Abstract
Background and Purpose
The link between type 2 diabetes mellitus (T2DM) and depression is bidirectional. However, the possibility that metabolic disorders may elicit anxiogenic‐like/depressive‐like symptoms or alter the efficacy of antidepressant drugs remains poorly documented. This study explored the influence of T2DM on emotionality and proposed a therapeutic strategy that might be used in depressed diabetic patients.
Experimental Approach
Mice were fed a high‐fat diet (HFD) and subjected to a full comprehensive metabolic and behavioural analysis to establish correlations between metabolic and psychiatric disorders. In vivo intra‐hippocampal microdialysis was also applied to propose a mechanism underpinning the phenotype of mice fed the HFD. Finally, we tested whether chronic administration of the selective 5‐HT reuptake inhibitor escitalopram or HFD withdrawal could reverse HFD‐induced metabolic and behavioural anomalies.
Key Results
The increased body weight, hyperglycaemia and impaired glucose tolerance in response to HFD were correlated with anxiogenic‐like/depressive‐like symptoms. Moreover, this phenotype was associated with decreased extracellular 5‐HT levels in the hippocampus which may result from increased sensitivity of the dorsal raphe 5‐HT1A autoreceptor. Interestingly, the beneficial effect of prolonged administration of escitalopram was abolished in HFD‐fed mice. On the contrary, HFD withdrawal completely reversed metabolic impairments and positively changed symptoms of anxiety, although some behavioural anomalies persisted.
Conclusions and Implications
Our data provide clear‐cut evidence that both pathologies are finely correlated and associated with impaired 5‐HT mediated neurotransmission in the hippocampus. Further experiments are warranted to define the most adequate strategy for the treatment of such co‐morbidity.
Linked Articles
This article is part of a themed section on Updating Neuropathology and Neuropharmacology of Monoaminergic Systems. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v173.13/issuetoc
Abbreviations
- [5‐HT]ext
extracellular 5‐HT levels
- 8‐OH‐DPAT
8‐hydroxy‐2‐(di‐n‐propylamino)tetralin
- HFD
high‐fat diet
- HPC
ventral hippocampus
- IPGTT
intraperitoneal glucose tolerance test
- MD
major depression
- NSF
novelty suppressed feeding
- OF
open field
- OGTT
oral glucose tolerance test
- SSRI
selective serotonin reuptake inhibitor
- ST
splash test
- STD
standard diet
- T2DM
type 2 diabetes mellitus
- TST
tail suspension test
Tables of Links
| TARGETS |
|---|
| GPCRs |
| 5‐HT1A receptors |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
According to the World Health Organization, diabetes and depression are each estimated to affect 350 million people, and both pathologies will constitute the greatest healthcare burdens in the next years. Clinical studies have yielded converging evidence regarding a bidirectional link between both pathologies. Indeed, depressive disorders developing earlier in life lead to an increased risk of diabetes (Knol et al., 2006), and 10 to 30% of diabetic patients suffer from major depression (MD) (Anderson et al., 2001; Ali et al., 2006). Such a prevalence is a serious medical and public health concern because these coexisting pathologies impose substantial economic costs, and their effects on disability, productivity and quality of life further accentuate these costs (Egede et al., 2010). Interestingly, these epidemiological data are supported by recent findings in animal models of type 1 (T1DM) and type 2 diabetes mellitus (T2DM) showing that metabolic impairments induced by streptozotocin, a long‐term high‐fat diet (HFD) or a Western diet, elicit depressive‐like behaviours (Miyata et al., 2004; Ho et al., 2012; Gupta et al., 2014; André et al., 2014). It has also been reported that HFD exacerbates behavioural anomalies in various animal models of depression (Abildgaard et al., 2011; Liu et al., 2014). Conversely, the central injection of insulin or adiponectin, a hormone exerting anti‐diabetic and insulin‐sensitizing action, produces antidepressant‐like behavioural effects (Ho et al., 2012; Liu et al., 2012; Gupta et al., 2014). Collectively, these findings emphasize the existence of common brain circuits and signalling pathways (Ho et al., 2013) between both metabolic and psychiatric disorders. However, there is little research that explicitly demonstrates whether T2DM affects specific symptoms of MD. Likewise, the mechanisms by which T2DM negatively affects emotionality remain unknown.
Biological evidence suggests that MD results from changes in 5‐HT‐mediated activity in various brain regions, including the ventral hippocampus (HPC) and the frontal cortex (Hamon and Blier, 2013). Diabetes and brain insulin signalling have been associated with modifications of the 5‐HT system. For example, the destruction of insulin secreting beta cells in the rat pancreas produced a significant attenuation of 5‐HT levels in the brain (Curzon and Fernando, 1977). Consistent with the latter observation, in vivo microdialysis studies reported decreased extracellular 5‐HT levels in the HPC of T1DM animal models (Kino et al., 2004; Yamato et al., 2004). However, whether impairment in energy homeostasis induced by a long‐term HFD might influence the activity of the brain 5‐HT system or the therapeutic activity of antidepressant drugs is poorly documented. Indirect evidence, however, is in favour of an attenuation of serotonergic tone because decreased levels of free tryptophan were detected in the CNS of patients with T2DM (Kloiber et al., 2010; Herrera‐Marquez et al., 2011). Accordingly, a significant decrease in plasma or brain levels of 5‐HT has been reported in rodents after prolonged HFD exposure (Kim et al., 2013; Derkach et al., 2015), but these neurochemical changes have never been confirmed using in vivo microdialysis.
Selective 5‐HT reuptake inhibitors (SSRIs) represent the most commonly prescribed antidepressants. These pharmacological agents, including escitalopram or sertraline, are effective in treating MD in patients with T2DM (Williams et al., 2007; Gehlawat et al., 2013). However, other clinical studies have led to the opposite conclusions (Anderson et al., 2010; Gois et al., 2014), as a significant proportion of patients with T2DM suffering from MD do not achieve remission with SSRI treatment. Among the factors that might explain such a non‐response to antidepressant drugs, patient age, the severity of the psychiatric/metabolic disorder or the ability of 5‐HT related antidepressant drugs to further destabilize glucose homeostasis (Ghaeli et al., 2004; Raeder et al., 2006; Williams et al., 2007) are possible explanations.
Given the relatively few attempts to investigate the link between HFD‐induced T2DM and MD, the present study aimed to elucidate possible correlations between both disorders in mice. We applied an original approach consisting of the integration of metabolic and emotional parameters into separate z‐scores in the same animal. Full quantifiable assessment of metabolism and emotionality is possible when the same animal is exposed to multiple tests covering a wide range of representative symptoms. This set of converging observations defines a syndrome, and the z‐scores can therefore be assimilated into the clinical characterization of the human pathologies. In addition to behavioural assessments, we also examined the functional consequences of HFD‐induced T2DM on the activity of the 5‐HT system and determined whether chronic SSRI treatment or HFD withdrawal had a positive and reciprocal influence on metabolism and emotionality.
Methods
Animals, diets and drugs
All animal care and experimental procedures complied with the European directive 2010/63/UE and were approved by the French Ministry of Research and the local ethical committees (C2EA Grand Campus de Dijon N°105). Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Seven‐week‐old C57/Bl6 male mice (Elevages Janvier Farms, Saint‐Quentin Fallaviers, France) were housed, five mice per cage under standard conditions (12:12 h light–dark cycle, lights on at 7 AM, 22°C, 60% relative humidity). After 1 week of acclimatization, mice were randomly assigned to receive free access to a standard diet (STD A04; SAFE diets, Augy, France), an HFD (D12451; Research Diets Inc., New Brunswick, NJ, USA) or a 60% fructose‐enriched diet (U8960 version 015 SAFE diets) for up to 16 weeks (Table 1). We verified that tryptophan amounts – the precursor for 5‐HT synthesis – were equivalent between STD and HFD (Table 1). Body weight was monitored weekly. In a specific experiment, STD and HFD mice were subcutaneously and chronically treated (4 weeks), with the SSRI escitalopram (Biotrend Chemikalien GmbH, Switzerland) at the active dose of 10 mg∙kg−1∙day−1 (Guiard et al., 2012). Mice received their daily injection of escitalopram (volume 100 μL∙10 g−1 of mouse) at 5 PM and were tested the next day between 9 and 12 PM.
Table 1.
Composition of the fructose and high‐fat diets
| Diets Energy (kcal%) | STD | HFD | 60% fructose |
|---|---|---|---|
| Proteins | 26.2 | 20 | 21.2 |
| Carbohydrates | 60 | 35 | 65.8 |
| Lipids | 3.1 | 45 | 13 |
| Energy content (kcal∙kg−1 dry diet) | 2791 | 4727 | 3466 |
| Tryptophan content (mg∙kg−1 dry diet) | 1.9 | 2.1 | 2.2 |
STD, standard diet; HFD, high‐fat diet.
Group sizes
The exact group size (n) for each experimental group/condition is provided in the Figure legends, and ‘n’ refers to independent values, not replicates. For each experimental protocol assessing behavioural and metabolic parameters, independent cohorts of mice were used with a minimum of 10 animals per group. However, group sizes vary from one protocol to another because mice displaying serious skin injuries in response to HFD were discarded for ethical reasons and to avoid possible interferences with the behavioural tests.
Randomization
Where possible, we sought to randomize animals. In particular, for the pharmacological experiments, each cage contained mice administered with the treatment or its vehicle.
Blinding
The operator in charge of the treatment did not perform the experiments, and the animal identification was made after the tests using ear tags.
Metabolic parameters
Glucose tolerance test: Animals were individually housed, weighed and fasted for 4 h with free access to water. Blood glucose levels were measured from tail prick (Accu‐check Performa glucometer; Roche, Boulogne‐Billancourt, France) at basal (0 min), 15, 30, 45, 60, 90 and 120 min after oral [oral glucose tolerance test (OGTT), for HFD experiments] or i.p. [intraperitoneal glucose tolerance test (IPGTT), for fructose experiments] administration of glucose (2 g∙kg−1). The IPGTT was preferred for fructose‐fed animals (and their respective controls) because its oral administration is known to alter intestinal glucose transport (Tobin et al., 2008). Both the OGTT and IPGTT were calculated using area under the curve (AUC) with the coordinate axis set at 100. For plasma insulin levels, blood samples were collected through tail prick in heparinized capillary tubes (Microvette CB 300 K2E, Sarstedt, Nümbrecht, Germany). Blood was centrifuged (2500× g for 10 min), and plasma was collected and stored at −80°C before analysis. Insulin was measured using the AlphaLISA method according to manufacturer's instructions (Human insulin kit, PerkinElmer, Waltham, MA, USA, AL204C). Separate cohorts of animals were used to test the effect of long‐term (12 and 16 weeks) HFD exposure.
Behavioural tests
All behavioural tests were performed in the morning to avoid differences in locomotor activity and other variables affected by circadian rhythm. Previous studies have indicated that certain test variables are sensitive, whereas others are resistant, to test order (McIlwain et al., 2001). Bearing this in mind, performance was evaluated from the least to the most stressful test, thereby decreasing the chance that one test might affect the behaviour evaluated in the subsequent paradigm. Importantly, because previous handling and testing has been described to reduce exploratory activity and emotionality in mice (Voikar et al., 2004), animals were tested once in each paradigm. Finally, given that it has been demonstrated that the interval between behavioural tests could be as little as 1 day, with a weak effect on overall performance (Paylor et al., 2006), in the present study, a 2‐day recovery period between each test was provided. It is noteworthy that reducing the inter‐test interval reduces the possible effects of time of dietary/drug administration on tests.
Open field (OF) was performed in 40 × 40 cm2 Plexiglas boxes (Mouse Open Field Arena ENV‐510; Med Associates Inc., St. Albans, VT, USA) during a 30 min session period. Activity chambers were computer interfaced for data analysis (SOF‐811; Med Associates Inc.), and two regions were defined by grid lines that divided each box into the centre and periphery, with each of the four lines being 11 cm from each wall.
The tail suspension test (TST) was performed using the BIOSEB's TST system (Bioseb, Vitrolles, France) during a 6 min session. Immobility time was scored as an index of resignation. Movements in terms of energy and power in motion were measured to ensure the absence of any locomotor bias.
The splash test (ST) was performed for a 5 min period as previously described (David et al., 2009). After squirting 200 μL of a 10% sucrose solution on the mouse's snout, grooming time was scored by a single experimenter as an index of self‐care.
Novelty suppressed feeding (NSF) was performed in a white plastic box (30 × 60 cm). Mice were food deprived for 24 h before testing and then placed in the corner of the box with their respective food pellet on a white square filter paper at the centre of the arena under a bright light (~60 W) placed about 60–80 cm above the food pellet. Latency to begin eating was scored by a single experimenter, with a cut‐off time of 10 min. Upon return to the home cage, the total amount of food intake was measured for a 5 min period to ensure the absence of differences in hunger/motivation.
Metabolic and emotionality z‐scores
Z‐normalization across complementary measures of metabolism and emotionality‐related behaviours assessed from different paradigms was applied after each experimental protocol. Simple mathematical tools were used to normalize data from each individual raw metabolic and behavioural data to the mean of the STD groups within each experimental cohort. Data were then integrated into a single value, named metabolic and emotionality z‐scores respectively. Their values were obtained by subtracting the average of observations in a population from an individual raw value and then dividing this difference by the population SD as described previously (Guilloux et al., 2011). This type of normalization allows data on different scales to be compared. The metabolic z‐score included final body weight, glycaemia and insulinaemia and the AUC of the glucose tolerance test. The emotionality z‐score included the parameters measured in the OF (centre entries, centre time and centre‐to‐total‐distance ratio), TST (immobility time) and ST (grooming time). It is noteworthy that several parameters were calculated in the OF to evaluate anxiety. However, to avoid any weighted effect of this test compared with the paradigms in which only one parameter was evaluated (TST, ST, NSF or metabolic parameters), we averaged these normalized behavioural parameters in the OF to obtain a single value per mouse and per behavioural test.
In vivo intracerebral microdialysis
After 16 weeks on STD or HFD diets, anesthetized mice (chloral hydrate, 400 mg∙kg−1, i.p.) were stereotaxically implanted with concentric microdialysis probes (effective membrane length 2.0 mm) in the HPC. Coordinates from bregma, according to the mouse brain atlas (Paxinos and Franklin's, 1997), were as follows (in millimeters): anteroposterior: −2.2; lateral: ±2; and ventral: 2 mm. On the next day, mice were connected to a swivel system, and the probes were connected to a microinjection pump, allowing a continuous perfusion of artificial CSF at a flow rate of 1.5 μL∙min−1. A 2 h perfusion was performed to allow for stabilization of extracellular 5‐HT concentrations ([5‐HT]ext). Then, microdialysate samples were collected every 15 min for 2 h. At the end of the microdialysis, samples were kept at −80°C until 5‐HT content analysis was performed by HPLC (XL‐ODS, 4.6 × 7.0 mm, particle size 3 μm; Beckman Coulter, Palo Alto, CA, USA), coupled with an amperometric detector (1049A, Hewlett‐Packard, Les Ulis, France). For 5‐HText, AUC values (% of baseline) were calculated during the 120 min post‐treatment period. The sensitivity limit for 5 ‐HT was ~0.5 fmol∙sample−1 (signal‐to‐noise ratio = 2). The amount of 5‐HT in dialysate samples was calculated by measuring the peak heights relative to the external standards. At the end of the experiments, localization of microdialysis probes was verified histologically.
8‐OH‐DPAT‐induced hypothermia
Body temperature was measured from 9 to 12 AM by gently inserting a microprobe thermometer (Ugo Basile, Varese, Italy) into the mice rectum (Bill et al., 1991). Digital recordings of the temperatures were obtained with an accuracy of ±0.1°C, as indicated in the technical specifications. Temperatures were measured before (t‐20, t‐10 and t0 min) and after treatment with 8‐hydroxy‐2‐(di‐n‐propylamino)tetralin (8‐OH‐DPAT, Sigma‐Aldrich, Saint Quentin Fallavier, France; 100 μg∙kg−1; s.c.; SOURCE). This dose was chosen based on an initial report showing its ability to induce hypothermia and also on electrophysiological experiments in which it completely inhibited the firing rate of 5‐HT neurons in response to the stimulation of the 5‐HT1A autoreceptor (Rainer et al., 2012). In this test, data were calculated as a percent of decreased body temperature after 8‐OH‐DPAT administration relative to the temperature obtained at t0.
Data analysis
Data were analysed using StatView 5.0 software (SAS Institute Inc., Cary, NC, USA). Results were expressed as means ± SEM. Statistical analyses were performed by two‐tailed unpaired Student's t‐test, one‐way or two‐way ANOVA, followed by Tukey's post hoc test when the F‐value was significant. The linear relationship between metabolic and emotionality z‐scores was analysed by the Pearson's r after a Shapiro–Wilk normality test. In the NSF experiments, latency to feed was presented in survival curves using the Kaplan–Meier method. In agreement with the guidance on appropriate statistical tests (Curtis et al., 2015), the threshold for statistical significance was defined throughout the manuscript at P < 0.05. A complete statistical summary analysis for behavioural and metabolic data is provided in Supporting Information Table 1.
Results
Development of T2DM promotes anxiogenic‐like/depressive‐like behaviours
To study the effects of metabolic disorder on anxiogenic‐like/depressive‐like behaviours, mice were fed an HFD either for 12 or 16 weeks. As expected, the HFD diet progressively increased body weight over the weeks, along with fasting hyperglycaemia, hyperinsulinaemia and glucose intolerance (Supporting Information Figure 1A–D). To integrate these metabolic impairments, we established metabolic z‐scores that normalize each individual raw data to the mean of the control group (STD) and integrate all parameters into a single value. Increased metabolic z‐scores were observed after both 12 and 16 weeks of an HFD in comparison with their respective STD groups (Supporting Information Figure 1E). The longer the HFD, the more pronounced the metabolic z‐score, suggesting a time‐dependent effect of an HFD on the induction of metabolic disorders (z‐score HFD 12 weeks: 2.63 ± 0.16 vs. HFD 16 weeks: 5.80 ± 1.11; P < 0.05, unpaired t‐test).
HFD‐fed mice were tested for anxiogenic‐like/depressive‐like symptoms using complementary behavioural tests. In the OF, 12 weeks of an HFD did not alter the number of centre entries, while it significantly decreased the time spent in this compartment (Figure 1A and B, left panels). In the 16 week HFD‐fed mice, both the number of entries and the time spent in the centre of the arena were decreased (Figure 1A and B, right panels). To eliminate putative bias, we verified that after 12 and 16 weeks, the HFD did not change the total ambulatory distance travelled (12 weeks: STD 2690 ± 148 vs. HFD: 2670 ± 295 cm; P > 0.05; 16 weeks: STD: 2492 ± 332 vs. HFD: 2667 ± 336 cm; P > 0.05; unpaired t‐test). In the TST, neither 12 nor 16 weeks of an HFD modified the immobility time in comparison with their respective STD groups (Figure 1C). Owing to the increased body weight in HFD mice, we also ascertained that the energy (Supporting Information Figure 2A) and the physical driving force during the TST (Supporting Information Figure 2B) were not affected. Finally, in the ST, although the HFD did not modify the grooming time after 12 weeks, this parameter was significantly reduced after 16 weeks (Figure 1D).
Figure 1.
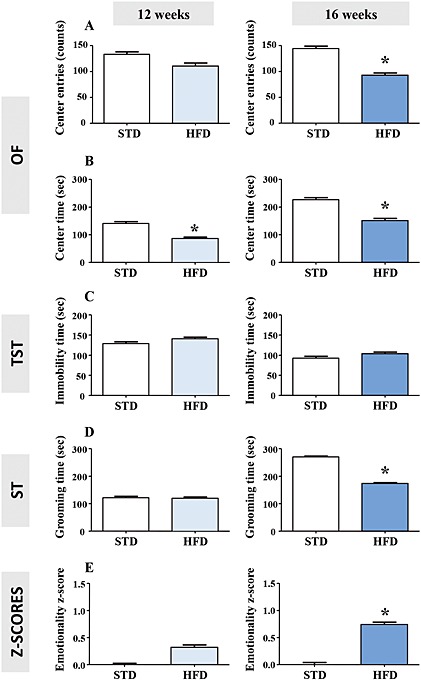
Long‐term (12 and 16 weeks) HFD induces anxiogenic‐like/depressive‐like phenotype. Centre entries (A) and time spent in the centre (B) in the OF, immobility time in the TST (C), grooming time in the ST (D), emotionality z‐score (E) integrating all these parameters (centre entries and time in the OF, immobility time in the TST and grooming time in the ST) in mice fed an STD (n = 14 and 10) or HFD (n = 12 and 10) for 12 (left panels) or 16 (right panels) weeks. *P < 0.05, significantly different from STD; two‐tailed Student's t‐test.
The integration of individual behavioural data revealed an increased emotionality z‐score after 16 weeks of an HFD (Figure 1E). A trend towards an increased emotionality z‐score was already observed after 12 weeks of an HFD, although it was not statistically significant (P = 0.06). Similar to metabolic z‐scores, the longer the HFD, the more pronounced the emotionality z‐scores, suggesting a time‐dependent effect of the HFD on the induction of anxiogenic‐like/depressive‐like behavioural anomalies (z‐score HFD 12 weeks: 0.32 ± 0.15 vs. HFD 16 weeks: 0.74 ± 0.17; P < 0.05, unpaired t‐test). A significant positive correlation between metabolic and emotionality z‐scores was unveiled after both 12 (Pearson r = 0.3949; P < 0.05; Figure 2A) and 16 weeks (Pearson r = 0.6187; P < 0.05; Figure 2B) of an HFD.
Figure 2.
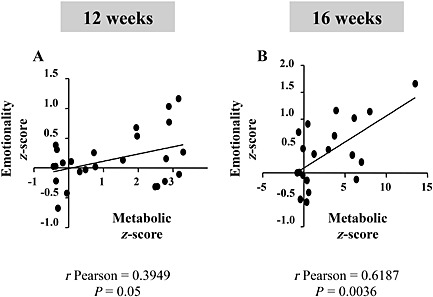
HFD‐induced T2DM metabolic disorders correlates with anxiogenic‐like/depressive‐like phenotype. Correlation between metabolic and emotionality z‐scores in mice fed the HFD for 12 (A) or 16 (B) weeks. Metabolic z‐score parameters included: final body weight, fasting glycaemia and insulinaemia, AUC during the OGTT. Emotionality z‐score parameters included: centre entries and time in the OF, immobility time in the TST and grooming time in the ST. All animals tested in Figure 1 were included in this correlation analysis.
To further understand the relationship between metabolic disorders and the development of anxiogenic‐like/depressive‐like behaviours, we used another model of diet‐induced T2DM. Enriched fructose diet intake is a recognized model of diet‐induced T2DM which does not alter body weight (Samuel, 2011). As expected, in another cohort of mice fed a fructose‐enriched diet, fasting hyperglycaemia and glucose intolerance were accompanied with lower body weight compared with controls (Supporting Information Figure 3A–D). Overall, a higher metabolic z‐score was detected in fructose‐fed mice compared with controls (Supporting Information Figure 3E). Surprisingly, despite the development of these T2DM‐like characteristics, the fructose diet did not elicit anxiogenic‐like/depression‐like behaviours, as revealed by the lack of significant differences between the emotionality z‐scores of STD‐fed and fructose‐fed mice (Supporting Information Figure 3F–I).
HFD impairs 5‐HT transmission
Because the brain 5‐HT system plays a major role in anxiety and depression, we next determined whether the behavioural effects of the HFD were accompanied with modifications in the 5‐HT tone of the HPC. The choice of this brain region was based on the fact that it is located at a crossroad of the limbic system, sending notably functional projections in the prefrontal cortex, the amygdala and the hypothalamus (Fanselow and Dong, 2010; Radley and Sawchenko, 2011). Using in vivo intracerebral microdialysis in freely moving mice, we observed a significant decrease in basal extracellular 5‐HT levels ([5‐HT]ext) in the HPC of HFD‐fed mice for 16 weeks (Figure 3A). Given that the somatodendritic 5‐HT1A autoreceptors located on 5‐HT neurons of the dorsal raphe nucleus exert a negative feedback control on 5‐HT release at the 5‐HT nerve terminals, we examined its functional activity in the 8‐OH‐DPAT‐induced hypothermia paradigm. Although both STD‐fed and HFD‐fed mice displayed a hypothermic response following the injection of the 5‐HT1A receptor agonist, this effect was significantly greater in HFD‐fed mice (Figure 3B). These data suggest that the decreased [5‐HT]ext observed in the HPC could be the consequence of an increased expression and/or function of the raphe 5‐HT1A autoreceptors.
Figure 3.
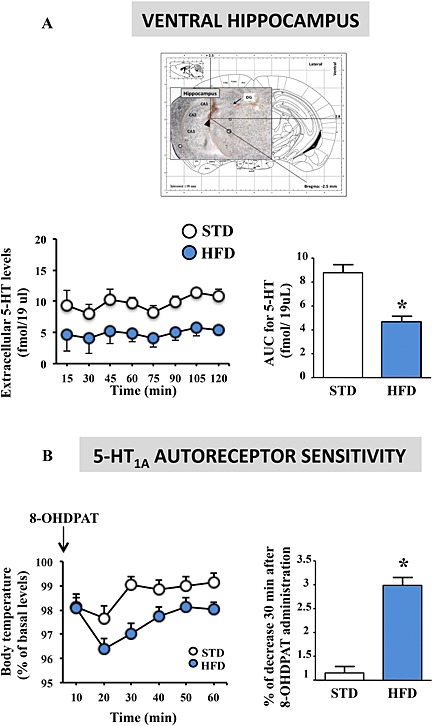
Long‐term (16 weeks) HFD impairs 5‐HT transmission. Basal 5‐HT extracellular levels (fmol∙sample−1) in the HPC (left panel) and AUC for 5‐HT extracellular levels over the 0–150 min period in mice fed the STD (n = 7) or HFD (n = 5) for 16 weeks (right panel) (A). Changes in body temperature induced by the 5‐HT1A receptor agonist 8‐OH‐DPAT (100 μg∙kg−1 s.c.). Data are expressed as percentage (%) of basal rectal temperature in mice fed an STD (n = 14) or HFD (n = 10) for 16 weeks (left panel) and percentage of decreased rectal temperature 30 min after 8‐OH‐DPAT administration (right panel) (B). Basal body temperatures were 36.2 ± 1 and 35.9 ± 0.8°C in STD‐fed and HFD‐fed mice respectively. *P < 0.05, significantly different from STD; two‐tailed Student's t‐test.
HFD impairs the anxiolytic‐like/antidepressant‐like effects of chronic escitalopram treatment
Currently available antidepressant drugs mainly exert their therapeutic action through the enhancement of 5‐HT neurotransmission. Thus, we investigated whether HFD‐induced attenuation of hippocampal 5‐HT levels influenced the anxiolytic and antidepressant‐like activities of the SSRI escitalopram. Mice were fed an STD or HFD for 16 weeks while being under daily administration of escitalopram (10 mg∙kg−1; Figure 4A) during the last 4 weeks. Overall, escitalopram treatment did not alter metabolic parameters (Figure 4B, C and E), although a trend towards an increase in fasting insulinaemia (P = 0.08) was detected in HFD‐fed mice (Figure 4D). However, this had no significant impact on the calculated metabolic z‐score (Figure 4F). These data showed that long‐term escitalopram treatment did not improve or worsen the T2DM‐like metabolic disorders of HFD‐fed mice.
Figure 4.
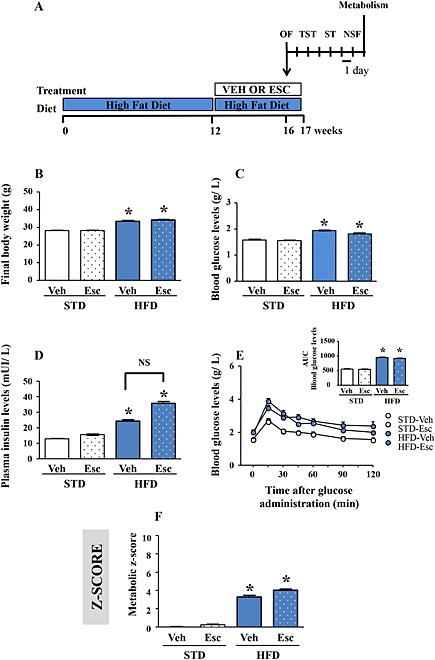
Chronic escitalopram treatment does not modify metabolic parameters. Experimental protocol. Mice were fed as STD or HFD (STD vs. HFD) for 16 weeks and received during the last 4 weeks a s.c. injection of vehicle (Veh) or escitalopram (Esc; 10 mg·kg−1·day−1) (A). Final body weight (B), fasting blood glucose (C) and insulin (D) levels, change in blood glucose levels during an OGTT (E; inset: AUC of the glycaemia over the 120 min) and metabolic z‐score (F; parameters included are as follows: final body weight, fasting glycaemia and insulinaemia, AUC during the OGTT) in mice fed an STD treated with vehicle or escitalopram (n = 13 and 15), or fed an HFD treated with vehicle or escitalopram (n = 10 and 15). *P < 0.05, significantly different from corresponding STD value; one‐way ANOVA followed by Tukey's post hoc test.
Regarding behaviours, the effects of escitalopram on HFD‐induced anxiogenic‐like/depressive‐like symptoms were specifically tested in the OF and the ST, given their sensitivity to such hyperlipidic food. The NSF test was also used to further study escitalopram's activity. As expected, in STD‐fed animals, escitalopram increased the number of centre entries in the OF and the grooming time in the ST. These responses were however not observed in HFD‐fed mice (Figure 5A and B). In the NSF, although escitalopram alone failed to modify the latency to feed, it potentiated the ability of the HFD to increase this parameter, thereby suggesting a potentiation of the anxiogenic‐like/depressive‐like response in this paradigm (Figure 5C and Supporting Information Figure 5A). With regard to home cage consumption, we showed that food intake was not different between groups (STD‐vehicle: 7.03 ± 0.5; STD‐escitalopram: 6.31 ± 0.61; HFD‐vehicle: 6.12 ± 1.98; and HFD‐escitalopram: 4.55 ± 0.68 mg∙g−1 of body weight; P > 0.05; two‐way ANOVA). Altogether, these data indicate that escitalopram failed to exert appreciable anxiolytic‐like and antidepressant‐like effects in HFD‐fed mice. Moreover, the NSF data suggest that escitalopram treatment may even reinforce the anxiogenic‐like/depressive‐like symptoms of HFD‐fed mice with T2DM. Interestingly, in HFD‐fed mice, a higher emotionality z‐score was detected in response to escitalopram compared with vehicle (Figure 5D).
Figure 5.
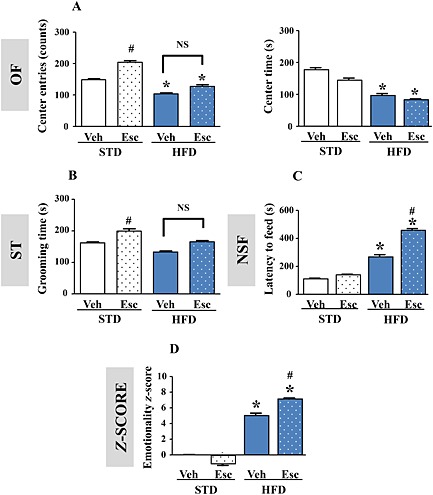
Chronic escitalopram treatment fails to reverse HFD‐induced anxiogenic‐like/depressive‐like phenotype. Centre entries (left panel) and time spent in the centre (right panel) in the OF (A), grooming time in the ST (B), latency to feed in the NSF test (C) and emotionality z‐score (D) integrating all these parameters (centre entries and time in the OF, grooming time in the ST and latency to feed in the NSF) in mice fed an STD treated with vehicle (Veh) or escitalopram (Esc; 10 mg·kg−1·day−1) (n = 13 and 15) or fed an HFD treated with vehicle (Veh) or escitalopram (Esc; 10 mg·kg−1·day−1) (n = 10 and 15). *P < 0.05, significantly different from corresponding STD, #P < 0.05, significantly different from corresponding Veh; NS P > 0.05. One‐way ANOVA followed by Tukey's post hoc test.
HFD withdrawal reverses metabolic disorders but not anxiogenic‐like/depressive‐like behaviours
Lastly, we investigated whether reversing metabolic impairments would ameliorate anxiogenic‐like/depressive‐like anomalies. A reinstatement of the STD regimen for 1 month was performed after 12 weeks of an HFD, while two other groups were maintained on the HFD or STD for 16 weeks (Figure 6A). The switch from the HFD to the STD reversed HFD‐induced increases in body weight and T2DM‐like disorders (Supporting Information Figure 4A–E).
Figure 6.
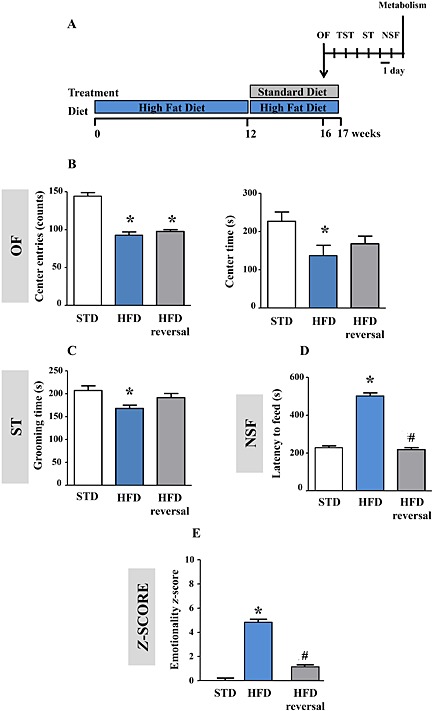
Withdrawal of HFD reverses anxiogenic‐like/depressive‐like phenotype. Experimental protocol. Mice were fed an STD or HFD (STD vs. HFD) for 16 weeks. One group was fed an HFD for 12 weeks before a reinstatement of an STD regimen during the last 4 weeks (HFD reversal) (A). Centre entries (left panel) and time spent in the centre (right panel) in the OF (B), grooming time in the ST (C), latency to feed in the NSF test (D) and emotionality z‐score (E) integrating all these parameters (centre entries and time in the OF, grooming time in the ST and latency to feed in the NSF) in mice fed an STD (n = 10) or HFD (n = 10) for 16 weeks or an HFD for 12 weeks followed by an STD for 4 weeks (HFD reversal n = 12). *P < 0.05, significantly different from STD. #P < 0.05, significantly different from HFD; one‐way ANOVA followed by Tukey's post hoc test.
In light of these metabolic improvements, behavioural performances were also explored. In the OF, both HFD and HFD‐reversal groups showed a decrease in the number of centre entries compared with STD‐fed mice (Figure 6B, left panel). However, while the time in the centre was decreased in the HFD compared with the STD group, this difference was not observed in the HFD‐reversal group (Figure 6B, right panel). Similarly, in the ST, the grooming time was significantly decreased in the HFD group but not in the HFD‐reversal group relative to the controls (Figure 6C). In the NSF, although the HFD increased the latency to feed, this parameter remained unchanged in the HFD‐reversal group relative to the controls (Figure 6D and Supporting Information Figure 5B). Importantly, food intake after the test in the animals' home cage, measured over 5 min, was not different between the groups (STD: 6.81 ± 1.08; HFD: 5.04± 1.61 HFD reversal: 7.03 ± 0.79 mg∙g−1 of body weight; P > 0.05; one‐way ANOVA). Overall, the emotionality z‐scores indicated that HFD reversal restored normal behaviour compared with HFD‐fed mice (Figure 6E).
Discussion
The induction of T1DM notably in response to streptozotocin administration is known to alter emotionality in rodents (Ho et al., 2012; Gupta et al., 2014). In the present study, we extended this observation to T2DM because we found that metabolic disorders observed after prolonged exposure to an HFD elicited anxiogenic‐like/depressive‐like symptoms. Interestingly, chronic antidepressant administration which did not modify HFD‐induced metabolic impairment failed to exert therapeutic‐like activities in mice with T2DM. Conversely, HFD withdrawal, which allowed for complete recovery of metabolic parameters, reversed the behavioural impairments observed in HFD‐fed mice. These findings illustrate that the induction of T2DM, even transitory, was sufficient to alter emotionality and promote antidepressant treatment non‐response. Although the mechanisms underpinning the physiopathology of such co‐morbidity remain unknown, we propose that changes in the activity of the hippocampal 5‐HT transmission might give rise to the behavioural anomalies reported herein.
Evidence demonstrates that HFD induces T2DM in rodents (see Islam and Loots du, 2009). In agreement with this finding, our results indicated that such a diet produced an increase in body weight, along with fasting hyperglycaemia, hyperinsulinaemia and glucose intolerance. Because diabetes is multimodal, quantifiable assessment of glucose homeostasis is possible when different metabolic parameters can be measured in the same animal. Based on the z‐score method, we thus normalized each parameter from the average of the corresponding values observed in the control group fed an STD diet and integrated these values into a single score. Therefore, we obtained a metabolic z‐score which was significantly increased after 12 and 16 weeks of an HFD compared with their respective control groups. In a similar manner, we characterized the global impact of diet on mice behaviour with an emotionality z‐score used as a relevant index of depression severity (Guilloux et al., 2011; Petit et al., 2014). We revealed a robust effect of food on anxiogenic‐like/depressive‐like symptoms, as manifested by higher emotionality z‐scores in HFD‐fed mice, particularly after 16 weeks. These results suggest that metabolic impairments precede the onset of mood‐related symptoms. A more detailed analysis revealed increased anxiety in the OF after 12 and 16 weeks of an HFD, as previously reported by using distinct behavioural paradigms (Abildgaard et al., 2011; Del Rosario et al., 2012; Sharma and Fulton, 2013; Papazoglou et al., 2015; Sivanathan et al., 2015). Regarding the paradigms assessing depressive‐like symptoms, increased carelessness in the ST was also detected specifically after 16 weeks. We should interpret the latter results with caution because decreased grooming in response to the application of sucrose on the mouse coat could result from loss of motivation towards this palatable substance. In support of this hypothesis, decreased sucrose consumption associated with altered nucleus accumbens dopaminergic neurotransmission was reported in HFD‐fed mice or rats (Sharma and Fulton, 2013; Papazoglou et al., 2015). Nevertheless, the fact that grooming time was also reduced in HFD‐fed mice compared with controls in response to the application of agar, a non‐palatable substance (data not shown), suggests that the observed effect in the ST might be specific and related to a depressive‐like state. Finally, it is noteworthy that 12 or 16 weeks of an HFD did not affect despair, as measured in the TST. This absence of response could be because such a paradigm was initially validated to unveil antidepressant‐like activity rather than pathological states. Moreover, the TST evaluates hopelessness, which represents only one symptom of depression and which is not necessarily affected by an HFD. This strengthens our approach involving the assessment of distinct but complementary behaviours. Overall, our study emphasizes the fact that anxiety is likely the first behavioural impairment that emerges in response to metabolic disorders. We cannot, however, definitively rule out the occurrence of depressive‐like abnormalities.
It is important to note that mice fed an HFD displayed impaired glucose homeostasis along with an increase in body weight. Whether one or both of these metabolic deregulations are responsible for altered emotional behaviour is currently unknown. Therefore, we went on to use an enriched fructose diet, which is particularly interesting in the context of our study because it induces T2DM without body weight gain (Samuel, 2011). Here, we reported that although prolonged consumption of fructose reproduced many of the metabolic changes seen in HFD‐fed mice, it failed to produce an anxiogenic‐like/depressive‐like phenotype. Therefore, the nature of the diet responsible for the development of T2DM differentially affects emotional responses in mice. This could explain why not all patients with diabetes develop mood disorders. Moreover, in agreement with previous clinical and preclinical studies, our findings reinforce the idea that body weight gain, potentially leading to obesity, represents an important factor in the aetiology of MD (Licinio and Wong, 2003; Capuron et al., 2011; Sharma and Fulton 2013; André et al., 2014). Hence, the phenotype induced by prolonged HFD exposure could be due to changes in leptin action and not necessarily to glucose metabolism. In support of this hypothesis, there is clear evidence that HFD enhances circulating leptin and induces leptin resistance, while recent data indicate that this hormone is responsible for HFD‐induced depressive‐like behaviours (Yamada et al., 2011).
Despite these data, the mechanisms by which an HFD might have elicited anxiogenic‐like/depressive‐like behaviours are presently unclear. Of the various factors implicated in co‐morbid diabetes and depression, inflammation has received much attention (Leonard, 2013). Indeed, activation of inflammatory processes negatively affects the morphological integrity of the hippocampus, a brain region involved in the anxiogenic/depressive phenotype. As an example of the putative role of T2DM on adult hippocampal neuroplasticity, an HFD has been shown to decrease local levels of brain‐derived neurotrophic factor and related neurogenesis (Lindqvist et al., 2006; Park et al., 2010) or synaptodendritic connections (Arnold et al., 2014) in a similar manner to that observed in various animal models of depression (David et al., 2009). Because impaired hippocampal neuroplasticity has been attributed to an attenuation of 5‐HT neurotransmission (Mahar et al., 2014), we then questioned the extent to which HFD‐induced T2DM modified the extracellular levels of this neurotransmitter. In agreement with previous microdialysis experiments performed in a rat model of T1DM (Kino et al., 2004; Yamato et al., 2004), we showed that HFD‐fed mice displayed lower basal hippocampal extracellular 5‐HT concentrations. These neurochemical data are consistent with studies showing that an HFD significantly decreased brainstem 5‐HT levels (Kimbrough and Weekley, 1984) and blood/brain tryptophan levels in rats (Kloiber et al., 2010; Herrera‐Marquez et al., 2011). Exaggerated stimulation of MAO activity – the enzyme responsible for 5‐HT degradation – or the indoleamine 2,3‐dioxygenase – the enzyme that metabolizes tryptophan along the kynurenine pathway – are possible explanations for such neurochemical changes (André et al., 2014; Dinel et al., 2014; Gupta et al., 2014). Here, we investigated another possible cause of 5‐HT deficiency. Given that the firing activity of the 5‐HT system, along with the release of 5‐HT in the hippocampus, is limited by a negative feedback control mediated by somatodendritic 5‐HT1A autoreceptors in the raphe (Blier and De Montigny, 1990), we then tested the sensitivity of this element in HFD‐fed mice using the 8‐OH‐DPAT‐induced hypothermia paradigm. Our results indicated that HFD‐fed mice displayed a more robust hypothermic response to 8‐OH‐DPAT than controls, providing clear‐cut evidence that the impairment of peripheral metabolism was associated with a hypersensitisation or up‐regulation of the inhibitory 5‐HT1A autoreceptor. Interestingly, recent data in rats demonstrated that HFD‐induced anhedonia was associated with an impairment of 5‐HT‐mediated hippocampal GSK3β phosphorylation (Papazoglou et al., 2015). Nevertheless, even if prolonged HFD intake decreases hippocampal 5‐HT levels, such neurochemical changes do not necessarily explain all the behavioural anomalies reported herein. Indeed, there is compelling evidence that increased anxiety, as reported here in response to HFD, is related to an excess of 5‐HT neurotransmission (Hamon, 1994). It is therefore possible that opposite changes could be observed in other brain regions, such as the frontal cortex or the amygdala. In agreement with this hypothesis, it has been proposed that excess 5‐HT2C receptor‐mediated transmission in the frontal cortex favours anxiety (Martin et al., 2015).
In separate cohorts, we subjected mice to the paradigms that were previously altered by the HFD (i.e. the OF and the ST) and evaluated the effects of prolonged administration of the SSRI escitalopram. In control STD‐fed mice, we clearly showed that escitalopram produced anxiolytic‐like and antidepressant‐like effects, as previously reported in relevant models of depression (Sanchez et al., 2003). However, such responses were completely abolished in HFD‐fed mice, suggesting a non‐response to the antidepressant. To confirm this observation, we investigated mice behaviour in the NSF. Although chronic escitalopram failed to elicit anxiolytic‐like/antidepressant‐like activities in control mice fed an STD diet, as previously reported in basal conditions with various SSRIs (David et al., 2009; Rainer et al., 2012), it potentiated the ability of the HFD to promote deleterious effects in this paradigm. Collectively, these results are consistent with previous data demonstrating that the antidepressant‐like activities of the SSRI fluoxetine were reduced or even completely abolished in mice administered with streptozotocin (Kamei et al., 2003; Myata et al., 2004). With regard to the effects of HFD, only one study reported that unpredictable chronic mild stress‐induced behavioural changes were all reversed by fluoxetine in STD‐fed mice but not in HFD‐fed mice (Isingrini et al., 2010). SSRI non‐response in T2DM is also supported by a number of clinical studies showing associations between low rates of remission with SSRI treatment and T2DM (Anderson et al., 2010; Bryan et al., 2010; Gois et al., 2014). At the molecular levels, it is possible that the basal hypersensitivity of the 5‐HT1A autoreceptor played an important role in the escitalopram non‐response by hindering its ability to enhance 5‐HT levels in the hippocampus. Alternatively, we postulated that long‐term escitalopram treatment might have further destabilized glucose homeostasis, thereby leading to counter‐productive effects on anxiogenic‐like/depressive‐like behaviours. In line with this idea, antidepressants have been associated with an increased risk of diabetes in humans (Knol et al., 2007; Brown et al., 2008), potentially resulting from the deleterious effect of 5‐HT on beta pancreatic cells (Levkovitz et al., 2007; Isaac et al., 2013, De Long et al., 2015). However, our results pointed out that chronic escitalopram administration had no major effect on HFD‐induced metabolic impairments. As previously mentioned, our results did not allow us to definitively exclude the possibility that obesity played an important role in the phenotype of HFD‐fed mice, and this is further supported by the fact that depressed obese patients or rodents show little or no therapeutic benefit with different classes of antidepressant drugs (Kloiber et al., 2007), including tricyclics (Uher et al., 2009) and SSRIs (Guo and Lu, 2014) such as fluoxetine (Lin et al., 2014; Papakostas et al., 2005). However, although a higher body mass index and obesity can predict poor response to antidepressant drugs, a recent study challenged this hypothesis for escitalopram (Uher et al., 2009). Finally, the possibility that an HFD alters the pharmacokinetic properties of escitalopram can be also advanced. Indeed, the treatment non‐response reported here could result from changes not only in drug distribution but also in drug metabolism and excretion in overweight mice. For example, in obese patients, a higher percentage of adipose tissue may influence the distribution of drugs. Although there are only a few studies evaluating the influence of body weight on the serum levels of antidepressant drugs, a significant effect of body weight on the clearance of antidepressant drugs (including citalopram) and their volume of distribution has been reported (Bies et al., 2004). However, despite these data, Unterecker and colleagues recently reported that body weight did not affect the pharmacokinetics of different antidepressant drugs, including escitalopram (Unterecker et al., 2011).
Finally, given the lack of beneficial effects of the SSRI escitalopram in HFD‐fed mice, we evaluated the influence of HFD withdrawal, which effectively reversed metabolic impairments, on behavioural performances. Interestingly, of the three behavioural parameters measured in HFD‐reversal mice, a complete recovery of performance was detected in the ST and NSF, suggesting that such a procedure reversed anxiogenic‐like/depressive‐like symptoms, as recently observed in rats (Papazoglou et al., 2015). The latter results strengthen our hypothesis that T2DM and mood disorders are closely interrelated. However, it is noteworthy that the anxiety measured in the OF persisted. Along with our initial observation that this trait was the first symptom to emerge in response to an HFD, it would seem that anxiety is particularly sensitive to metabolic changes.
In conclusion, there is a controversy regarding whether T2DM and MD are causally linked. Our present study provides clear‐cut evidence, using an original approach based on the z‐score method, that both pathologies are well correlated, notably when T2DM is induced by a prolonged HFD. Beyond this association, T2DM exerts another negative influence on emotionality because it may attenuate escitalopram‐induced anxiolytic‐like/antidepressant‐like activities. Whether or not such effects can be extended to other SSRIs and to different classes of antidepressant drugs has yet to be determined. Considering the prevalence of T2DM and MD, and their consequences on morbidity, mortality and quality of life, the optimization of current antidepressant treatment is clearly needed.
Author contributions
J. Z. and G. Q. performed the behavioural experiments and analysed the related data. X. F. and L. P. provided their skills in the field of metabolism to characterize the metabolic status of our animal cohorts. B. P. G. and X. F. contributed to the design of the study. J. Z., G. Q., D. J., L. P., X. F. and B. P. G. actively participated in the discussions to prepare this manuscript and gave their final approval of the version to be submitted.
Conflict of interest
None.
Supporting information
Figure S1 Long‐term (12 and 16 weeks) HFD induces T2DM‐like metabolic disorders. Final body weight (A), fasting blood glucose (B) and insulin (C) levels, change in blood glucose level during an OGTT (D; inset: AUC of the glycaemia over the 120 minutes) and metabolic z‐score (E, parameters included: final body weight, fasting glycaemia and insulinaemia, AUC during the OGTT) in mice fed a STD (white bars; n=14 and 10) or HFD (sky/dark blue bars; n=12 and 10) diet for 12 (left panels) or 16 (right panels) weeks. *p<0.05 vs. STD, Two‐tailed student's t test.
Figure S2 Long‐term (12 and 16 weeks) HFD induces anxiogenic/depressive‐like phenotype. Energy (A) and physical driving force (B) in the Tail Suspension Test (TST) in mice fed a STD (white bars; n=14 and 10) or HFD (sky/dark blue bars; n=12 and 10) for 12 (left panels) or 16 (right panels) weeks. Two‐tailed student's t test.
Figure S3 Long‐term (12 weeks) intake of fructose enriched diet induces metabolic disorders without effects on emotionality. Final body weight (A), fasting blood glucose (B) and insulin (C) levels, change in blood glucose levels during an OGTT (D; inset: AUC of the glycaemia over the 120 minutes) and metabolic z‐score (E, parameters included: final body weight, fasting glycaemia and insulinaemia, AUC during the OGTT) in mice fed a STD (white bars; n=15) or fructose (black bars; n=15) diet. Center entries and time spent in the center in the Open Field (F), immobility time in the Tail Suspension Test (TST, G), grooming time in the Splash Test (H) and emotionality z‐score (I, parameters included: center entries and time in the OF, immobility time in the TST and grooming time in the ST) in mice fed a STD (white bars) or fructose diet (black bars) for 12 weeks. *p<0.05 vs. STD, Two‐tailed student's t test.
Figure S4 Withdrawal of high‐fat diet reverses metabolic disorders. Final body weight (A), fasting blood glucose (B) and insulin (C) levels, change in blood glucose level during an OGTT (D; inset: AUC of the glycaemia over the 120 minutes) and metabolic z‐score (E, parameters included: final body weight, fasting glycaemia and insulinaemia, AUC during the OGTT) in mice fed a STD (white bars; n=10) or HFD (black bars; n=10) for 16 weeks or a HFD for 12 weeks and STD for 4 weeks (gray bars; n=12). *p<0.05 vs. STD, #p< 0.05 vs. HFD; One‐way ANOVA followed by Tukey's post‐hoc test.
Figure S5 Cumulative survival curve of animals that have not eaten over 10 min during the novelty suppressed paradigm. Effects of escitalopram (A) or HFD‐withdrawal (B).
Table S1 Complete statistical summary analysis for behavioural and metabolic data.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
Authors are thankful to K. Ly, A. Lefranc, S. Lieby (Dijon) and P. Robert (Châtenay‐Malabry) for animal care and weight monitoring. This work was supported by the SFD (Société Française du Diabète, B. P. G.). J. Z. was supported by the program Ciência sem Fronteiras funded by the National Council for Scientific and Technological Development (CNPq).
Zemdegs, J. , Quesseveur, G. , Jarriault, D. , Pénicaud, L. , Fioramonti, X. , and Guiard, B. P. (2016) High‐fat diet‐induced metabolic disorders impairs 5‐HT function and anxiety‐like behavior in mice. British Journal of Pharmacology, 173: 2095–2110. doi: 10.1111/bph.13343.
References
- Abildgaard A, Solskov L, Volke V, Harvey BH, Lund S, Wegener G (2011). A high‐fat diet exacerbates depressive‐like behavior in the Flinders Sensitive Line (FSL) rat, a genetic model of depression. Psychoneuroendocrinology 36: 623–633. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013). The Concise Guide to PHARMACOLOGY 2013/14: G protein‐coupled receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K (2006). The prevalence of co‐morbid depression in adults with type 2 diabetes: a systematic review and meta‐analysis. Diabet Med 23: 1165–1173. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ (2001). The prevalence of comorbid depression in adults with diabetes: a meta‐analysis. Diabetes Care 24: 1069–1078. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Gott BM, Sayuk GS, Freedland KE, Lustman PJ (2010). Antidepressant pharmacotherapy in adults with type 2 diabetes: rates and predictors of initial response. Diabetes Care 33: 485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André C, Dinel AL, Ferreira G, Laye S, Castanon N (2014). Diet‐induced obesity progressively alters cognition, anxiety‐like behavior and lipopolysaccharide‐induced depressive‐like behavior: focus on brain indoleamine 2,3‐dioxygenase activation. Brain Behav Immun 41: 10–21. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H et al. (2014). High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis 67: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bies RR, Feng Y, Lotrich FE, Kirshner MA, Roose S, Kupfer DJ et al. (2004). Utility of sparse concentration sampling for citalopram in elderly clinical trial subjects. J Clin Pharmacol 44: 1352–1359. [DOI] [PubMed] [Google Scholar]
- Bill DJ, Knight M, Forster EA, Fletcher A (1991). Direct evidence for an important species difference in the mechanism of 8‐OH‐DPAT‐induced hypothermia. Br J Pharmacol 103: 1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C (1990). Differential effect of gepirone on presynaptic and postsynaptic serotonin receptors: single‐cell recording studies. J Clin Psychopharmacol 10 (3 Suppl): 13S–20S. [DOI] [PubMed] [Google Scholar]
- Brown LC, Majumdar SR, Johnson JA (2008). Type of antidepressant therapy and risk of type 2 diabetes in people with depression. Diabetes Res Clin Pract 79: 61–67. [DOI] [PubMed] [Google Scholar]
- Bryan C, Songer T, Brooks MM, Rush AJ, Thase ME, Gaynes B et al. (2010). The impact of diabetes on depression treatment outcomes. Gen Hosp Psychiatry 32: 33–41. [DOI] [PubMed] [Google Scholar]
- Capuron L, Poitou C, Machaux‐Tholliez D, Frochot V, Bouillot JL, Basdevant A et al. (2011). Relationship between adiposity, emotional status and eating behaviour in obese women: role of inflammation. Psychol Med 41: 1517–1528. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G, Fernando JC (1977). Drugs altering insulin secretion: effects on plasma and brain concentrations of aromatic amino acids and on brain 5‐hydroxytryptamine turnover. Br J Pharmacol 60: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I et al. (2009). Neurogenesis‐dependent and ‐independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Long NE, Stepita RA, Taylor VH, Holloway AC (2015). Major depressive disorder and diabetes: does serotonin bridge the gap? Curr Diabetes Rev 11: 71–78. [DOI] [PubMed] [Google Scholar]
- Del Rosario A, McDermott MM, Panee J (2012). Effects of a high‐fat diet and bamboo extract supplement on anxiety‐ and depression‐like neurobehaviours in mice. Br J Nutr 108: 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach KV, Bondareva VM, Chistyakova OV, Berstein LM, Shpakov AO (2015). The effect of long‐term intranasal serotonin treatment on metabolic parameters and hormonal signaling in rats with high‐fat diet/low‐dose streptozotocin‐induced type 2 diabetes. Int J Endocrinol 2015: 245459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinel AL, Andre C, Aubert A, Ferreira G, Laye S, Castanon N (2014). Lipopolysaccharide‐induced brain activation of the indoleamine 2,3‐dioxygenase and depressive‐like behavior are impaired in a mouse model of metabolic syndrome. Psychoneuroendocrinology 40: 48–59. [DOI] [PubMed] [Google Scholar]
- Egede LE, Grubaugh AL, Ellis C (2010). The effect of major depression on preventive care and quality of life among adults with diabetes. Gen Hosp Psychiatry 32: 563–569. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlawat P, Gupta R, Rajput R, Gahlan D, Gehlawat VK (2013). Diabetes with comorbid depression: role of SSRI in better glycemic control. Asian J Psychiatr 6: 364–368. [DOI] [PubMed] [Google Scholar]
- Ghaeli P, Shahsavand E, Mesbahi M, Kamkar MZ, Sadeghi M, Dashti‐Khavidaki S (2004). Comparing the effects of 8‐week treatment with fluoxetine and imipramine on fasting blood glucose of patients with major depressive disorder. J Clin Psychopharmacol 24: 386–388. [DOI] [PubMed] [Google Scholar]
- Gois C, Dias VV, Carmo I, Duarte R, Ferro A, Santos AL et al. (2014). Treatment response in type 2 diabetes patients with major depression. Clin Psychol Psychother 21: 39–48. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Mansari ME, Murphy DL, Blier P (2012). Altered response to the selective serotonin reuptake inhibitor escitalopram in mice heterozygous for the serotonin transporter: an electrophysiological and neurochemical study. Int J Neuropsychopharmacol 15: 349–361. [DOI] [PubMed] [Google Scholar]
- Guilloux JP, Seney M, Edgar N, Sibille E (2011). Integrated behavioral z‐scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods 197: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Lu XY (2014). Leptin receptor deficiency confers resistance to behavioral effects of fluoxetine and desipramine via separable substrates. Transl Psychiatry 4: e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D, Radhakrishnan M, Kurhe Y (2014). Insulin reverses anxiety‐like behavior evoked by streptozotocin‐induced diabetes in mice. Metab Brain Dis 29: 737–746. [DOI] [PubMed] [Google Scholar]
- Hamon M (1994). Neuropharmacology of anxiety: perspectives and prospects. Trends Pharmacol Sci 15: 36–39. [DOI] [PubMed] [Google Scholar]
- Hamon M, Blier P (2013). Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry 45: 54–63. [DOI] [PubMed] [Google Scholar]
- Herrera‐Marquez R, Hernandez‐Rodriguez J, Medina‐Serrano J, Boyzo‐Montes de Oca A, Manjarrez‐Gutierrez G (2011). Association of metabolic syndrome with reduced central serotonergic activity. Metab Brain Dis 26: 29–35. [DOI] [PubMed] [Google Scholar]
- Ho N, Balu DT, Hilario MR, Blendy JA, Lucki I (2012). Depressive phenotypes evoked by experimental diabetes are reversed by insulin. Physiol Behav 105: 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N, Sommers MS, Lucki I (2013). Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev 37: 1346–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac R, Boura‐Halfon S, Gurevitch D, Shainskaya A, Levkovitz Y, Zick Y (2013). Selective serotonin reuptake inhibitors (SSRIs) inhibit insulin secretion and action in pancreatic beta cells. J Biol Chem 288: 5682–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C (2010). Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One 5: e10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Loots du T (2009). Experimental rodent models of type 2 diabetes: a review. Methods Find Exp Clin Pharmacol 31: 249–261. [DOI] [PubMed] [Google Scholar]
- Kamei J, Miyata S, Morita K, Saitoh A, Takeda H (2003). Effects of selective serotonin reuptake inhibitors on immobility time in the tail suspension test in streptozotocin‐induced diabetic mice. Pharmacol Biochem Behav 75: 247–254. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). NC3Rs Reporting Guidelines Working Group. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Bae S, Lim KM (2013). Impact of high fat diet‐induced obesity on the plasma levels of monoamine neurotransmitters in C57BL/6 Mice. Biomol Ther (Seoul) 21: 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough TD, Weekley LB (1984). The effect of a high‐fat diet on brainstem and duodenal serotonin (5‐HT) metabolism in Sprague–Dawley and Osborne‐Mendel rats. Int J Obes 8: 305–310. [PubMed] [Google Scholar]
- Kino M, Yamato T, Aomine M (2004). Simultaneous measurement of nitric oxide, blood glucose, and monoamines in the hippocampus of diabetic rat: an in vivo microdialysis study. Neurochem Int 44: 65–73. [DOI] [PubMed] [Google Scholar]
- Kloiber S, Ising M, Reppermund S, Horstmann S, Dose T, Majer M et al. (2007). Overweight and obesity affect treatment response in major depression. Biol Psychiatry 62: 321–326. [DOI] [PubMed] [Google Scholar]
- Kloiber S, Kohli MA, Brueckl T, Ripke S, Ising M, Uhr M et al. (2010). Variations in tryptophan hydroxylase 2 linked to decreased serotonergic activity are associated with elevated risk for metabolic syndrome in depression. Mol Psychiatry 15: 736–747. [DOI] [PubMed] [Google Scholar]
- Knol MJ, Geerlings MI, Egberts AC, Gorter KJ, Grobbee DE, Heerdink ER (2007). No increased incidence of diabetes in antidepressant users. Int Clin Psychopharmacol 22: 382–386. [DOI] [PubMed] [Google Scholar]
- Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F (2006). Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta‐analysis. Diabetologia 49: 837–845. [DOI] [PubMed] [Google Scholar]
- Leonard BE (2013). Inflammation as the cause of the metabolic syndrome in depression. Mod Trends Pharmacopsychiatri 28: 117–126. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Ben‐Shushan G, Hershkovitz A, Isaac R, Gil‐Ad I, Shvartsman D et al. (2007). Antidepressants induce cellular insulin resistance by activation of IRS‐1 kinases. Mol Cell Neurosci 36: 305–312. [DOI] [PubMed] [Google Scholar]
- Licinio J, Wong ML (2003). The interface of obesity and depression: risk factors for the metabolic syndrome. Rev Bras Psiquiatr 25: 196–197. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chen CC, Wong J, McIntyre RS (2014). Both body weight and BMI predicts improvement in symptom and functioning for patients with major depressive disorder. J Affect Disord 161: 123–126. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P et al. (2006). High‐fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol 13: 1385–1388. [DOI] [PubMed] [Google Scholar]
- Liu J, Guo M, Zhang D, Cheng SY, Liu M, Ding J et al. (2012). Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant‐like activity. Proc Natl Acad Sci U S A 109: 12248–12253. doi: 10.1073/pnas.1202835109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhai X, Li H, Ji L (2014). Depression‐like behaviors in mice subjected to co‐treatment of high‐fat diet and corticosterone are ameliorated by AICAR and exercise. J Affect Disord 156: 171–177. [DOI] [PubMed] [Google Scholar]
- Mahar I, Bambico FR, Mechawar N, Nobrega JN (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev 38: 173–192. [DOI] [PubMed] [Google Scholar]
- Martin CB, Martin VS, Trigo JM, Chevarin C, Maldonado R, Fink LH et al. (2015). 5‐HT2C receptor desensitization moderates anxiety in 5‐HTT deficient mice: from behavioral to cellular evidence. Int J Neuropsychopharmacol 18. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C (2010). Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva‐Paylor LA, Paylor R (2001). The use of behavioral test batteries: effects of training history. Physiol Behav 73: 705–717. [DOI] [PubMed] [Google Scholar]
- Miyata S, Hirano S, Kamei J (2004). Diabetes attenuates the antidepressant‐like effect mediated by the activation of 5‐HT1A receptor in the mouse tail suspension test. Neuropsychopharmacology 29: 461–469. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Iosifescu DV, Iosifescu DV, Burns AM, Nierenberg AA et al (2005). Obesity among outpatients with major depressive disorder. Int J Neuropsychopharmacol 8: 59–63. [DOI] [PubMed] [Google Scholar]
- Papazoglou IK1, Jean A, Gertler A, Taouis M, Vacher CM (2015). Hippocampal GSK3β as a Molecular Link Between Obesity and Depression. Mol Neurobiol 52: 363–374. doi: 10.1007/s12035-014-8863-x [DOI] [PubMed] [Google Scholar]
- Park HR, Park M, Choi J, Park KY, Chung HY, Lee J (2010). A high‐fat diet impairs neurogenesis: involvement of lipid peroxidation and brain‐derived neurotrophic factor. Neurosci Lett 482: 235–239. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, Davenport AP, McGrath JC, Peters JA, Southan C, Spedding M, Yu W, Harmar AJ; NC‐IUPHAR. (2014) The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos and Franklin's (1997). The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego. [Google Scholar]
- Paylor R, Spencer CM, Yuva‐Paylor LA, Pieke‐Dahl S (2006). The use of behavioral test batteries, II: effect of test interval. Physiol Behav 87: 95–102. [DOI] [PubMed] [Google Scholar]
- Petit AC, Quesseveur G, Gressier F, Colle R, David DJ, Gardier AM et al. (2014). Converging translational evidence for the involvement of the serotonin 2A receptor gene in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 54: 76–82. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE (2011). A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci 31: 9683–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeder MB, Bjelland I, Emil Vollset S, Steen VM (2006). Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. J Clin Psychiatry 67: 1974–1982. [DOI] [PubMed] [Google Scholar]
- Rainer Q, Nguyen HT, Quesseveur G, Gardier AM, David DJ, Guiard BP (2012). Functional status of somatodendritic serotonin 1A autoreceptor after long‐term treatment with fluoxetine in a mouse model of anxiety/depression based on repeated corticosterone administration. Mol Pharmacol 81: 106–112. [DOI] [PubMed] [Google Scholar]
- Samuel VT (2011). Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab 22: 60–65. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Gruca P, Papp M (2003). R‐citalopram counteracts the antidepressant‐like effect of escitalopram in a rat chronic mild stress model. Behav Pharmacol 14: 465–470. [DOI] [PubMed] [Google Scholar]
- Sharma S, Fulton S (2013). Diet‐induced obesity promotes depressive‐like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond) 37: 382–389. [DOI] [PubMed] [Google Scholar]
- Sivanathan S1, Thavartnam K1, Arif S1, Elegino T1, McGowan PO2 (2015). Chronic high fat feeding increases anxiety‐like behaviour and reduces transcript abundance of glucocorticoid signalling genes in the hippocampus of female rats. Behav Brain Res 286: 265–270. doi: 10.1016/j.bbr.2015.02.036 [DOI] [PubMed] [Google Scholar]
- Tobin V, Le Gall M, Fioramonti X, Stolarczyk E, Blazquez AG, Klein C et al. (2008). Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin‐resistant mice. Diabetes 57: 555–562. [DOI] [PubMed] [Google Scholar]
- Uher R, Mors O, Hauser J, Rietschel M, Maier W, Kozel D et al. (2009). Body weight as a predictor of antidepressant efficacy in the GENDEP project. J Affect Disord 118: 147–154. [DOI] [PubMed] [Google Scholar]
- Unterecker S, Deckert J, Pfuhlmann B (2011). No influence of body weight on serum levels of antidepressants. Ther Drug Monit 33: 730–734. [DOI] [PubMed] [Google Scholar]
- Voikar V, Vasar E, Rauvala H (2004). Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav 3: 27–38. [DOI] [PubMed] [Google Scholar]
- Williams MM, Clouse RE, Nix BD, Rubin EH, Sayuk GS, McGill JB et al. (2007). Efficacy of sertraline in prevention of depression recurrence in older versus younger adults with diabetes. Diabetes Care 30: 801–806. [DOI] [PubMed] [Google Scholar]
- Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K et al. (2011). Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology 152: 2634–2643. [DOI] [PubMed] [Google Scholar]
- Yamato T, Misumi Y, Yamasaki S, Kino M, Aomine M (2004). Diabetes mellitus decreases hippocampal release of neurotransmitters: an in vivo microdialysis study of awake, freely moving rats. Diabetes Nutr Metab 17: 128–136. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Long‐term (12 and 16 weeks) HFD induces T2DM‐like metabolic disorders. Final body weight (A), fasting blood glucose (B) and insulin (C) levels, change in blood glucose level during an OGTT (D; inset: AUC of the glycaemia over the 120 minutes) and metabolic z‐score (E, parameters included: final body weight, fasting glycaemia and insulinaemia, AUC during the OGTT) in mice fed a STD (white bars; n=14 and 10) or HFD (sky/dark blue bars; n=12 and 10) diet for 12 (left panels) or 16 (right panels) weeks. *p<0.05 vs. STD, Two‐tailed student's t test.
Figure S2 Long‐term (12 and 16 weeks) HFD induces anxiogenic/depressive‐like phenotype. Energy (A) and physical driving force (B) in the Tail Suspension Test (TST) in mice fed a STD (white bars; n=14 and 10) or HFD (sky/dark blue bars; n=12 and 10) for 12 (left panels) or 16 (right panels) weeks. Two‐tailed student's t test.
Figure S3 Long‐term (12 weeks) intake of fructose enriched diet induces metabolic disorders without effects on emotionality. Final body weight (A), fasting blood glucose (B) and insulin (C) levels, change in blood glucose levels during an OGTT (D; inset: AUC of the glycaemia over the 120 minutes) and metabolic z‐score (E, parameters included: final body weight, fasting glycaemia and insulinaemia, AUC during the OGTT) in mice fed a STD (white bars; n=15) or fructose (black bars; n=15) diet. Center entries and time spent in the center in the Open Field (F), immobility time in the Tail Suspension Test (TST, G), grooming time in the Splash Test (H) and emotionality z‐score (I, parameters included: center entries and time in the OF, immobility time in the TST and grooming time in the ST) in mice fed a STD (white bars) or fructose diet (black bars) for 12 weeks. *p<0.05 vs. STD, Two‐tailed student's t test.
Figure S4 Withdrawal of high‐fat diet reverses metabolic disorders. Final body weight (A), fasting blood glucose (B) and insulin (C) levels, change in blood glucose level during an OGTT (D; inset: AUC of the glycaemia over the 120 minutes) and metabolic z‐score (E, parameters included: final body weight, fasting glycaemia and insulinaemia, AUC during the OGTT) in mice fed a STD (white bars; n=10) or HFD (black bars; n=10) for 16 weeks or a HFD for 12 weeks and STD for 4 weeks (gray bars; n=12). *p<0.05 vs. STD, #p< 0.05 vs. HFD; One‐way ANOVA followed by Tukey's post‐hoc test.
Figure S5 Cumulative survival curve of animals that have not eaten over 10 min during the novelty suppressed paradigm. Effects of escitalopram (A) or HFD‐withdrawal (B).
Table S1 Complete statistical summary analysis for behavioural and metabolic data.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


