range of particulate carrier system used for targeted drug delivery to colon, which mainly includes microparticles, microcapsules, nanoparticles, nanocapsules to overcome the disadvantages of conventional drug delivery systems like increased risk of systemic adverse drug reactions. To target drugs only to their desired gastro-intestinal regions is really a great challenge. Therefore, there is the need to design such a delivery system, with a more effective drug targeting. Micro particulate carriers like polymeric micro particles are found to have lots of applications in ulcerative colitis patients (Collnot et al., 2012). Advances in the understanding of pathogenesis of ulcerative colitis and mechanism of action of drug brought novel ideas for drug targeting to specific site of action.
At present, the etiology of disease is not fully understood but it has been hypothesized that various factors such as genetic, Gut/environmental, psychosomatic, autoimmune, epidemiological are responsible for the development of ulcerative colitis (Molodecky and Kaplan, 2010). Gut/environmental factors include immune/epithelial interactions, bacterial infections, and epithelial barrier functions. Epidemiological studies include dietary habits, smoking habits, intake of drugs, hormonal status, variations due to different climates, and changes due to social circumstances. The inflammatory factors can be examined through different cell signaling pathways, inflammatory mediators such as tumor necrosis factor α (TNF-α), Interleukin-1, Interleukin-6, Interleukin-12, Interleukin-4, Interleukin-10 and Interleukin-11, and Eicosanoids profiles (Carter et al., 2004). Severe form of ulcerative colitis leads to colon cancer. Chronic inflammation generated by the production of reactive oxygen species leads to generate dysplasia, which further turns into CAC, i.e., colitis associated colorectal cancer, which is a severe form of ulcerative colitis (Rogler, 2014). Therefore, there is the high risk of colon cancer in patients with ulcerative colitis (Navaneethan et al., 2013). This risk is increasing every year. Urban and western industrialized regions are mainly affected by these diseases rather than rural areas (Thippeswamy et al., 2011). Ulcerative colitis is generally authenticated by mucosal inflammation which generally extends from the proximal region of colon to rectum. In ulcerative colitis, with the influx of neutrophils in lamina propia, there is the localized collection of pus cells surrounded by inflamed tissue and depletion of mucin followed by production of inflammatory mediators like cytokines. Conversely, CD does not restrict to a confined region and can occur in any part of GIT, where the accompanying inflammation is described as irregular/patchy, segmented, and transmural. Most commonly, the terminal ileum exhibits early lesions on or near Peyer’s patches. In earlier studies, it has been reported that smoking is beneficial in ulcerative colitis, whereas it shows a negative effect on Crohn’s disease (Botoman et al., 1998).
There are various conventional and unconventional therapies used for the treatment of ulcerative colitis include aminosalicylates, glucocorticoids, immunomodulators, etc. These therapies need to be administered frequently to patients, which reduces patient compliance and can cause systemic side-effects. Thus, microparticles are found to be a promising approach for controlled and site specific drug delivery, which minimize dosage frequency and improve patient compliance.
1.1. Signs and symptoms of ulcerative colitis
The chief initial symptoms of onset of ulcerative colitis consists of severe abdominal pain, tenesmus, anorexia, bloody or mucous diarrhea in contrast to Crohn’s disease, which does not show any bloody diarrhea (Lee et al., 2013, Liang, 2002). The severe symptoms include weight loss, tachycardia, anemia, rectal bleeding, and bowel distension (Meier and Sturm, 2011). According to the severity of the disease, ulcerative colitis is divided into different categories i.e., extensive, distal, proctosigmoiditis, ulcerative proctitis. The schematic diagram which briefly explains the ulcerative colitis is given in Fig. 1.
Figure 1.
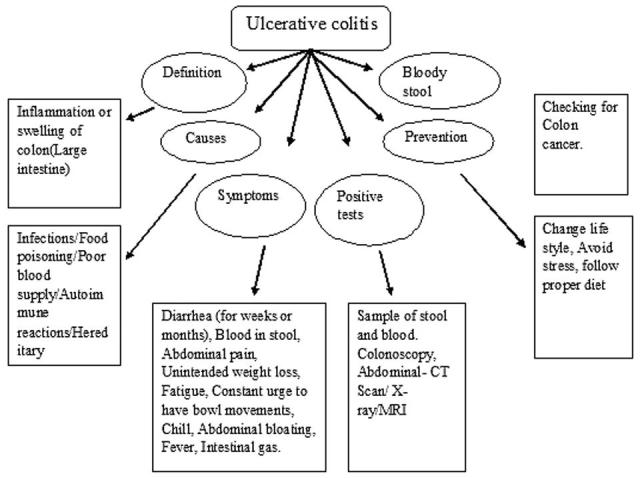
A schematic diagram for ulcerative colitis.
The global estimates of UC are known to be varied by geographic, environmental, socio-economic characteristics and are increasing day by day (Eisen and Sandler, 1994). Most of the peak incidences occur in the young people between the age of 10–40 years. It has been estimated that up to 2,40,000 people in UK, 1.4 million in United States and 2.2 million in Europe are affected by ulcerative colitis. Inflammatory bowel disease can affect at a person of any age and can continue throughout their life as it does not have any permanent cure (Cosnes et al., 2011). Therefore, there is the need of administering drug for life time for the maintenance of health related quality of life (Lautenschläger et al., 2013b). In UC, sites of inflammation extend to the more proximal regions of the colon over time. Usually, rectum is also involved. Whereas in CD, site of inflammation is usually distal ileum Table 1 shows comparison of Crohn’s disease vs. ulcerative colitis (Botoman et al., 1998).
Table 1.
Comparision of Crohn’s disease vs. ulcerative colitis.
| S. No. | Features | Ulcerative colitis | Crohn’s |
|---|---|---|---|
| 1. | Site of action | Extending from rectum proximally to entire colon only | Any part of GIT (mainly ileum is involved |
| 2. | Symptoms | Diarrhea, wt. loss, malnutrition and other extraintestinal manifestations (JSEM), smoking improves condition | Diarrhea, abdominal pain, wt. loss, malnutrition, growth failures in kids, smoking make it worse |
| 3. | Pathology and complications | Mucosal inflammation, if get severe can cause colon cancer. Fistulas, abscesses and strictures absent | Transmural inflammation, non caseating granulomas, fistulas, abscesses, perianal involvement and strictures are common. Toxic megacolon absent |
| 4. | Cytokine response | Associated with Th17 | Associated with Th2 |
| 5. | Distribution | Continuous distribution | Discontinuous |
| 6. | Drugs used | 5-Amino salicylic acid, | Hydrocortisone, |
| Sulfasalazine, | Budesonide, | ||
| Balsalazide, Infliximab, | Prednisolone, | ||
| Azathioprine and mercaptopurine | Sulfasalazine, Olsalazine, | ||
| Mesalazine and Balsalazide, Infliximab |
1.2. Epidemiology of ulcerative colitis
In western Europe, Asia and North America, UC has an annual incidence of approximately 24.3 per 100,000 population per year, 6.3 per 100,000 population per year and 19.2 per 100,000 population per year, respectively Whereas, Crohn’s disease has less incidence as compared to UC i.e., approximately 12.7 per 100,000 population per year in Europe, 5.0 population per year in Asia and 20.2 per 100,000 population in North America. All around the globe, IBD is becoming a global disease because of increasing incidences and prevalences of it with respect to time. There are very lower incidences in Asia, Africa and South America (Molodecky et al., 2012). In the United States, it has been estimated that more than 1 million individuals are suffering from IBD, among which, 100,000 are children. IBD occupied 5th rank among the most prevalent gastrointestinal diseases (Stein et al., 1998).
1.3. Multifactorial pathogenesis of ulcerative colitis
Ulcerative colitis (UC) is a chronic, relapsing intestinal inflammatory disorder of the colon, with variable distribution but limited to the distal bowel (distal colitis and proctitis) in 60% of cases. UC has multifactorial etiology that may result in either primary immunological dysfunction or an inappropriate pathological immunological response to an environment. e.g., commensal intestinal microorganisms. The first cause is the dysregulation of immune system which further leads to excessive immune responses to normal microflora. Secondly, epithelial cell abnormalities and changes in composition of gut microflora facilitate an abnormal mucosal immune response (Strober et al., 2007). Thirdly, impaired gene expression i.e., mutation of gene named CARD15/NOD2, located on Chr 16 and other genes named OCTN 1 and 2 on Chr 5, in case of Crohn’s disease (Ardizzone and Porro, 2002).
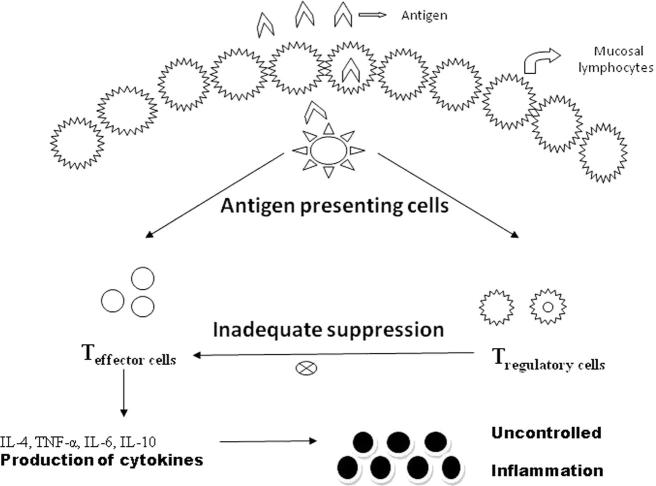
The etiology of idiopathic inflammatory bowel diseases like ulcerative colitis and Crohn’s disease is found to be an enigma for the Gastroenterologists and immunologists. Ulcerative colitis is a local inflammatory bowel disorder in which continuous mucosal inflammation extends from the rectum toward the cecum, and is generally associated with excess IL-13 production whereas, Crohn’s disease is a relapsing inflammatory disorder which may involve any part of the gastrointestinal tract, but mainly terminal ileum and colon are involved having discontinuous ulceration and inflammation involving granulomas, which is associated with excess of production of IL-12/IL-23 and IFN-γ/IL-17 as shown in Fig. 2 (Fuss et al., 2004). Fig. 2 shows the diagrammatic scheme of pathogenesis of ulcerative colitis by following pathway of T-Lymphocyte activation. Previous studies reported that alterations in intestinal barrier function may play a crucial role in the development of ulcerative colitis. Some authors reported that abnormally increased intestinal permeability is the etiologic factor in ulcerative colitis and contributes to disease perpetuation. Thus disrupted barrier function of epithelial wall leads to an increase in the permeability of mucosa for luminal antigens, bacteria/microorganisms, and loss of water and electrolytes, thereby, stimulating the inflammatory process (Stein et al., 1998). Due to this disruption of barrier, various electrolytes and water have been lost from the body via epithelium. In addition to this, the polarity of damaged intestinal epithelial cells has also been lost which results in apical expression of transferring receptor protein, whose expression is generally enhanced on both apical and basolateral region of enterocytes in inflammatory mucosa of IBD patients (Harel et al., 2011).
Figure 2.
Diagrammatic scheme of pathogenesis of inflammatory bowel disease by following pathway of T-Lymphocyte activation.
2. Major challenges in the treatment of ulcerative colitis
The major challenge in the treatment of ulcerative colitis is the reduction of drug related side-effects by doing site specific drug delivery to colon. Furthermore, long term medication produces lots of side-effects, which negatively affect the quality of life of ulcerative colitis suffered patients. To design a delivery system, which delivers the maximum amount of drug to specific site at the right time in the body that increases efficacy, compliance is really a great challenge (Kim and Pack, 2006). There are many drugs which are used for the treatment of inflammatory bowel diseases showing adverse effects like peptic ulcers, diarrhea, nephro and hepatotoxicity, glaucoma, vomiting, Cushing’s syndrome, etc. (Buchman, 2001, Ransford and Langman, 2002, Rutgeerts et al., 2005, Papadakis et al., 2005).
Over the past two decades the major challenge for scientists is to target the drugs specifically to the colonic region of GIT (Sowmya et al., 2012). Another challenge for children with IBD, mainly Crohn’s disease, is the impairment of skeletal and growth development due to lack of balanced nutrition. In addition, inflammatory mediators such as cytokines cause some mutations in hormonal axes, which directly influence growth. To overcome these problems, proper nutrition and appropriate anti-inflammatory therapy are found to be the best option.
3. Various drugs used for the treatment of ulcerative colitis
The cure of UC depends upon the severity of disease, its subtype, and its pre-existing illness. The most commonly used drugs for its treatment are anti-inflammatory agents which mainly include 5-amino salicylates (mainly used in UC) like olsalazine, mesalazine and balsalazide, which is used for the cure of mild to moderate attacks and to maintain remission in UC and immunosuppressive agents which mainly include azathioprine, 6-mercaptopurine, methotrexate, cyclosporine, tacrolimus calcineurin inhibitors (Isaacs et al., 2005). Other class of drugs which can also be used are corticosteroids, like prednisolone and anti-TNF-α-antibodies for moderate to severe conditions of Ulcerative Colitis (Taylor and Irving, 2011). In case of refractory and fulminate disease stages, surgery may serve as a better option for IBD patients (Neurath and Travis, 2012).
3.1. Conventional/unconventional drug therapy for UC
3.1.1. Conventional drug therapy
3.1.1.1. Amino salicylates
Sulfasalazine is the first class drug of antibiotics, consists of 5-aminosalicylic acid (5-ASA) and sulphapyridine in which 5-ASA shows anti-inflammatory properties, while sulfapyridine shows anti-bacterial properties. The exclusive mechanism of action of 5-aminosalicylates involves the stimulation of class of nuclear receptors which generally control the inflammation and cell proliferation. It helps in reducing the production of chemoattractant leukotrienes and inhibits the cellular release of interleukin-1 and 2 (Zhou et al., 1999).
It also inhibits the transcriptional activity of nuclear-factor κB, that regulates the genes responsible for immunity and inflammation. It is extensively metabolized in liver, therefore, it is having poor systemic bioavailability. Baron et al. (1962) reported that this class of drugs induce remission in 40–80% of patients (Riley et al., 1988, Baron et al., 1962). It is used in the form of oral sustained release tablet or granule and locally applied in the form of suppositories, enemas etc., but its usage is limited because of sulphapyridine-related intolerance (Egan et al., 1999). Most of studies reported that ciprofloxacin is the commonly used antibiotic for moderate to severe UC (Turunen et al., 1998). Table 2 shows list of drugs used for the treatment of ulcerative colitis. Approximately 80% of remission can be achieved within first 2–4 weeks after having Ist line therapy.
Table 2.
List of drugs used for the treatment of ulcerative colitis.
| Classes of drugs | Trade name | Marketed formulations | Adverse effects | Dose | ||
|---|---|---|---|---|---|---|
| 1 | Aminosalicylates | Sulfasalazine | Azulfidine | Delayed release tablet | Agranulocytosis, pancreatitis, interstitial nephritis | 4–6 g/day divided qid |
| Mesalamine | Asacol | Eudragit S 100 coated tablet (dissolves at pH 7) | Hepatitis, male infertility, arthralgia, pneumonitis | 2–4 g/day divided bid-qid | ||
| Olsalazine (it should be taken after having meals) | Dipentum | Hard gelatine capsules | Stomach upset, bloating, loss of appetite, blurred vision, headache, pain in joints, dizziness | 1.5–3 g/day divided bid qid | ||
| Balsalazide | Colazal | Tablet | Headache, abdominal pain, upset stomach, diarrhea, vomiting, joint pain, difficulty falling or staying asleep, tiredness | 2.25 g 3 times daily for 8–12 week | ||
| 2 | Corticosteroids | Budesonide | Entocort | Eudragit L coated beads | Dry or irritated mouth or throat, cough, difficult or painful speech, neck pain, stomach pain | 9 mg/day |
| Prednisolone | Deltasone, Orasone | Tablets of 2.5, 5, 10, 20 and 50 mg and oral solution/syrup | Hyperglycemia, hypertension, electrolyte disturbances | 5–50 mg per day | ||
| Dexamethasone | Dexasone, Diodex, Decadron | Tablets, Elixirs and solutions | Cataracts, osteoporosis, myopathy | Tablets – 0.25–6 mg | ||
| Elixir – 0.5 mg/ml | ||||||
| Solution – 0.5, 1 mg/5 ml | ||||||
| 3 | Immunosuppressive agents | Azathioprine, Methotrexate | Imuran | Tablet | Hyperglycemia, hypertension, electrolyte disturbances, cataracts | 50 mg |
| Rheumatrex, Traxell | Solution given i.v | 25 mg/ml | ||||
| 4 | Antimicrobial agents | Metronidazole | Flagyl | Extended release tablets 750 mg, capsule 375 mg, cream 0.75 and 1%, lotion 0.75%, gel 0.75% and 1% injection | Urticaria, glossitis; long-term use may develop paresthesia | 750 mg orally three times daily for 5–10 days |
| Ciprofloxacin | Cipro, Proquin XR | Tablets 250, 500 and 750 mg | Diarrhea, vomiting and rash. Other side effects (e.g. headache, abdominal pain, pain in extremities, injection site reaction, cardiovascular, Gastrointestinal, etc.) In less than 1% of the patients | 500 mg twice daily | ||
| Extended released (XR) 500 and 1000 mg | ||||||
| Microcapsules for suspension 250 mg/5 ml | ||||||
| Injections 200 mg/100 ml | ||||||
| 5 | Inhibitors of TNF-α | Infliximab | Remicade | Powder for intravenous injection | Acute infusion reactions | 100 mg |
| Adalimubab | Humira | Prefilled glass syringe | Serum sickness, increase in serious infections (e.g. sepsis, pneumonia, tuberculosis) | 20 mg/0.4 ml and 40 mg/0.8 ml | ||
3.1.1.2. Glucocorticoids
These drugs act via glucocorticoid receptor in the cell nucleus and modulate immune response but due to steroidal nature of these drugs, they can develop corticoid dependency and corticoid resistance which limit their applicability in ulcerative colitis (Ford et al., 2011). Such findings need steroid-sparing medication in IBD. In previous studies, it has been found that budesonide 9 mg per day when formulated as a pH and time dependent dosage form is much effective than oral daily dose of 4000 mg of 5-ASA for remission induction in IBD. These drugs are used for mild to moderate conditions of IBD. But in severe form of IBD and for patients who do not respond to corticosteroids, Immunosuppressive drugs like azathioprine, 6-mercaptopurine, methotrexate, cyclosporine, tacrolimus etc. are the next line of drugs and play an important role for IBD. But safety profile of this therapy is still conflicting (Isaacs et al., 2005).
3.1.2. Unconventional drug therapy
3.1.2.1. Biological therapies
The recent insights into the techniques of molecular biology lead to find new discoveries/therapies for the management of inflammatory bowel disease. These agents are classified as biological therapies which include gene therapy, recombinant peptides and proteins, antibody based therapies like monoclonal antibodies, which are mainly TNF-α inhibitors i.e., Infliximab, a chimeric IgG1 comprising 75% humans and 25% murine sequences is currently used for the treatment of CD and biological preparations such as immunoglobulins and vaccines (Ardizzone and Porro, 2002). TNF-α inhibitors like infliximab (previously known as cA2, adalimumab and certolizumab pegol play an important role in the treatment for severe chronic disease stages. The mechanism of action of infliximab is to inhibit the biological activities of TNF-α or interaction of TNF-α with its receptors. In addition to infliximab, other two drugs i.e. adalimumab and certolizumab have also been approved by FDA for IBD and having the mechanism same as that of infliximab (Siegel et al., 1995). The treatment for IBD requires a balance between high therapeutic efficacy and reduced adverse drug reactions. A promising strategy to enhance the therapeutic benefit of established drugs is the utilization of particle carrier systems, which release active agents specifically at the site of intestinal inflammation.
3.1.2.2. Immunomodulators
New immunomodulators developed for the management of IBD are thalidomide, tacrolimus, mycophenolate mofetil. The mechanism of action of thalidomide is the reduction of TNF-α production by increasing the destruction of TNF-α messenger RNA encoding protein, which inhibits transcription and translation (Sandborn et al., 2001). It also inhibits the production of IL-12, responsible for the regulation of cellular immune responses (Moreira et al., 1993, Moller et al., 1997).
4. Role of microparticulate carrier for targeted drug delivery to colon for ulcerative colitis
Polymeric microparticles have turned out to be a promising approach as a targeted drug delivery system for the treatment of ulcerative colitis. The main aim for the targeted drug strategy is to target the maximum concentration of active agents in inflamed intestinal tissues by using selective delivery to achieve therapeutic efficacy while simultaneously reducing adverse effects. In addition to this, such targeted delivery system must meet the conditions for complete biodegradation and high biocompatibility without pro-inflammatory properties (Lautenschläger et al., 2013a). Controlled release drug delivery system is one of the most efficient methods to overcome most of the difficulties associated with other methods of administration. Controlled release drug delivery includes carriers such as polymer-based disks, microparticles, nanoparticles, pellets in which drug gets encapsulated and release at controlled rates for relatively long periods of time. Such kind of systems often show several advantages over other methods of administration.
-
•
First advantage is the drug release rates can be adjusted according to the needs of a specific application; for example, providing a constant rate of delivery or pulsatile release.
-
•
Second, controlled release systems also protect drugs, especially proteins, from degradation that are otherwise rapidly destroyed by the body. Finally, by using controlled release systems, frequent (daily) dosing can be replaced by giving once per month injection, which ultimately increases patient comfort and compliance.
In recent years, Particulate drug carriers include microparticulate, nanocarriers have been gaining an immense importance due to their wide advantages over existing systems (Gilhotra et al., 2009). These particulate systems are unique in terms of their particle size, as optimum particle size of carrier for localization of drug should be in the range of 5–15 μm to increase the residence time of drug in the colonic region. Particle size of this range is able to adhere at the site of action and get accumulated in the targeted region. But in some studies, it has been reported that for IBD therapy, microparticles should be in size range of 10–300 μm to target specifically to the inflamed region of colon (Collnot et al., 2012). Selection of carrier is very important for particular drug i.e., either hydrophilic or lipophilic. It depends on the diseased conditions as well as on the physico-chemical nature of drug. Among recent techniques used for colon specific delivery, micro and nano-particles are well known for achieving site specificity, increasing drug stability via encapsulation. Optimal size range of carrier system for proper localization and accumulation in targeted region is between 4 and 15 μm. Most of the drugs used in the treatment of IBD show strong adverse effects due to systemic bioavailability. But targeting drug to the localized area of intestine can reduce the systemic side-effects.
5. Various microparticulate polymeric approaches used for targeting drugs to colon for the treatment of ulcerative colitis
Colon targeted drug delivery via pH dependent, time dependent, microflora or enzyme activated system, mucoadhesive and pressure controlled based system have attracted great interest for the treatment of systemic as well as local disease (Patel et al., 2007).
5.1. pH dependent polymeric approaches
The most commonly used pH dependent polymers are CAP (Cellulose acetate phthalates), CAT (cellulose acetate phthalate), HPMCP 50 and 55, (Hydroxypropylmethyl cellulose phthalate), Eudragit S 100, Eudragit L (Copolymers of methacrylic acid and methacrylate), Eudragit FS, Eudragit P4135 F, For the development of effective delivery system, the threshold pH of polymer and their solubility in different pH conditions should be well known (Newton et al., 2012). According to previous reported studies, there are various fluctuations in pH of colon due to some reasons. In normal healthy individuals, there is increase in pH from duodenum (pH 6.6 ± 0.5) to terminal ileum (pH 7.5 ± 0.4) and a decrease in cecum (pH 6.4 ± 0.4), then a slow rise from right to left colon with final value (pH 7.0 ± 0.7). But it has been reported that these polymers are unable to release the drug to large intestine as these are pH dependent and leads to premature release in small intestine or very less amount release in colon because of varying transit time of small intestine (Ashford et al., 1993). Therefore, multiparticulate drug delivery systems formulated as single layered or multi-layered product have a great potential for the reproducible drug release in inflamed sites of the colon due to less intersubject variations (Chourasia and Jain, 2003, Singh, 2007).
Some examples for this delivery system are 5-amino salicylic acid coated with Eudragit S 100, marketed as Asacol tablet has been prepared for its pH dependent release (Schroeder et al., 1987). Lialda, a locally acting delayed release tablet under the common name mesalamine, has been approved in January, 2007, in which drug is completely dispersed in lipophilic matrix. According to another study by Mahkam and Vakhshouri (2010), 5-aminosalicylic acid loaded methacrylic acid/perlite composites have been prepared which show pH dependent release.
Some other marketed formulations of multiparticulate pH dependent systems are commercially available such as Budenofalk and Entocort. In budenofalk, pH dependent polymers like Eudragit RL, Eudragit S, Eudragit L, etc. were used whereas, in Entocort, delayed release polymers like ethylcellulose were used for time dependent release (Rodríguez et al., 1998). In other study, Makhlof et al. (2009), prepared PLGA coated budesonide loaded nanospheres for sustained release at pH 7.4.
But, due to lack of site specificity of pH dependent polymers in colon, there exists a controversy for reliability of targeting method. To remove such limitation, there is the need of combining two polymers i.e., one is delayed release/time controlled and other is pH dependent, so that maximum amount of drug releases in the colon and show its therapeutic effect. Hashem et al. (2013), combined both time and pH dependent system for targeting prednisolone microspheres to colon. He found that Eudragit S, an enteric coated polymer starts dissolving at pH more than 6, which causes the lack of site specificity of drug at colonic region. Therefore, he combined both Eudragit S 100 and ethylcellulose, as only 20% drug released in small intestine while due to delayed release polymer, rest 80% will release in colon.
5.2. Time-dependent polymeric systems
Time dependent systems such as sustained release or delayed release system found to be a better approach for site specific system. As transit time of gastrointestinal tract is varied in diseased conditions, which ultimately lead to either premature release of drug in small intestine or very less amount will be released in the colon (Lee and wilson, 2003, Hinton et al., 1969). For the successful drug delivery, one should have the knowledge of gastric transit time of dosage form. It varies between 15 and 180 min. in case of non-disintegrating single dosage form, where as it is more constant for small intestine in between 3–4 h and average transit time in the colon is 47 h in women and 33 h in men. But there are some intersubject variations in GI transit times that depend on amount of food intake, peristalsis movement in stomach. Due to these variations, time dependent system alone is not suitable for ideal drug delivery to colon. For overcoming such limitation of this system, two approaches were combined i.e., pH dependent and time controlled which serve to improve site specific drug delivery to colon (Fukui et al., 2000).
El-Gibaly (2002), formulated ketoprofen-loaded Zn pectinate gel (ZPG) microparticles along with pectin mixtures in form of tablets and found that there was the extended release of drug from ZPG microparticles with pectinate microparticles, which contained 2–3% w/v pectin, 2.75% w/v Zn (CH3COO)2 and 2.5% w/v drug. It was also concluded that untableted ZPG microparticles retard the release of ketoprofen in simulated intestinal fluid (pH 7.4), which made it a promising controlled-release carriers for colon-targeted drug delivery.
5.3. Biodegradable polymeric systems
Various attempts have focussed on the recent applications of polysaccharides for colon specific drug delivery. The extensive growth of colonic microflora is the characteristic feature of colon which explored it for site specific colonic drug delivery. This vast anaerobic microflora further produces no. of hydrolytic as well as reproductive enzymes such as nitroreductase, azoreductase, glucuronidase, deaminase, xylosidase (Scheline, 1973) which made it suitable for site specific drug delivery. Most commonly used polysaccharides which are degraded by these colonic enzymes are chitosan and chondroitin (obtained from animal origin), pectin, guar-gum and inulin (from plant origin), alginates (from algal), dextran (from microbial origin), amylase. Because of its excellent and fascinating features such as biodegradability, high stability, safe, non-toxicity, cheap, natural origin, gel forming and wide distribution in the world made it suitable for colon targeted drug delivery (Philip and Philip, 2010). Description of polysaccharides is given below.
5.3.1. Guar gum
Guar gum is an outstanding eco friendly non ionic polysaccharide obtained from the seeds of Cyamopsis tetragonolobus belonging to the leguminous family . Chemically, it consists of mannose backbone i.e., d-Mannopyranosyl along with galactose side branching i.e., d-glucopyranosyl side branching.
Patel et al. (2007) developed guar gum microspheres of mebeverine hydrochloride for local release of drug in the colon in the form of tableted microspheres (Patel et al., 2007). He found that in vitro study of microspheres may lead to premature release in upper GIT. Therefore, they compressed microspheres into tablets so that mebeverine remain intact in tableted form in lower GIT. As it reached to colon, enteric coating removed and microspheres were dispersed and showed its therapeutic beneficial in colon targeting.
In another study, Sarkar et al., formulated guar gum microspheres by emulsion cross linking technique, which is further coated with Eudragit S 100. He found that 7–11% of drug was released in first four hours while 80% of drug released in 8–10 h, that shows its maximum drug release in desired colonic region.
5.3.2. Chitosan
Chitosan a functional linear co-polymer consisting of 2-amino-2-deoxy-d-glucose and 2-acetamido-2-deoxy-d-glucose unit links with β-(1–4) bonds is the most abundantly found natural polysaccharide after cellulose, derived from chitin. It carries positive charge and reacts with the negatively charged surface including DNA and polymers to achieve site specific drug delivery. As chitosan is having glycosidic linkages, it undergoes glycosidic hydrolysis by microbial enzymes present in the colon which leads to loss of mechanical strength and molecular weight (Ghandehari et al., 1997). In one study, Saboktakin et al., 2011 prepared carboxymethyl starch–chitosan nanoparticles for targeted drug delivery system to colon by complex coacervation process and found that average drug entrapment was 81 ± 1.86% in nanoparticle because of higher crosslinked gel like structure (Saboktakin et al., 2011). In this study, it was concluded that the amount of drug released from nanoparticles decreased with increase the cross linking time. Table 3 shows design approach by using different polymers.
Table 3.
Various advantages, disadvantages and applications of methods used for ulcerative colitis.
| Polymer | Formulation and design approach | References |
|---|---|---|
| Eudragit P-4135 F, a new pH sensitive polymer, dissolve at pH > 7.2 | Enteric coating polymer used to prepare microparticles of tacrolimus, an immunosuppressant drug used mostly in ulcerative colitis | Lamprecht et al. (2005) |
| Eudragit L30 D-55, copolymer of methacrylic acid and ethyl acrylate (dissolves at pH 5.5). Eudragit FS 30 D, copolymer of methacrylic acid, methacrylate and methylmethacrylate (dissolves at pH 7 or above) | Enteric coated HPMC capsules of paracetamol designed to achieve intestinal targeting | Cole et al. (2002) |
| Eudragit L100 (dissolve pH > 6) | Enteric coated tablets of mesalazine for the treatment of Crohn’s disease | Tromm et al. (1998) |
| Ethylcellulose, inert hydrophobic polymer and Eudragit S 100 soluble at pH 7 | Enteric coated time released-matrix tablets for colon targeting | Alvarez-Fuentes et al. (2004) |
| Ethylcellulose, hydrophobic and hydroxypropylmethyl cellulose phthalate | Combination of ethylcellulose and HPMC used for the preparation of empty pressure-controlled colon delivery capsules of Fluorescein | Jeong et al. (2001) |
Gawde et al., 2012 formulated mucoadhesive microspheres of deflazacort for the treatment of ulcerative colitis by using chitosan, as a mucoadhesive polymer with the coating of Eudragit S 100. He found that chitosan coating of deflazacort improves the bioavailability of the drug by crosslinking it with glutaraldehyde.
In another study, Sareen et al. (2014) formulated curcumin loaded Eudragit-coated chitosan microspheres by emulsion crosslinking method. He concluded that curcumin loaded chitosan microspheres without Eudragit S 100 coating showed burst release in first 4 h, while Eudragit coated microspheres prevent the drug to release early and showed controlled release of drug up to 12 h, which confirms the potential of delivery system for ulcerative colitis.
Chandra et al. (2012), developed satranidazole loaded Eudragit microspheres by oil in oil solvent evaporation method. In this article, it was concluded that coating of Eudragit prevents drug release in stomach, while maximum release was at pH 7.4.
Rasool et al. (2012), tried to develop the modified release dosage form of metoprolol, a cardioselective β-blocker to reduce the dosage frequency as well as side-effects. Eudragit FS was used for pH dependent release in intestine. In this study, it was concluded that according to drug release data, zero order model was found to be best suited and shows controlled release delivery. Table 4 shows a brief description of reported microparticles formulated by using various polymers by different methods (see Table 5).
Table 4.
Various polymers used for the treatment of ulcerative colitis.
| Microparticle formulation | Polymer used | Size | Method | Inference | References | |
|---|---|---|---|---|---|---|
| 1. | PEG-functionalized microparticles | Fluorescent chitosan, PEG and PLGA | Diameter of 3000–300 nm | Solvent evaporation technique | Fluoresceinamine-labeled-PEG-functionalized microparticles showed increased translocation through inflamed mucosa | Lautenschläger et al. (2013b) |
| 2. | Multiparticulate system | Cellulose acetate butyrate coated by Eudragit S | 60–110 μm | Emulsion solvent evaporation technique | With the use of hydrophobic polymer i.e., CAB, hydrophobic drug i.e., Ondansetron and budesonide get diffuse in the controlled manner | Rodríguez et al. (1998) |
| 3. | Eudragit coated chitosan-prednisolone conjugate microspheres | Chitosan, succinyl prednisolone, Eudragit L100 | 1.5–26.6 μm | Emulsification and evaporation method | Prednisolone loaded Ch–SP-MS/EuL reduces the toxic side-effects of drug | Oosegi et al. (2008) |
| 4. | Ethyl cellulose coated pectin alginate microspheres | Ethyl cellulose, pectin, alginate | 500–700 μm | Ionotropic-external gelation technique | Ethyl cellulose-coated pectin microspheres of 5-fluorouracil prevents the drug release in the upper part of GIT | Ramana and Krishna (2011) |
| 5. | Ethyl cellulose coated gelatine 5-Amino salicylic acid microspheres | Gelatin, ethyl cellulose | 50–400 μm | Solvent evaporation method | As gelatin is hydrophilic, coating of ethyl cellulose makes it a delayed drug delivery system in which 30% drug will release from microspheres within first 6 h, which makes it suitable for maximum drug delivery to colon | Atyabi et al. (2010) |
| 6. | Magnetic microspheres | Eudragit S100, ethyl cellulose, chitosan | 153–200 μm | Solvent evaporation technique | Magnetic microspheres | Kakar et al. (2012) |
| 7. | Biodegradable mesalamine containing microspheres | PLGA coated mesalamine microspheres | 1.15 μm | Emulsification solvent evaporation method | PLGA-mesalamine microspheres provides the controlled drug delivery as compared to suspension of the same drug, which shows effective targeted drug delivery to inflamed site in ulcerative colitis | Mahajan et al. (2011) |
| 8. | Naproxen sodium using sodium alginate and Eudragit S100 as mucoadhesive and pH-sensitive polymer | Eudragit S-100 and sodium alginate | 454.26 μm | By cross-linking with CaCl2 | Enteric coated microspheres showed a longer residence time in colon after removing its coating due to better mucoadhesion properties of sodium alginate | Chawla et al. (2012) |
| 9. | 5-Florouracil microspheres for colon delivery | Chitosan, Eudragit S 100 | 206.23 μm | Emulsion dehydration method and emulsion solvent evaporation method | Eudragit S 100 coated chitosan microspheres reduces the side-effects of drug caused by its absorption from upper part of GIT when given in conventional dosage form | Raj et al. (2013) |
| 10. | Budesonide containing microparticles | Eudragit RS/Eudragit RL 70:30 (w/w) | 110 μm | Solvent evaporation/spray drying technique | Eudragit RS shows better protection of drug from gastric acidity than those of Eudragit RS: Eudragit RL | Cortesi et al. (2012) |
| 11. | Albendazole microspheres | Eudragit RL | 220 μm | Solvent evaporation method | By using Eudragit RL, drug gets targeted to the terminal ileum and colonic regions | Jain et al. (2004) |
Table 5.
List of brief description of reported microparticles formulated by using various polymers by different methods.
| S. No. | Methods | Advantages | Disadvantages | Applications |
|---|---|---|---|---|
| 1. | Spray drying | 1. Process is rapid | 1. High temperature is required | This technique is rapid, fast. PLGA-microparticles loaded with thyrotropin releasing hormone was produced by Takada et al. (1995) |
| 2. Formation of porous microparticles | 2. Yield is very less due to the sticking of microparticles to the drying chamber | |||
| 3. Complete evaporation of organic solvent | 3. It can change the polymorphism of spray dried drugs | |||
| 4. Operation is feasible under aseptic conditions | 4. Cost effective | |||
| 5. Both hydrophobic and hydrophilic polymer can be used | 5. Highly viscous fluids cannot be spray dried | |||
| 6. Ideal for sterile product manufacturing | ||||
| 2. | Single Emulsion technique | 1. Simple method | 1. Chemical crosslinker is toxic and if added in excess, should be subjected to centrifugation, washing and separation, which makes it a lengthy process | This method is used to prepare microspheres containing protein and peptide drugs. Kawashima et al. (1999) prepared nanospheres by using single emulsion technique in which insulin solution was dissolved in organic solvent |
| 2. In expensive | ||||
| 3. | Double emulsification | 1. Controlled release | 1. Stability problem is the major disadvantage | Currently, a product named, leuprolide acetate (a luteinizing hormone-releasing hormone (LHRH) agonist was encapsulated in form of w/o/w by using PLGA (75/25) polymer having MW 14 K is in market |
| 2. Used for hydrophilic drugs, proteins, vaccines | 2. Coalescence | |||
| 4. | Solvent evaporation | 1. Suitable for microencapsulation of lipophilic drugs like peptide for sustained delivery | 1. Sometimes in w/o type of emulsions, removal of oil from final product is complicated | Preparation of drug containing Poly (dl-lactide) microparticles by solvent evaporation method |
| 2. Cost effective | ||||
| 3. Use of organic solvents like DCM which is toxic, and need its complete removal from final product | ||||
| 4. Sometimes, protein get denatured and formed aggregates | ||||
| 5. | Interfacial polymerization | 1. Fast, rapid | 1. Microcapsules formed by this method are fragile and difficult to handle | Watnasirichaikul et al. (2000), prepared insulin nanoparticles using this technique |
| 2. Much controlled approach | 2. Due to large w/o interface, enzymes or proteins get inactivated | |||
| 3. Efficient | 3. It’s hard to control the polymerization reaction | |||
| 4. Large no. of washing steps are required for the complete removal of monomers and other by products | ||||
6. Mechanism of microparticle uptake in ulcerative colitis
The mechanism of microparticles for proper site specific drug delivery is based on complete knowledge of mechanism of drug as well as disease. For improved localization and increased residence time of drug at the site of inflammation, optimum particle size should be between 4 and 15 μm (Coppi et al., 2001, Coppi et al., 2002, Lamprecht et al., 2001a, Lamprecht et al., 2001b). Particle size in that range gets accumulated in the inflamed sites even without macrophage uptake. Particles below size range of 10 μm increase residence time at the inflamed region, while with further size reduction, clearance get minimized at size of approximately 100 nm. The first important layer covering colonic mucosa is the mucus gel layer. To achieve high localization in Payer’s patches, intestinal lymphoid tissue and lamina propria, there is the need to overcome such barrier/layer. After crossing such mucosal layer, particles have to be translocated across M cells and enterocytes depending upon size of particles. The mechanism of this size dependent translocation across colonocytes involves following process: (1) Particles of size range <500 nm show endocytotic uptake through endocytosis. (2) Particles of size <5 μm adsorbed by M cells of Peyer’s patches show lymphatic uptake. (3) By using mucoadhesive coating polymers, bioadhesion of microparticles/nanoparticles is increased. In case of UC, as the disease severity increases, protective mucus layer starts becoming thinner. This pathophysiology of mucus layer increases the mucosal permeability and helps in the proper location at its inflamed sites. According to Nixon et al., microparticles having size range of 2–5 μm remain in payer patches for longer period of time and show less systemic distribution and improve local inflammation (Lamprecht et al., 2001b). Fig. 3 shows (1) Uninflammed mucosa: (a) Particles of size <500 nm show endocytotic uptake, (b) particles of size <5 nm show lymphatic uptake. (2) Inflammed mucosa: Increased particle uptake due to leaky epithelium.
Figure 3.
(1) Uninflammed mucosa: (a) Particle size <500 nm shows endocytotic uptake, (b) particle size <5 nm shows lymphatic uptake. (2) Inflammed mucosa: Increased particle uptake due to leaky epithelium.
7. Various methods of preparation of microparticles
Microparticles should be biocompatible and biodegradable and have the ability to deliver drug specifically to the confined area rather than other sites. It depends upon the properties of polymer used, method of preparation and various other factors which effect its surface properties. There are various methods used for the preparation of microparticles as mentioned below.
7.1. Spray drying technique
This technique is used for the preparation of polymeric blended microspheres having the size range from 1 to 100 μm. In this method, polymer is dissolved in volatile organic solvent like DCM or acetone. Then core material is dispersed in this polymeric solution. This dispersed phase is atomized in a spray chamber under hot air, which leads to the formation of fine mist from which solvent evaporates instantaneously. Thus microparticles get separated by cyclone separator, present just beside spray chamber, while whole solvent is removed by vacuum drying.
Mathew et al. (2008); prepared the ketoprofen loaded PCL [poly(epsilon-caprolactone)] and cellulose acetate butyrate (CAB) by using this technique. Varshosaz et al. (2011), prepared the microcapsules of budesonide with different molecular weight dextran at different ratios and found that 1:10 drug to polymer ratio is effective in targeting drug to colon when compared with mesalazine suspension.
7.2. Single emulsion technique
In this method, drug is dissolved in aqueous medium, which acts as dispersed phase, is dropped into oil phase, which acts as continuous phase. A single emulsion will form, which is further stabilized by addition of either crosslinker or by heating. The most commonly used chemical crosslinkers are formaldehyde, glutaraldehyde, epichlorohydrin, etc. Example of this technique is Gelatin A microspheres by Jeevana and Sunitha (2009).
Raj et al., formulated and evaluated 5-Flourouracil loaded chitosan microspheres with the coating of Eudragit S100 by using oil in oil emulsification method using core:coat ratio is 5:1. It was concluded that with the increase in polymer concentration, rate of drug release decreased. Thus, Eudragit S 100 coated chitosan microspheres prepared by single emulsification technique provided controlled release drug delivery.
Dashora and Jain (2009), formulated pectin-prednisolone microspheres for the treatment of ulcerative colitis by using single emulsion-dehydration technique. In this study, two types of microspheres, one is simple prednisolone loaded pectin microspheres; while other is Eudragit coated pectin-prednisolone microspheres were prepared. It was concluded from this study that Eudragit coated microspheres released drug from 6.25% to 8.95% in simulated gastric fluid and simulated intestinal fluid respectively after 4 h, which is less as compared to simple pectin-prednisolone microspheres.
Bhat et al. (2013), prepared enteric coated gelatin capsules containing 5-FU loaded guar gum microspheres by using water in oil emulsification method. In this study, in vitro drug release was conducted at simulated gastric fluid at pH 1.2 and simulated intestinal fluid at pH 7.4 and it was concluded that capsule loaded microspheres showed low initial burst release when compared to microsphere formulation.
7.3. Double emulsification technique
In pharmaceutical industry, Double emulsification technique is mostly used for the hydrophilic drugs, proteins, vaccines, vitamins, enzymes for controlled release. In this technique, two emulsions are formed, one is primary and other is secondary. For the preparation of primary emulsion, protein aqueous solution is dispersed in lipophilic continuous phase for the encapsulation of protein contained in dispersed aqueous phase. This primary phase is then homogenized and dropped into aqueous solution of poly vinyl alcohol, which acts as secondary phase. Addition of primary phase to secondary leads to the formation of double emulsion. The possible mechanism for the drug release is diffusion process (Mathew et al., 2008).
Nakase et al. (2000) developed oral drug delivery system to target macrophages with poly(dl-lactic acid) microspheres containing dexamethasone, as a model drug by using solvent evaporation with double emulsification method. This study concluded that microspheres showed therapeutic effect in ulcerative colitis by inhibiting proinflammatory cytokines.
Meissner et al. (2007), prepared low molecular weight heparin loaded P4135F microparticles by using double emulsion technique with solvent evaporation method for the treatment of ulcerative colitis.
7.4. Solvent evaporation technique
This technique is carried out for the preparation of microcapsules either by o/w or w/o or o/o type of emulsion. In this method, core material is dispersed/dissolved in polymer solution. This phase is then dropped into continuous phase, in which either water or oil is used as vehicle to obtain microcapsules. For the complete removal of organic solvent, heating is necessary. Then, freeze dried the microparticles for further use. Mateović-Rojnik et al., 2005 prepared Eudragit RS 100 microspheres containing ketoprofen as model drug by solvent evaporation method.
7.5. Polymerization techniques
The polymerization techniques are generally classified as (i) normal polymerization, (ii) interfacial polymerization.
7.5.1. Normal polymerization
Various techniques are used for carrying out normal polymerization such as suspension, emulsion and bulk techniques. In suspension technique, heating of one or more monomers as droplets dispersion is carried out in a continuous aqueous phase. This technique is also known as bead or pearl polymerization. In bulk polymerization, heating of one or more monomers in the presence of catalyst takes place for the initiation of polymerization. This type of polymerization is best for the formation of pure polymers. The main example of emulsion polymerization is nylon microcapsules (Whateley, 1996).
7.5.2. Interfacial polymerization
In this method, two reactive monomers, one is dissolved while other is dispersed separately in two immiscible liquids. A brisk reaction occurs at the interface between two solutions, which creates a thin interfacial polymer film.
8. Mechanism of drug release of microparticles
Theoretically, there are different mechanisms of drug release of microparticulate systems.
8.1. Diffusion controlled reservoir system
In this system, a controlling membrane is present around drug, through which drug starts diffusing out. Here, release rate is not affected by matrix degradation. After complete diffusion, membrane gets eroded. Fig. 4 shows diffusion of drug through controlling membrane.
Figure 4.
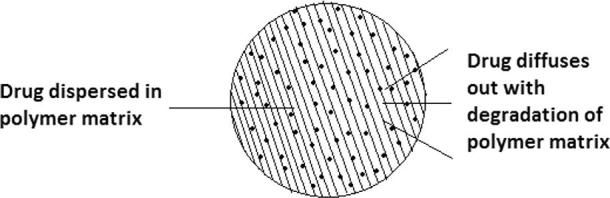
Mechanism of drug release through controlling membrane.
8.2. Diffusion controlled monolithic system
In this system, drug is dispersed in polymer matrix. With degradation of polymer matrix, drug starts diffusing out. Here, release rate is highly affected by matrix degradation. Fig. 5 shows diffusion of drug through polymer matrix.
Figure 5.
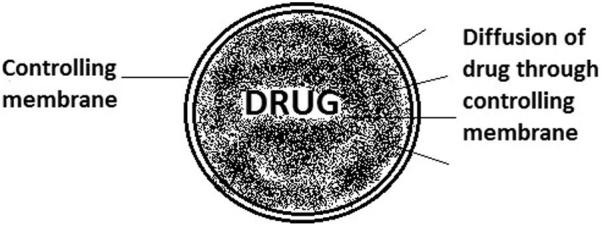
Mechanism of drug release through polymer matrix.
8.3. Degradation controlled monolithic system
In this system, drug is uniformly dispersed in polymer matrix and rate of diffusion depends upon degradation of matrix. Generally, rate of diffusion is slow as compared with degradation of matrix. The formula for calculating release of sphere is governed by following equation:
where Mt is amount of drug released at time t; M∞ is amount at time t∞ for total erosion (Keerthi et al.)
8.4. Conclusion and future prospectives
With increasing new technologies, a significant progress has been made in developing a truly selective system for targeting drug to site of action in ulcerative colitis. However, there is the lack of commercial products especially biologic drugs for the treatment of ulcerative colitis. In this respect, Foong et al. (2010) formulated infliximab loaded PLGA microspheres for the treatment of Crohn’s disease fistulae. At present, no microencapsulated probiotic cells exist in market. Thus, search is increasing on new delivery strategies that can provide the therapeutic beneficial to colitis suffered patients.
It has been concluded from above study that microparticulate carrier system appears to be the most promising approach by specifically accumulating in inflamed intestinal region. Combined approach of both pH dependent and time dependent particulate systems is strongly appealing to use in a truly colon specific drug delivery system. It may reduce the side-effects of drug caused by absorption from stomach when given in form of tablets. But still, there are several issues, which need to be sorted out with regard to stability during gastric and small intestinal passage by using this particulate carrier system.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alvarez-Fuentes J., Fernández-Arévalo M., González-Rodríguez M., Cirri M., Mura P. Development of enteric-coated timed-release matrix tablets for colon targeting. J. Drug Target. 2004;12(9–10):607–612. doi: 10.1080/10611860400013501. [DOI] [PubMed] [Google Scholar]
- Ardizzone S., Porro G.B. Inflammatory bowel disease: new insights into pathogenesis and treatment. J. Intern. Med. 2002;252(6):475–496. doi: 10.1046/j.1365-2796.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- Ashford M., Fell J.T., Attwood D., Sharma H., Woodhead P.J. An in vivo investigation into the suitability of pH dependent polymers for colonic targeting. Int. J. Pharm. 1993;95(1):193–199. [Google Scholar]
- Atyabi F., Vahabzadeh R., Dinarvand R. Preparation of ethylcellulose coated gelatin microspheres as a multiparticulate colonic delivery system for 5-aminosalicilic acid. Iran. J. Pharm. Res. 2010:81–86. [Google Scholar]
- Baron J., Connell A., Lennard-Jones J., Avery Jones F. Sulphasalazine and salicylazosulphadimidine in ulcerative colitis. Lancet. 1962;279(7239):1094–1096. doi: 10.1016/s0140-6736(62)92080-9. [DOI] [PubMed] [Google Scholar]
- Bhat Subramanya K., Keshavayya J., Kulkarni Venkatrao H., Venugoapala Reddy K.R., Kulkarni Anandrao R., Kulkarni Preeti V. Preparation characterization and in-vitro release studies of enteric coated gelatin capsules containing guar gum microspheres for targeted delivery of 5-fluorouracil to colon. Der Pharma Chem. 2013;5(3):221–231. [Google Scholar]
- Botoman V.A., Bonner G.F., Botoman D.A. Management of inflammatory bowel disease. Am. Fam. Physician. 1998;57(1):57–68. 71-2. [PubMed] [Google Scholar]
- Buchman A.L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001;33(4):289–294. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Carter M.J., Lobo A.J., Travis S.P. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl. 5):v1–v16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra Dinesh, Yadav Indranil Kumar, Singh Hari Pratap, Jain D.A. Design and development of satranidazole microspheres for colon targeted drug delivery. Int. J. Pharm. Chem. Sci. 2012;1(3) [Google Scholar]
- Chawla Anuj, Sharma Pooja, Pawar Pravin. Eudragit S-100 coated sodium alginate microspheres of naproxen sodium: formulation, optimization and in vitro evaluation. Acta Pharm. 2012;62:529–545. doi: 10.2478/v10007-012-0034-x. [DOI] [PubMed] [Google Scholar]
- Chourasia M., Jain S.K. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharm. Sci. 2003;2003(6):33–66. [PubMed] [Google Scholar]
- Cole E.T., Scott R.A., Connor A.L., Wilding I.R., Petereit H.-U., Schminke C. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharm. 2002;231(1):83–95. doi: 10.1016/s0378-5173(01)00871-7. [DOI] [PubMed] [Google Scholar]
- Collnot E.-M., Ali H., Lehr C.-M. Nano-and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J. Control. Release. 2012;161(2):235–246. doi: 10.1016/j.jconrel.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Coppi G., Iannuccelli V., Leo E., Bernabei M.T., Cameroni R. Chitosan–alginate microparticles as a protein carrier. Drug Dev. Ind. Pharm. 2001;27(5):393–400. doi: 10.1081/ddc-100104314. [DOI] [PubMed] [Google Scholar]
- Coppi G., Iannuccelli V., Bernabei M., Cameroni R. Alginate microparticles for enzyme peroral administration. Int. J. Pharm. 2002;242(1):263–266. doi: 10.1016/s0378-5173(02)00171-0. [DOI] [PubMed] [Google Scholar]
- Cortesi Rita, Ravani Laura, Menegatti Enea, Esposito Elisabetta, Ronconi F. Eudragit® microparticles for the release of budesonide: a comparative study. Indian J. Pharm. Sci. 2012;74(5):415–421. doi: 10.4103/0250-474X.108416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosnes J., Gower-Rousseau C., Seksik P., Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Dashora A., Jain C.P. Development and characterization of pectin-prednisolone microspheres for colon targeted delivery. Int. J. ChemTech Res. 2009;1(3):51–757. [Google Scholar]
- Egan L.J., Mays D.C., Huntoon C.J., Bell M.P., Pike M.G., Sandborn W.J. Inhibition of interleukin-1-stimulated NF-κB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J. Biol. Chem. 1999;274(37):26448–26453. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- Eisen G.M., Sandler R.S. Update on the epidemiology of IBD. Prog. Inflamm. Bowel Dis. 1994;15:1–8. [Google Scholar]
- El-Gibaly I. Oral delayed-release system based on Zn-pectinate gel (ZPG) microparticles as an alternative carrier to calcium pectinate beads for colonic drug delivery. Int. J. Pharm. 2002;232(1–2):199–211. doi: 10.1016/s0378-5173(01)00903-6. [DOI] [PubMed] [Google Scholar]
- Foong K.S., Patel R., Forbes A., Day R.M. Anti-tumor necrosis factor-alphaloaded Microspheres as a prospective novel treatment for Crohn's disease fistulae. Tissue Eng. Part C Methods. 2010;16(16):855–864. doi: 10.1089/ten.TEC.2009.0599. [DOI] [PubMed] [Google Scholar]
- Ford A.C., Bernstein C.N., Khan K.J., Abreu M.T., Marshall J.K., Talley N.J. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am. J. Gastroenterol. 2011;106(4):590–599. doi: 10.1038/ajg.2011.70. [DOI] [PubMed] [Google Scholar]
- Friend D.R. New oral delivery systems for treatment of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2005;57(2):247–265. doi: 10.1016/j.addr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fukui E., Miyamura N., Uemura K., Kobayashi M. Preparation of enteric coated timed-release press-coated tablets and evaluation of their function by in vitro and in vivo tests for colon targeting. Int. J. Pharm. 2000;204(1):7–15. doi: 10.1016/s0378-5173(00)00454-3. [DOI] [PubMed] [Google Scholar]
- Fuss I.J., Heller F., Boirivant M., Leon F., Yoshida M., Fichtner-Feigl S. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 2004;113(10):1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawde Preeti, Agrawal Shikha, Jain Prabhat. Development of mucoadhesive microsphere for colon delivery. Int. J. Pharm. Biol. Arch. 2012;3(3):440–442. [Google Scholar]
- Ghandehari H., Kopeckova P., Kopecek J. There are various methods for the preparation of nanoparticles which includes ionic gelation method, complex coacervation, emulsion cross linking and spray drying. Biomaterials. 1997;18:861–872. [Google Scholar]
- Gilhotra Ritu Mehra, Bhardwaj Vishv Priya, Mishra D.N. A comparative review of recently developed particulate drug carrier systems. Taxonomy upgrade extras. Target. Drug Deliv. Syst. 2009;7(3) [Google Scholar]
- Harel E., Rubinstein A., Nissan A., Khazanov E., Nadler Milbauer M., Barenholz Y., Tirosh B. Enhanced transferrin receptor expression by proinflammatory cytokines in enterocytes as a means for local delivery of drugs to inflamed gut mucosa. PLoS One. 2011;6:e24202. doi: 10.1371/journal.pone.0024202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem Fahima M., Shaker Dalia S., Nasr Mohamed, Ragaey Reem. In vitro and in vivo evaluation of combined time and pH dependent oral colon targeted prednisolone microspheres. Br. J. Pharm. Res. 2013;3(3):20–434. [Google Scholar]
- Hinton J., Lennard-Jones J., Young A. A new method for studying gut transit times using radioopaque markers. Gut. 1969;10(10):842–847. doi: 10.1136/gut.10.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs K.L., Lewis J.D., Sandborn W.J., Sands B.E., Targan S.R. State of the art: IBD therapy and clinical trials in IBD. Inflamm. Bowel Dis. 2005;11(S1):S3–S12. doi: 10.1097/01.mib.0000184852.84558.b2. [DOI] [PubMed] [Google Scholar]
- Jain Sunil K., Rai Gopal, Saraf D.K., Agrawal G.P. Albendazole microspheres for colonic delivery. Pharm. Technol. 2004;28(12):66–71. [Google Scholar]
- Jeevana B., Sunitha J.G. Development and evaluation of gelatin microspheres of tramadol hydrochloride. J. Young Pharm. 2009;1(1):24–27. [Google Scholar]
- Jeong Y.-I., Ohno T., Hu Z., Yoshikawa Y., Shibata N., Nagata S. Evaluation of an intestinal pressure-controlled colon delivery capsules prepared by a dipping method. J. Control. Release. 2001;71(2):175–182. doi: 10.1016/s0168-3659(01)00211-5. [DOI] [PubMed] [Google Scholar]
- Kakar S., Batra D., Singh R., Nautiyal U. Magnetic microspheres as magical novel drug delivery system: a review. J. Acute Dis. 2012;2(1):1–12. [Google Scholar]
- Kawashima Y., Yamamoto H., Takeuchi H., Fujioka S., Hino T. Pulmonary delivery of insulin with nebulized DL-lactide/glycolide copolymer (PLGA) nanospheres to prolong hypoglycemic effect. J. Cont. Rel. 1999;62:279–287. doi: 10.1016/s0168-3659(99)00048-6. [DOI] [PubMed] [Google Scholar]
- Keerthi, T.S., Vinupama, S., Kamatha, Shwetha S., Singh, Prashanth, Senthil Kumar, S.K. Biodecomposable polymeric microspheres: review. Int. Bull. Drug Res. 1(2), 81.
- Kim K.K., Pack D.W. Springer; 2006. Microspheres for Drug Delivery. BioMEMS and Biomedical Nanotechnology. pp. 19–50. [Google Scholar]
- Lamprecht A., Schäfer U., Lehr C.-M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001;18(6):788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- Lamprecht A., Ubrich N., Yamamoto H., Schäfer U., Takeuchi H., Maincent P. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2001;299(2):75–81. [PubMed] [Google Scholar]
- Lamprecht A., Yamamoto H., Takeuchi H., Kawashima Y. A pH-sensitive microsphere system for the colon delivery of tacrolimus containing nanoparticles. J. Control. Release. 2005;104(2):337–346. doi: 10.1016/j.jconrel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Lautenschläger C., Schmidt C., Fischer D., Stallmach A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2013 doi: 10.1016/j.addr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Lautenschläger C., Schmidt C., Lehr C.-M., Fischer D., Stallmach A. PEG-functionalized microparticles selectively target inflamed mucosa in inflammatory bowel disease. Eur. J. Pharm. Biopharm. 2013;85(3):578–586. doi: 10.1016/j.ejpb.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Lee W.W., Wilson C.G., Mukherji G. Marcel Dekker; New York: 2003. Time-dependent Systems for Colonic Delivery. [Google Scholar]
- Lee J.K., Tang D.H., Mollon L., Armstrong E.P. Cost-effectiveness of biological agents used in ulcerative colitis. Best Pract. Res. Clin. Gastroenterol. 2013;27(6):949–960. doi: 10.1016/j.bpg.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Liang D. Ulcerative colitis: diagnosis and management. Hosp. Physician. 2002:53. [Google Scholar]
- Mahajan N.M., Sakarkar D.M., Manmode A.S. Preparation and characterization of meselamine loaded PLGA nanoparticles. Int. J. Pharm. Pharm. Sci. 2011;3(4) [Google Scholar]
- Mahkam Mehrdad, Vakhshouri Laleh. Colon-specific drug delivery behavior of pH-responsive PMAA/perlite composite. Int. J. Mol. Sci. 2010;11(4):1546–1556. doi: 10.3390/ijms11041546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlof A., Tozuka Y., Takeuchi H. pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur. J. Pharm. Biopharm. 2009;72(1):1–8. doi: 10.1016/j.ejpb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Mateović-Rojnik Tatjana, Frlan Rok, Bogataj Marija, Bukovec Peter, Mrhar Aleš. Effect of preparation temperature in solvent evaporation process on Eudragit RS microsphere properties. Chem. Pharm. Bull. 2005;53(1):143–146. doi: 10.1248/cpb.53.143. [DOI] [PubMed] [Google Scholar]
- Mathew Sam T., Devi Gayathri S., Prasanth V.V., Vinod B. NSAIDs as microspheres. Int. J. Pharmacol. 2008;6(1) [Google Scholar]
- Meier J., Sturm A. Current treatment of ulcerative colitis. World J. Gastroenterol. 2011;17(27):3204–3212. doi: 10.3748/wjg.v17.i27.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner Y., Ubrich N., El Ghazouani F., Maincent P., Lamprecht A. Low molecular weight heparin loaded pH-sensitive microparticles. Int. J. Pharm. 2007;335(1–2):147–153. doi: 10.1016/j.ijpharm.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Moller D.R., Wysocka M., Greenlee B.M., Ma X., Wahl L., Flockhart D.A. Inhibition of IL-12 production by thalidomide. J. Immunol. 1997;159(10):5157–5161. [PubMed] [Google Scholar]
- Molodecky N.A., Kaplan G.G. Environmental risk factors for inflammatory bowel disease. Gastroenterol. Hepatol. 2010;6(5):339. [PMC free article] [PubMed] [Google Scholar]
- Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Moreira A.L., Sampaio E.P., Zmuidzinas A., Frindt P., Smith K.A., Kaplan G. Thalidomide exerts its inhibitory action on TNF-α by enhancing m RNA degradation. J. Exp. Med. 1993;177(6):1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Hiroshi, Okazaki Kazuichi, Tabata Yasuhiko, Uose Suguru, Ohana Masaya, Uchida Kazushige, Matsushima Yumi, Kawanami Chiharu, Oshima Chikashi, Ikada Yoshito, Chiba Tsutomu. Development of an oral drug delivery system targeting immune-regulating cells in experimental inflammatory bowel disease: a new therapeutic strategy. J. Pharmacol. Exp. Ther. 2000;292:5–21. [PubMed] [Google Scholar]
- Navaneethan U., Jegadeesan R., Gutierrez N.G., Venkatesh P.G., Hammel J.P., Shen B. Progression of low-grade dysplasia to advanced neoplasia based on the location and morphology of dysplasia in ulcerative colitis patients with extensive colitis under colonoscopic surveillance. J. Crohns Colitis. 2013;7(12):e684–e691. doi: 10.1016/j.crohns.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Neurath M.F., Travis S.P. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61(11):1619–1635. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- Newton A.M.J., Prabakaran L., Jayaveera K. Pectin-HPMC E15LV Vs pH sensitive polymer coating films for delayed drug delivery to colon: a comparison of two dissolution models to assess colonic targeting performance in-vitro. Int. J. Appl. Res. Nat. Prod. 2012;5(3):1–16. [Google Scholar]
- Oosegi T., Onishi H., Machida Y. Gastrointestinal distribution and absorption behavior of Eudragit-coated chitosan–prednisolone conjugate microspheres in rats with TNBS-induced colitis. Int. J. Pharm. 2008;348(1):80–88. doi: 10.1016/j.ijpharm.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Papadakis K.A., Shaye O.A., Vasiliauskas E.A., Ippoliti A., Dubinsky M.C., Birt J. Safety and efficacy of adalimumab (D2E7) in Crohn’s disease patients with an attenuated response to infliximab. Am. J. Gastroenterol. 2005;100(1):75–79. doi: 10.1111/j.1572-0241.2005.40647.x. [DOI] [PubMed] [Google Scholar]
- Patel M., Shah T., Amin A. Therapeutic opportunities in colon-specific drug-delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2007;24(2) doi: 10.1615/critrevtherdrugcarriersyst.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- Philip A.K., Philip B. Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med. J. 2010;25(2):79. doi: 10.5001/omj.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj Behin Sundara, Shanthi, Nair Rajesh Sreedharan, Samraj Punitha Isaac, Vidya Formulation and evaluation of coated microspheres for colon targeting. J. Appl. Pharm. Sci. 2013;3(8):68–S74. [Google Scholar]
- Ramana G., Krishna Chaitanya A. Preparation and in-vitro characterization of ethyl cellulose coated pectin alginate microspheres of 5-fluorouracil for colon targeting. J Appl. Pharm. Sci. 2011;1(08):70–76. [Google Scholar]
- Ransford R., Langman M. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002;51(4):536–539. doi: 10.1136/gut.51.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool Fatima, Ahmad Mahmood, Murtaza Ghulam, Khan Haji M.S., Khan Shujaat Ali. Eudragitæ fs based colonic microparticles of metoprolol tartrate. Acta Pol. Pharm. 2012;69(2):347–353. [PubMed] [Google Scholar]
- Riley S., Mani V., Goodman M., Herd M., Dutt S., Turnberg L. Comparison of delayed release 5 aminosalicylic acid (mesalazine) and sulphasalazine in the treatment of mild to moderate ulcerative colitis relapse. Gut. 1988;29(5):669–674. doi: 10.1136/gut.29.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez M., Vila-Jato J.L., Torres D. Design of a new multiparticulate system for potential site-specific and controlled drug delivery to the colonic region. J. Control. Release. 1998;55(1):7–77. doi: 10.1016/s0168-3659(98)00029-7. [DOI] [PubMed] [Google Scholar]
- Rogler Gerhard. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014;345(2):235–241. doi: 10.1016/j.canlet.2013.07.032. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Sandborn W.J., Feagan B.G., Reinisch W., Olson A., Johanns J. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- Saboktakin M.R., Tabatabaie R.M., Maharramov A., Ramazanov M.A. Synthesis and in vitro evaluation of carboxymethyl starch–chitosan nanoparticles as drug delivery system to the colon. Int. J. Biol. Macromol. 2011;48(3):381–385. doi: 10.1016/j.ijbiomac.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Hanauer S.B., Katz S., Safdi M., Wolf D.G., Baerg R.D. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121(5):1088–1094. doi: 10.1053/gast.2001.28674. [DOI] [PubMed] [Google Scholar]
- Sareen R., Jain N., Rajkumari A., Dhar K.L. pH triggered delivery of curcumin from Eudragit-coated chitosan microspheres for inflammatory bowel disease: characterization and pharmacodynamic evaluation. Drug Deliv. 2014 doi: 10.3109/10717544.2014.903534. (in Press) [DOI] [PubMed] [Google Scholar]
- Scheline R.R. Metabolism of foreign compounds by gastrointestinal microorganisms. Pharmacol. Rev. 1973;25(4):451. [PubMed] [Google Scholar]
- Schroeder K.W., Tremaine W.J., Ilstrup D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987;317(26):625–629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- Siegel S.A., Shealy D.J., Nakada M.T., Le J., Woulfe D.S., Probert L. The mouse/human chimeric monoclonal antibody cA2 neutralizes TNF in vitro and protects transgenic mice from cachexia and TNF lethality in vivo. Cytokine. 1995;7(1):15–25. doi: 10.1006/cyto.1995.1003. [DOI] [PubMed] [Google Scholar]
- Singh B.M. Modified-release solid formulations for colonic delivery. Recent Pat. Drug Delivery Formulation. 2007;1:53–63. doi: 10.2174/187221107779814122. [DOI] [PubMed] [Google Scholar]
- Sowmya Cherukuri, Reddy Chappidi Suryaprakash, Vishnu Priya Neelaboina, Sandhya Reddipalli, Komaragiri Keerthi. Colon specific drug delivery systems: a review on pharmaceutical approaches with current trends. Int. Res. J. Pharm. 2012;3(7):45. [Google Scholar]
- Stein J., Ries J., Barrett K.E. Disruption of intestinal barrier function associated with experimental colitis: possible role of mast cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1998;274(1):G203–G209. doi: 10.1152/ajpgi.1998.274.1.G203. [DOI] [PubMed] [Google Scholar]
- Strober W., Fuss I., Mannon P. The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 2007;117(3):514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S., Uda Y., Toguchi H., Ogawa Y. Application of a spray drying technique in the production of TRH-containing injectable sustained-release microparticles of biodegradable polymers. PD J PhAarm. Sci. Technol. 1995;49(4):180–184. [PubMed] [Google Scholar]
- Talaei F., Atyabi F., Azhdarzadeh M., Dinarvand R., Saadatzadeh A. Overcoming therapeutic obstacles in inflammatory bowel diseases: a comprehensive review on novel drug delivery strategies. Eur. J. Pharm. Sci. 2013;49(4):712–722. doi: 10.1016/j.ejps.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Taylor K.M., Irving P.M. Optimization of conventional therapy in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2011;8(11):646–656. doi: 10.1038/nrgastro.2011.172. [DOI] [PubMed] [Google Scholar]
- Thippeswamy B.S., Mahendran S., Biradar M.I., Raj P., Srivastava K., Badami S. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur. J. Pharmacol. 2011;654(1):100–105. doi: 10.1016/j.ejphar.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Tromm A., Griga T., May B. Oral mesalazine for the treatment of Crohn’s disease: clinical efficacy with respect to pharmacokinetic properties. Hepatogastroenterology. 1998;46(30):3124–3135. [PubMed] [Google Scholar]
- Turunen U.M., Färkkilä M.A., Hakala K., Seppälä K., Sivonen A., Ögren M. Long-term treatment of ulcerative colitis with ciprofloxacin: a prospective, double-blind, placebo-controlled study. Gastroenterology. 1998;115(5):1072–1078. doi: 10.1016/s0016-5085(98)70076-9. [DOI] [PubMed] [Google Scholar]
- Varshosaz Jaleh, Ahmadi Fatemeh, Emami Jaber, Tavakoli Naser, Minaiyan Mohsen, Mahzouni Parvin, Dorkoosh Farid. Microencapsulation of budesonide with dextran by spray drying technique for colon-targeted delivery: an in vitro/in vivo evaluation in induced colitis in rat. J. Microencapsul. 2011;28(1):2–73. doi: 10.3109/02652048.2010.529947. [DOI] [PubMed] [Google Scholar]
- Watnasirichaikul S., Davies N.M., Rades T. Preparation of biodegradable insulin nanocapsules from biocompatible microemulsions. Pharm. Res. 2000;9:176684–176689. doi: 10.1023/a:1007574030674. [DOI] [PubMed] [Google Scholar]
- Whateley, T.L., 1996. Microcapsules: preparation by interfacial polymerization and interfacial complexation and their application. In: Benita, S. (Ed.), Microencapsulation: Methods and Industrial Applications, pp. 349–375.
- Zhou S., Fleisher D., Pao L., Li C., Winward B., Zimmermann E. Intestinal metabolism and transport of 5-aminosalicylate. Drug Metab. Dispos. 1999;27(4):479–485. [PubMed] [Google Scholar]