Abstract
Whole-exome sequencing (WES) is increasingly being utilized to diagnose individuals with undiagnosed disorders. Developmental delay and short stature are common clinical indications for WES. We performed WES in three families, using proband-parent trios and two additional affected siblings. We identified a syndrome due to an autosomal-recessively inherited deficiency of transketolase, encoded by TKT, on chromosome 3p21. Our series includes three families with a total of five affected individuals, ranging in age from 4 to 25 years. Two families of Ashkenazi Jewish ancestry were homozygous for an 18 base pair in-frame insertion in TKT. The third family was compound heterozygous for nonsense and missense variants in TKT. All affected individuals had short stature and were developmentally delayed. Congenital heart defects were noted in four of the five affected individuals, and there was a history of chronic diarrhea and cataracts in the older individuals with the homozygous 18 base pair insertion. Enzymatic testing confirmed significantly reduced transketolase activity. Elevated urinary excretion of erythritol, arabitol, ribitol, and pent(ul)ose-5-phosphates was detected, as well as elevated amounts of erythritol, arabitol, and ribitol in the plasma of affected individuals. Transketolase deficiency reduces NADPH synthesis and nucleic acid synthesis and cell division and could explain the problems with growth. NADPH is also critical for maintaining cerebral glutathione, which might contribute to the neurodevelopmental delays. Transketolase deficiency is one of a growing list of inborn errors of metabolism in the non-oxidative part of the pentose phosphate pathway.
Keywords: transketolase deficiency, TKT, pentose phosphate pathway, congenital heart disease, neurodevelopmental disability
Main Text
The use of whole-exome sequencing (WES) to diagnose individuals with undiagnosed disorders, particularly those that are familial and likely to have an inherited basis, is increasing in clinical practice.1, 2, 3 Developmental delay is a common clinical indication for WES, due to the genetic heterogeneity and incomplete knowledge of all the genes responsible for developmental delay; a definitive genetic etiology for the developmental delay was found in 34% of individuals analyzed with clinical WES at one institution.4
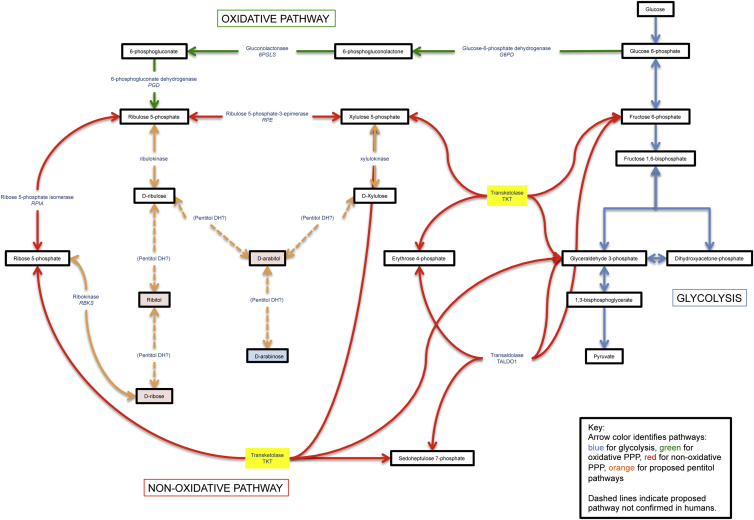
We describe a syndrome identified through WES in five affected individuals from three unrelated families, all of whom have proportional short stature, developmental delay, and congenital heart disease. Transketolase (TKT) is a reversible, thiamine-dependent enzyme in the pentose phosphate pathway (PPP) (Figure 1). The PPP consists of an oxidative phase, which generates NADPH for use in reductive biosynthesis, and a non-oxidative phase, which interconverts pentose sugars so they can be used for nucleotide biosynthesis and as intermediates of the glycolytic pathway. Depending on the cell type and its requirement for NADPH, ribose-5P, or ATP, the flow of glucose-6P is either through the oxidative or the non-oxidative branches.
Figure 1.
Pentitol Pathway
Yellow highlighting reflects the affected TKT enzyme. Metabolites in excess are in red, and deficient metabolites are in blue.
Numerous inborn errors of the PPP have been identified in both branches of the pathway (Figure 1). Within the oxidative branch, glucose 6-phosphate dehydrogenase (G6PD) is the first enzyme in the pathway. G6PD deficiency is the most common enzyme deficiency in humans and is associated with hemolysis and jaundice upon exposure to certain foods, illness, and medications.5 Within the non-oxidative branch, a ribose 5-phosphate isomerase deficiency was identified in one person presenting with leukoencephalopathy and peripheral neuropathy.6 Also within the non-oxidative branch, deficiencies of transaldolase are associated with a variable phenotype that includes liver disease, hepatosplenomegaly, hemolytic anemia, dysmorphic features, and congenital heart disease.7, 8, 9
We analyzed 2,625 individuals with neurodevelopmental disorders referred for clinical WES by using the SureSelect XT2 All Exon V4 or the Clinical Research Exome kit (Agilent Technologies), comparing both siblings and their parents. Three probands and two additional family members from three independent families all had homozygous or compound-heterozygous rare variants in TKT (MIM: 606781). The parents of all probands gave informed consent, and the studies were approved by the institutional review board of Columbia University. WES data were generated, sequences aligned, and variants filtered as previously described.10 In brief, variants were filtered on the basis of inheritance patterns, gene lists of interest, phenotype, and population frequencies (<1%), and were manually reviewed. Resources including the Human Gene Mutation Database (HGMD), the 1000 Genomes database, the NHLBI Exome Variant Server, OMIM, PubMed, ClinVar, and GeneDx’s internal database were used to evaluate genes and variants of interest. We confirmed identified sequence variants of interest with conventional di-deoxy DNA sequence analysis by using an ABI3730 (Life Technologies) and a new DNA preparation for all family members studied.
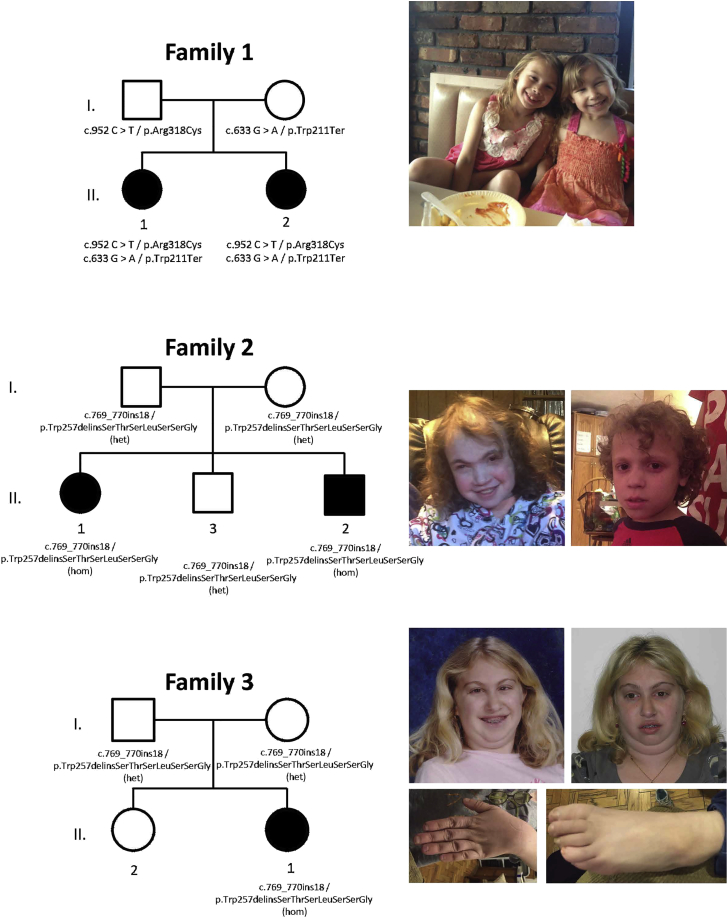
In the five affected individuals reported, there was an average of ∼13 GB of sequence per sample. Mean coverage of captured regions was ∼177× per sample, and >98.4% of the targeted sequences had at least 10× coverage, an average of >92% had a base call quality of Q30 or greater, and the overall average mean quality score was <Q36. A list of the number of variants for each family after filtering is provided in Table S1. In family 1, both affected siblings have two compound-heterozygous variants, c.633G>A (p.Trp211Ter) and c.952C>T (p.Arg318Cys) (GenBank: NM_001135055.2). The mother is heterozygous for the p.Trp211Ter variant, and the father is heterozygous for the p.Arg318Cys variant. The p.Trp211Ter variant is predicted to lead to nonsense-mediated decay. The p.Arg318Cys variant is predicted to be deleterious by SIFT, PolyPhen, CADD, and SVM. In families 2 and 3, both of Ashkenazi heritage, all three affected individuals are homozygous for c.769_770insCTACCTCCTTATCTTCTG (p.Trp257delinsSerThrSerLeuSerSerGly), and parents in both families are heterozygous carriers. The c.769_770insCTACCTCCTTATCTTCTG TKT variant (which, for simplicity we refer to as c.769_770ins18) causes an in-frame deletion of a single conserved tryptophan residue and an insertion of seven amino acid residues at codon 257.
The 1000 Genomes database and NHLBI Exome Variant Server did not detect any of the variants in approximately 6,000 individuals of European and African American ancestry, in the Database of Single Nucleotide Polymorphisms (dbSNP), or in our local database. The p.Trp257delinsSerThrSerLeuSerSerGly variant and the p.Trp211Ter variant were not present in the Exome Aggregation Consortium (ExAC) Browser, and the p.Arg318Cys variant was heterozygous in two individuals of European ancestry. In our own internal database of 22,700 individuals sequenced with WES, the p.Trp211Ter variant was not present, the p.Arg318Cys was seen at 0.01% frequency (heterozygous in four individuals from two separate European families not in this paper), and the p.Trp257delinsSerThrSerLeuSerSerGly variant was seen at 0.02% frequency (heterozygous in three individuals from two separate families not in this paper); of the 1,126 Ashkenazi Jewish adults in the database, two carried one of these variants, for a carrier frequency of 0.16% in Ashkenazi Jews.
The clinical characteristics of the five affected individuals with TKT homozygous or compound-heterozygous variants are summarized in Table 1, and the pedigrees are shown in Figure 2. The proband in family 1 (1.II.1) is an 8 year old girl, born at 37 weeks with a birth weight of 2.27 kg (−2.1 SD), length of 44.5 cm (−2.2 SD), and head circumference of 31.1 cm (−2.3 SD). She had problems breastfeeding due to low oral motor tone. Developmentally, she was delayed in her early milestones and sat without support at 7 months, walked at 17.5 months, and had little speech until three years of age. Due to concerns of hyperactivity, distractibility, and speech and language delays, a formal developmental assessment was performed at five years of age and demonstrated a full scale intelligence quotient (FSIQ) of 87, verbal IQ of 86, and processing speed of 85. A cardiac echocardiogram was performed after the proband’s sister was born with congenital heart disease and showed trace aortic regurgitation and a right coronary artery with an anomalous origin. On physical examination at 7 years of age, she remained under the first percentile for weight and height. Growth hormone levels were normal. She is mildly dysmorphic with hypertelorism. Results of a chromosome microarray were normal.
Table 1.
Clinical Characteristics of Individuals with Transketolase Deficiency
|
Family 1 |
Family 2 |
Family 3 |
|||
|---|---|---|---|---|---|
| II.1 | II.2 | II.1 | II.2 | II.1 | |
| Mutations in TKT | c.633G>A, p.Trp211Ter and c.952C >T, p.Arg318Cys | c.633G>A, p.Trp211Ter and c.952C>T, p.Arg318Cys | c.769_770ins18, p.Trp257delinsSerThrSerLeuSerSerGly | c.769_770ins18, p.Trp257delinsSerThrSerLeuSerSerGly | c.769_770ins18, p.Trp257delinsSerThrSerLeuSerSerGly |
| Ancestry | European | European | Russian, Ashkenazi Jewish | Russian, Ashkenazi Jewish | Ashkenazi Jewish |
| Sex | F | F | F | M | F |
| Current age (years) | 8 | 5 | 20 | 11 | 24 |
| Developmental delay and/or intellectual disability | + (FSIQ = 87, VIQ = 86) | + | + | + | + (FSIQ = 75) |
| Speech and language | delayed speech and language | delayed speech | non-verbal | non-verbal | delayed speech |
| Short stature | + | + | + | + | + |
| CHD | anomalous coronary artery | VSD, ASD | VSD, PFO, PDA | 2 VSDs | ASD, VSD |
| Hypotonia | low oral motor tone in infancy | − | − | + | + |
| Ophthalmic findings | − | − | bilateral cataracts, severe blepharoconjunctuvitis, uveitis | − | bilateral cataracts, strabismus, anterior uveitis |
| Psychiatric | ADHD | − | stereotypic behavior, self-injurious behavior, OCD | − | ADHD, OCD |
| Additional phenotypes | mild hearing loss | − | loose stools | loose stools, sebhorrheic dermatitis | insulin dependent diabetes, pancreatic exocrine dysfunction, hepatomegaly, kidney cysts, secondary amenorrhea |
CHD, congenital heart disease; VSD, ventricular septal defect; ASD, atrial septal defect; PDA, patent ductus arteriosus; PFO, patent foramen ovale; ADHD, attention deficit hyperactivity disorder; OCD, obsessive compulsive disorder; FSIQ, full scale intelligence quotient; VIQ, verbal intelligence quotient; +, present; −, absent.
Figure 2.
Pedigrees and Photographs of Three Families Affected by TKT Variants
The proband’s younger sister (1.II.2) is similarly affected. She was born at almost 37 weeks with a birth weight of 2.62 kg (−1.5 SD), length of 44 cm (−2.4 SD), and head circumference of 31cm (−2.4 SD). At birth, she was small and edematous with anasarca. Cardiac echo showed a muscular ventricular septal defect, patent ductus arteriosus, and an atrial septal defect. Developmentally, she was delayed and walked at 18 months and talked at 3 years. At 5 years of age her height and weight are both in the first percentile. She is mildly dysmorphic with hypertelorism.
The proband in family 2 (2.II.1) is a 20 year old woman, born at 40 weeks’ gestation with a birth weight of 2.96 kg (−.9 SD), length of 47 cm (−1 SD), and head circumference of 34.3 cm (−0.3 SD). In infancy, she was found to have a ventricular septal defect, patent foramen ovale, and a patent ductus arteriosus. At 13 years, she developed bilateral cataracts requiring surgical removal. Additional medical problems include continued severe blepharoconjunctuvitis and uveitis, as well as a history of loose stools. Developmentally, she is significantly delayed and non-verbal with skills estimated at the level of a 6 year old. She demonstrates self-injurious and irritable behavior, as well as stereotypical behavior and obsessive-compulsive tendencies. At 20 years of age, her adult height, weight, and head circumference are all well below the first percentile, with a height of 127.9 cm (−5.4 SD), weight of 37.6 kg (−3.0 SD), and a head circumference of 51.5 cm (−2.6SD). Growth hormone, growth hormone binding protein, insulin-like growth factor-1, and insulin-like growth factor binding protein-3 were all normal. She has dysmorphic features, including posteriorly rotated ears with flattened superior helices, broad and full eyebrows with synophrys, and a mildly flattened nasal bridge with a slight underbite. A chromosome microarray and molecular testing for MECP2 were normal.
Her brother (2.II.2) is an 11 year old boy, born at 38.5 weeks’ gestation with a birth weight of 2.89 kg (−1.1 SD) and length of 48.3 cm (−0.6 SD). He had two ventricular septal defects. Additional medical problems include hypotonia requiring bilateral ankle-foot orthotics, chronic loose stools, and seborrheic dermatitis. He has severe developmental delay and is non-verbal. At 10 years and 4 months, his growth was below the first percentile, with a height of 105.9 cm (−5.2 SD), weight of 18 kg (−3.4 SD), and a head circumference of 48 cm (−3.8 SD). He has some mildly dysmorphic features, including a flattened, broad, and slightly upturned nose, along with a flattened philtrum and a thin upper lip. The family is Ashkenazi Jewish.
The proband in family 3 (3.II.1) is a 24 year old woman, born full term and small for gestational age with a birth weight of 2.46 kg (−1.8 SD), length of 45.72 cm (−1.6 SD), and a head circumference of 32.8 cm (−1.8 SD). Cardiac septal defects were present at birth. Her developmental milestones were delayed. She walked at 24 months and talked at 30 months. At 7 years of age, she was diagnosed with attention deficit hyperactivity disorder. When she was 12, she was diagnosed with bilateral cataracts and persistent strabismus. At 16, she had a FSIQ of 75, with immature behavior and demeanor and weaknesses in reading social cues. She demonstrated perseverative behaviors including skin picking and nail biting. At age 18, she was diagnosed with exocrine pancreatic insufficiency, pancreatitis, and insulin-dependent diabetes mellitus. She has persistent hepatomegaly and multiple small kidney cysts. At age 20, she was diagnosed with anterior uveitis, with pigment deposits in her lens, reduced visual acuity in both eyes, and unilateral optic nerve edema. Currently, she is at or under the first percentile with her weight (40.23 kg, −3.6 SD), height (128.8 cm, −8 SD), and head circumference (51.5 cm, −2.6 SD), with a low anterior and posterior hairline, a prominent forehead, thick hair, prominent eyes, hypotelorism, a broad nasal tip and narrow palate, small ears, and small hands and feet. Growth hormone levels were normal. The family is Ashkenazi Jewish. She had a chromosome microarray analysis that showed a mosaic 23.4 MB interstitial deletion of 13q12.3–13q14.3.
To functionally assess whether these rare variants affect TKT activity, we directly tested TKT activity in cell extracts from all five individuals and detected complete absence or low residual activities (Table 2). We also quantified metabolites of the PPP in the urine and plasma. Urinary excretion of sugars, polyols, and sugar-phosphates was measured and demonstrated significant elevations of erythritol, arabitol, ribitol, ribose-5-phosphate, and ribulose/xylulose-5-phosphate and mild elevations of ribose, galactitol, and lactose in individuals 1.II.1, 1.II.2, and 3.II.1 (Table S2). In the plasma of all individuals, erythritol, arabitol, and ribitol were elevated and myo-inositol was reduced (Table 2).
Table 2.
Aberrant Sugar and Polyol Metabolites in Plasma and Deficient Transketolase Enzymatic Activity
|
Subject |
Control Values | |||||
|---|---|---|---|---|---|---|
| I.1 | I.2 | II.1 | II.2 | III.1 | ||
| Age (years) | 8 | 5 | 20 | 11 | 24 | – |
| Plasma Polyol and Sugar Metabolites (μmol/L) | ||||||
| Threitol | <5 | <5 | 6 (↑) | <5 | 7 (↑) | 0–5 |
| Erythritol | 28 (↑) | 28 (↑) | 60 (↑) | 28 (↑) | 56 (↑) | 0–5 |
| Arabitol | 18 (↑) | 18 (↑) | 111 (↑) | 93 (↑) | 44 (↑) | 0–5 |
| Ribitol | 13 (↑) | 14 (↑) | 80 (↑) | 45 (↑) | 40 (↑) | 0–5 |
| Myo-inositol | 18 (↓) | 17 (↓) | 30 | 19 (↓) | 19 (↓) | 21–49 |
| Xylulose | <5 | <5 | <5 | <5 | <5 | 0–5 |
| Ribose | <5 | <5 | 25 (↑) | 29 (↑) | interferencea | 0–5 |
| Xylitol | <5 | <5 | <5 | <5 | <5 | 0–5 |
| Enzyme Studies (nmol/mg Protein/Minute) | ||||||
| Erythrocyte transketolase | – | – | minimal detectable activity | minimal detectable activity | – | – |
| Lymphoblast transketolase | 4.7 | 4.8 | – | – | – | 12.7–31.3 |
| Fibroblast transketolase | – | – | – | – | no detectable activity | 12.1–17.8 |
Sugars and polyols were analyzed in urine and plasma by gas-chromatography as described.11 Transketolase activity was measured enzymatically in lymphoblast, fibroblast, and erythrocyte extracts as previously described.12 ↑, elevated;↓, decreased.
The peak in the chromatogram is disturbed by another peak and therefore cannot be quantified.
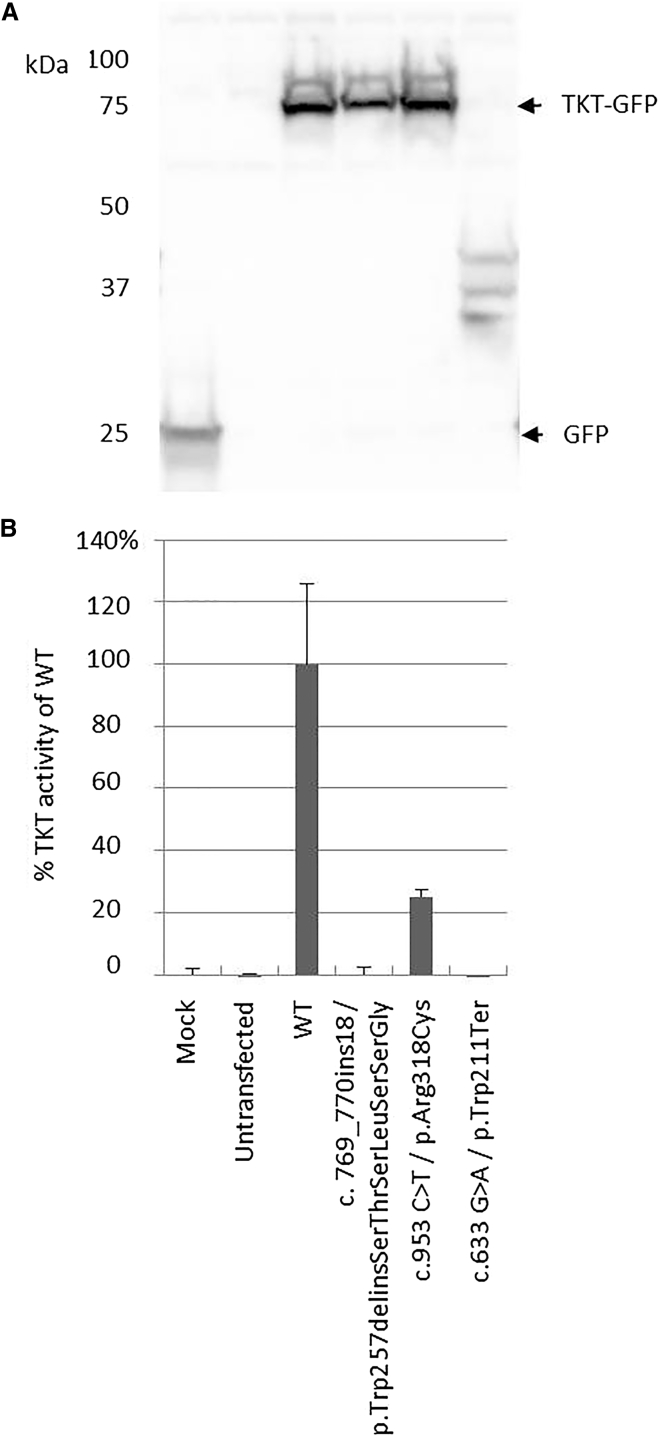
To study the effect of the TKT variants, we transiently transfected vectors containing each of the three variants (p.Arg318Cys, p.Trp211Ter, and p.Trp257delinsSerThrSerLeuSerSerGly) in HEK293 cells. Western blotting confirmed the presence of GFP or GFP-TKT fusion proteins in all transfected cells. The p.Trp211Ter transfected cells showed degradation of TKT proteins, indicating nonsense-mediated decay (Figure 3). The missense substitution p.Arg318Cys was found to have residual TKT activity of 25% when compared to wild-type transfectants, whereas both the p.Trp211Ter and the p.Trp257delinsSerThrSerLeuSerSerGly transfected cells had no residual activity. This confirms the pathogenic nature of all three variants.
Figure 3.
Ectopic Expression of TKT Variants Alters TKT Activity
(A) To study the effect of the TKT variants, we constructed two vectors (pCMV6-AN-GFP-TKT [wild-type, WT] and pCMV6-AN-GFP-TKT Trp257delinsSerThrSerLeuSerSerGly [delins]) and introduced the missense and nonsense variants in the WT construct. HEK293 cells were transfected in triplicate with the different TKT constructs, with Fugene HD reagent (Promega), and harvested by trypsinization 24 hr after transfections. To prove the success of the transient transfections, accumulation of the TKT-GFP fusion protein was studied by SDS-PAGE and western blotting. Immunodetection of the TKT-GFP fusion protein was carried out with rabbit polyclonal anti-GFP primary antibody (Abcam), polyclonal goat anti-rabbit immunoglobulins/HRP secondary antibody (Dako), and enhanced chemiluminescent substrates (Lumi-LightPLUS Western Blotting Substrate; Roche Applied Science). We analyzed all triplicate samples by western blotting for the presence of the GFP-TKT fusion proteins by using an antibody against GFP (indicated by arrows). A representative analysis of the triplicate samples is shown. The fusion protein with an apparent mass of 75 kDa is present in all transfectants except in the mock, the untransfected, and the p.Trp211Ter transfected HEK293 cells. In the latter, degraded TKT fusion proteins are present.
(B) TKT activity was measured in both untransfected and transfected HEK cells. After 30 min of incubation at 37°C, 2.5 mM ribose-5-phosphate and 0.5 mM xylulose-5-phosphate was added and the decrease of NADH was photometrically determined.
All transfections were performed in triplicate and TKT activity was measured in duplicate per transfectant. The activities are corrected for the basal activity in the mock cells and as the relative activity compared to the WT transfected HEK293 cells. Error bars represent the SE of the triplicate transfections.
Possibly, the measured residual activity in erythrocytes is from another protein with the same function, e.g., TKT-like protein (TKTL1 or TKTL2), and these proteins are not expressed in fibroblasts, explaining why individual 3.II.1 had no detectable TKT activity in fibroblasts. The low TKT activity in some tissues might explain in part why TKT deficiency is still compatible with life even though TKT is an essential enzyme.
We describe an inherited genetic disease due to transketolase deficiency and associated with short stature, developmental delay, and congenital heart disease. TKT is a thiamine-dependent reversible enzyme in the PPP. TKT catalyzes the transfer of a two-carbon unit from xylulose-5-phosphate to one of two substrates: ribose-5-phosphate producing glyceraldehyde-3-phosphate and sedoheptulose-7-phosphate or erythrose-4-phosphate producing glyceraldehyde-3-phosphate and fructose-6-phosphate (Figure 1). The end result of the reaction is the conversion of the pentose phosphates to intermediates used in the glycolytic pathway. TKT is an important enzyme of the PPP, necessary for NADPH synthesis and nucleic acid synthesis and cell division, and might explain the problems with growth we observe in the individuals reported. NADPH is also critical for maintaining cerebral glutathione, disturbance of which might contribute to the neurodevelopmental delays.
In all five affected individuals, we demonstrated elevated concentrations of polyols (erythritol, arabitol, and ribitol) in urine and plasma and sugar-phosphates (ribose-5-phosphate and xylulose/ribulose-5-phosphate) in urine. Other inborn errors in the PPP, such as RPI and TALDO deficiency, can also lead to accumulation of polyols and sugar-phosphates, and TALDO deficiency has also been associated with congenital heart disease, including atrial septal defect, patent ductus arteriosis, and patent foramen ovale.9 The source of the accumulated erythritol is unknown, given that there are no clear elevations of sedoheptulose or erythrose-4-phosphate, although this could be due to the limit of sensitivity of the assay.
Because of the TKT block, xylulose-5-phosphate, ribulose-5-phosphate, and ribose-5-phosphate can only be synthesized via the oxidative branch of the PPP (Figure 1), and the activity of the non-oxidative branch is markedly reduced, resulting in a reduced influx of intermediates in the glycolytic pathway and reduced ATP production.
After multiple unsuccessful attempts to create a Tkt knockout mouse, Xu and colleagues concluded that Tkt-null embryos die at or before the morula stage.13 40% of the Tkt+/− mice had 50% of enzymatic activity and exhibited postnatal growth retardation with normal growth hormone levels. The small female Tkt+/− mice had reduced fertility, markedly reduced adipose tissue, and smaller livers, kidneys, hearts, and brains.
In animal models, activation of TKT by benfotiamine, a lipid-soluble thiamine derivative, prevented diabetic retinopathy and inhibited hyperglycemia-induced vascular damage related to the increased accumulation of glycolytic metabolites.14 It is possible that benfotiamine could be used therapeutically in individuals with TKT deficiency with some residual activity, and this should be investigated.
At present, a routine diagnostic work-up of individuals with short stature, developmental delay, and congenital heart disease does not include assessment of polyols or sugar-phosphate, suggesting that other individuals with TKT deficiency have yet to be diagnosed, particularly in the Ashkenazi Jewish community. We recommend that TKT deficiency be included in the differential diagnosis of children with short stature, developmental delay, and congenital heart disease.
TKT deficiency is one of a growing list of inborn errors of metabolism in the non-oxidative portion of the PPP. With the increase in utilization of WES and other genetic and metabolic testing, we anticipate that more individuals might be identified with inborn errors in this pathway, allowing further elucidation of these phenotypes.
Conflicts of Interest
K. R., M.T.C., A.B., and K.G.M. are employees of GeneDx.
Acknowledgments
We gratefully acknowledge the contributions of the individuals with transketolase deficiency and their families. We are also grateful to Margaret Au from Cedars-Sinai Medical Center for helping us obtain some of the clinical information for family 3 and to Julie Scuffins from GeneDx for assisting with bioinformatics. This work was supported in part by funding from the Simons Foundation.
Published: June 2, 2016
Footnotes
Supplemental Data include two tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.03.030.
Accession Numbers
The accession numbers for the TKT variants reported here are ClinVar: SCV000264337, SCV000264338, and SCV000264339.
Web Resources
Supplemental Data
References
- 1.Lee H., Deignan J.L., Dorrani N., Strom S.P., Kantarci S., Quintero-Rivera F., Das K., Toy T., Harry B., Yourshaw M. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA. 2014;312:1880–1887. doi: 10.1001/jama.2014.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y., Muzny D.M., Reid J.G., Bainbridge M.N., Willis A., Ward P.A., Braxton A., Beuten J., Xia F., Niu Z. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y., Muzny D.M., Xia F., Niu Z., Person R., Ding Y., Ward P., Braxton A., Wang M., Buhay C. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iglesias A., Anyane-Yeboa K., Wynn J., Wilson A., Truitt Cho M., Guzman E., Sisson R., Egan C., Chung W.K. The usefulness of whole-exome sequencing in routine clinical practice. Genet. Med. 2014;16:922–931. doi: 10.1038/gim.2014.58. [DOI] [PubMed] [Google Scholar]
- 5.Cappellini M.D., Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 6.Huck J.H., Verhoeven N.M., Struys E.A., Salomons G.S., Jakobs C., van der Knaap M.S. Ribose-5-phosphate isomerase deficiency: new inborn error in the pentose phosphate pathway associated with a slowly progressive leukoencephalopathy. Am. J. Hum. Genet. 2004;74:745–751. doi: 10.1086/383204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyaid W., Al Harbi T., Anazi S., Wamelink M.M., Jakobs C., Al Salammah M., Al Balwi M., Alfadhel M., Alkuraya F.S. Transaldolase deficiency: report of 12 new cases and further delineation of the phenotype. J. Inherit. Metab. Dis. 2013;36:997–1004. doi: 10.1007/s10545-012-9577-8. [DOI] [PubMed] [Google Scholar]
- 8.Leduc C.A., Crouch E.E., Wilson A., Lefkowitch J., Wamelink M.M., Jakobs C., Salomons G.S., Sun X., Shen Y., Chung W.K. Novel association of early onset hepatocellular carcinoma with transaldolase deficiency. JIMD Rep. 2014;12:121–127. doi: 10.1007/8904_2013_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wamelink M.M., Struys E.A., Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J. Inherit. Metab. Dis. 2008;31:703–717. doi: 10.1007/s10545-008-1015-6. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka A.J., Cho M.T., Millan F., Juusola J., Retterer K., Joshi C., Niyazov D., Garnica A., Gratz E., Deardorff M. Mutations in SPATA5 Are Associated with Microcephaly, Intellectual Disability, Seizures, and Hearing Loss. Am. J. Hum. Genet. 2015;97:457–464. doi: 10.1016/j.ajhg.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen G., Muskiet F.A., Schierbeek H., Berger R., van der Slik W. Capillary gas chromatographic profiling of urinary, plasma and erythrocyte sugars and polyols as their trimethylsilyl derivatives, preceded by a simple and rapid prepurification method. Clin. Chim. Acta. 1986;157:277–293. doi: 10.1016/0009-8981(86)90303-7. [DOI] [PubMed] [Google Scholar]
- 12.Kaasinen E., Rahikkala E., Koivunen P., Miettinen S., Wamelink M.M., Aavikko M., Palin K., Myllyharju J., Moilanen J.S., Pajunen L. Clinical characterization, genetic mapping and whole-genome sequence analysis of a novel autosomal recessive intellectual disability syndrome. Eur. J. Med. Genet. 2014;57:543–551. doi: 10.1016/j.ejmg.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z.P., Wawrousek E.F., Piatigorsky J. Transketolase haploinsufficiency reduces adipose tissue and female fertility in mice. Mol. Cell. Biol. 2002;22:6142–6147. doi: 10.1128/MCB.22.17.6142-6147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammes H.P., Du X., Edelstein D., Taguchi T., Matsumura T., Ju Q., Lin J., Bierhaus A., Nawroth P., Hannak D. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.