Abstract
Endocannabinoids and their receptors play a modulatory role in the control of dopamine transmission in the basal ganglia. However, this influence is generally indirect and exerted through the modulation of GABA and glutamate inputs received by nigrostriatal dopaminergic neurons, which lack cannabinoid CB1 receptors although they may produce endocannabinoids. Additional evidence suggests that CB2 receptors may be located in nigrostriatal dopaminergic neurons, and that certain eicosanoid‐related cannabinoids may directly activate TRPV1 receptors, which have been found in nigrostriatal dopaminergic neurons, thus allowing in both cases a direct regulation of dopamine transmission by specific cannabinoids. In addition, CB1 receptors form heteromers with dopaminergic receptors which provide another pathway to direct interactions between both systems, in this case at the postsynaptic level. Through these direct mechanisms or through indirect mechanisms involving GABA or glutamate neurons, cannabinoids may interact with dopaminergic transmission in the basal ganglia and this is likely to have important effects on dopamine‐related functions in these structures (i.e. control of movement) and, particularly, on different pathologies affecting these processes, in particular, Parkinson's disease, but also dyskinesia, dystonia and other pathological conditions. The present review will address the current literature supporting these cannabinoid–dopamine interactions at the basal ganglia, with emphasis on aspects dealing with the physiopathological consequences of these interactions.
Linked Articles
This article is part of a themed section on Updating Neuropathology and Neuropharmacology of Monoaminergic Systems. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v173.13/issuetoc
Abbreviations
- Δ9‐THC
Δ9‐tetrahydrocannabinol
- FAAH
fatty acid amide hydrolase
Table of Links
| TARGETS |
|---|
| GPCRs a |
| Adenosine A2A receptors |
| Cannabinoid CB1 receptors |
| Cannabinoid CB2 receptors |
| Dopamine D1 receptors |
| Dopamine D2 receptors |
| Enzymes b |
| FAAH, fatty acid amide hydrolase |
| Monoacylglycerol lipase |
| Ion Channels c |
| TRPV1 channels |
| Transporters d |
| Dopamine transporter, DAT |
| Nuclear hormone receptors e |
| PPAR‐α |
| PPAR‐γ |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,d,eAlexander et al., 2013a, 2013b, 2013c, 2013d, 2013e).
Overview of the endocannabinoid signalling system and its interaction with neurotransmitter systems
It is well established that the endocannabinoid system, formed by different signalling lipids, the enzymes involved in their synthesis and degradation, and their target receptors, plays a modulatory function in important processes of the CNS. This includes the control of movement (see Fernández‐Ruiz, 2009), learning and memory (see Zanettini et al., 2011), emotional behaviour (see McLaughlin and Gobbi, 2012), nociception (see Guindon and Hohmann, 2009), brain reward (see Solinas et al., 2008), feeding behaviour (see Kirkham, 2009) and emesis (see Parker et al., 2011), among others. This modulatory function is exerted through the ability of endocannabinoids and their receptors to participate in the retrograde signalling in different synapses located in those brain structures that regulate these processes (Castillo et al., 2012). This is facilitated by the presynaptic location of cannabinoid CB1 receptors, the key neuronal cannabinoid receptor type, that allow endocannabinoids to directly modulate the function of most of neurotransmitters including glutamate, GABA, opioid peptides, acetylcholine and 5‐HT (see Heifets and Castillo, 2009; Kano et al., 2009). This function is particularly important in the case of glutamatergic and GABAergic synapses, in which, through well‐defined processes of short‐ and long‐lasting synaptic depression, it prevents an excess of excitation or inhibition, respectively, (Lovinger, 2008) that may lead to pathological conditions if prolonged and/or enhanced.
Dopamine has been also linked to the action of cannabinoids (see Fernández‐Ruiz et al., 2010; El Khoury et al., 2012). However, the different subpopulations of dopaminergic neurons within the CNS and, in particular, those neurons whose cell bodies are located in the substantia nigra and that project to the caudate‐putamen, the so‐called nigrostriatal dopaminergic neurons, do not appear to contain cannabinoid CB1 receptors (see Fernández‐Ruiz, 2009; Fernández‐Ruiz et al., 2010), the cannabinoid receptor type mostly involved in the control of synaptic activity. CB1 receptors are also absent from other dopaminergic neuronal subpopulations (e.g. mesocorticolimbic neurons), although this does not exclude possible interactions between cannabinoids and dopamine in the control of those behaviours (e.g. brain reward, motivation, emotion) regulated by these neurons in physiological and physiopathological conditions (e.g. addiction). However, this has been the subject of a recent review (Fernández‐Ruiz et al., 2010) and will not be addressed in the present one, which will concentrate exclusively in these interactions at the level of the basal ganglia.
Nigrostriatal dopaminergic neurons exert a regulatory action on different effector neurons within the basal ganglia thus influencing the control of movement. Although these neurons do not contain CB1 receptors, they are significantly affected by either the activation or the blockade of the endocannabinoid system, leading to important changes in the motor activity (Fernández‐Ruiz, 2009; Fernández‐Ruiz et al., 2010). It is generally accepted that these effects are exerted through CB1 receptors located in other neuronal subpopulations (i.e. GABAergic, glutamatergic and opioidergic neurons). These neurons are located in the close vicinity of, and connected with, dopaminergic neurons (see van der Stelt and Di Marzo, 2003). It is also important to note that these midbrain dopaminergic neurons, although lacking CB1 receptors, may produce and release endocannabinoid ligands from their somas and dendrites (as shown for the midbrain dopaminergic neurons located in the ventral‐tegmental area; Melis et al., 2004; Riegel and Lupica, 2004), thus facilitating the retrograde signalling function of these transmitters and CB1 receptors in excitatory and inhibitory synapses (reviewed in Seutin, 2005). Lastly, even though most of the cannabinoid effects on dopaminergic transmission are indirect and exerted through GABA‐ and/or glutamate‐containing neurons, there are some recent studies that propose additional or alternative mechanisms that involve a closer relationship between the endocannabinoid and the dopaminergic systems (see below).
Cannabinoid–dopamine interactions at the basal ganglia
As mentioned above, there is solid anatomical, biochemical, physiological and pharmacological evidence that supports the idea that dopamine is the key regulatory transmitter in the control of movement exerted at the basal ganglia level (see Smith and Villalba, 2008). The activation of dopaminergic transmission in this circuitry produces hyperkinesia, whereas its inhibition results in a reduction of movement. By contrast, activation of the endocannabinoid system has been associated with motor inhibition and even catalepsy (see Fernández‐Ruiz, 2009), so that it has been widely speculated that the hypokinetic effect of cannabinoid agonists might be produced through a reduction in dopaminergic activity, given their ability to modify the action of several substances acting on the dopamine system. For example, cannabinoid agonists potentiated reserpine‐induced hypokinesia (Moss et al., 1981) and dopamine receptor antagonist‐induced catalepsy (Anderson et al., 1996), whereas they reduced quinpirole‐induced hyperlocomotion (Marcellino et al., 2008) and amphetamine‐induced hyperactivity (Gorriti et al., 1999) in laboratory rodents (for a complete summary of the behavioural data associated with the activation of CB1 receptor‐mediated signals within the basal ganglia, see Fernández‐Ruiz and Gonzáles, 2005; Fernández‐Ruiz, 2009). Based on these data, several authors have proposed the idea of an inverse correlation between the two transmitter systems, with a reduced endocannabinoid tone accompanied by increased dopaminergic activity occurring in hyperkinetic conditions, and the opposite associated with a reduction in movement (see Fernández‐Ruiz, 2009). However, there are recent reports of a long‐lasting activation of striatal dopaminergic function, reflected in an enhanced tyrosine hydroxylase expression, by CB1 receptor agonists (Bosier et al., 2012). The inverse correlation between both systems has been proposed for physiological conditions and also for pathological events, for example, Parkinson's disease, the most prevalent disorder affecting the basal ganglia (Obeso et al., 2008). The endocannabinoid system becomes hyperactivated in Parkinson's disease in parallel to the dopamine deficiency produced by the progressive degeneration of nigrostriatal dopaminergic neurons, resulting in the occurrence of motor symptoms such as bradykinesia, rigidity and tremor (see Fernández‐Ruiz, 2009). As will be discussed in the last section, this inverse correlation may serve for the development of cannabinoid‐based therapies for this disease.
As mentioned above, the most intriguing aspect of this pharmacological interaction between both systems is that it occurs in the absence of CB1 receptors on the dopaminergic neurons (Herkenham et al., 1991a), which would imply that the mechanism enabling this interaction would be largely, if not exclusively, indirect and based on the necessary mediation of GABA‐ and/or glutamate‐containing neurons that do contain these receptors (see Fernández‐Ruiz, 2009; Fernández‐Ruiz et al., 2010). However, three recent experimental observations have challenged this classic idea. First, certain eicosanoid‐derived cannabinoids, including anandamide, N‐arachidonoyl‐dopamine (NADA) and AM404, have been found to bind and activate TRPV1 receptors (see Starowicz et al., 2007). Also, these receptors have been located in dopaminergic neurons within the basal ganglia (Mezey et al., 2000), allowing a direct action of these endocannabinoid/endovanilloid compounds on dopaminergic transmission. Second, there is also recent evidence that indicates that CB1 receptors are able to form heteromers with other metabotropic receptors, including the dopamine D1 and D2 receptor types located, among others, in striatal projection neurons, enabling both systems to directly interact at postsynaptic level (see Ferré et al., 2009). These studies have provided interesting novel insights in terms of the function and therapeutic potential of the endocannabinoid signalling in the basal ganglia, as well as its interaction with dopaminergic transmission, from both basic and clinical perspectives. Lastly, CB2 receptors have recently been identified in nigrostriatal dopaminergic neurons in the human brain (García et al., 2015), enabling endocannabinoids to act through the other major cannabinoid receptor type to directly modulate dopaminergic transmission, although the distribution of this receptor type in the brain is much more restricted than that of the CB1 receptor and is frequently associated with pathological conditions (Fernández‐Ruiz et al., 2007). These mechanisms will be addressed in more detail below, after examining the classical indirect mechanism first proposed to explain the endocannabinoid–dopamine interactions (see Figure 1 for a representative diagram of these interactions).
Figure 1.
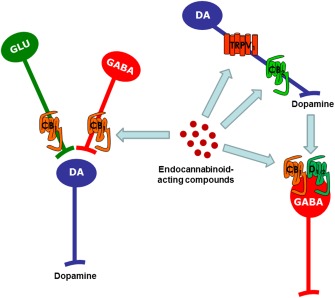
Summary of the different neuronal mechanisms proposed to explain the interactions between the endocannabinoid signalling system and dopaminergic transmission at the level of the basal ganglia.
Effects of cannabinoids on dopaminergic transmission exerted through CB1 receptors located in GABAergic and glutamatergic neurons
As mentioned above, the abundant presence of endocannabinoid elements, that is, CB1 receptors and their endogenous ligands, in the basal ganglia (Herkenham et al., 1991b; Mailleux and Vanderhaeghen, 1992; Tsou et al., 1998; Bisogno et al., 1999; Breivogel and Sim‐Selley, 2009), supports the idea that the endocannabinoid system plays an important modulatory role in the function of these brain structures (see Fernández‐Ruiz, 2009). It is generally accepted that those substances that enhance the endocannabinoid activity, preferentially the direct agonists of the CB1 receptor, generate a dose‐dependent motor inhibition in laboratory animals that may even produce catalepsia with the highest doses (see Fernández‐Ruiz, 2009). This has been also observed in human smokers of cannabis and is associated with a detrimental effect on striatal dopaminergic functioning (Kowal et al., 2011). Similar results were obtained by administering the so‐called indirect cannabinoid agonists that are inhibitors of the endocannabinoid inactivation processes, for example, the enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase and the endocannabinoid transporter (Fernández‐Ruiz, 2009). These hypokinetic effects were generally reversed by the administration of rimonabant or other CB1 receptor antagonists, supporting the idea that this receptor type is the key cannabinoid receptor involved in motor effects of cannabinoid compounds. In addition, rimonabant and other antagonists of CB1 receptors produce by themselves a certain degree of hyperlocomotion, because many of them are inverse agonists (see Fernández‐Ruiz, 2009), whereas mice lacking CB1 receptors exhibited several motor anomalies (see Valverde et al., 2005), supporting the key role played by these receptors (for a complete summary of the behavioural data associated with the activation/inhibition of CB1 receptor‐mediated signals within the basal ganglia, see Fernández‐Ruiz and Gonzáles, 2005; Fernández‐Ruiz, 2009).
A priori, the motor effects of cannabinoid agonists were explained as the normal consequence of their activity on those neuronal subpopulations that contain CB1 receptors within the basal ganglia circuitry. Striatal projection GABAergic neurons and subthalamonigral glutamatergic neurons were the first CB1 receptor‐containing neurons identified in relation with the motor effects of cannabinoids (Herkenham et al., 1991a; Mailleux and Vanderhaeghen, 1992; Tsou et al., 1998; Fusco et al., 2004). Further studies, conducted mostly with immunohistochemical procedures, demonstrated that CB1 receptors were also located in corticostriatal glutamatergic afferences (Köfalvi et al., 2005; Uchigashima et al., 2007) and in some subpopulations of striatal GABA interneurons (Fusco et al., 2004; Uchigashima et al., 2007). In all cases, the neurons containing CB1 receptors are GABAergic or glutamatergic neurons, thus supporting the idea that the first event associated with the activation of these receptors is an alteration in the activity of GABA and glutamate synapses but not the dopaminergic synapses. The changes in this neurotransmitter would occur secondarily to a primary effect on GABA or glutamate transmission, and they would be due to the connection of dopaminergic transmission with these neurons. However, as mentioned above, it is also possible that dopaminergic neurons located in the substantia nigra may be responsible for producing endocannabinoids for the activation of CB1 receptors located in GABAergic or glutamatergic neurons, as found for dopaminergic neurons located in the ventral tegmental area (Melis et al., 2004; Riegel and Lupica, 2004). In addition, endocannabinoids may be also produced by striatal‐projecting neurons in order to target CB1 receptors located in corticostriatal glutamatergic neurons and inhibit glutamate release, a response that appears to be regulated by the interaction of D2 and adenosine A2A receptors located in striatal cholinergic interneurons (Tozzi et al., 2011). All these findings are supported by the different anatomical studies mentioned above, but also by numerous pharmacological, electrophysiological and neurochemical studies that addressed the interaction of cannabinoid agonists with substances acting on the dopamine system, in relation to the motor effects in laboratory animals, studies that have been mentioned in the above section.
Effects of eicosanoid‐related cannabinoids exerted through TRPV1 receptors located in dopaminergic neurons
As mentioned above, further investigations have, however, provided new elements to re‐evaluate the idea that the effects of endocannabinoids on dopaminergic transmission in the basal ganglia are necessarily indirect and mediated by CB1 receptors located in GABA‐ or glutamate‐containing neurons. For example, it is now well known that anandamide and some of its analogues, for example, AM404, but not classic cannabinoids such as. Δ9‐tetrahydrocannabinol (Δ9‐THC), may behave as full agonists for the TRPV1 receptors (see Starowicz et al., 2007). These receptors have been identified in the basal ganglia located, among other markers, in nigrostriatal dopaminergic neurons (Mezey et al., 2000). The activation of these receptors with capsaicin or with other potential vanilloid ligands produced hypokinesia in rats (Di Marzo et al., 2001). Anandamide produced the same behavioural effect accompanied by a reduction in the activity of dopaminergic terminals in the striatum (de Lago et al., 2004), and this effect was reversed by capsazapine, thus supporting that it is exerted by the activation of TRPV1 receptors (de Lago et al., 2004). Further in vitro studies using perfused striatal fragments confirmed the activity of anandamide and the lack of effect of classic cannabinoids, such as Δ9‐THC, that do not bind to vanilloid‐like receptors, indicating that the TRPV1, rather than the CB1 receptor, is the key target involved in these effects (de Lago et al., 2004). Other authors reported that the activation of TRPV1 receptors in the substantia nigra pars compacta, rather than producing an inhibition, stimulated dopamine release, although these effects seem to be mediated by TRPV1 receptors located in glutamatergic neurons, rather than by those located in dopaminergic terminals (Marinelli et al., 2003; 2007).
Another interesting compound active at the TRPV1 receptor is NADA, an arachidonic acid derivative with properties of endocannabinoid and endovanilloid ligands (Starowicz et al., 2007). NADA seems to be synthesized through the conjugation of an arachidonic acid molecule directly with dopamine (Hu et al., 2009), excluding earlier suggestions that it would be synthesized through the hydroxylation of N‐arachidonoyl‐tyrosine followed by decarboxylation, by the same enzymes as those involved in dopamine synthesis. Its physiological significance is yet poorly understood, but some evidence suggests that it can serve as an antioxidant and neuroprotective compound (Bobrov et al., 2008). In addition, making the issue even more complex, a further study by Ferreira et al. (2009) revealed that N‐acyldopamines, such as NADA, were able to control the activity of dopaminergic terminals in the striatum via ion channels other than TRPV1 receptors, an effect that was not observed with anandamide or capsaicin. Importantly, NADA was likely to be synthesized in the substantia nigra in conditions of hyperactivity (Marinelli et al., 2007).
Another recent observation that makes the issue even more complex suggests that anandamide may inhibit the dopamine transporter function by a receptor‐independent mechanism, an effect found in heterologous cells and synaptosomal preparations and mimicked by the anandamide analogue methanandamide, not by arachidonic acid (Oz et al., 2010). In addition, inhibition of FAAH or COX‐2 failed to alter the effect of anandamide, thus indicating that this effect is not related to the metabolism of this endocannabinoid (Oz et al., 2010). Authors also found that the effect was not attenuated by Pertussis toxin, excluding the involvement of CB1, CB2 or GPR55 receptors, but not excluding that of TRPV1 receptors. Other authors also reported an inhibition of the dopamine transporter by different cannabinoid ligands in the rodent striatum (Price et al., 2007; Pandolfo et al., 2011). The inhibition was seen with the non‐selective cannabinoid agonists WIN55,212‐2 and O‐2545, and also with cannabidiol and NADA, but not with anandamide and 2‐arachidonoyl glycerol (Pandolfo et al., 2011). The effect was also seen with various CB1 receptor antagonists/inverse agonists such as AM251 (Pandolfo et al., 2011). As expected, authors concluded that these effects were likely to be CB1 receptor‐independent (Pandolfo et al., 2011).
Interaction of CB1 and dopamine receptors at the postsynaptic level
As mentioned above, CB1 receptors do not appear to be located in dopaminergic neurons, with the only exception of a study that described direct interactions of the CB1 receptor with the D2 presynaptic receptor, which would be only possible if both receptors are located in the same neurons (O'Neill et al., 2009). However, most of the authors believe that CB1 receptors are not located on dopaminergic neurons, but in striatal GABAergic projection neurons (striatonigral and striatopallidal pathways, respectively), in which they co‐localize with D1 or D2 receptors (Hermann et al., 2002; Martín et al., 2008). This may facilitate postsynaptic interactions between endocannabinoids and dopamine at the level of G‐protein/adenylyl cyclase signal transduction (Giuffrida et al., 1999; Meschler and Howlett, 2001; Nguyen et al., 2012). In addition, there is strong evidence supporting the formation of heteromers between CB1 and D2 receptors, and also adenosine A2A receptors (see Ferré et al., 2009; Brugarolas et al., 2014). These CB1, D2 and A2A receptor heteromers were found in the dendritic spines of GABAergic neurons projecting to the globus pallidus, but their functional properties and their role in striatal function still need further investigation (see Ferré et al., 2009). This type of postsynaptic mechanism facilitates the direct interaction between cannabinoids and dopamine allowing, in this case, a bidirectional regulation, endocannabinoids to dopamine and vice versa. Thus, on one side, the motor effects of CB1 receptor agonists have been associated with an activation of signalling via the neuronal phosphoprotein DARPP‐32, which has been linked to intracellular responses elicited by D1 and D2 receptors in the striatal projection neurons, whereas the genetic inactivation of DARPP‐32 resulted in an attenuation in the motor effects of cannabinoids (Andersson et al., 2005). On the other side, D2 receptors controlled anandamide production in the striatum. This may serve as an inhibitory feedback mechanism counteracting dopamine‐induced facilitation of psychomotor activity (Giuffrida et al., 1999), as well as controlling Gi/o protein availability for CB1 receptors (González et al., 2009) and facilitating endocannabinoid‐mediated long‐term synaptic depression of GABAergic neurons (Kreitzer and Malenka, 2007), an effect also seen in the ventral tegmental area (Pan et al., 2008). A similar interaction of endocannabinoids with D1 receptors has been recently proposed (Martín et al., 2008) and this proposal has been extended to glutamatergic synapses in which dopamine and its receptors also promote endocannabinoid‐mediated synaptic depression (see Lovinger and Mathur, 2012). In fact, the changes in corticostriatal glutamatergic synapses derived from the deficiency in dopamine occurring in Parkinson's disease have been proposed as a key factor in the pathogenesis of this disease (Lovinger and Mathur, 2012). Similarly, the formation of receptor heteromers (e.g. CB1, D1/D2, A2A) in striatal neurons may be of interest from a pharmacological point of view for the treatment of Parkinson's disease symptoms, in particular, levodopa‐induced dyskinesias. However, a recent study has demonstrated that levodopa disrupts the crosstalk between A2A‐CB1‐D2 receptors in experimental models of Parkinson's disease in rodents (Pinna et al., 2014) and primates (Bonaventura et al., 2014).
Location of CB2 receptors in nigrostriatal dopaminergic neurons
Recent evidence indicates that the TRPV1 receptor is not the only neuronal receptor other than the CB1 receptor that may be involved in the action of cannabinoids in the basal ganglia. Some recent studies showed that CB2 receptors, a receptor type preferentially associated with glial elements within the CNS, particularly when these become overactive in conditions of brain damage (see Fernández‐Ruiz et al., 2007), may be also present in neurons of the basal ganglia in primates, in particular, in the pallidothalamic‐projecting neurons (Lanciego et al., 2011). In addition, we have just found, using post mortem human tissues, that CB2 receptors were also located in nigrostriatal dopaminergic neurons (García et al., 2015), which supports the idea that those cannabinoids that target the CB2 receptor may influence the activity of these dopaminergic neurons through effects on their neuronal firing and/or the control of synaptic activity. Although this has not been investigated yet in dopaminergic neurons located in the substantia nigra, such effects have been recently described for dopaminergic neurons located in the neighbouring ventral tegmental area (Zhang et al., 2014). These authors identified CB2 receptors in these dopaminergic neurons in mice and demonstrated that their activation functionally modulated dopaminergic neuronal excitability and related behavioural consequences, for example, drug self‐administration (Zhang et al., 2014), so it is probable that this also occurs with the CB2 receptors located in nigral neurons. At present, the most important observation related to the presence of CB2 receptors in nigrostriatal dopaminergic neurons is their marked reduction in the substantia nigra of Parkinson's disease patients (García et al., 2015), which supports the possibility that this receptor may be used as a biomarker of nigral degeneration in this disease.
Relevance of cannabinoid–dopamine interactions in the basal ganglia in pathological conditions
The ability of the endocannabinoid signalling system to modulate dopaminergic transmission at the basal ganglia, by acting indirectly at CB1 receptors located in neurons for other neurotransmitters, or directly at TRPV1 or CB2 receptors located in dopaminergic neurons or through postsynaptic interactions between CB1 and D1/D2 receptors, enables this system to be pharmacologically manipulated in order to normalize dopaminergic transmission and, subsequently, to alleviate dopamine‐related motor symptoms, in conditions of dopamine deficiency, overactivity or dysregulation as those that occur in various basal ganglia disorders (see van der Stelt and Di Marzo, 2003; Fernández‐Ruiz, 2009; García‐Arencibia et al., 2009; Pisani et al., 2011). To date, most studies have concentrated on Parkinson's disease, the major basal ganglia disorder characterized by the progressive death of nigral dopaminergic neurons and dopaminergic denervation of the striatum, and have addressed the issue mainly at the preclinical level, using different models of experimental Parkinsonism (see Fernández‐Ruiz, 2009; García‐Arencibia et al., 2009; Pisani et al., 2011). The issue has been also studied at the clinical level in patients affected by Parkinson's disease or by other pathological conditions related to the basal ganglia function, such as Gilles de la Tourette's syndrome, dystonia and dyskinesia. However, the few clinical trials conducted so far have not revealed many positive results (Frankel et al., 1990; Sieradzan et al., 2001; Fox et al., 2002; Müller‐Vahl et al., 2002; 2003; Jabusch et al., 2004; Mesnage et al., 2004; Fabbrini et al., 2007).
The preclinical studies using models of experimental Parkinsonism have investigated both agonists and antagonists for the CB1 receptor, used alone or as coadjuvants, and have concentrated first in the alleviation of specific motor symptoms (see Brotchie, 2003; Fernández‐Ruiz, 2009; García‐Arencibia et al., 2009; Pisani et al., 2011). There is also evidence that cannabinoids may serve to delay and arrest the progression of this disease (see Brotchie, 2003; Fernández‐Ruiz, 2009; García‐Arencibia et al., 2009; Pisani et al., 2011), although this potential will not be addressed here.
As regards the Parkinsonian symptoms that may be potentially alleviated by manipulating the endocannabinoid system, one relevant example is the tremor that is associated with the frequent overactivity of the subthalamic nucleus occurring in Parkinson's disease. CB1 receptor agonists have been investigated for the reduction of tremor, with positive results in experimental Parkinsonism (Sañudo‐Peña et al., 1999), providing a neurobiological support for the anecdotal data (e.g. surveys) that indicated that Parkinsonian patients who self‐medicated with cannabis obtained benefits in the reduction of tremor (see Venderová et al., 2004). However, the few clinical studies conducted to validate the potential of CB1 receptor agonists against tremor in patients did not confirm these positive effects (Frankel et al., 1990).
Another Parkinsonian symptom investigated in relation to the activity of the CB1 receptor is bradykinesia. Blockade of CB1 receptors with rimonabant or with other antagonists reduced akinesia and motor inhibition in experimental models of Parkinson's disease (Fernández‐Espejo et al., 2005; González et al., 2006; Kelsey et al., 2009; García et al., 2011), although a few studies showed conflicting results (Di Marzo et al., 2000; Meschler et al., 2001). However, again the only clinical trial conducted with CB1 receptor antagonists in Parkinsonian patients did not confirm the positive effects found in experimental models, although the study was conducted with a group of patients who were all good responders to levodopa (Mesnage et al., 2004). It is possible that the blockade of CB1 receptors would be more effective in patients who are weak responders to levodopa or in disease states in which the classic dopaminergic therapy does not work. If this possibility were to be confirmed, it would represent an important advance in the development of novel antiParkinsonian agents. This can be concluded from the preclinical studies that demonstrated that rimonabant was more effective when used at low doses (González et al., 2006; Kelsey et al., 2009) and in very advanced phases of the disease characterized by extreme nigral damage (Fernández‐Espejo et al., 2005), conditions that were not completely reproduced in the clinical trial. In addition, these studies also demonstrated that the positive effects of rimonabant (González et al., 2006), as well as of other antagonists such as Δ9‐tetrahydrocannabivarin (García et al., 2011), were independent of dopaminergic transmission and related to an enhancement of glutamatergic transmission at the striatal level (García‐Arencibia et al., 2008; García et al., 2011). It is important to note that the usefulness of CB1 receptor antagonists in this disease agrees with the pharmacological strategy derived from the results of several studies demonstrating up‐regulation of CB1 receptors and other elements of this signalling system in Parkinson's disease (Mailleux and Vanderhaeghen, 1993; Di Marzo et al., 2000; Lastres‐Becker et al., 2001; Gubellini et al., 2002). As mentioned above, there is an imbalance between dopamine, which goes down, and endocannabinoids, which go up, in the basal ganglia once nigral damage is already evident, which supports the potential of CB1 receptor antagonists in this disease. This type of response has been observed in rats treated acutely with reserpine (Di Marzo et al., 2000) or chronically with dopaminergic antagonists (Mailleux and Vanderhaeghen, 1993), or after the damage of nigrostriatal neurons with 6‐hydroxydopamine (Mailleux and Vanderhaeghen, 1993; Gubellini et al., 2002) or MPTP (Lastres‐Becker et al., 2001) in different laboratory animals. It was also found in patients (Lastres‐Becker et al., 2001; Pisani et al., 2005). In support of this concept of imbalance, classic dopaminergic replacement therapy with levodopa reversed this endocannabinoid overactivity (Lastres‐Becker et al., 2001; Maccarrone et al., 2003). By contrast, Kreitzer and Malenka (2007) demonstrated that endocannabinoid retrograde signalling was absent in the indirect pathway in experimental Parkinsonism, and they found benefits for Parkinsonian motor deficits in these experimental models with a combination of D2 agonists and inhibitors of endocannabinoid degradation which elevated the endocannabinoid tone. This emphasizes the complexity of the basal ganglia circuitry, due to the multiplicity of neuronal sites for the generation of endocannabinoids and CB1 receptor‐mediated signals.
The occurrence of dyskinesia associated with prolonged therapy of dopaminergic replacement with levodopa represents the major complicating factor in the treatment of patients affected by Parkinson's disease (Fabbrini et al., 2007; Iravani and Jenner, 2011). Numerous studies conducted in the last 15 years have demonstrated that it can be pharmacologically reduced with certain cannabinoid compounds, although this finding is controversial, because of the opposing effects exerted by the different targets activated by the active cannabinoids. For example, CB1 receptor agonists have shown antidyskinetic effects (Ferrer et al., 2003; Martinez et al., 2012) and a normalization of the signalling mechanisms (e.g. cAMP/PKA activation) involved in the dyskinetic anomalies (Martinez et al., 2012). However, the clinical validation of this potential of CB1 receptor agonists has produced controversial results (Sieradzan et al., 2001; Carroll et al., 2004). This controversy has been also found in the preclinical studies, for example, the benefits of the activation of CB1 receptors against levodopa‐induced dyskinesia were not found with the so‐called indirect cannabinoid agonists, e.g. FAAH inhibitors, presumably because they are also able to activate TRPV1 receptors in addition to CB1 receptors (Morgese et al., 2007). In fact, only when combined with a TRPV1 receptor antagonist, were FAAH inhibitors able to show antidyskinetic properties, thus indicating that CB1 and TRPV1 receptors work in opposite directions to control levodopa‐induced dyskinesia (Morgese et al., 2007). On the other hand, another conflicting result derived from studies showing that CB1 receptor antagonists also reduced and/or delayed levodopa‐induced dyskinesia (see Fabbrini et al., 2007). Their administration in combination with levodopa produces some interesting synergies in relation with motor symptoms but also with disease progression (Gutiérrez‐Valdez et al., 2013). Again, this indicates the complexity of the neuronal circuitry in which both CB1 agonists and antagonists may provide the same type of therapeutic benefit, a fact presumably related to the presence of these receptors in both excitatory and inhibitory synapses within the basal ganglia circuitry. Lastly, a recent study added more complexity by suggesting that certain cannabinoids (e.g. anandamide) may reduce levodopa‐induced dyskinesias by activating PPAR‐γ (Martinez et al., 2015). Beneficial effects were also reported in relation with oleoyl‐ethanolamide, an endocannabinoid‐related lipid, which is an endogenous ligand for PPAR‐α receptor, but authors attributed its antidyskinetic effects to the blockade of TRPV1 receptors rather than the activation of PPAR‐α receptors (González‐Aparicio and Moratalla, 2014).
Lastly, it is also important to consider the therapeutic benefits that the antagonists of TRPV1 receptors can offer for the treatment of motor defects in Parkinson's disease, given their well‐demonstrated role in regulating dopamine release from nigral neurons (de Lago et al., 2004). For example, they are necessary for unmasking the anti‐dyskinetic effects of FAAH inhibitors or other cannabinoid agonists able to directly or indirectly activate TRPV1 receptors (Morgese et al., 2007). However, given that they are located in the neuronal subpopulation that degenerates in this disease (Carroll et al., 2004), it is necessary to assume that this target would experience a loss of efficacy in parallel to the progression of the disease, a highly relevant consideration in a disorder whose first motor symptoms appear when an important loss of dopaminergic neurons has already occurred.
Concluding remarks
In this article, we have reviewed the established findings and the recent advances in cannabinoid–dopamine interactions paying emphasis in a process in which dopamine has been proposed as a key regulatory neurotransmitter, the function of the basal ganglia and the control of movement. We have explored the mechanisms underlying these interactions, which demonstrate how compounds active at the endocannabinoid system can interfere with this process. In most of the cases, we have concluded that dopaminergic neurons do not contain CB1 receptors but that these receptors are located on neurons present in regions innervated by dopaminergic neurons, which allows relevant bidirectional interactions. However, we have also presented evidence indicating that endocannabinoid–dopamine interactions are not exerted exclusively by indirect pathways, and that additional direct mechanisms may also facilitate these interactions, for example, through TRPV1 and CB2 receptors located in dopaminergic neurons as well as through postsynaptic interactions of CB1 receptors with D1/D2 receptors. Lastly, we have reviewed those diseases characterized by either deficiency or dysregulation of dopaminergic transmission, such as Parkinson's disease, and in which cannabinoids might be of therapeutic potential possibly through actions that facilitate, among others, a normalization of dopaminergic transmission.
Conflict of interest
The authors declare that they have no conflict of interest in relation to this review article.
Acknowledgements
This work has been supported by grants from CIBERNED (CB06/05/0089), MINECO (SAF2009/11847 and SAF2012/39173), Michael J. Fox Foundation and CAM (S2011/BMD‐2308). These agencies had no further role in study design, the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. Authors are indebted to all coworkers who participated in the studies of our group mentioned in this review, and to Yolanda García‐Movellán for administrative assistance.
García, C. , Palomo‐Garo, C. , Gómez‐Gálvez, Y. , and Fernández‐Ruiz, J. (2016) Cannabinoid–dopamine interactions in the physiology and physiopathology of the basal ganglia. Br J Pharmacol, 173: 2069–2079. doi: 10.1111/bph.13215.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013a). The Concise Guide to PHARMACOLOGY 2013/14: G Protein‐Coupled Receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013b). The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA et al (2013c). The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol 170: 1607–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013d). The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol 170: 1706–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013e). The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol 170: 1652–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JJ, Kask AM, Chase TN (1996). Effects of cannabinoid receptor stimulation and blockade on catalepsy produced by dopamine receptor antagonists. Eur J Pharmacol 295: 163–168. [DOI] [PubMed] [Google Scholar]
- Andersson M, Usiello A, Borgkvist A, Pozzi L, Dominguez C, Fienberg AA et al (2005). Cannabinoid action depends on phosphorylation of dopamine‐ and cAMP‐regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. J Neurosci 25: 8432–8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernández‐Ruiz J et al (1999). Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun 256: 377–380. [DOI] [PubMed] [Google Scholar]
- Bobrov MY, Lizhin AA, Andrianova EL, Gretskaya NM, Frumkina LE, Khaspekov LG et al (2008). Antioxidant and neuroprotective properties of N‐arachidonoyldopamine. Neurosci Lett 431: 6–11. [DOI] [PubMed] [Google Scholar]
- Bonaventura J, Rico AJ, Moreno E, Sierra S, Sánchez M, Luquin N et al (2014). L‐DOPA‐treatment in primates disrupts the expression of A2A adenosine‐CB1 cannabinoid‐D2 dopamine receptor heteromers in the caudate nucleus. Neuropharmacology 79: 90–100. [DOI] [PubMed] [Google Scholar]
- Bosier B, Muccioli GG, Mertens B, Sarre S, Michotte Y, Lambert DM et al (2012). Differential modulations of striatal tyrosine hydroxylase and dopamine metabolismo by cannabinoid agonists as evidence for functional selectivity in vivo. Neuropharmacology 62: 2328–2336. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Sim‐Selley LJ (2009). Basic neuroanatomy and neuropharmacology of cannabinoids. Int Rev Psychiatry 21: 113–121. [DOI] [PubMed] [Google Scholar]
- Brotchie JM (2003). CB1 cannabinoid receptor signalling in Parkinson's disease. Curr Opin Pharmacol 3: 54–61. [DOI] [PubMed] [Google Scholar]
- Brugarolas M, Navarro G, Martínez‐Pinilla E, Angelats E, Casadó V, Lanciego JL et al (2014). G‐protein‐coupled receptor heteromers as key players in the molecular architecture of the central nervous system. CNS Neurosci Ther 20: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C et al (2004). Cannabis for dyskinesia in Parkinson disease: a randomized double‐blind crossover study. Neurology 63: 1245–1250. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y (2012). Endocannabinoid signaling and synaptic function. Neuron 76: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM (2000). Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson's disease. FASEB J 14: 1432–1438. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Lastres‐Becker I, Bisogno T, De Petrocellis L, Milone A, Davis JB et al (2001). Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur J Pharmacol 420: 123–131. [DOI] [PubMed] [Google Scholar]
- El Khoury MA, Gorgievski V, Moutsimilli L, Giros B, Tzavara ET (2012). Interactions between the cannabinoid and dopaminergic systems: evidence from animal studies. Prog Neuropsychopharmacol Biol Psychiatry 38: 36–50. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG (2007). Levodopa‐induced dyskinesias. Mov Disord 22: 1379–1389. [DOI] [PubMed] [Google Scholar]
- Fernández‐Espejo E, Caraballo I, de Fonseca FR, El Banoua F, Ferrer B, Flores JA et al (2005). Cannabinoid CB1 antagonists possess antiparkinsonian efficacy only in rats with very severe nigral lesion in experimental parkinsonism. Neurobiol Dis 18: 591–601. [DOI] [PubMed] [Google Scholar]
- Fernández‐Ruiz J (2009). The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol 156: 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Ruiz J, Gonzáles S (2005). Cannabinoid control of motor function at the basal ganglia. Handb Exp Pharmacol 168: 479–507. [DOI] [PubMed] [Google Scholar]
- Fernández‐Ruiz J, Romero J, Velasco G, Tolón RM, Ramos JA, Guzmán M (2007). Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci 28: 39–45. [DOI] [PubMed] [Google Scholar]
- Fernández‐Ruiz J, Hernández M, Ramos JA (2010). Cannabinoid‐dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16: e72–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Goldberg SR, Lluis C, Franco R (2009). Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology 56: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SG, Lomaglio T, Avelino A, Cruz F, Oliveira CR, Cunha RA et al (2009). N‐acyldopamines control striatal input terminals via novel ligand‐gated cation channels. Neuropharmacology 56: 676–683. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A (2003). Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa‐induced dyskinesias. Eur J Neurosci 18: 1607–1614. [DOI] [PubMed] [Google Scholar]
- Fox SH, Kellett M, Moore AP, Crossman AR, Brotchie JM (2002). Randomised, double‐blind, placebo‐controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov Disord 17: 145–149. [DOI] [PubMed] [Google Scholar]
- Frankel JP, Hughes A, Lees AJ, Stern GM (1990). Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatry 53: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco FR, Martorana A, Giampa C, De March Z, Farini D, D'Angelo V et al (2004). Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse 53: 159–167. [DOI] [PubMed] [Google Scholar]
- García C, Palomo‐Garo C, García‐Arencibia M, Ramos J, Pertwee R, Fernández‐Ruiz J (2011). Symptom‐relieving and neuroprotective effects of the phytocannabinoid Δ9‐THCV in animal models of Parkinson's disease. Br J Pharmacol 163: 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García MC, Cinquina V, Palomo‐Garo C, Rábano A, Fernández‐Ruiz J (2015). Identification of CB2 receptors in human nigral neurons that degenerate in Parkinson's disease. Neurosci Lett 587: 1–4. [DOI] [PubMed] [Google Scholar]
- García‐Arencibia M, Ferraro L, Tanganelli S, Fernández‐Ruiz J (2008). Enhanced striatal glutamate release after the administration of rimonabant to 6‐hydroxydopamine‐lesioned rats. Neurosci Lett 438: 10–13. [DOI] [PubMed] [Google Scholar]
- García‐Arencibia M, García C, Fernández‐Ruiz J (2009). Cannabinoids and Parkinson's disease. CNS Neurol Disord Drug Targets 8: 432–439. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D (1999). Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2: 358–363. [DOI] [PubMed] [Google Scholar]
- González B, Paz F, Florán L, Aceves J, Erlij D, Florán B (2009). Cannabinoid agonists stimulate [3H]GABA release in the globus pallidus of the rat when Gi protein‐receptor coupling is restricted: role of dopamine D2 receptors. J Pharmacol Exp Ther 328: 822–828. [DOI] [PubMed] [Google Scholar]
- González S, Scorticati C, Garcia‐Arencibia M, de Miguel R, Ramos JA, Fernández‐Ruiz J (2006). Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson's disease. Brain Res 1073–1074: 209–219. [DOI] [PubMed] [Google Scholar]
- González‐Aparicio R, Moratalla R (2014). Oleoylethanolamide reduces L‐DOPA‐induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson's disease. Neurobiol Dis 62: 416–425. [DOI] [PubMed] [Google Scholar]
- Gorriti MA, Rodriguez de Fonseca F, Navarro M, Palomo T (1999). Chronic (‐)‐Δ9‐tetrahydrocannabinol treatment induces sensitization to the psychomotor effects of amphetamine in rats. Eur J Pharmacol 365: 133–142. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D et al (2002). Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci 22: 6900–6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG (2009). The endocannabinoid system and pain. CNS Neurol Disord Drug Targets 8: 403–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez‐Valdez AL, García‐Ruiz R, Anaya‐Martínez V, Torres‐Esquivel C, Espinosa‐Villanueva J, Reynoso‐Erazo L et al (2013). The combination of oral L‐DOPA/rimonabant for effective dyskinesia treatment and cytological preservation in a rat model of Parkinson's disease and L‐DOPA‐induced dyskinesia. Behav Pharmacol 24: 640–652. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE (2009). Endocannabinoid signaling and long‐term synaptic plasticity. Annu Rev Physiol 71: 283–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK (1991a). Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res 547: 267–274. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Melvin LS, Johnson MR, de Costa DR et al (1991b). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11: 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B (2002). Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience 109: 451–460. [DOI] [PubMed] [Google Scholar]
- Hu SS, Bradshaw HB, Benton VM, Chen JS, Huang SM, Minassi A et al (2009). The biosynthesis of N‐arachidonoyl dopamine (NADA), a putative endocannabinoid and endovanilloid, via conjugation of arachidonic acid with dopamine. Prostaglandins Leukot Essent Fatty Acids 81: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani MM, Jenner P (2011). Mechanisms underlying the onset and expression of levodopa‐induced dyskinesia and their pharmacological manipulation. J Neural Transm 118: 1661–1690. [DOI] [PubMed] [Google Scholar]
- Jabusch HC, Schneider U, Altenmüller E (2004). Δ9‐tetrahydrocannabinol improves motor control in a patient with musician's dystonia. Mov Disord 19: 990–991. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno‐Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M (2009). Endocannabinoid‐mediated control of synaptic transmission. Physiol Rev 89: 309–380. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Harris O, Cassin J (2009). The CB1 antagonist rimonabant is adjunctively therapeutic as well as monotherapeutic in an animal model of Parkinson's disease. Behav Brain Res 203: 304–307. [DOI] [PubMed] [Google Scholar]
- Kirkham TC (2009). Cannabinoids and appetite: food craving and food pleasure. Int Rev Psychiatry 21: 163–171. [DOI] [PubMed] [Google Scholar]
- Kowal MA, Colzato LS, Hommel B (2011). Decreased spontaneous eye blink rates in chronic cannabis users: evidence for striatal cannabinoid‐dopamine interactions. PLoS ONE 6: e26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA et al (2005). Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci 25: 2874–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC (2007). Endocannabinoid‐mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature 445: 643–647. [DOI] [PubMed] [Google Scholar]
- de Lago E, de Miguel R, Lastres‐Becker I, Ramos JA, Fernández‐Ruiz JJ (2004). Involvement of vanilloid‐like receptors in the effects of anandamide on motor behavior and nigrostriatal dopaminergic activity: in vivo and in vitro evidence. Brain Res 1007: 152–159. [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Barroso‐Chinea P, Rico AJ, Conte‐Perales L, Callén L, Roda E et al (2011). Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol 25: 97–104. [DOI] [PubMed] [Google Scholar]
- Lastres‐Becker I, Cebeira M, de Ceballos M, Zeng B‐Y, Jenner P, Ramos JA et al (2001). Increased cannabinoid CB1 receptor binding and activation of GTP‐binding proteins in the basal ganglia of patients with Parkinson's syndrome and of MPTP‐treated marmosets. Eur J Neurosci 14: 1827–1832. [DOI] [PubMed] [Google Scholar]
- Lovinger DM (2008). Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol 184: 435–477. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Mathur BN (2012). Endocannabinoids in striatal plasticity. Parkinsonism Relat Disord 18: S132–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Gubellini P, Bari M, Picconi B, Battista N, Centonze D et al (2003). Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J Neurochem 85: 1018–1025. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ (1992). Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience 48: 655–668. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ (1993). Dopaminergic regulation of cannabinoid receptor mRNA levels in the rat caudate‐putamen: an in situ hybridization study. J Neurochem 61: 1705–1712. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I et al (2008). Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology 54: 815–823. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G et al (2003). Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci 23: 3136–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Florenzano F, Fezza F, Viscomi MT, van der Stelt M et al (2007). N‐arachidonoyl‐dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology 32: 298–308. [DOI] [PubMed] [Google Scholar]
- Martín AB, Fernandez‐Espejo E, Ferrer B, Gorriti MA, Bilbao A, Navarro M et al (2008). Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor‐mediated motor behaviors. Neuropsychopharmacology 33: 1667–1679. [DOI] [PubMed] [Google Scholar]
- Martinez A, Macheda T, Morgese MG, Trabace L, Giuffrida A (2012). The cannabinoid agonist WIN55212‐2 decreases L‐DOPA‐induced PKA activation and dyskinetic behavior in 6‐OHDA‐treated rats. Neurosci Res 72: 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AA, Morgese MG, Pisanu A, Macheda T, Paquette MA, Seillier A et al (2015). Activation of PPAR‐γ receptors reduces levodopa‐induced dyskinesias in 6‐OHDA‐lesioned rats. Neurobiol Dis 74: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Gobbi G (2012). Cannabinoids and emotionality: a neuroanatomical perspective. Neuroscience 204: 134–144. [DOI] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL (2004). Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci 24: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschler JP, Howlett AC (2001). Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology 40: 918–926. [DOI] [PubMed] [Google Scholar]
- Meschler JP, Howlett AC, Madras BK (2001). Cannabinoid receptor agonist and antagonist effects on motor function in normal and 1‐methyl‐4‐phenyl‐1,2,5,6‐tetrahydropyridine (MPTP)‐treated non‐human primates. Psychopharmacology (Berl) 156: 79–85. [DOI] [PubMed] [Google Scholar]
- Mesnage V, Houeto JL, Bonnet AM, Clavier I, Arnulf I, Cattelin F et al (2004). Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson's disease. Clin Neuropharmacol 27: 108–110. [DOI] [PubMed] [Google Scholar]
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R et al (2000). Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1‐like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A 97: 3655–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgese MG, Cassano T, Cuomo V, Giuffrida A (2007). Anti‐dyskinetic effects of cannabinoids in a rat model of Parkinson's disease: role of CB1 and TRPV1 receptors. Exp Neurol 208: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DE, McMaster SB, Rogers J (1981). Tetrahydrocannabinol potentiates reserpine‐induced hypokinesia. Pharmacol Biochem Behav 15: 779–783. [DOI] [PubMed] [Google Scholar]
- Müller‐Vahl KR, Schneider U, Koblenz A, Jobges M, Kolbe H, Daldrup T et al (2002). Treatment of Tourette's syndrome with Δ9‐tetrahydrocannabinol (THC): a randomized crossover trial. Pharmacopsychiatry 35: 57–61. [DOI] [PubMed] [Google Scholar]
- Müller‐Vahl KR, Schneider U, Prevedel H, Theloe K, Kolbe H, Daldrup T et al (2003). Δ9‐Tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6‐week randomized trial. J Clin Psychiatry 64: 459–465. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Wang H, Verdurand M, Zavitsanou K (2012). Differential treatment regimen‐related effects of HU210 on CB1 and D2‐like receptor functionality in the rat basal ganglia. Pharmacology 89: 64–73. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Marin C, Rodriguez‐Oroz C, Blesa J, Benitez‐Temiño B, Mena‐Segovia J et al (2008). The basal ganglia in Parkinson's disease: current concepts and unexplained observations. Ann Neurol 64: S30–S46. [DOI] [PubMed] [Google Scholar]
- O'Neill C, Evers‐Donnelly A, Nicholson D, O'Boyle KM, O'Connor JJ (2009). D2 receptor‐mediated inhibition of dopamine release in the rat striatum in vitro is modulated by CB1 receptors: studies using fast cyclic voltammetry. J Neurochem 108: 545–551. [DOI] [PubMed] [Google Scholar]
- Oz M, Jaligam V, Galadari S, Petroianu G, Shuba YM, Shippenberg TS (2010). The endogenous cannabinoid, anandamide, inhibits dopamine transporter function by a receptor‐independent mechanism. J Neurochem 112: 1454–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS (2008). D2 dopamine receptor activation facilitates endocannabinoid‐mediated long‐term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP‐protein kinase A signaling. J Neurosci 28: 14018–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfo P, Silveirinha V, dos Santos‐Rodrigues A, Venance L, Ledent C, Takahashi RN et al (2011). Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur J Pharmacol 655: 38–45. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rock EM, Limebeer CL (2011). Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol 163: 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al; NC‐IUPHAR (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl Acids Res 42 (Database Issue): D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna A, Bonaventura J, Farré D, Sánchez M, Simola N, Mallol J et al (2014). L‐DOPA disrupts adenosine A2A‐cannabinoid CB1‐dopamine D2 receptor heteromer cross‐talk in the striatum of hemiparkinsonian rats: biochemical and behavioral studies. Exp Neurol 253: 180–191. [DOI] [PubMed] [Google Scholar]
- Pisani A, Fezza F, Galati S, Battista N, Napolitano S, Finazzi‐Agro A et al (2005). High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson's disease patients. Ann Neurol 57: 777–779. [DOI] [PubMed] [Google Scholar]
- Pisani V, Madeo G, Tassone A, Sciamanna G, Maccarrone M, Stanzione P et al (2011). Homeostatic changes of the endocannabinoid system in Parkinson's disease. Mov Disord 26: 216–222. [DOI] [PubMed] [Google Scholar]
- Price DA, Owens WA, Gould GG, Frazer A, Roberts JL, Daws LC et al (2007). CB1‐independent inhibition of dopamine transporter activity by cannabinoids in mouse dorsal striatum. J Neurochem 101: 389–396. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR (2004). Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci 24: 11070–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sañudo‐Peña MC, Tsou K, Walker JM (1999). Motor actions of cannabinoids in the basal ganglia output nuclei. Life Sci 65: 703–713. [DOI] [PubMed] [Google Scholar]
- Seutin V (2005). Dopaminergic neurones: much more than dopamine? Br J Pharmacol 146: 167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM (2001). Cannabinoids reduce levodopa‐induced dyskinesia in Parkinson's disease: a pilot study. Neurology 57: 2108–2111. [DOI] [PubMed] [Google Scholar]
- Smith Y, Villalba R (2008). Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord 23: S534–S547. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D (2008). The endocannabinoid system in brain reward processes. Br J Pharmacol 154: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Nigam S, Di Marzo V (2007). Biochemistry and pharmacology of endovanilloids. Pharmacol Ther 114: 13–33. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V (2003). The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol 480: 133–150. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Di Filippo M, Tantucci M, Costa C, Borsini F et al (2011). The distinct role of medium spiny neurons and cholinergic interneurons in the D2/A2A receptor interaction in the striatum: implications for Parkinson's disease. J Neurosci 31: 1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo‐Peña MC, Mackie K, Walker JM (1998). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M (2007). Subcellular arrangement of molecules for 2‐arachidonoyl‐glycerol‐mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci 27: 3663–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde O, Karsak M, Zimmer A (2005). Analysis of the endocannabinoid system by using CB1 cannabinoid receptor knockout mice. Handb Exp Pharmacol 168: 117–145. [DOI] [PubMed] [Google Scholar]
- Venderová K, Růzicka E, Vorísek V, Visnovský P (2004). Survey on cannabis use in Parkinson's disease: subjective improvement of motor symptoms. Mov Disord 19: 1102–1106. [DOI] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S (2011). Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci 5: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ et al (2014). Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine‐related behavior in mice. Proc Natl Acad Sci U S A 111: E5007–E5015. [DOI] [PMC free article] [PubMed] [Google Scholar]


