Abstract
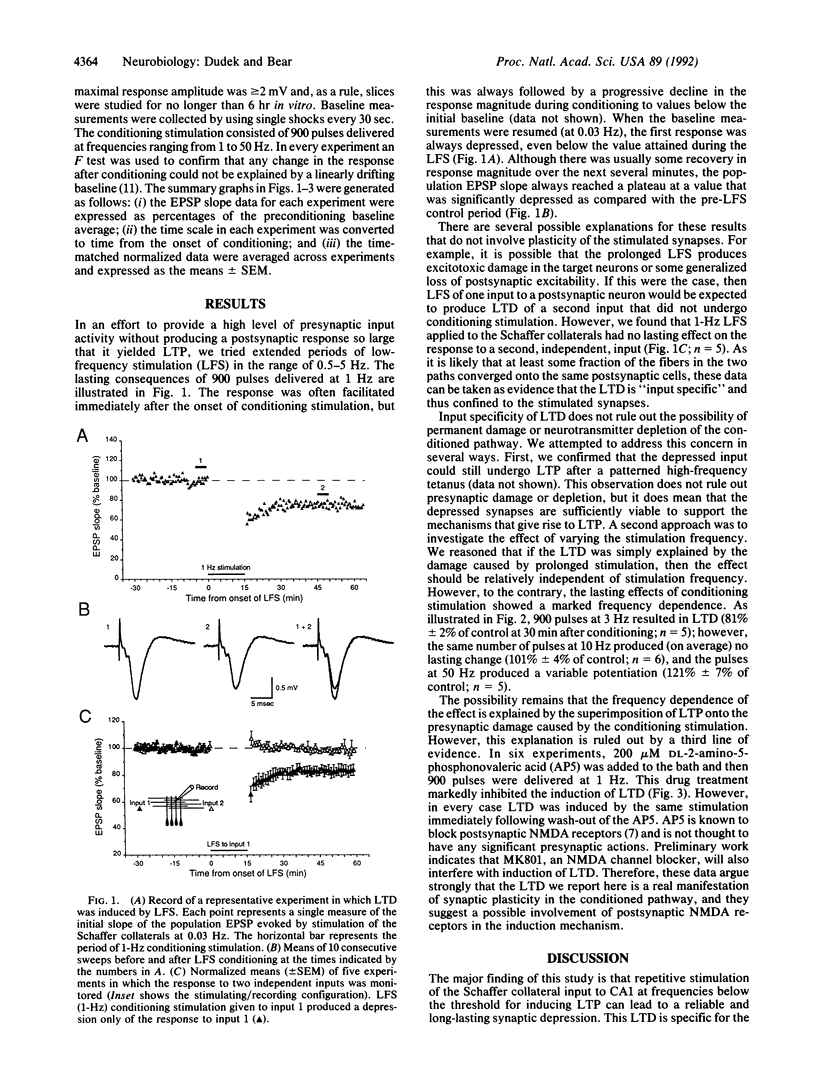
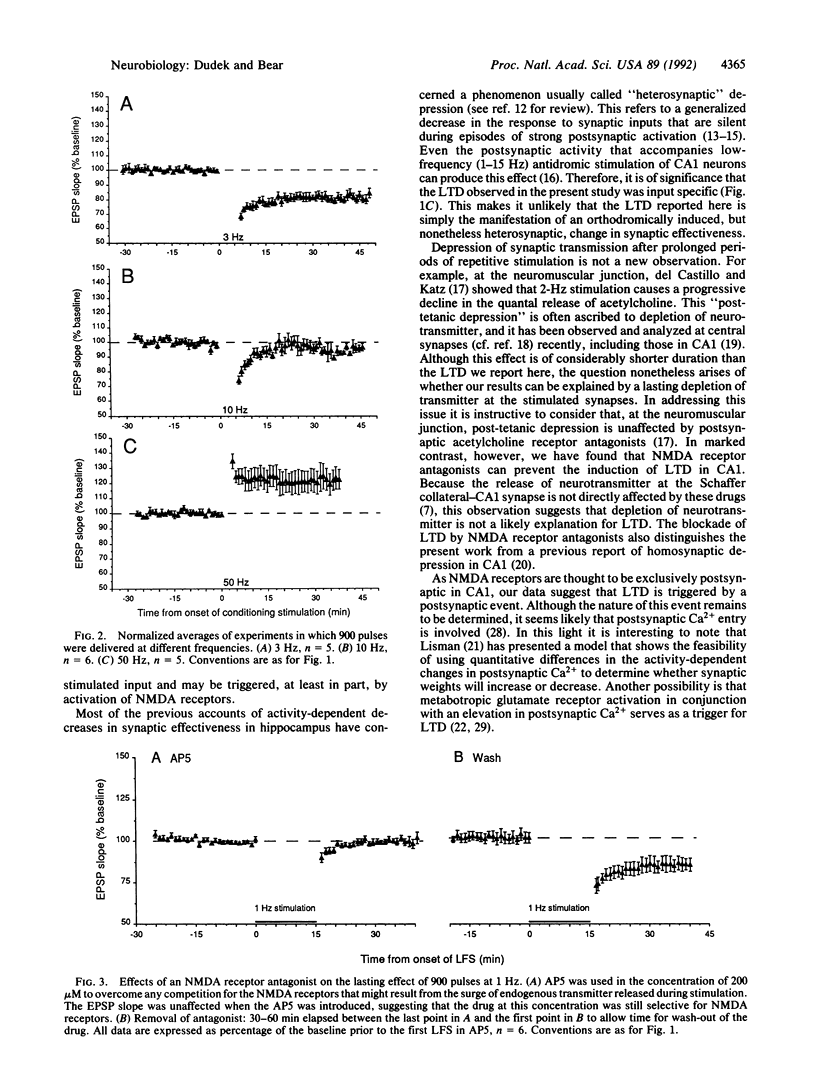
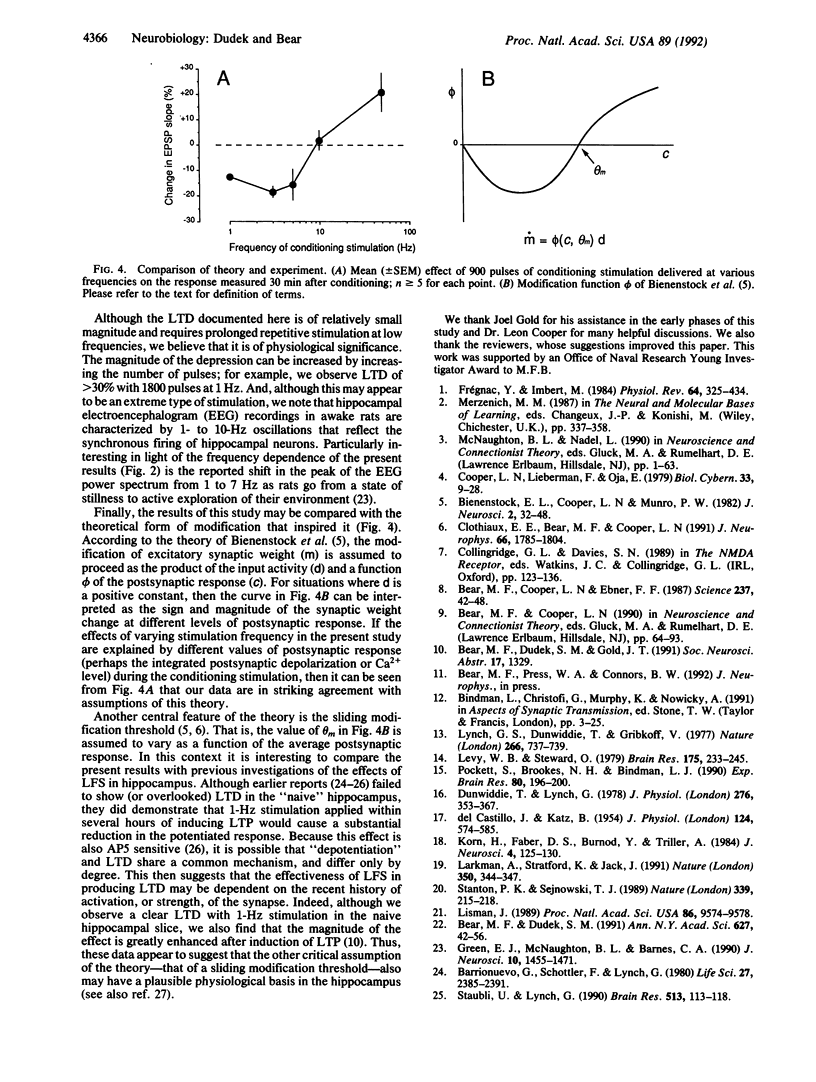
We tested a theoretical prediction that patterns of excitatory input activity that consistently fail to activate target neurons sufficiently to induce synaptic potentiation will instead cause a specific synaptic depression. To realize this situation experimentally, the Schaffer collateral projection to area CA1 in rat hippocampal slices was stimulated electrically at frequencies ranging from 0.5 to 50 Hz. Nine hundred pulses at 1-3 Hz consistently yielded a depression of the CA1 population excitatory postsynaptic potential that persisted without signs of recovery for greater than 1 hr after cessation of the conditioning stimulation. This long-term depression was specific to the conditioned input, ruling out generalized changes in postsynaptic responsiveness or excitability. Three lines of evidence suggest that this effect is accounted for by a modification of synaptic effectiveness rather than damage to or fatigue of the stimulated inputs. First, the effect was dependent on the stimulation frequency; 900 pulses at 10 Hz caused no lasting change, and at 50 Hz a synaptic potentiation was usually observed. Second, the depressed synapses continued to support long-term potentiation in response to a high-frequency tetanus. Third, the effects of conditioning stimulation could be prevented by application of NMDA receptor antagonists. Thus, our data suggest that synaptic depression can be triggered by prolonged NMDA receptor activation that is below the threshold for inducing synaptic potentiation. We propose that this mechanism is important for the modifications of hippocampal response properties that underlie some forms of learning and memory.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrionuevo G., Schottler F., Lynch G. The effects of repetitive low frequency stimulation on control and "potentiated" synaptic responses in the hippocampus. Life Sci. 1980 Dec 15;27(24):2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Cooper L. N., Ebner F. F. A physiological basis for a theory of synapse modification. Science. 1987 Jul 3;237(4810):42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Dudek S. M. Stimulation of phosphoinositide turnover by excitatory amino acids. Pharmacology, development, and role in visual cortical plasticity. Ann N Y Acad Sci. 1991;627:42–56. doi: 10.1111/j.1749-6632.1991.tb25912.x. [DOI] [PubMed] [Google Scholar]
- Bienenstock E. L., Cooper L. N., Munro P. W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982 Jan;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröcher S., Artola A., Singer W. Intracellular injection of Ca2+ chelators blocks induction of long-term depression in rat visual cortex. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):123–127. doi: 10.1073/pnas.89.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clothiaux E. E., Bear M. F., Cooper L. N. Synaptic plasticity in visual cortex: comparison of theory with experiment. J Neurophysiol. 1991 Nov;66(5):1785–1804. doi: 10.1152/jn.1991.66.5.1785. [DOI] [PubMed] [Google Scholar]
- Cooper L. N., Liberman F., Oja E. A theory for the acquisition and loss of neuron specificity in visual cortex. Biol Cybern. 1979 Jun 29;33(1):9–28. doi: 10.1007/BF00337414. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T., Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol. 1978 Mar;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Development of neuronal selectivity in primary visual cortex of cat. Physiol Rev. 1984 Jan;64(1):325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Fujii S., Saito K., Miyakawa H., Ito K., Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991 Jul 26;555(1):112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Gold R., Kappos L. Faziale Myokymie durch pontine Läsionen und zentrales Fieber bei multipler Sklerose--Kasuistik. Schweiz Rundsch Med Prax. 1991 Nov 19;80(47):1327–1329. [PubMed] [Google Scholar]
- Green E. J., McNaughton B. L., Barnes C. A. Exploration-dependent modulation of evoked responses in fascia dentata: dissociation of motor, EEG, and sensory factors and evidence for a synaptic efficacy change. J Neurosci. 1990 May;10(5):1455–1471. doi: 10.1523/JNEUROSCI.10-05-01455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Y., Colino A., Selig D. K., Malenka R. C. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992 Feb 7;255(5045):730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- Korn H., Faber D. S., Burnod Y., Triller A. Regulation of efficacy at central synapses. J Neurosci. 1984 Jan;4(1):125–130. doi: 10.1523/JNEUROSCI.04-01-00125.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A., Stratford K., Jack J. Quantal analysis of excitatory synaptic action and depression in hippocampal slices. Nature. 1991 Mar 28;350(6316):344–347. doi: 10.1038/350344a0. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979 Oct 19;175(2):233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Linden D. J., Dickinson M. H., Smeyne M., Connor J. A. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991 Jul;7(1):81–89. doi: 10.1016/0896-6273(91)90076-c. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. S., Dunwiddie T., Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977 Apr 21;266(5604):737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Pockett S., Brookes N. H., Bindman L. J. Long-term depression at synapses in slices of rat hippocampus can be induced by bursts of postsynaptic activity. Exp Brain Res. 1990;80(1):196–200. doi: 10.1007/BF00228861. [DOI] [PubMed] [Google Scholar]
- Stanton P. K., Sejnowski T. J. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature. 1989 May 18;339(6221):215–218. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- Staubli U., Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1-5 Hz stimulation. Brain Res. 1990 Apr 9;513(1):113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]



