Abstract
Background and Purpose
One important syndrome of psychiatric disorders in humans is catalepsy. Here, we created mice with different predispositions to catalepsy and analysed their pharmacological and behavioural properties.
Experimental Approach
Two mouse lines, B6‐M76C and B6‐M76B, were created by transfer of the main locus of catalepsy containing the 5‐HT1A receptor gene to the C57BL/6 genetic background. Behaviour, brain morphology, expression of key components of the serotoninergic system, and pharmacological responses to acute and chronic stimulation of the 5‐HT1A receptor were compared.
Key Results
B6‐M76B mice were not cataleptic, whereas 14% of B6‐M76C mice demonstrated catalepsy and decreased depressive‐like behaviour. Acute administration of the 5‐HT1A receptor agonist 8‐OH‐DPAT resulted in dose‐dependent hypothermia and in decreased locomotion in both lines. Chronic 8‐OH‐DPAT administration abolished the 5‐HT1A receptor‐mediated hypothermic response in B6‐M76C mice and increased locomotor activity in B6‐M76B mice. In addition, 5‐HT metabolism was significantly reduced in the hippocampus of B6‐M76C mice, and this effect was accompanied by an increased expression of the 5‐HT1A receptor.
Conclusions and Implications
Our findings indicate that transfer of the main locus of hereditary catalepsy containing the 5‐HT1A receptor from CBA mice to the C57BL/6 genetic background led to increased postsynaptic and decreased presynaptic functional responses of the 5‐HT1A receptor. This characteristic establishes the B6‐M76C line as an attractive model for the pharmacological screening of 5‐HT1A receptor‐related drugs specifically acting on either pre‐ or postsynaptic receptors.
Linked Articles
This article is part of a themed section on Updating Neuropathology and Neuropharmacology of Monoaminergic Systems. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v173.13/issuetoc
Abbreviations
- 5‐HIAA
5‐hydroxyindoleacetic acid
- 8‐OH‐DPAT
8‐hydroxy‐2‐(di‐n‐propylamino) tetralin
- ASC
antidepressant‐sensitive catalepsy
- BDNF
brain‐derived neurotrophic factor
- EPM
elevated plus‐maze test
- FST
forced swim test
- OF
open field test
- Slc6a4
gene encoding 5‐HT transporter
- Tph2
tryptophan hydroxylase 2
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Enzymes c |
| 5‐HT1A receptor | MAO‐A |
| 5‐HT2A receptor | Tph2 |
| Catalytic receptors b | Transporters d |
| p75 (TNFRSF16) | Slc6a4 |
| TrkB |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,dAlexander et al., 2015a, 2015b, 2015c, 2015d).
Introduction
Catalepsy (tonic immobility, immobility reflex and animal hypnosis) is characterized by muscular rigidity leading to prolonged immobility and an inability to correct an externally imposed awkward posture. Under physiological conditions, catalepsy can be obtained in some vertebrates as a kind of passive defensive behaviour against a predator (Dixon, 1998). In humans, excessive catalepsy‐like dyskinesia is a pathological symptom occurring in schizophrenia, mood disorders (e.g. depression) and Parkinsons' disease (Klemm, 1989). It has been shown that the brain serotoninergic (5‐HT) system is crucially involved in the mechanisms of catalepsy in mice. For example, pharmacological activation of the 5‐HT1A receptor with 8‐OH‐DPAT attenuates catalepsy induced by neuroleptics (Wadenberg and Hillegaart, 1995; Wadenberg, 1996) as well as hereditary catalepsy in rats and mice (Kulikov et al., 1994; Popova et al., 1994; Popova and Kulikov, 1995). In addition, decreased expression of the gene for the 5‐HT2A receptor was found in the frontal cortex of all mice predisposed to catalepsy when compared with catalepsy‐resistant mice of the AKR strain (Naumenko et al., 2010). Moreover, irreversible inhibition of tryptophan hydroxylase 2 (Tph2), the key enzyme for 5‐HT synthesis, by p‐chlorophenylalanine significantly reduces catalepsy in rodents (Popova and Kulikov, 1995; Wadenberg and Hillegaart, 1995; Popova, 1997). More recently, the involvement of brain‐derived neurotrophic factor (BDNF) in the mechanisms of hereditary catalepsy in mice has also been demonstrated (Tikhonova et al., 2009; Naumenko et al., 2012; Naumenko et al., 2014).
In rodents, catalepsy can be induced by administration of the antipsychotic drug haloperidol, and such animals can be used as an appropriate model for analysis of extrapyramidal dysfunctions (Wadenberg, 1996). In contrast, a drug‐free or hereditary catalepsy is a relatively rare phenomenon, even though it might represent a suitable model for the analysis of genetic and molecular mechanisms involved in the psychopathology of catalepsy as well as for the development of novel anti‐cataleptic pharmacological substances. In mice with predisposition to hereditary catalepsy, a cataleptic episode can be evoked by pinching the skin at the scruff of the neck (pinch‐induced catalepsy) (Ornstein and Amir, 1981). It is noteworthy that the predisposition to pinch‐induced catalepsy undergoes significant genetic variability. It has been shown that pinching never induces catalepsy in intact C57BL/6, DBA/2 and AKR mouse strains. In contrast, about 50% of CBA/Lac mice showed pronounced immobility for more than 60 s after 4–5 pinches (Kulikov et al., 1989; Kulikov et al., 1993). Previously, we demonstrated that hereditary catalepsy in CBA mice can be markedly increased by prolonged selective breeding of the backcrosses between the CBA and catalepsy‐resistant AKR strain to produce mice with a high predisposition to catalepsy. About 80% of mice of the resulting Antidepressant‐Sensitive Catalepsy (ASC) line demonstrated cataleptic immobility. In addition to increased catalepsy, ASC mice showed depressive‐like behaviour and immune deficiency (Alperina et al., 2007). It is noteworthy that chronic pharmacological treatment of ASC mice with classic antidepressants, including imipramine, fluoxetine and acute i.c.v. administration of BDNF, significantly reduced catalepsy, suggesting that ASC mice are a suitable model for verification of antidepressive drugs (Tikhonova et al., 2006; Tikhonova et al., 2010; Naumenko et al., 2012).
Using a combination of selective breeding, genetic recombination and quantitative trait loci analysis, the main locus of predisposition to catalepsy was mapped on the105.89–118.83 Mbp fragment of mouse chromosome 13 (Kulikov et al., 2003; Kondaurova et al., 2006; Kulikov et al., 2008a). Among others, this locus contains a gene encoding the 5‐HT1A receptor. Transfer of the CBA‐derived 105.89–118.83 Mbp fragment of chromosome 13 to the genome of the AKR mouse line resulted in the creation of the recombinant AKR.CBA‐D13Mit76 line demonstrating pronounced catalepsy (50%), a high level of intermale aggression and learning deficits in the Morris water maze (Kulikov et al., 2014), which can be restored after an acute i.c.v. administration of BDNF (Kulikov et al., 2014). In addition to changes in behaviour, AKR.CBA‐D13Mit76 mice are characterized by decreased expressions of the key genes of the brain serotonergic system and reduced functional activity of 5‐HT2A receptors in comparison with mice of the parental AKR strain (Naumenko et al., 2014). However, no significant changes in 5‐HT levels or its metabolism in the brain of AKR.CBA‐D13Mit76 mice were observed (Sinyakova et al., 2014).
Even though the involvement of the 105.89–118.83 Mbp fragment of chromosome 13 in catalepsy was convincingly shown, the role of this locus in the regulation of brain function and pathological behaviour as well as underlying mechanisms are still not known. Therefore, the results obtained with the AKR.CBA‐D13Mit76 line are difficult to compare with other animal models, because the AKR genotype is not generally utilized as a genetic background for transgenic mice. While the catalepsy‐resistant C57BL/6 strain is widely used as a genetic background in numerous mouse models as well as in multiple pharmacological screenings, we decided to transfer the main locus of predisposition to catalepsy containing the 5‐HT1A receptor gene from the catalepsy‐prone strain CBA to the genome of catalepsy‐resistant C57BL/6 mice. To perform this task, we created the B6.CBA‐D13Mit76C (B6‐M76C) and B6.CBA‐D13Mit76B (B6‐M76B) recombinant lines on the genetic C57BL/6 background; these two lines were distinguished by the CBA‐derived and C57BL/6‐derived fragments of chromosome 13 containing the 5‐HT1A receptor gene. These lines were then compared for the occurrence of catalepsy, for behavioural differences as well as for their brain morphology. In addition, we compared these mouse lines with respect to their behavioural and hypothermic responses after acute and chronic pharmacological stimulation of 5‐HT1A receptors with the selective receptor agonist 8‐OH‐DPAT. Finally, we evaluated the expression of key genes of the brain serotonergic and BDNF systems as well as the brain level of 5‐HT and its main metabolite 5‐hydroxyindoleacetic acid (5‐HIAA) in both lines.
Methods
Breeding of mouse lines
Two recombinant lines were generated by transferring the CBA‐derived fragment 105.89–118.83 Mbp of chromosome 13 including the main locus of catalepsy to the C57BL/6 (B6) genome (Kulikov et al., 2003). Males generated of this catalepsy‐prone AKR.CBA‐D13Mit76 recombinant mouse line containing the CBA‐derived fragment in the AKR genome were mated with females of inbred mouse strain B6 to obtain the F1 hybrids (Kulikov et al., 2008a). The latter were used to create the recombinant line. After eight successive backcrossings of the F1 hybrids to the B6 strain, the heterozygous backcrosses were intercrossed to generate B6.CBA‐D13Mit76C (B6‐M76C) and B6.CBA‐D13Mit76B (B6‐M76B), containing the CBA‐derived and B6‐derived alleles of D13Mit76 and AKR‐derived and B6‐derived alleles of D13Mit78 in the B6 genome respectively.
Genotyping
DNA samples were extracted from tail tip tissue as previously described (Kulikov et al., 2003). Briefly, a piece of tail tip was digested overnight with 1 mL of buffer containing Tris–HCl, pH 7.9, 0.1 M, NaCl, 0.1 M, EDTA, 0.1 M, 0.5% SDS and 100 mg·mL−1 proteinase K, and DNA was extracted with 1 mL of phenol–chloroform mixture (1:1) and kept at −20°C. DNA samples were genotyped by PCR with two polymorphic microsatellites D13Mit76 and D13Mit78, mapped at 110.56 and 118.83 Mbp chromosome 13 respectively. Genomic DNA (50 ng in 5 μL) was mixed with 5 μL of corresponding primers (to a final concentration of 250 pM for each) (Table 1), 1 U of TaqDNA‐polymerase (Sibenzyme, Novosibirsk, Russia) and PCR buffer was added to make up to 20 μL, the final reaction volume. The PCR protocol was (a) 96°C, 5 min, (b) 35 cycles (92°C, 40 s; 60°C, 30 s; and 72°C, 30 s) and (c) 72°C, 2 min (Moisan et al., 1996). Mouse genotype was determined by electrophoreses of corresponding PCR products on 3% agarose gels. In order to generate B6‐M76C, the line of mice carrying the D13Mit76 marker c/c genotype (c‐allele derived from CBA) and D13Mit78 marker a/a genotype (a‐allele derived from AKR) were chosen. For B6‐M76B, line was generated from mice carrying D13Mit76 marker b/b genotype (b‐allele derived from C57BL/6) and genotype D13Mit78 marker b/b were chosen (Figure 1A and B).
Table 1.
Primer sequences, annealing temperatures and PCR product length
| Gene | Sequence | Annealing temperature, °C | Product length, bp |
|---|---|---|---|
| D13Mit76 marker | F5′‐atgcacctgtctaaatgtgtgc‐3′ | 60 | 109 |
| R5′‐agagggactgtgggactgtg‐3′ | |||
| D13Mit78 marker | F5′‐acagcacgggtttatcatcc‐3′ | 60 | 229 |
| R5′‐tatgcctgccaggcttctat‐3′ | |||
| BDNF | F5′‐ tagcaaaaagagaattggctg −3′ | 59 | 255 |
| R5′‐ tttcaggtcatggatatgtcc −3′ | |||
| TrkB | F5′‐cattcactgtgagaggcaacc‐3′ | 63 | 175 |
| F5′‐atcagggtgtagtctccgttatt‐3′ | |||
| p75 | F5′‐ acaacacccagcacccagga −3′ | 62 | 171 |
| R5′‐cacaaccacagcagccaaga −3′ | |||
| MAO‐A | F5′‐aatgaggatgttaaatgggtagatgttggt‐3′ | 62 | 138 |
| R5′‐cttgacatattcaactagacgctc‐3′ | |||
| 5‐HT 1A receptor | F5′‐gactgccaccctctgccctatatc‐3′ | 62 | 200 |
| R5′‐tcagcaaggcaaacaattccag‐3′ | |||
| 5‐HT 2A receptor | F5′‐agaagccaccttgtgtgtga‐3′ | 61 | 169 |
| R5′‐ttgctcattgctgatggact‐3′ | |||
| Tph2 | F5′‐cattcctcgcacaattccagtcg‐3′ | 61 | 239 |
| R5′‐agtctacatccatcccaactgctg‐3′ | |||
| Slc6a4 | F5′‐aagccccaccttgactcctcc‐3′ | 57 | 198 |
| R5′‐ctccttcctctcctcacatatcc‐3′ | |||
| Polr2a | F5′‐gttgtcgggcagcagaatgtag‐3′ | 63 | 188 |
| R5′‐tcaatgagaccttctcgtcctcc‐3′ |
Figure 1.
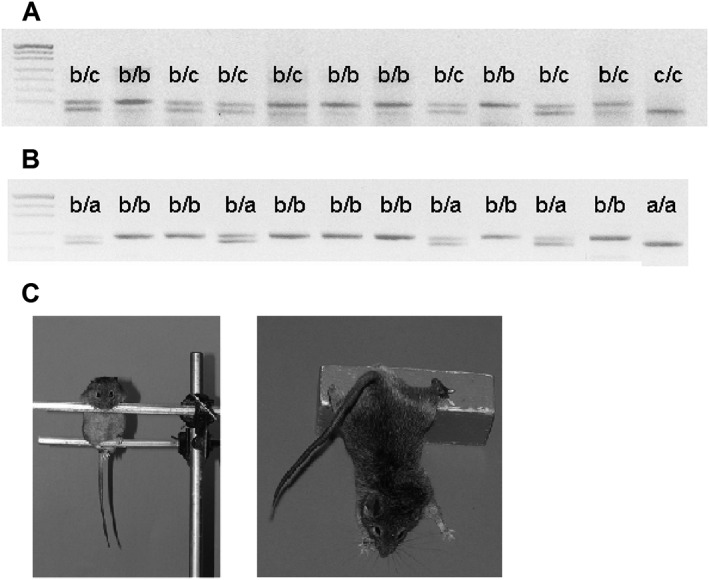
(A) Representative picture of the gel after electrophoresis of PCR products amplified with primers for D13Mit76 microsatellite used for selection of B6‐M76C mice carrying the c/c genotype marker (where c means c‐allele derived from CBA) and B6‐M76B mice carrying the b/b genotype marker (where b means b‐allele derived from C57BL/6). (B) Electrophoresis of PCR products amplified using primers for D13Mit78 microsatellite used for selection of B6‐M76C mice carrying the a/a genotype marker (where a means a‐allele derived from AKR) and B6‐M76B mice carrying the b/b genotype marker. (C) Representative photographs showing mice of the B6‐M76C line during a catalepsy episode (see also Supporting Information Videos S1 and S2).
Animals and experimental procedures
The breeding of new lines was performed in the frame of the basic research project no. 0324–2015‐0004 and conducted in the Centre for Genetic Resources Laboratory Animals (RFMEFI61914X0005 and RFMEFI62114X0010). Adult male mice of B6‐M76C and B6‐M76B lines were used for behavioural and pharmacological experiments. Animals were housed in groups of 7–8 per cage under standard conditions (20–22°C, free access to food and water, 12 h light/dark cycle). Two days before the experiments, mice were weighed and isolated in individual cages to remove the group effect. In all experiments, mice of different genotypes were aligned by weight. All experiments were performed in a double‐blind fashion. The brain morphology of the B6‐M76C (n = 5) and B6‐M76B (n = 6) mice was compared by MRI tomography.
In behavioural experiments, B6‐M76C (n = 16) and B6‐M76B (n = 9) mice were tested in the open field (OF), elevated plus‐maze (EPM) and forced swim tests (FST) and then for the occurrence of pinch‐induced catalepsy (see discussion below). Two days later, randomly chosen animals of each genotype (n = 8) were decapitated; the frontal cortex, hippocampus and midbrain area were dissected, frozen in liquid nitrogen and stored at −80°C until RNA isolation. An increased number of mice of B6‐M76C genotype (n = 16) was used for more precise evaluation of the percentage of cataleptics in this line.
In pharmacological experiments, the effects of acute i.p. administration of 0.1 and 1 mg·kg−1 of 8‐OH‐DPAT on the behaviour in the OF as well as on the hypothermic response (see below) were investigated in males divided into six groups (three groups for each genotype) and treated with vehicle, 0.1 mg·kg−1 and 1 mg·kg−1 of 8‐OH‐DPAT (n = 8 per genotype per drug). In addition, the effects of chronic administration of 8‐OH‐DPAT (14 days, 1 mg·kg−1, i.p. ) on the behaviour in the OF and 5‐HT1A receptor‐mediated hypothermic responses were investigated in male groups (n = 8 per genotype per drug).
Moreover, 5‐HT metabolism in the frontal cortex, hippocampus, striatum and midbrain area of B6‐M76C and B6‐M76B mice (n = 8 per genotype) was studied using HPLC. All experimental procedures were in compliance with the EC Directive 86/609/EEC for animal experiments and were approved by the Institutes' Ethics Committee. All efforts were made to minimize the number of animals used and their suffering. The researchers were blind to the protocol of the experiments. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath & Lilley, 2015).
Pharmacological analysis of the 5‐HT1A receptor functional activity
The functional activity of 5‐HT1A receptors was estimated by quantification of the hypothermic response obtained after acute administration of the 5‐HT1A agonist 8‐OH‐DPAT (1 mg·kg−1, i.p.) (Overstreet et al., 1996; Naumenko et al., 2011). The body temperature was measured by means of a KJT thermocouple (Hanna Instruments, Singapore) with Cooper Constantan Rectal Probes for mice (Physitemp Instruments, Clifton NJ, USA) 20 min after drug or saline administration. For estimation of hypothermic effect after chronic 8‐OH‐DPAT administration, two temperature values were analysed; the first one was measured in animals undergoing a 14 day treatment procedure before and the second one 20 min after acute 8‐OH‐DPAT administration to mice of experimental and sham groups.
Pinch‐induced catalepsy
The catalepsy test was performed according to the procedure described by Kulikov et al. (1989, 1993). Animals were firmly pinched between two fingers for 5 s at the scruff of their neck, placed on parallel bars with the forepaws 5 cm above the hind legs and then gently released. The catalepsy duration was recorded in s from the moment when an animal was released to the moment when the animal shifted its front paws from the initial position on the upper bar or made gross body or head movements. The trial ended either when a mouse started to move or after 120 s of immobility. Each animal was submitted to 10 successive trials with 2 min intervals. Mice were kept in the home cages between the trials. The mouse was considered to be cataleptic if the time of immobility was longer than 20 s in at least three of the 10 trials.
Open field
The OF test was carried out in a circle arena (40 cm in diameter) surrounded by a white plastic wall (25 cm high) and illuminated through the mat and semi‐transparent floor with two halogen lamps of 12 W each placed 40 cm under the floor (Kulikov et al., 2008b). The mouse was placed near the wall, and its movements were tracked for 5 min with a digital camera (Sony, Tokyo, Japan). The area was carefully cleaned after each test. The video stream from the camera was analysed frame by frame using the original EthoStudio software (Kulikov and Popova, 2008). The horizontal locomotor activity (distance run) and time in the centre were measured automatically. The number of rearing events was measured manually.
Elevated plus‐maze
The EPM was performed using a device consisting of two crosswise open and closed arms (35 × 7 cm). The closed arms had side walls 30 cm in length and 20 cm high. The mouse was placed in the centre of the maze facing an enclosed arm. Behaviour was tracked for 5 min with a digital camera. The time spent in the closed arms, the time spent in the centre, the number of times it entered the closed and open arms and the number of peeks and head dippings were measured.
Forced swim test
Mice were placed in a clear plastic box (30 × 30 × 30 cm) filled with water at a temperature of 25°C. Total immobility time was recorded after 2 min of adaptation during 4 min.
Real‐time PCR
Total RNA was extracted with Trizol (Bio Rad, Hercules, CA, USA), and 1 μg of the total RNA was taken for cDNA synthesis with a random hexanucleotide mixture (Kulikov et al., 2005). The number of copies of 5‐HT1A, 5‐HT2A receptors, MAO A (MAO‐A), Tph2, genes encoding the 5‐HT transporter (Slc6a4), BDNF, TrkB and p75 receptor cDNAs was evaluated with SYBR Green real‐time quantitative PCR using selective primers (Table 1). The 50, 100, 200, 400, 800, 1600, 3200 and 5000 copies of genomic DNA were used as external standards for all the genes studied. The gene expression was presented as a relative number of cDNA copies compared with 100 copies of Polr2a cDNA (Kulikov et al., 2005; Naumenko and Kulikov, 2006; Naumenko et al., 2008).
HPLC
Concentrations of 5‐HT and 5‐HIAA were assessed with HPLC. The chromatography system contained the following: Lune C 18(2) column (5 μm particle size, L × i.d. 150 × 2 mm; Phenomenex, Torrance, CA, USA) and Nucleosil C 8 guard column (10 × 4.6 mm; Superlco Analytical, Sigma‐Aldrich, St. Louis, MO, USA), electrochemical detection (500 mV, Coulochem III; ESA Inc., Chelmsford, MA, USA), flowcell (BAS Inc., Lafayette, IN, USA), delivery module LC‐20AD (Shimadzu Corporation, Kyoto, Japan) and autosampler Optimas (Spark, Emmen, Holland). The mobile phase contained KH2PO4 (100 mM, pH = 4.5), 0.1 mM Na2EDTA, 1.4 mM 1‐octanesulfonic acid sodium salt (Sigma, St. Louis, MO, USA) and methanol (8% vol; Vekton Ltd., Voronezh, Russia). The flow rate was 0.6 mL·min−1. Tissue samples were homogenized in 200 μL of 0.8 M HClO4 (Sigma). The homogenates were centrifuged for 15 min at 15 000× g (4°C), and the supernatant was transferred to a clear tube and was diluted twice in Milli‐Q H2O. The pellet was diluted in 0.1 M NaOH (Vekton Ltd.,Voronezh, Russia), and protein concentration was estimated by the Bradford protein assay. Solutions containing 2 ng of 5‐HT and 5‐HIAA were used as an external standard. The concentration of biogenic amines in samples was estimated by comparison of the magnitudes of corresponding picks with the respective external standards using the MultiChrome v.1.5 software (Ampersand Ltd., Moscow, Russia) and expressed in ng·mg−1 of the total protein in sample estimated using the Bradford assay. The intensity of 5‐HT metabolism was evaluated by the ratio of 5‐HIAA/5‐HT.
MRI
MRI experiments were performed on a horizontal 11.7 T magnet (BioSpec 117/16 USR; Bruker, Karlsruhe, Germany). During the MRI experiment, each mouse received isoflurane in oxygen mix (1.5%, flow rate 200 mL·min−1) for anaesthesia. Animals were placed in the prone position on the animal bed, which was then slid into the magnet bore. A respiratory pillow placed underneath the lower torso was used to monitor respiration (SA Instruments, Stony Brook, NY, USA). All images of the mouse brain were obtained with a transmitter volume (500.3 MHz; distribution, 72/89 mm) and receiver surface (500.3 MHz; 20 × 20 mm) 1H radiofrequency coils. High‐resolution T2‐weighted images of the mouse brain (slice thickness = 0.5 mm, inter‐slice gap = 0.5 mm, field of view = 2 × 2 cm, matrix = 256 × 256, number of averages = 2 and scan duration = 3 min and 44 s) were recorded by TurboRARE (rapid with relaxation enhancement) with the following pulse sequence parameters: TE = 11 ms, TEeff = 33 ms, TR = 3.5 s and RARE factor = 8. The T2‐weighted images were obtained in three plane orientations: axial, sagittal and coronal. For quantification, one or two slices from each projection were used: the axial orientation (−0.28 and −2.3 mm to bregma), the sagittal orientation (level of bregma) and the coronal orientation (2.5 mm from the dorsal surface of the brain). The brain structures were restricted using the manufacturers' Region of Interest Tool software in ParaVision 5.0 and a standard mouse brain atlas by Hof et al.,(2000). The areas of all structures and the brain size in each slice were calculated by one slice as the number of pixels multiplied by the size of one pixel in mm2. For measurements, the sizes of the hippocampus, midbrain and cortex in the coronal slice the sum of right and left parts of these structures were used. Total volume of the brain was estimated using 17 slices of sagittal orientation (slice thickness: 0.5 mm, inter‐slice gap: 0 mm) and calculated as a sum of the areas of slices multiplied by 0.5 mm.
Statistical analysis
All experimental results are presented as mean ± SEM and compared by one‐way or two‐way ANOVA. The percentage of cataleptic mice was compared using Fishers' arcsine conversion. In all statistical analyses, P < 0.05 was considered as a threshold for statistical significance. When F achieved P < 0.05 and there was no significant variance in homogeneity, statistically significant effects were analysed using post hoc Fishers' test for group comparison. The Dixon's Q‐test was used for identification and rejection of outliers. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Results
Transferring the distal fragment of chromosome 13 results in increased catalepsy
A drug‐free hereditary catalepsy represents a promising model for investigating the mechanisms underlying different kinds of catalepsy‐associated psychopathology (Kulikov et al., 2006; Kulikov et al., 2008b). In order to create a novel mouse model of catalepsy, we transferred of CBA‐derived 105.89–118.83 Mbp fragment of chromosome 13 to the genome of catalepsy‐resistant C57BL/6 mice. This mouse strain was selected because the C57BL/6 genome is the most widely used genetic background for numerous mouse models. The transferring of the CBA‐derived fragment of chromosome 13 to the B6 genome was controlled using two polymorphic microsatellites D13Mit76 (110.56 Mbp) and D13Mit78 (118.83 Mbp) (Figure 1A and B). The heterozygous backcrosses of the eighth generation were intercrossed to generate B6‐M76C and B6‐M76B containing the CBA‐derived and B6‐derived alleles of D13Mit76 and AKR‐derived and B6‐derived alleles of D13Mit78 in the B6 genome respectively.
Both new lines (i.e. B6‐M76C and B6‐M76B) were subjected to the catalepsy test. In the case of the B6‐M76B line, we did not obtain any cataleptic response, while 14% of animals from the B6‐M76C line showed pronounced catalepsy (Figure 1C and Supporting Information Videos S1 and S2). These data confirm that the transfer of the main catalepsy locus located in the 105.89–118.83 Mbp fragment of chromosome 13 to the catalepsy‐resistant line leads to the development of cataleptic behaviour.
Behavioural effects of transferring the distal fragment of chromosome 13
To study the possible relationship between catalepsy and different kinds of behaviour, we performed detailed analysis of various behavioural aspects, in particular those used as read‐outs for psychiatric and neurological disorders. For that, mice of both lines were subjected to the OF test, EPM test and FST.
The OF test is widely used to evaluate locomotor activity (total distance traveled in the OF arena) (van Gaalen and Steckler, 2000; Prut and Belzung, 2003) and exploratory behaviour (number of rearing events) in rodents (Crusio, 2001). The results of the OF test revealed that B6‐M76B and B6‐M76C mouse lines did not differ by the distance travelled, by the number of rearings and time in the centre in the OF (Table 2).
Table 2.
Behaviour analysis of B6‐M76B and B6‐M76C mice in the OF test
| Behaviour parameter | B6‐M76B (n = 9) | B6‐M76C (n = 16) |
|---|---|---|
| Distance run, cm | 1136.56 ± 115.86 | 1197.14 ± 110.35 |
| Time in the centre, % | 0.11 ± 0.03 | 0.08 ± 0.01 |
| Number of rearings | 13.22 ± 2.90 | 16.69 ± 2.61 |
Data are presented as a mean ± SEM.
No significant differences between B6‐M76C and B6‐M76B lines were found.
The EPM test is routinely applied to study the response of animals to stimuli that cause fear and anxiety. It is considered that animals avoid entering the open arms of the maze because they are normally afraid of the open and illuminated areas. Therefore, the number of entries into the open arms as well as the time spent in them is utilized as parameters reflecting the degree of anxiety (Pellow et al., 1985; Ramos et al., 1997). In the EPM test, mice of the lines investigated did not differ by number of entries in the open arms, time spent in the open and in the closed arms, by number of peeks and by number of head dippings. These results suggest that B6‐M76B and B6‐M76C lines are similar in their manifestation of anxiety. In contrast, the number of entries in the closed arms was significantly reduced in the B6‐M76C mice in comparison with the B6‐M76B mice (Table 3).
Table 3.
Behaviour analysis of B6‐M76C and B6‐M76B mice in the EPM
| Behavior parameter | B6‐M76B (n = 9) | B6‐M76C (n = 16) |
|---|---|---|
| Number entering in the open arms | 3.6 ± 1 | 1.9 ± 0.6 |
| Time in the open arms, s | 48.5 ± 27.0 | 97.0 ± 29.3 |
| Number entering in the closed arms | 7.1 ± 1.0 | 3.1 ± 0.6 ** |
| Time in the closed arms, s | 210.7 ± 31.2 | 173.5 ± 32.0 |
| Number of peeks | 1.8 ± 0.5 | 1.2 ± 0.4 |
| Number head deeps | 7.9 ± 1.9 | 6.0 ± 1.6 |
Data are presented as a mean ± SEM.
P < 0.02 compared with B6‐M76B (presented in italics).
To evaluate the possible association between the chromosome 13 fragment with depressive‐like behaviour, the B6‐M76B and B6‐M76C lines were subjected to the FST. Increased immobility in the FST is a generally accepted as an indicator of increased depressive‐like behaviour. This idea is mainly based on the observation that classical antidepressants decrease the immobility time in this test (Steru et al., 1985; Borsini and Meli, 1988). Results obtained in the FST revealed that mice of the recombinant B6‐M76C line demonstrate significantly decreased immobility time in comparison with animals of the B6‐M76B line (Figure 2), indicating a decreased depressive‐like behaviour in B6‐M76C mice.
Figure 2.
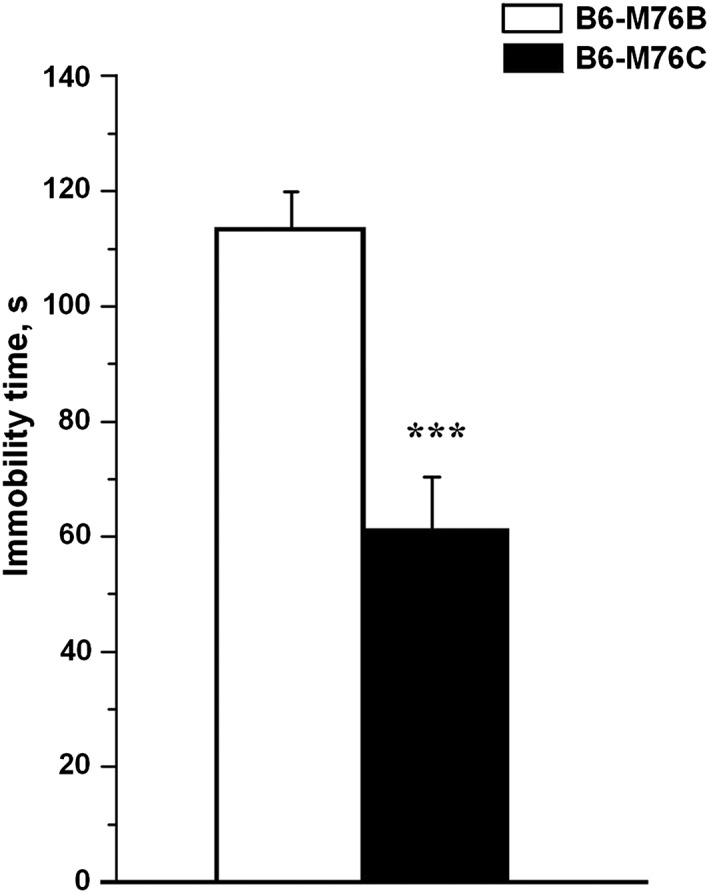
Immobility time in the FST in B6‐M76B (n = 9) and B6‐M76C (n = 16) males. Data are presented as mean ± SEM. *** P < 0.001.
These results show that the transferring of the main locus of catalepsy to the B6 background did not alter either the locomotor and exploration activities or the anxiety state in mice. In contrast, B6‐M76C mice demonstrated decreased depressive‐like behaviour.
Effects of acute and chronic 8‐OH‐DPAT treatment on 5‐HT1A receptor‐mediated hypothermic response
The main locus of catalepsy transferred to the C57BL/6 mice contains a gene encoding for the 5‐HT1A receptor. Among other effects, stimulation of this receptor is known to induce pronounced hypothermia (Naumenko et al., 2011). Next we compared the effect of acute and chronic administration of the 5‐HT1A receptor agonist 8‐OH‐DPAT on the hypothermic response in both recombinant mouse lines. A single i.p. administration of 8‐OH‐DPAT at concentration of 0.1 or 1 mg·kg−1 (acute stimulation) produced a significant dose‐dependent hypothermic effect in mice of both lines, with no significant interline differences (Figure 3A). In contrast, chronic administration of 8‐OH‐DPAT resulted in considerable differences in the hypothermic response induced by the subsequent acute 5‐HT1A receptor stimulation between B6‐M76B and B6‐M76C mice (Figure 3B). It is noteworthy that the initial body temperature of the B6‐M76C animals chronically treated with 8‐OH‐DPAT was significantly higher than that of the animals in the control group. In contrast, no differences in the initial body temperature between chronically treated and non‐treated B6‐M76B were observed (Figure 3B). Significant differences in the hypothermic response between the two lines were also found after acute injection of 1 mg·kg−1 8‐OH‐DPAT to the chronically treated animals: mice of the B6‐M76B line showed a pronounced decrease in body temperature, while this hypothermic response was completely abolished in the catalepsy‐prone B6‐M76C line.
Figure 3.
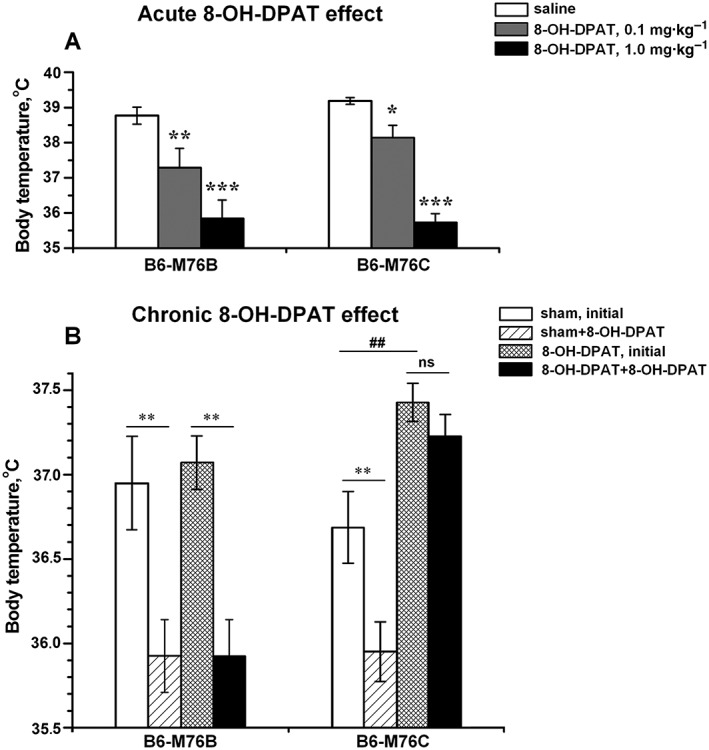
Effect of (A) acute (0.1 and 1 mg·kg−1, i.p.) and (B) chronic (1 mg·kg−1, i.p., 14 days) administration of 5‐HT1A receptor agonist 8‐OH‐DPAT on the body temperature in B6‐M76B and B6‐M76C mice. Data are presented as mean ± SEM. In experiments for analysis of the acute 8‐OH‐DPAT effects (A), body temperature was measured 20 min after 8‐OH‐DPAT or saline administration (n = 8 per genotype per drug).* P < 0.05; ** P < 0.01; *** P < 0.001 versus saline group. In the experiment for the chronic 8‐OH‐DPAT effects (B), mice were treated with 8‐OH‐DPAT (1 mg·kg−1 – 8‐OH‐DPAT group) or saline (sham group) for 14 days. To reveal the effect of chronic 8‐OH‐DPAT treatment on the 5‐HT1A receptor‐mediated hypothermic response, after 14 days of treatment, all groups received a single i.p. injection of 8‐OH‐DPAT (1 mg·kg−1), and the body temperature was measured before and 20 min after this injection (n = 8 per genotype per drug). ** P < 0.01, ## P < 0.01 versus initial temperature of sham group. ns, non‐significant differences.
Effects of acute and chronic 8‐OH‐DPAT treatment on locomotor and exploratory activity
Having demonstrated an interline difference in the 5‐HT1A receptor‐induced hypothermia, we next compared the behavioural effects of acute and chronic administration of 8‐OH‐DPAT between B6‐M76B and B6‐M76C mice using the OF test. As shown in Figure 4A and B, acute i.p. administration of 8‐OH‐DPAT (0.1 or 1 mg·kg−1) resulted in a dose‐dependent decrease in locomotor (distance run) as well as in exploratory (number of rearings) activities in both lines, although the changes in the explorative activity (in particular at the lower dosage of 8‐OH‐DPAT) were more noticeable in the B6‐M76C mice (Figure 4B). It is noteworthy that the acute administration of 8‐OH‐DPAT does not affect the time spent by animals of either line in the centre of the arena (not shown).
Figure 4.
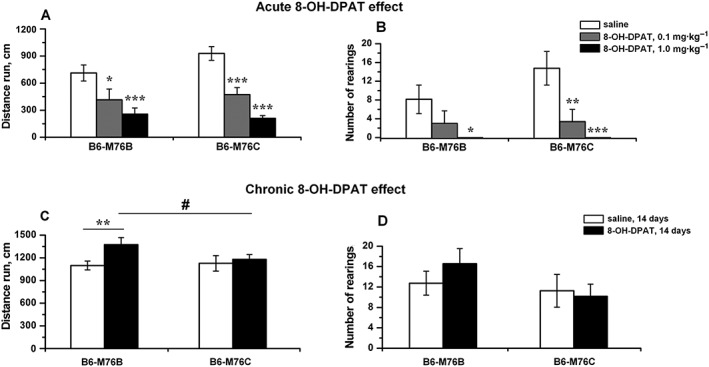
Effect of acute (A and B: 0.1 and 1 mg·kg−1, i.p.) and chronic (C and D: 1 mg·kg−1, i.p., 14 days) administration of 5‐HT1A receptor agonist 8‐OH‐DPAT on locomotor activity (distance run) (A, C) and exploration activity (number of rearings) (B, D) in the OF test in B6‐M76B and B6‐M76C mice. Data are presented as mean ± SEM. In the experiment for the analysis of the acute 8‐OH‐DPAT effects, the behaviour of mice in the OF test was estimated 20 min after 8‐OH‐DPAT or saline administration (n = 8 per genotype per drug). * P < 0.05; ** P < 0.01; *** P < 0.001 versus saline group. In experiments analysing the effects of chronic 8‐OH‐DPAT administration, the OF test was performed after daily injection of 8‐OH‐DPAT (1 mg kg−1 – 8‐OH‐DPAT group) or saline (sham group) for 14 days (n = 8 per genotype per drug); ** P < 0.01 versus saline (sham) group; # P < 0.05 versus B6‐M76B mice of 8‐OH‐DPAT group.
Then we investigated whether the prolonged treatment of B6‐M76B and B6‐M76C animals with 8‐OH‐DPAT (14 days, 1 mg·kg−1, i.p. injections) has line‐specific behavioural consequences. In these experiments, we observed that chronic 8‐OH‐DPAT administration significantly increases the locomotor activity only in B6‐M76B mice, while no changes in the distance run were observed in animals of the B6‐M76C line (Figure 4C). More importantly, the increase in locomotor activity induced by chronic 8‐OH‐DPAT treatment was significantly higher in the B6‐M76B line than in the B6‐M76C animals (Figure 4C). In contrast, chronic 8‐OH‐DPAT treatment did not significantly modify the explorative behavior of either line. Also, no interline differences were obtained on analysis of the number of rearings (Figure 4D). The effects of genotype and drug were also not significant for the time spent in the centre of the arena (not shown).
Comparison of the brain morphology between B6‐M76B and B6‐M76C mice using MRI
In order to reveal possible mechanisms underlying the aforementioned differences between B6‐M76B and B6‐M76C lines, we first compared their brain morphology. MRI‐based measurement of brain structures is an important indicator of pending cognitive decline in humans suffering from neurodegenerative diseases. Also in mouse models of neurodegenerative disorders, neuroimaging is considered to be a useful tool for phenotyping specific diseases and monitoring disease progression (Kooy et al., 2001; Waerzeggers et al., 2010). In the present study, morphology of the following brain structures was investigated: thalamus, corpus callosum, diencephalon region (including thalamus and hypothalamus), hippocampus (right and left parts), ventricle system, midbrain (right and left parts) and cortex (right and left parts) (Figure 5). Results of the MRI experiments are also summarized in Table 4. Detailed morphological analysis of MRI data revealed that the diencephalon of the B6‐M76C mice is significantly smaller than in the B6‐M76B mice. In addition, we found that the total volume of the brain as well as of the brain area in the axial orientations (−2.3 mm from bregma) were reduced in B6‐M76C mice when compared with B6‐M76B mice. At the same time, thalamus, hippocampus, midbrain, corpus callosum and brain areas in coronal (2.5 mm from the surface of brain) as well as in sagittal orientations (bregma) did not significantly differ between these lines. Taken together, these results demonstrate that the transferring of the main catalepsy locus produces brain shrinkage associated with a profound decrease in the size of the diencephalon, a brain structure implicated in mechanisms of psychiatric disorders.
Figure 5.
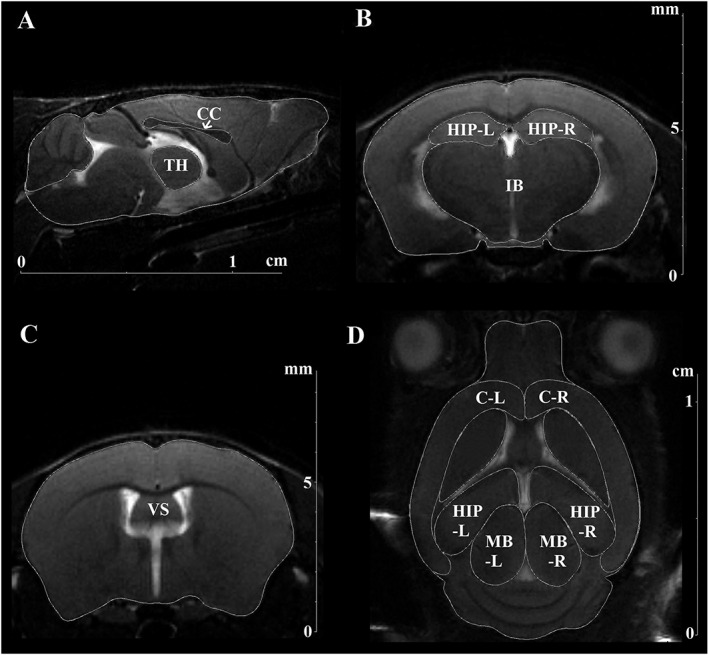
The T2‐weighted MRI images of mouse brain obtained using 11.7T BioSpec 117/16 USR (Bruker) tomograph. The brain structures investigated in the study are designated as follows: (A) sagittal slice (bregma). Thalamus (TH), corpus callosum (CC). (B) Axial slice (−2.3 mm to bregma). Diencephalon region (IB), hippocampus (right/left parts) (HIP‐R, HIP‐L). (C) Axial slice (−0.28 mm to bregma). Ventricle system (VS). (D) Coronal slice (−2.5 mm from the dorsal surface of the brain). Cortex (C‐R, C‐L), hippocampus (HIP‐R, HIP‐L) and midbrain (MB‐R, MB‐L). n = 5 for B6‐M76C and n = 6 for B6‐M76B mice.
Table 4.
Sizes of the brain structures measured using MRI in the B6‐M76B and B6‐M76C lines
| Slice | Bregma (mm) | Structure (mm2) | B6‐M76B | B6‐M76C | F 1,9 | P |
|---|---|---|---|---|---|---|
| Non‐cataleptic | Cataleptic | |||||
| Sagittal | 0 | Brain | 67.186 ± 0.777 | 66.554 ± 0.224 | 0.51 | |
| Thalamus | 4.102 ± 0.070 | 4.059 ± 0.055 | 0.213 | |||
| Corpus callosum | 1.433 ± 0.056 | 1.331 ± 0.079 | 1.165 | >0.05 | ||
| Axial | −2.3 | Brain | 50.834 ± 0.318 | 49.103 ± 0.347 | 13.53 | <0.01 |
| Diencephalon total | 17.207 ± 0.123 | 16.380 ± 0.105 | 24.99 | <0.001 | ||
| Hippocampus right | 2.050 ± 0.105 | 2.105 ± 0.054 | 0.189 | |||
| Hippocampus left | 1.943 ± 0.137 | 2.016 ± 0.030 | 0.228 | |||
| −0.28 | Brain | 43.070 ± 0.671 | 41.568 ± 0.332 | 3.52 | >0.05 | |
| Ventricle | 1.738 ± 0.085 | 1.825 ± 0.166 | 0.244 | >0.05 | ||
| Coronal | −2.5 | Brain | 91.518 ± 0.942 | 88.447 ± 1.107 | 4.52 | >0.05 |
| Hippocampus sum | 12.972 ± 0.568 | 12.752 ± 0.277 | 0.106 | |||
| Midbrain sum | 12.831 ± 0.265 | 12.256 ± 0.293 | 2.124 | >0.05 | ||
| Cortex sum | 24.228 ± 0.454 | 23.639 ± 0.309 | 1.050 | >0.05 | ||
| Sagittal | Volume (mm 3) | 429.427 ± 2.990 | 413.806 ± 2.452 | 15.42 | <0.01 |
Data are presented as the means ± SEMs of the values obtained in an independent group of animals (n = 5 for B6‐M76C and n = 6 for B6‐M76B mice). The sizes of brain structures (area, mm2) were calculated in the one slice of axial, sagittal and coronal orientations with different instance from bregma. Total volume of the brain was estimated using 17 slices of sagittal orientation (volume, mm3). Significant differences are in italics.
Molecular effects of transferring the main cataleptic locus
The pharmacological experiments described above revealed significant interline differences in hypothermic response as well as in locomotor activity after chronic stimulation of the 5‐HT1A receptor with 8‐OH‐DPAT. Therefore, we next compared the brain level of 5‐HT and its main metabolite 5‐HIAA in B6‐M76B and B6‐M76C mice. The 5‐HT level in the cortex, midbrain, hippocampus and striatum did not differ significantly between the lines investigated. The level of 5‐HIAA was also similar in all structures tested (Figure 6). However, the 5‐HIAA/5‐HT ratio, which reflects the efficiency of 5‐HT metabolism was significantly decreased in the hippocampus, but not in the midbrain and striatum of B6‐M76C line in comparison with B6‐M76B animals (Figure 6E).
Figure 6.
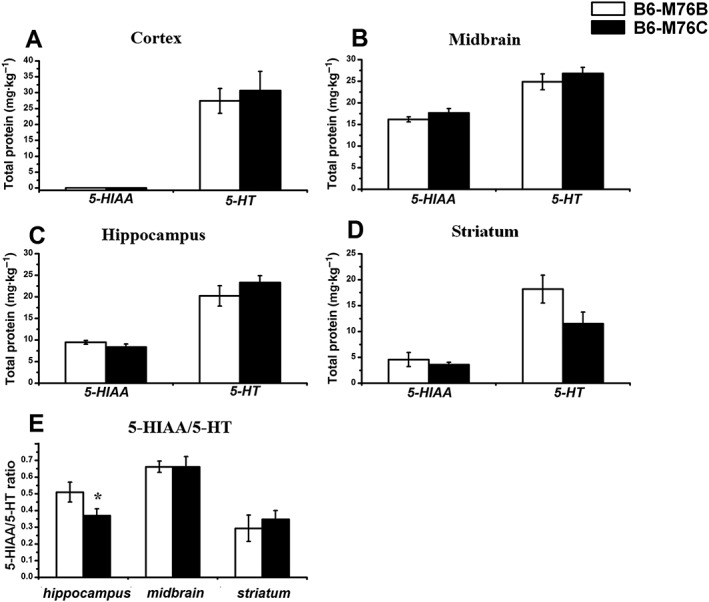
Concentrations of 5‐HT and its main metabolite 5‐HIAA in the different brain areas of B6‐M76C and B6‐M76B lines are shown. (A) cortex, (B) midbrain, (C) hippocampus and (D) striatum. (E) 5‐HIAA/5‐HT ratio reflecting the efficiency of 5‐HT metabolism. Data are presented as mean ± SEM (n = 8 per genotype). * P < 0.05 versus B6‐M76B mice.
In addition to the direct measurement of 5‐HT and 5‐HIAA concentrations using HPLC, we compared the expression of the key genes of the brain serotonergic system, including the 5‐HT1A receptor, 5‐HT2A receptor, 5‐HT transporter (Slc6a4), Tph2, the rate‐limiting enzyme for 5‐HT biosynthesis in the brain, and MAO‐A, an enzyme that degrades monoaminergic neurotransmitters, such as dopamine, noradrenaline and 5‐HT. The expression of these genes was determined in the frontal cortex, hippocampus and midbrain using quantitative real‐time PCR. These experiments demonstrated that the expression of the 5‐HT1A receptor gene was significantly higher in the hippocampus of the B6‐M76C mice, while the amount of 5‐HT1A receptor mRNA in the cortex and midbrain was similar in both lines (Figure 7A). Also, the level of the 5‐HT2A receptor mRNA was similar in both lines in all brain regions investigated (Figure 7B). In addition, the gene expression analysis revealed that the 5‐HT transporter Slc6a4 mRNA level in the midbrain of B6‐M76C mice was significantly decreased in comparison with the B6‐M76B animals (Figure 7C). The expression of Tph2 and MAO‐A genes was also significantly reduced in the midbrain of B6‐M76C mice (Figure 7C). Decreased expression levels of Slc6a4, Tph2 and MAO‐A genes in the midbrain in combination with a reduced 5‐HIAA/5‐HT ratio in the hippocampus suggest a reduced functionality of the brain serotonergic system in B6‐M76C. At the same time, an increase in the 5‐HT 1A receptor gene expression observed in the hippocampus of B6‐M76C mice seems to represent a compensatory mechanism for enhancing this dysregulated 5‐HT signalling.
Figure 7.
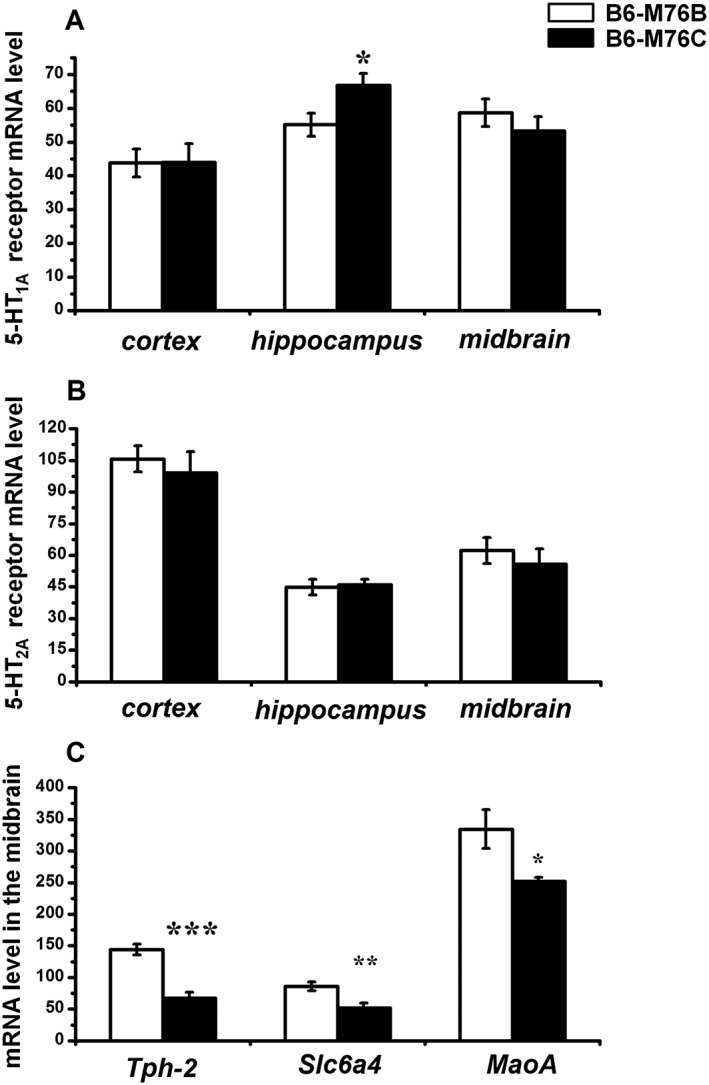
Expression of genes encoding for the 5‐HT1A (A) and 5‐HT2A (B) receptors in the frontal cortex, hippocampus and midbrain of B6‐M76B and B6‐M76C mice. Expression of genes encoding for Slc6a4, Tph2 and MAO‐A in the midbrain (C) of B6‐M76B and B6‐M76C animals. Gene expression is presented as the number of cDNA copies with respect to 100 cDNA copies of Polr2a. All values are presented as mean ± SEM (n = 8/ per genotype). * P < 0.05; ** P < 0.01; *** P < 0.001 versus B6‐M76B mice.
Finally, we analysed the effect of the transfer of the distal fragment of chromosome 13 on the expression of the genes involved in neurotrophic signalling. In this experiment, we did not find any significant changes in the expression of BDNF or in the expression levels of the main BDNF receptors TrkB and p75 in the brain of B6‐M76B and B6‐M76C mice (not shown).
Discussion
The main locus of a predisposition to catalepsy is mapped in the distal fragment 105.89–118.83 Mbp of chromosome 13, and its CBA‐derived allele determines about 20% of this trait penetrance, while other 30% of trait penetrance is defined by 29 polygenes wildly distributed in the whole genome (Kulikov et al., 2003; Kulikov et al., 2008a). In the present study, we showed that the transfer of this fragment from CBA mice to the catalepsy‐resistant B6 genetic background led to the development of catalepsy in the resulting B6‐M76C line. It is noteworthy that the transfer of the 105.89–118.83 Mbp fragment of chromosome 13 derived from B6 animals resulted in the catalepsy‐resistant B6‐M76B line. These findings provide additional experimental confirmation for the critical involvement of the distal fragment of chromosome 13 in the development of gene‐based catalepsy. Interestingly, that 14% of B6‐M76C mice demonstrated cataleptic‐like immobility tightly correlated with the 20% of the trait penetrance proposed for the CBA‐derived allele. Previously, it was shown that transferring the CBA‐derived fragment of chromosome 13 containing the main locus of catalepsy to the genetic background of the AKR strain also results in a mouse line with catalepsy. The resulting AKR.CBA‐D13Mit76 mice showed trait penetrance for cataleptics (approximately 50%), is similar to the parental strain CBA. This can be explained by the fact that the AKR genome contains additional genes enhancing the effect of the main genes of catalepsy in the CBA‐derived allele (Kulikov et al., 2008a).
Predisposition to catalepsy is often associated with profound changes in behaviour (Kolpakov et al., 2004; Bazovkina et al., 2005; Kondaurova et al., 2010). Our observation that mice of the B6‐M76B and B6‐M76C recombinant lines did not differ with regard to the distance travelled, time in the centre and the number of vertical postures in the OF test suggests that a predisposition to catalepsy in the B6‐M76C line is not associated with deficits in locomotor activity. In addition, time spent in the open arms in the EPM test did not differ significantly between these two lines, demonstrating that transfer of the main locus of predisposition to catalepsy to the B6 background does not produce any changes in the exploratory activity or in anxiety state under basal conditions. These data are in accordance with the results obtained in catalepsy‐prone AKR.CBA‐D13Mit76 mice (Bazovkina et al., 2005; Kondaurova et al., 2010). Interestingly, we observed a reduction in the number of entries in the closed arms in the EPM test in B6‐M76C mice, which can be an indirect indication of increased susceptibility to stress. At the same time, animals of the B6‐M76C line demonstrated a significantly shorter immobility time in the FST, a generally accepted indicator of depressive behaviour used to investigate the activity of antidepressant drugs (Steru et al., 1985; Borsini and Meli, 1988), suggesting a decreased depressive‐like behaviour in B6‐M76C mice.
Because the main locus of predisposition to catalepsy contains a gene encoding for the 5‐HT1A receptor, animals of the B6‐M76C and B6‐M76B lines should contain different 5‐HT1A receptor gene alleles derived from the CBA and C57BL/6 strain respectively. It has been shown that acute stimulation of the 5‐HT1A receptor with a selective agonist 8‐OH‐DPAT significantly attenuate catalepsy and locomotor activity in rodents (Kulikov et al., 1994; Bazovkina et al., 2010; Naumenko et al., 2012) and produce a strong hypothermic effect (Naumenko et al., 2010). In the present study, we found that acute administration of 8‐OH‐DPAT produced a strong dose‐dependent hypothermia in both mouse lines. In contrast, the intensity of the hypothermic response evoked by a single 8‐OH‐DPAT injection in mice chronically treated with 8‐OH‐DPAT was significantly lower in B6‐M76C than in B6‐M76B animals. These results are in accordance with the data obtained in cataleptic‐prone strain CBA (Popova et al., 2010) and can be explained by the more effective desensitization of the postsynaptic 5‐HT1A receptors in B6‐M76C line. Taken together with the observed central role of postsynaptic 5‐HT1A receptors in mediating the 8‐OH‐DPAT‐induced hypothermia (Blier et al., 2002), our data suggest that in the B6‐M76C line, postsynaptic 5‐HT1A receptors undergo a more effective desensitization.
At the same time, chronic 8‐OH‐DPAT produced a significant increase in 5‐HT1A receptor‐mediated locomotor activity only in B6‐M76B mice. These results are in line with the well‐known anxiolytic effect of 8‐OH‐DPAT (Overstreet et al., 1996). In contrast, no changes in locomotor activity were observed in B6‐M76C mice after prolonged 8‐OH‐DPAT treatment, suggesting the existence of compensatory and/or adaptive mechanisms in this line. Similar effects were also observed in CBA mice (Popova et al., 2010). Taking into consideration the important role of presynaptic 5‐HT1A receptors in the regulation of locomotor activity (Faccidomo et al., 2008) and catalepsy (Naumenko et al., 2010), our data suggest presynaptic 5‐HT1A receptors have a reduced sensitivity in B6‐M76C mice. Thus, transfer of the fragment containing the 5‐HT1A receptor from CBA mice to the C57BL/6 genetic background led to an increase in postsynaptic and decrease in presynaptic functional responses mediated by 5‐HT1A receptors in B6‐M76C mice. This interesting feature sites B6‐M76C line as an attractive model for the pharmacological screening of 5‐HT1A receptor‐related drugs specifically acting on either the pre‐ or postsynaptic receptors.
The brain serotonergic system plays an important role in the regulation of catalepsy. It has been demonstrated that stimulation of the 5‐HT1A receptor with different agonists reduces haloperidol‐ and morphine‐induced catalepsy (VanderWende and Spoerlein, 1979) as well as hereditary catalepsy in rats and mice (Popova and Kulikov, 1995; Popova, 1997). Moreover, irreversible inhibition of Tph2, the rate‐limiting enzyme for 5‐HT biosynthesis in the brain by p‐chlorophenylalanine, attenuates both haloperidol‐induced and hereditary catalepsy in rats (Kostowski et al., 1972). Hereditary catalepsy in rats and CBA mice has been also shown to be associated with increased Tph2 activity and decreased 5‐HT2A receptor density in the striatum (Kulikov et al., 1992; Kulikov et al., 1995; Popova and Kulikov, 1995). The results of the present study provide additional evidence for the close association between catalepsy and the serotonergic system. We have found that the expression of the key genes involved in either synthesis or metabolism of 5‐HT, including Tph‐2, MAO‐A and the 5‐HT transporter Slc6a4, was significantly reduced in the midbrain of B6‐M76C mice. Unexpectedly, concentrations of 5‐HT and its main metabolite 5‐HIAA in this brain structure were not changed in B6‐M76C animals, suggesting the existence of compensatory mechanisms responsible for the stable biosynthesis of 5‐HT. Correspondingly, the metabolism of 5‐HT in the midbrain calculated as a ratio between 5‐HT and 5‐HIAA levels was also unaffected in B6‐M76C mice. This result is in agreement with the findings that CBA, ASC, AKR.CBA‐D13Mit76 and catalepsy‐resistant AKR mice did not differ with regard to the levels of 5‐HT and its metabolites in the midbrain (Sinyakova et al., 2014; Tikhonova et al., 2013). At the same time, 5‐HT metabolism was significantly lower in the hippocampus of B6‐M76C mice, the brain structure strongly supplied by multiple serotonergic projections, and this effect was accompanied by an increased expression of the 5‐HT1A receptor gene. An increased expression of 5‐HT1A receptors in the hippocampus of B6‐M76C mice paralleled by increased functional activity of the postsynaptic 5‐HT1A receptors (see discussion earlier) could be the main reason for the reduced depressive‐like behaviour observed in this line. Indeed, it has been shown that a decreased level of postsynaptic 5‐HT1A receptors in combination with impaired receptor‐mediated signalling is associated with depression (van Praag, 2004). In addition to 5‐HT, neurotrophic signalling, and in particular BDNF, was shown to be involved in the mechanism of catalepsy (Tikhonova et al., 2009; Naumenko et al., 2012; Naumenko et al., 2014). However, in the present study, we did not find any alteration in the levels of mRNA encoding BDNF and its main receptors TrkB and p75 in different brain regions of the B6‐M76C mice. This finding suggests that the development of catalepsy evoked by transferring the CBA‐derived fragment of chromosome 13 to a B6 genetic background is independent of the neurotrophic system.
Author contributions
K.E.A. wrote the paper and performed MRI analysis and statistical analysis; B.D.V. performed behavioural studies, pharmacological studies, mRNA isolation and reverse transcription reaction; A.A.E. performed MRI analysis; T.A.S. performed pharmacological studies; F.D.V. made HPLC; K.A.V. is in experimental design and the creation of the lines, N.V.S. in general supervision and proofreading of the manuscript, P.E. in general supervision, writing and proofreading the manuscript and K.E.M. in the creation of mouse lines, experimental design, genes expression estimation, statistical analysis and proofreading of the MS.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Video S1 Mice of catalepsy‐prone B6‐M76C line during catalepsy episode.
Video S2 Mice of catalepsy‐resistant B6‐M76B line in test for catalepsy.
Acknowledgements
The study was supported by the Russian Scientific Foundation grant no. 14‐25‐00038.
Kulikova, E. A. , Bazovkina, D. V. , Akulov, A. E. , Tsybko, A. S. , Fursenko, D. V. , Kulikov, A. V. , Naumenko, V. S. , Ponimaskin, E. , and Kondaurova, E. M. (2016) Alterations in pharmacological and behavioural responses in recombinant mouse line with an increased predisposition to catalepsy: role of the 5‐HT1A receptor. British Journal of Pharmacology, 173: 2147–2161. doi: 10.1111/bph.13484.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alperina EL, Kulikov AV, Popova NK, Idova GV (2007). Immune response in mice of a new strain ASC (antidepressants sensitive catalepsy). Bull Exp Biol Med 144: 221–223. [DOI] [PubMed] [Google Scholar]
- Bazovkina DV, Kulikova AV, Kondaurova EM, Popova NK (2005). Selection for the predisposition to catalepsy enhances depressive‐like traits in mice. Genetika 41: 1222–1228. [PubMed] [Google Scholar]
- Bazovkina DV, Terenina EE, Kulikov AV (2010). Effect of selective agonist of serotonin 5‐HT1A receptors on defensive behavior in mice with different predisposition to catalepsy. Bull Exp Biol Med 150: 225–228. [DOI] [PubMed] [Google Scholar]
- Blier P, Seletti B, Gilbert F, Young SN, Benkelfat C (2002). Serotonin 1A receptor activation and hypothermia in humans: lack of evidence for a presynaptic mediation. Neuropsychopharmacology 27: 301–308. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A (1988). Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 94: 147–160. [DOI] [PubMed] [Google Scholar]
- Crusio WE (2001). Genetic dissection of mouse exploratory behaviour. Behav Brain Res 125: 127–132. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AK (1998). Ethological strategies for defence in animals and humans: their role in some psychiatric disorders. Br J Med Psychol 71 (Pt 4): 417–445. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Bannai M, Miczek KA (2008). Escalated aggression after alcohol drinking in male mice: dorsal raphe and prefrontal cortex serotonin and 5‐HT(1B) receptors. Neuropsychopharmacology 33: 2888–2899. [DOI] [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR (2000). Comparative cytoarchitectonic atlas of the C57Bl/6 and 129 Sv mouse brains: Elsevier, Amsterdam. [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). NC3Rs Reporting Guidelines Working Group. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm WR (1989). Drug effects on active immobility responses: what they tell us about neurotransmitter systems and motor functions. Prog Neurobiol 32: 403–422. [DOI] [PubMed] [Google Scholar]
- Kolpakov VG, Kulikov AV, Alekhina TA, Chugui VF, Petrenko OI, Barykina NN (2004). Catatonia or depression: the GC rat strain as an animal model of psychopathology. Genetika 40: 827–834. [PubMed] [Google Scholar]
- Kondaurova EM, Bazovkina DV, Kulikov AV (2010). Study of the association between catalepsy, anxiety, aggression and depressive‐like behavior in congenic mice. Ross Fiziol Zh Im I M Sechenova 96: 464–471. [PubMed] [Google Scholar]
- Kondaurova EM, Bazovkina DV, Kulikov AV, Popova NK (2006). Selective breeding for catalepsy changes the distribution of microsatellite D13Mit76 alleles linked to the 5‐HT serotonin receptor gene in mice. Genes Brain Behav 5: 596–601. [DOI] [PubMed] [Google Scholar]
- Kooy RF, Verhoye M, Lemmon V, Van Der Linden A (2001). Brain studies of mouse models for neurogenetic disorders using in vivo magnetic resonance imaging (MRI). Eur J Hum Genet 9: 153–159. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Gumulka W, Cxlonkowski A (1972). Reduced cataleptogenic effects of some neuroleptics in rats with lesioned midbrain raphe and treated with p‐chlorophenylalanine. Brain Res 48: 443–446. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Bazovkina DV, Kondaurova EM, Popova NK (2008a). Genetic structure of hereditary catalepsy in mice. Genes Brain Behav 7: 506–512. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Bazovkina DV, Moisan MP, Mormede P (2003). The mapping of the gene of susceptibility to catalepsy in mice using polymorphic microsatellite markers. Dokl Biol Sci 393: 531–534. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Fursenko DV, Khotskin NV, Bazovkina DV, Kulikov VA, Naumenko VS et al. (2014). Spatial learning in the Morris water maze in mice genetically different in the predisposition to catalepsy: the effect of intraventricular treatment with brain‐derived neurotrophic factor. Pharmacol Biochem Behav 122: 266–272. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Kolpakov VG, Maslova GB, Kozintsev I, Popova NK (1994). Effect of selective 5‐HT1A agonists and 5‐HT2 antagonists on inherited catalepsy in rats. Psychopharmacology (Berl) 114: 172–174. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Kolpakov VG, Popova NK (2006). The genetic cataleptic (GC) rat strain as a model of depresive disorders In: Kalueff AV. (ed). Animal Models in Biological Psychiatry: Nova Science Publishers, Inc., New York, pp. 59–73. [Google Scholar]
- Kulikov AV, Kozlachkova E, Popova NK (1989). Genetic control of catalepsy in mice. Genetika 25: 1402–1408. [PubMed] [Google Scholar]
- Kulikov AV, Kozlachkova EY, Kudryavtseva NN, Popova NK (1995). Correlation between tryptophan hydroxylase activity in the brain and predisposition to pinch‐induced catalepsy in mice. Pharmacol Biochem Behav 50: 431–435. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Kozlachkova EY, Maslova GB, Popova NK (1993). Inheritance of predisposition to catalepsy in mice. Behav Genet 23: 379–384. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Kozlachkova EY, Popova NK (1992). Activity of tryptophan hydroxylase in brain of hereditary predisposed to catalepsy rats. Pharmacol Biochem Behav 43: 999–1003. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Naumenko VS, Voronova IP, Tikhonova MA, Popova NK (2005). Quantitative RT‐PCR assay of 5‐HT1A and 5‐HT2A serotonin receptor mRNAs using genomic DNA as an external standard. J Neurosci Methods 141: 97–101. [DOI] [PubMed] [Google Scholar]
- Kulikov AV, Popova NK (2008). Genetic control of catalepsy in mice In: Torres SL, Martin MS. (eds). Genetic predisposition to disease: Nova Science Publishers, Inc., New York, pp. 215–236. [Google Scholar]
- Kulikov AV, Tikhonova MA, Kulikov VA (2008b). Automated measurement of spatial preference in the open field test with transmitted lighting. J Neurosci Methods 170: 345–351. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan MP, Courvoisier H, Bihoreau MT, Gauguier D, Hendley ED, Lathrop M et al. (1996). A major quantitative trait locus influences hyperactivity in the WKHA rat. Nat Genet 14: 471–473. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Bazovkina DV, Kondaurova EM, Zubkov EA, Kulikov AV (2010). The role of 5‐HT2A receptor and 5‐HT2A/5‐HT1A receptor interaction in the suppression of catalepsy. Genes Brain Behav 9: 519–524. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Kondaurova EM, Bazovkina DV, Tsybko AS, Il'chibaeva TV, Popova NK (2014). On the role of 5‐HT(1A) receptor gene in behavioral effect of brain‐derived neurotrophic factor. J Neurosci Res 92: 1035–1043. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Kondaurova EM, Bazovkina DV, Tsybko AS, Tikhonova MA, Kulikov AV et al. (2012). Effect of brain‐derived neurotrophic factor on behavior and key members of the brain serotonin system in genetically predisposed to behavioral disorders mouse strains. Neuroscience 214: 59–67. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Kondaurova EM, Popova NK (2011). On the role of brain 5‐HT7 receptor in the mechanism of hypothermia: comparison with hypothermia mediated via 5‐HT1A and 5‐HT3 receptor. Neuropharmacology 61: 1360–1365. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Kulikov AV (2006). Quantitative assay of 5‐HT(1A) serotonin receptor gene expression in the brain. Mol Biol (Mosk) 40: 37–44. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Osipova DV, Kostina EV, Kulikov AV (2008). Utilization of a two‐standard system in real‐time PCR for quantification of gene expression in the brain. J Neurosci Methods 170: 197–203. [DOI] [PubMed] [Google Scholar]
- Ornstein K, Amir S (1981). Pinch‐induced catalepsy in mice. J Comp Physiol Psychol 95: 827–835. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH, Knapp DJ, Crews FT, Janowsky DS (1996). Further selection of rat lines differing in 5‐HT‐1A receptor sensitivity: behavioral and functional correlates. Psychiatr Genet 6: 107–117. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. NC‐IUPHAR(2014). The IUPHAR/BPS guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucleic Acids Res 42: D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M (1985). Validation of open: closed arm entries in an elevated plus‐maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167. [DOI] [PubMed] [Google Scholar]
- Popova NK (1997). Serotonin in genetically determined types of defensive behavior. Zh Vyssh Nerv Deiat Im I P Pavlova 47: 350–357. [PubMed] [Google Scholar]
- Popova NK, Kulikov AV (1995). On the role of brain serotonin in expression of genetic predisposition to catalepsy in animal models. Am J Med Genet 60: 214–220. [DOI] [PubMed] [Google Scholar]
- Popova NK, Kulikov AV, Avgustinovich DF, Vishnivetskaia GB, Kolpakov VG (1994). Participation of brain 5‐HT1A serotonin receptors in regulating hereditary catalepsy. Biull Eksp Biol Med 118: 633–635. [PubMed] [Google Scholar]
- Popova NK, Naumenko VS, Cybko AS, Bazovkina DV (2010). Receptor‐genes cross‐talk: effect of chronic 5‐HT(1A) agonist 8‐hydroxy‐2‐(di‐n‐propylamino) tetralin treatment on the expression of key genes in brain serotonin system and on behavior. Neuroscience 169: 229–235. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C (2003). The open field as a paradigm to measure the effects of drugs on anxiety‐like behaviors: a review. Eur J Pharmacol 463: 3–33. [DOI] [PubMed] [Google Scholar]
- Ramos A, Berton O, Mormede P, Chaouloff F (1997). A multiple‐test study of anxiety‐related behaviours in six inbred rat strains. Behav Brain Res 85: 57–69. [DOI] [PubMed] [Google Scholar]
- Sinyakova NA, Kulikova EA, Kulikov AV (2014). Serotonin and dopamine brain metabolism in mice with different predisposition to catalepsy. Zh Vyssh Nerv Deiat Im I P Pavlova 64: 686–692. [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P (1985). The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85: 367–370. [DOI] [PubMed] [Google Scholar]
- Tikhonova MA, Alperina EL, Tolstikova TG, Bazovkina DV, Di VY, Idova GV et al. (2010). Effects of chronic fluoxetine treatment on catalepsy and the immune response in mice with a genetic predisposition to freezing reactions: the roles of types 1A and 2A serotonin receptors and the tph2 and SERT genes. Neurosci Behav Physiol 40: 521–527. [DOI] [PubMed] [Google Scholar]
- Tikhonova MA, Kulikov AV, Bazovkina DV, Kulikova EA, Tsybko AS, Bazhenova EY et al. (2013). Hereditary catalepsy in mice is associated with the brain dysmorphology and altered stress response. Behav Brain Res 243: 53–60. [DOI] [PubMed] [Google Scholar]
- Tikhonova MA, Kulikov AV, Naumenko VS, Morozova MV, Bazovkina DV, Popova NK (2009). Intracerebral administration of brain‐derived neurotrophic factor (BDNF) reduces severity of cataleptic freezing in mice with genetic predisposition to catalepsy. Bull Exp Biol Med 148: 889–891. [DOI] [PubMed] [Google Scholar]
- Tikhonova MA, Lebedeva VV, Kulikov AV, Bazovkina DV, Popova NK (2006). Effect of imipramine on the behavior and cerebral 5‐HT1A serotonin receptors in mice genetically predisposed to catalepsy. Bull Exp Biol Med 141: 48–50. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Steckler T (2000). Behavioural analysis of four mouse strains in an anxiety test battery. Behav Brain Res 115: 95–106. [DOI] [PubMed] [Google Scholar]
- van Praag HM (2004). Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry 28: 891–907. [DOI] [PubMed] [Google Scholar]
- VanderWende C, Spoerlein MT (1979). Morphine‐induced catalepsy in mice. Modification by drugs acting on neurotransmitter systems. Neuropharmacology 18: 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg ML (1996). Serotonergic mechanisms in neuroleptic‐induced catalepsy in the rat. Neurosci Biobehav Rev 20: 325–339. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Hillegaart V (1995). Stimulation of median, but not dorsal, raphe 5‐HT1A autoreceptors by the local application of 8‐OH‐DPAT reverses raclopride‐induced catalepsy in the rat. Neuropharmacology 34: 495–499. [DOI] [PubMed] [Google Scholar]
- Waerzeggers Y, Monfared P, Viel T, Winkeler A, Jacobs AH (2010). Mouse models in neurological disorders: applications of non‐invasive imaging. Biochim Biophys Acta 1802: 819–839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Mice of catalepsy‐prone B6‐M76C line during catalepsy episode.
Video S2 Mice of catalepsy‐resistant B6‐M76B line in test for catalepsy.


