Abstract
Small size at birth is linked with lifelong adverse health implications. However, small size is only a proxy for the pathological process of interest, intrauterine growth restriction. We examined the extent to which information on intrauterine growth patterns improved prediction of childhood anthropometry, above and beyond birth weight alone. We obtained fetal weights estimated via serial ultrasound for 478 children in the Scandinavian Successive Small-for-Gestational-Age Births Study (1986–1988). Size at birth was classified using birth weight-for-gestational-age z scores and conditional fetal growth z scores (reflecting growth between 25 weeks’ gestation and birth) using internal references. Conditional z scores were also expressed as residuals of birth weight z scores. Growth measures were linked with age-5-years anthropometric characteristics using linear regression. In univariable analyses, conditional fetal growth z scores were positively associated with z scores for child height, body mass index, total skinfold thickness, and head circumference (β = 0.24 (95% confidence interval (CI): 0.18, 0.31), β = 0.16 (95% CI: 0.09, 0.23), β = 0.08 (95% CI: 0.01, 0.16), and β = 0.37 (95% CI: 0.22, 0.52), respectively). However, conditional z scores were highly correlated with birth weight z scores (r = 0.9), and residuals explained minimal additional variation in anthropometric factors (null coefficients; adjusted R2 increases < 0.01). Information on the intrauterine trajectory through which birth weight was attained provided little additional insight into child growth beyond that obtained from absolute size at birth.
Keywords: birth weight, child growth, estimated fetal weight, growth assessment, intrauterine growth
Intrauterine growth restriction has conventionally been defined as a birth weight below the 10th percentile for sex and gestational age, or small-for-gestational-age (SGA) birth (1). It is well established that being born SGA has adverse implications for lifelong health, such as increased risk of metabolic syndrome in comparison with appropriately grown peers (2, 3). Although mechanisms for the increased risks are unclear, it is hypothesized that fetal undernutrition may lead to permanent changes in pancreatic islet β-cell development and/or activation of the hypothalamic-pituitary-adrenal axis (2).
However, not all infants with birth weights below the 10th population percentile are small because of intrauterine growth restriction. Infants can also be small at birth because they are constitutionally small (“small but healthy”) or because of a congenital anomaly. It has been estimated that up to 70% of SGA infants are constitutionally small, making SGA birth a poor surrogate for intrauterine growth restriction (4). As a result, many investigators have suggested that intrauterine growth restriction should be identified on the basis of measurements of fetal growth patterns, rather than birth weight (5–7).
We hypothesized that the intrauterine growth pattern through which an infant attained its weight at birth would be a better measure of child growth and adiposity than birth weight alone. In this study, we examined the incremental value of conditional fetal growth percentiles (which classify infants according to their intrauterine growth trajectories) in explaining child anthropometric characteristics at age 5 years, above and beyond absolute size at birth.
METHODS
Study population
The study population was drawn from women who participated in the Scandinavian Successive Small-for-Gestational-Age Births Study (1986–1988), which recruited women with singleton pregnancies of parity 1 or 2 prior to the 20th week of gestation in the counties of Trondheim and Bergen, Norway, and Uppsala, Sweden (8). The study investigators recruited a 10% random sample of eligible women in the participating regions (n = 561) and oversampled 1,384 women with 1 or more risk factors for having an SGA infant (a 50% sample of smokers (n = 598) and 100% of mothers with an increased risk due to obstetrical history (n = 786)).
All infants in the 10% random sample and infants born SGA in the high-risk group were eligible for follow-up at age 5 years. In the overall study, follow-up differed by study site (83% and 84% at the Norwegian sites, 52% in Uppsala), and losses to follow-up were primarily due to social inconvenience/lack of interest. The delivery and newborn characteristics of children lost to follow-up were not meaningfully different from those of children included in the final cohort (data available upon request). Signed informed consent was obtained from the women, and the protocol was approved by the respective local ethics committees.
Ultrasound measurements
Ultrasonographic fetal biometric measurements were obtained at the 17-, 25-, 33-, and 37-week study visits. Measurements were taken in triplicate by specially trained midwives, and the average of the 3 values was used (9). Fetal weight was estimated using the formula of Hadlock et al. (10). We assessed the accuracy of estimated fetal weight (EFW) measurements in the cohort by calculating the percentage of error between EFW and birth weight (% error = (EFW − birth weight)/birth weight × 100) in women who had an ultrasound scan within 3 days of delivery (n = 36). Gestational age was calculated using the date of the last menstrual period, unless the discrepancy between gestational age estimated from early ultrasound and that estimated from the last menstrual period was greater than 14 days, in which case the former was used. Because the 17-week ultrasonogram was used to establish gestational age, we did not also use it to assess growth. Using the same size measurements to establish both gestational age and growth would have introduced circular logic.
Birth weight z scores and fetal growth
We used the serial fetal weight measurements of liveborn, term (37–41 weeks, inclusive) deliveries in the 10% random sample subcohort with at least 1 ultrasound estimate of fetal weight to create reference values for fetal size (estimated weight-for-gestational-age z scores) and fetal growth (conditional fetal growth z scores). The references were created by expressing fetal weight as a function of gestational age using a multilevel model (11). This provided estimates of the population average pattern of growth throughout gestation, as well as the variability in growth within and between fetuses. Fetal weight was log-transformed to ensure that the variance in the model's residuals remained stable across gestation. Gestational age was expressed as a restricted cubic spline with 5 knots, which allowed us to model fetal growth in a smooth, nonlinear manner (12). Fetal sex was included as a covariate. We specified an unstructured covariance matrix (so that correlations between individual time periods were not constrained).
We calculated birth weight-for-gestational-age z scores for children in the age-5-years (age 5) cohort by comparing each child's birth weight with the mean value and standard deviation for sex and gestational age predicted by the multilevel model (i.e., expressing the child's birth weight in relation to the birth weights of others in the population of similar gestational age). SGA was defined as a birth weight z score below −1.28 for sex and gestational age based on the 10% random sample subcohort internal reference (corresponding to the 10th percentile).
Conditional fetal growth z scores were calculated using the methods of Royston (11). In this approach, an infant's expected weight at time t is predicted based on 1) its EFW at a previous point in the pregnancy (t − 1) in relation to the population average weight at t – 1; 2) the population average weight at time t; 3) the time interval between measurements; and 4) the extent to which fetal weight tends to “track” along the same percentile or z score throughout gestation, measured by the correlation between different time points. The infant's actual weight at time t is then expressed as a z score in relation to its predicted weight. Thus, an infant whose actual weight at time t was identical to its predicted weight would have a conditional z score of 0 (50th percentile), while an infant whose weight was 2 standard deviations below its predicted weight would have a conditional z score of −2 (2.5th percentile). Details on the calculation of conditional z scores are provided in Web Appendix 1 (available at http://aje.oxfordjournals.org/). Although alternative methods have been proposed for quantifying fetal growth, instead of fetal size (13, 14), conditional z scores are one of the more methodologically sound approaches, because they correctly account for the different sources of variability in fetal growth (which arise both between fetuses (because different fetuses grow at different rates) and within fetuses (due to measurement error in the assessment of fetal weight, as well as deviations from smooth growth trajectories over time)) and for regression to the mean (15).
For our primary analysis, we calculated conditional fetal growth z scores for weight at birth given EFW at the time of the 25-week study visit. As sensitivity analyses, we calculated conditional fetal growth z scores using 3 alternative definitions: 1) weight at birth given EFW at the 33-week study visit (late-onset growth restriction); 2) EFW at 33 weeks’ gestation given weight at the 25-week visit (early growth restriction); and 3) conditional SGA, defined as birth weight below a conditional z score of −1.28 (<10th conditional percentile) given EFW at both 33 weeks and 25 weeks. We created this latter classification to reduce the contribution of ultrasound measurement error by requiring that weight at birth be smaller than predicted based on 2 EFW measurements, rather than 1.
Anthropometric characteristics at age 5 years
Height (cm), weight (kg), subscapular skinfold thickness (mm), triceps skinfold thickness (mm), and head circumference (cm) were measured in triplicate at the age 5 study visit. Weight was recorded to the nearest 100 g using a standardized scale. Height was measured to the nearest 0.1 cm with the child standing with his/her back to the wall, with the head positioned such that the orbits were on the same horizontal level as the external acoustic meatus. Skinfold thickness was measured to the nearest 0.1 mm after 60 seconds using a Harpenden caliper. Total skinfold thickness was calculated as the sum of subscapular and triceps skinfold thicknesses. Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meters. Height and BMI were converted to age- and sex-specific z scores using World Health Organization growth charts (16). Total skinfold thickness and head circumference were standardized into age- and sex-specific z scores using internal references created from the 10% random sample subcohort.
Statistical analyses
We calculated the correlation between birth weight z score and conditional fetal growth z score using Pearson's r. Linear regression models were used to estimate the univariable association between fetal growth measurements (birth weight z scores or conditional fetal growth z scores) and each age 5 child anthropometric characteristic.
Multivariable linear regression was used to determine the incremental value of conditional fetal growth z scores, above and beyond birth weight z scores. To account for potential collinearity between these 2 types of z scores, we expressed conditional fetal growth z scores as residuals of a model regressing the conditional z scores on birth weight z scores (17). The residuals from this model represent the difference between each infant's actual conditional growth z score and that expected based on its birth weight z score. The residuals are, by definition, uncorrelated with birth weight z score and allow the variation due to conditional fetal growth patterns to be isolated. We assessed the incremental value of conditional fetal growth z scores by adding the residuals as an independent variable to models regressing each child anthropometric outcome on birth weight z score. We examined the resulting coefficient for the residuals as well as the change in the models' adjusted R2 values before and after adding the conditional fetal growth z score residuals (i.e., the amount of additional variance in child anthropometry explained by conditional growth z scores).
We explored the potential for nonlinear associations using restricted cubic splines and by grouping fetal growth z scores into fifths, regressing age 5 anthropometric values on the fifths, and plotting the resulting β coefficients with 95% confidence intervals. We added terms for interaction between birth weight and conditional fetal growth z score residuals to determine whether the association between fetal growth and child size differed according to absolute birth weight. Analyses were conducted using Stata, version 13 (StataCorp LP, College Station, Texas).
RESULTS
The 10% random sample subcohort of the Scandinavian Successive Small-for-Gestational-Age Births Study cohort included 434 term liveborn infants for creation of the internal reference charts. The mean number of weights per fetus (including birth weight) was 4.8 (range, 2–5). Validation of the ultrasound EFW among the 36 infants whose mothers underwent ultrasonography less than 3 days prior to delivery showed a median percent error of 1.6% (interquartile range, −3.6 to 4.2), with 92% (33/36) of measurements having less than 10% error. Estimates of the parameters of the multilevel model used to create the fetal growth and fetal weight reference values are provided in Web Appendix 2 (Web Tables 1 and 2).
A total of 478 children at the age 5 follow-up had ultrasound estimates of fetal weight (see Web Figure 1 for flow of participants). Of these children, 86% had 4 ultrasounds, 12% had 3 ultrasounds, and 2% had 1 or 2 ultrasounds. Their birth characteristics are shown in Table 1 according to birth weight and conditional fetal growth z scores. Thirty-three percent (156/478) of the cohort was SGA based on birth weight z scores, reflecting the oversampling of higher-risk pregnancies in this cohort. Twenty-five percent (118/478) were classified as SGA based on conditional fetal growth z scores. Women with infants classified as SGA by conditional fetal growth z scores were, on average, shorter in stature, leaner, and more likely to smoke than women whose infants were not conditional SGA. Conditional SGA infants were more likely to be delivered by cesarean section for fetal asphyxia and be followed by the pediatrics department, confirming the increased risks associated with poor intrauterine growth. They also had lower ponderal indices (weight (g)/length (m)3), abdominal circumferences, and head circumferences at birth.
Table 1.
Characteristics of Women and Infants in the Scandinavian Successive Small-for-Gestational-Age Births Study, 1986–1988
| Maternal or Newborn Characteristic | Conditional Fetal Growth z Score |
Birth Weight z Score |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <−1.28 (SGAa) |
≥−1.28 (Non-SGA) |
<−1.28 (SGA) |
≥−1.28 (Non-SGA) |
|||||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| No. of mother-child pairs | 118 | 360 | 156 | 322 | ||||||||
| Mothers | ||||||||||||
| Maternal age, years | 28.9 (4.8) | 29.0 (4.1) | 28.4 (4.4) | 29.2 (4.1) | ||||||||
| Maternal height, cm | 164 (5) | 167 (6) | 164 (5) | 167 (6) | ||||||||
| Prepregnancy body mass indexb | 20.8 (3.1) | 21.8 (2.7) | 20.6 (2.9) | 22.0 (2.7) | ||||||||
| Smoking at the start of pregnancy | ||||||||||||
| Light smoking (<20 cigarettes/day) | 68 | 58 | 120 | 33 | 98 | 63 | 90 | 28 | ||||
| Heavy smoking (≥20 cigarettes/day) | 11 | 9 | 21 | 6 | 12 | 8 | 20 | 6 | ||||
| Preexisting hypertension | 4 | 3.4 | 8 | 2.2 | 5 | 3.2 | 7 | 2.2 | ||||
| Infants | ||||||||||||
| Transfer to pediatric department | 18 | 15.3 | 16 | 4.4 | 20 | 12.8 | 14 | 4.4 | ||||
| Cesarean delivery for fetal asphyxia | 8 | 6.8 | 3 | 0.8 | 7 | 4.5 | 4 | 1.2 | ||||
| Gestational age at birth, days | 278 (18) | 282 (13) | 279 (18) | 282 | ||||||||
| Birth weight, g | 2,767 (467) | 3,633 (585) | 2,781 (490) | 3,728 (509) | ||||||||
| Ponderal indexc | 2.5 (0.2) | 2.8 (0.2) | 2.5 (0.2) | 2.8 (0.2) | ||||||||
| Abdominal circumference, cm | 29.6 (2.3) | 32.6 (2.7) | 29.5 (2.4) | 33.0 (2.4) | ||||||||
| Head circumference, cm | 33.4 (1.8) | 35.2 (1.7) | 33.4 (2.0) | 35.4 (1.4) | ||||||||
Abbreviations: SD, standard deviation; SGA, small for gestational age.
a Small size for gestational age was defined as z score <−1.28 (10th percentile).
b Weight (kg)/height (m)2.
c Weight (g)/length (m)3.
When infants were classified using birth weight z scores, the differences in characteristics between SGA and non-SGA births were similar to those observed using the conditional fetal growth z scores. Mothers of birth weight SGA infants were also shorter, leaner, and more likely to have smoked during pregnancy; their pregnancies had higher risks of complications, and they gave birth to smaller and leaner infants.
Conditional fetal growth and birth weight z scores were highly correlated (r = 0.90, P < 0.001). As shown in Figure 1, only 16 infants were classified as SGA by the conditional z scores alone, and 54 were classified as SGA by the population birth weight z scores alone; most infants who were conditionally SGA were also classified as SGA by the birth weight z scores (86%; 102/118). The 54 infants who were small in birth weight but not conditionally small tended to have birth weight z scores that were only slightly below the −1.28 (10th percentile) threshold compared with infants classified as SGA by both types of z scores (i.e., they were milder cases of SGA birth) (Figure 2). In other words, infants whose growth trajectory had dropped off sufficiently to be classified as conditional SGA ended up also being small in size. This is illustrated in Figure 3, which compares the growth of 10 randomly selected conditional SGA and 10 conditional non-SGA infants. The conditional SGA births, indicated in black, have birth weights that are systematically lower than the conditional non-SGA births.
Figure 1.
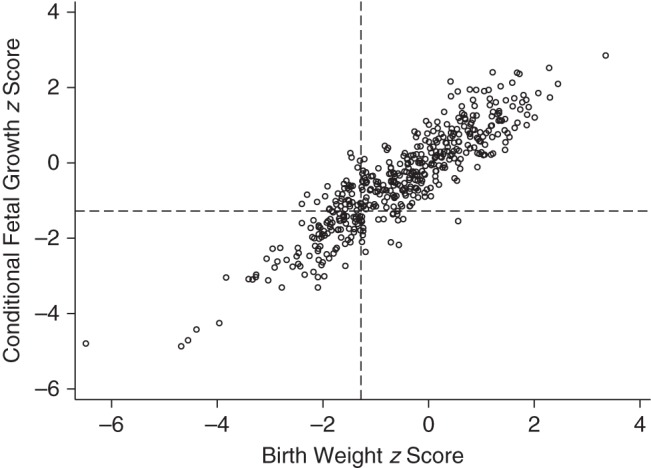
Correlation between conditional fetal growth z scores (weight at birth given estimated fetal weight at the time of a 25-week ultrasonogram) and birth weight-for-gestational-age z scores, Scandinavian Successive Small-for-Gestational-Age Births Study, 1986–1988. Dashed lines indicate a z score of −1.28, corresponding to the 10th percentile.
Figure 2.
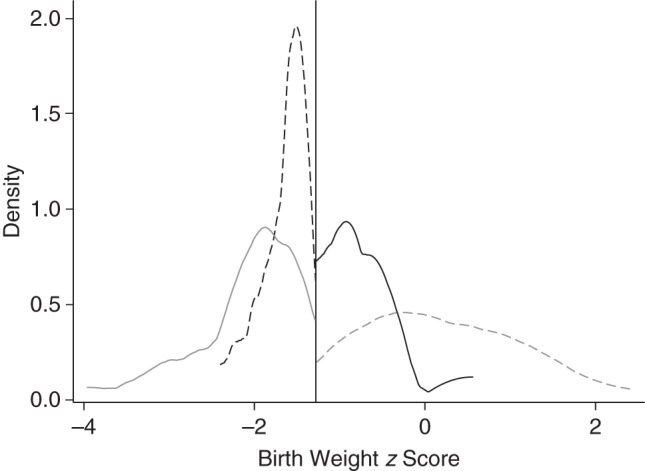
Birth weight z scores of infants born small for gestational age (SGA) (z score <−1.28, 10th percentile) as defined by birth weight and conditional fetal growth z scores, Scandinavian Successive Small-for-Gestational-Age Births Study, 1986–1988. The vertical line indicates a z score of −1.28, corresponding to the 10th percentile. The solid gray line indicates a designation of SGA birth according to both z scores; the dashed gray line indicates a designation of non-SGA birth according to both z scores; the solid black line indicates a designation of SGA birth according to conditional z scores only; and the dashed black line indicates a designation of SGA birth according to birth weight z score only.
Figure 3.
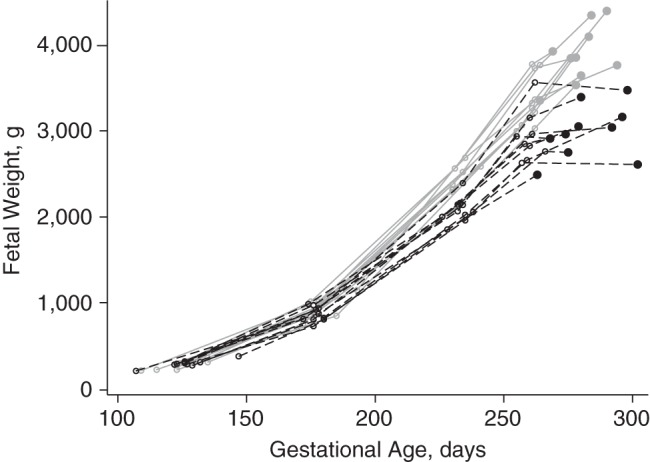
Fetal growth trajectories of 10 randomly selected infants born conditionally small for gestational age (SGA), defined as conditional fetal growth z score <−1.28, based on the change between estimated fetal weight at 25 weeks’ gestation and weight at birth (black dashed lines), and conditionally non-SGA, defined as conditional fetal growth z score ≥1.28 (solid gray lines), Scandinavian Successive Small-for-Gestational-Age Births Study, 1986–1988.
Average values for height, BMI, total skinfold thickness, and head circumference were 111 cm (standard deviation (SD), 5); 15.6 (SD, 1.5); 15.6 mm (SD, 3.4); and 51.9 cm (SD, 1.3), respectively (corresponding to z scores of 0.13 (SD, 1.0); 0.15 (SD, 1.0); −0.08 (SD, 1.0); and −0.26 (SD, 1.1), respectively). We found no evidence of nonlinear relationships between conditional fetal growth/birth weight z scores and age 5 anthropometric characteristics; as a result, z scores were included as linear terms. Figure 4 summarizes the crude relationships between conditional fetal growth z scores, birth weight z scores, and child anthropometric characteristics at age 5 years. Conditional fetal growth z score was positively associated with all 4 early childhood anthropometric outcomes; the strongest association was for head circumference and the weakest for total skinfold thickness (β = 0.24 (95% confidence interval (CI): 0.18, 0.31), β = 0.16 (95% CI: 0.09, 0.23), β = 0.08 (95% CI: 0.01, 0.16), and β = 0.37 (95% CI: 0.22, 0.52) for height, BMI, total skinfold thickness, and head circumference, respectively). However, these associations were very similar to those obtained after replacing conditional fetal growth z score with population birth weight z score (Figure 4, white circles), which was not unexpected given the high correlation between the two measures. Similar results were obtained using alternative conditional fetal growth z score definitions (Web Figure 2).
Figure 4.
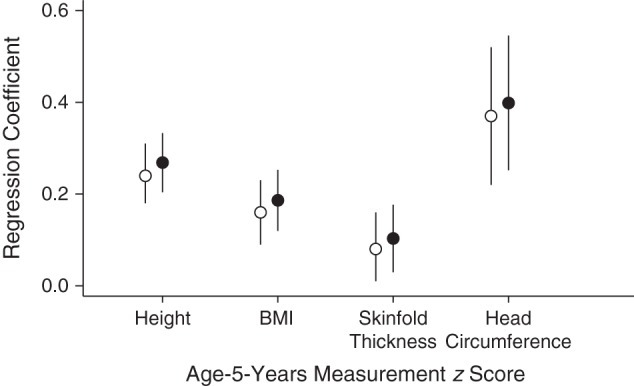
Univariable regression coefficients for the association between fetal growth measurements (birth weight z score (white circles) or birth weight conditional on 25-week estimated fetal weight z score (black circles)) and age-5-years anthropometric characteristics, Scandinavian Successive Small-for-Gestational-Age Births Study, 1986–1988. BMI, body mass index. Vertical bars, 95% confidence intervals.
The independent value of conditional fetal growth z scores in explaining variation in child anthropometry is shown in Table 2. Coefficients for the conditional fetal growth z score residuals were all close to zero, with 95% confidence intervals that included the null. Adjusted R2 values were virtually unchanged after addition of conditional fetal growth z score residuals to the univariable birth weight z score models (all increases in adjusted R2 < 0.01; values available on request), suggesting that they provided little incremental value. Findings were similar in our sensitivity analyses calculating conditional fetal growth z scores using alternative definitions (Table 2). A single exception was the coefficient for the conditional fetal growth z score residual for EFW at 33 weeks given EFW at 25 weeks in the model predicting age 5 head circumference z score, where a positive association was observed (β = 0.37, 95% CI: 0.10, 0.64). However, the increase in adjusted R2 in this model remained small (from 0.21 to 0.22). Terms for interaction between conditional fetal growth z score residuals and birth weight z scores were not statistically significant. A post hoc sensitivity analysis restricting the cohort to Norwegian study sites (because of their higher rates of follow-up) did not meaningfully change our findings (results available on request).
Table 2.
Variation in Children's Anthropometric Characteristics at Age 5 Years Explained by Conditional Fetal Growth z Score Residuals (Above and Beyond Birth Weight z Scores) in the Scandinavian Successive Small-for-Gestational-Age Births Study, 1986–1988
| Conditional Fetal Growth Residual | Age-5-Years Anthropometric Characteristic (z Score) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Height |
Body Mass Indexa |
Total Skinfold Thickness |
Head Circumference |
|||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Birth weight conditional on 25-week EFW | −0.05 | −0.21, 0.10 | −0.04 | −0.20, 0.12 | −0.07 | −0.25, 0.11 | 0.24 | −0.06, 0.54 |
| Birth weight conditional on 33-week EFW | −0.05 | −0.17, 0.06 | −0.001 | −0.12, 0.11 | −0.01 | −0.14, 0.12 | −0.05 | −0.31, 0.22 |
| 33-week EFW conditional on 25-week EFW | 0.02 | −0.10, 0.13 | −0.07 | −0.20, 0.05 | −0.08 | −0.21, 0.06 | 0.37 | 0.10, 0.64 |
| SGAb at birth conditional on both 25- and 33-week EFWs | 0.04 | −0.22, 0.30 | −0.03 | −0.30, 0.25 | 0.15 | −0.15, 0.45 | −0.07 | −0.62, 0.49 |
Abbreviations: CI, confidence interval; EFW, estimated fetal weight; SGA, small for gestational age.
a Weight (kg)/height (m)2.
b Small size for gestational age was defined as z score <−1.28 (10th percentile).
DISCUSSION
In this study, we found that fetal growth pattern (change in EFW) had very little incremental value in explaining child anthropometric characteristics at age 5 years above and beyond information on birth weight for gestational age. This finding appeared to be driven by the strong correlation between conditional fetal growth z scores and birth weight z scores. The result of a decreasing growth trajectory is that the infant is also smaller in size, so conditional fetal growth z scores provided similar information as birth weight z scores. Our findings suggest that information on birth weight alone may be sufficient to examine long-term anthropometric consequences of abnormal fetal growth.
Alternative explanations for our unanticipated results should be considered. First, EFW is known to have measurement error, which may have prevented accurate assessment of fetal growth patterns. However, the accuracy of EFW in our cohort compares favorably to that reported in a systematic review of EFW validation studies (18), so our findings reflect the performance of conditional percentiles in real-world clinical settings. Second, it is possible that the pathological process of growth restriction begins prior to 25 weeks’ gestation and that conditional percentiles should have been calculated conditioning on a first- or early second-trimester weight. Despite evidence of growth restriction in the first trimester (19), it seems unlikely that no further growth restriction occurs from 25 weeks onward (when the majority of fetal fat deposition occurs (14) and when monitoring for fetal growth restriction occurs in clinical practice).
Our findings agree with the literature in several ways. The positive association between birth weight and attained BMI in our cohort has consistently been reported (2), supporting the generalizability of associations in our cohort. Our finding that conditionally small infants tended also to be small in absolute size is similar to other evaluations of fetal growth patterns. Barker et al. (20) grouped a cohort of high-risk fetuses into “pathological” and “normal” growth trajectories and found that the average weight of the pathological group was over 1,000 g lower than that of fetuses with normal trajectories. As with our study, Barker et al. found that the pathological growth group had higher rates of maternal and neonatal complications, but they did not further evaluate the incremental value of trajectory grouping information above and beyond that provided by birth weight alone (20). Our research group has previously shown that conditional fetal growth percentiles calculated using birth weight conditional on a fetal weight estimated via 32-week ultrasound were not better able to predict neonatal mortality and serious neonatal morbidity than birth weight percentiles (21). Other approaches to measurement of fetal growth have likewise not been found to be markedly better in predicting newborn outcomes than size alone (22, 23). Nevertheless, our study examined the difference between fetal growth trajectory and birth weight measurements only in the context of child anthropometric outcomes, so it is possible that the incremental value of fetal growth trajectory data might differ for other health outcomes.
Few studies have examined patterns of fetal growth and anthropometry in early childhood (24). Among 438 children in the Project Viva cohort, child BMI at age 3 years was examined according to cross-tabulated categories of birth weight quartile and second-trimester EFW quartile (with growth classified on the basis of change in quartile between time periods) (25). While age 3 BMI z scores were significantly higher among infants who moved from the first quartile of second-trimester EFW to the fourth quartile of birth weight, the incremental value of growth measurements over birth weight alone was not examined. In the Generation R Study, a study of 6,464 children, Gishti et al. (26) found that increased weight gain in each of the second and third trimesters (based on change in standardized EFW) was positively associated with child BMI at age 6 years (but not childhood general or abdominal fat). The extent to which information on fetal growth trajectory provided new information above and beyond birth weight was also not examined, although the associations between fetal growth measures and child BMI appeared to be weaker than associations between birth weight and child BMI.
Although the Scandinavian Successive Small-for-Gestational-Age Births Study remains one of the largest serial ultrasound studies with long-term follow-up, a larger sample size would have enabled us to explore in more detail the characteristics and outcomes of infants classified as “conditional SGA only” or “birth weight SGA only,” as well as the potential interaction between size and weight. However, the key finding that most conditionally small infants are also small in absolute size would probably not have been altered. Losses to follow-up may have introduced selection bias, but follow-up in the Norwegian counties was excellent (>80%), and losses in Uppsala County were predominantly due to geographical distances/inconvenience, rather than systematic differences in participant characteristics. Nevertheless, replication of our findings in a large, contemporary cohort (such as the Generation R Study (19) or a future follow-up of the INTERGROWTH-21st Project's Fetal Growth Longitudinal Study (27)) would be valuable. Examining the 2 fetal growth measures in relation to child growth trajectories and anthropometry at older ages also is an important area for future research.
Strengths of this study included its unique sampling design, which enabled us to create population-based internal reference values using the 10% random sample. This ensured that our comparison of conditional fetal growth z scores with birth weight z scores was not obscured by differences introduced by the use of external reference charts from different populations. The oversampling of higher-risk pregnancies helped to increase statistical precision in our SGA groups. Rigorous data collection protocols reduced the potential for measurement error (as evidence by the relatively low error in EFWs (18)).
Although they are counter to our hypothesis and to current conceptualizations of fetal growth restriction (1), our findings suggest that detailed ultrasound trajectory data may be less useful than previously believed in identifying pathologically small infants. After using information on absolute size as a screening tool, other approaches altogether (like placental biomarkers) may prove better for discriminating between constitutionally small fetuses and growth-restricted fetuses (28).
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Obstetrics and Gynaecology, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada (Jennifer A. Hutcheon); Department of Public Health and General Practice, Faculty of Medicine, Norwegian University of Science and Technology, Trondheim, Norway (Geir W. Jacobsen); Department of Pediatrics and Department of Epidemiology, Biostatistics and Occupational Health, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Michael S. Kramer, Robert W. Platt); and Department of Laboratory Medicine, Children's and Women's Health, Faculty of Medicine, Norwegian University of Science and Technology, Trondheim, Norway (Marit Martinussen).
Collection of the study data was financed by the National Institute of Child Health and Human Development, US National Institutes of Health (contract 1-HD-4-2803). J.A.H. is the recipient of New Investigator awards from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. R.W.P. holds a Chercheur-National award from the Fonds de la Recherche du Québec–Santé.
Conflict of interest: none declared.
REFERENCES
- 1.Zhang J, Merialdi M, Platt LD et al. Defining normal and abnormal fetal growth: promises and challenges. Am J Obstet Gynecol. 2010;2026:522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;114:496–506. [DOI] [PubMed] [Google Scholar]
- 3.Levitt NS, Lambert EV, Woods D et al. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young South African adults: early programming of cortisol axis. J Clin Endocrinol Metab. 2000;8512:4611–4618. [DOI] [PubMed] [Google Scholar]
- 4.Royal College of Obstetricians and Gynaecologists. The investigation and management of the small-for-gestational-age fetus. London, United Kingdom: Royal College of Obstetricians and Gynaecologists; 2013. (Green-top Guideline no. 31). [Google Scholar]
- 5.Resnik R. One size does not fit all. Am J Obstet Gynecol. 2007;1973:221–222. [DOI] [PubMed] [Google Scholar]
- 6.Altman DG, Hytten FE. Intrauterine growth retardation: let's be clear about it. Br J Obstet Gynaecol. 1989;9610:1127–1132. [DOI] [PubMed] [Google Scholar]
- 7.Gillman MW. Epidemiological challenges in studying the fetal origins of adult chronic disease. Int J Epidemiol. 2002;312:294–299. [PubMed] [Google Scholar]
- 8.Bakketeig LS, Jacobsen G, Hoffman HJ et al. Pre-pregnancy risk factors of small-for-gestational age births among parous women in Scandinavia. Acta Obstet Gynecol Scand. 1993;724:273–279. [DOI] [PubMed] [Google Scholar]
- 9.Vik T, Vatten L, Jacobsen G et al. Prenatal growth in symmetric and asymmetric small-for-gestational-age infants. Early Hum Dev. 1997;48(1-2):167–176. [DOI] [PubMed] [Google Scholar]
- 10.Hadlock FP, Harrist RB, Sharman RS et al. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol. 1985;1513:333–337. [DOI] [PubMed] [Google Scholar]
- 11.Royston P. Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med. 1995;1413:1417–1436. [DOI] [PubMed] [Google Scholar]
- 12.Harrell FE Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;8015:1198–1202. [DOI] [PubMed] [Google Scholar]
- 13.Deter RL, Rossavik IK, Harrist RB et al. Mathematic modeling of fetal growth: development of individual growth curve standards. Obstet Gynecol. 1986;682:156–161. [PubMed] [Google Scholar]
- 14.Owen P, Donnet ML, Ogston SA et al. Standards for ultrasound fetal growth velocity. Br J Obstet Gynaecol. 1996;1031:60–69. [DOI] [PubMed] [Google Scholar]
- 15.Davis CE. The effect of regression to the mean in epidemiologic and clinical studies. Am J Epidemiol. 1976;1045:493–498. [DOI] [PubMed] [Google Scholar]
- 16.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 suppl):1200S–1228S. [DOI] [PubMed] [Google Scholar]
- 18.Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;251:80–89. [DOI] [PubMed] [Google Scholar]
- 19.Mook-Kanamori DO, Steegers EA, Eilers PH et al. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA. 2010;3036:527–534. [DOI] [PubMed] [Google Scholar]
- 20.Barker ED, McAuliffe FM, Alderdice F et al. The role of growth trajectories in classifying fetal growth restriction. Obstet Gynecol. 2013;1222:248–254. [DOI] [PubMed] [Google Scholar]
- 21.Hutcheon JA, Egeland GM, Morin L et al. The predictive ability of conditional fetal growth percentiles. Paediatr Perinat Epidemiol. 2010;242:131–139. [DOI] [PubMed] [Google Scholar]
- 22.Owen P, Harrold AJ, Farrell T. Fetal size and growth velocity in the prediction of intrapartum caesarean section for fetal distress. Br J Obstet Gynaecol. 1997;1044:445–449. [DOI] [PubMed] [Google Scholar]
- 23.Owen P, Khan KS. Fetal growth velocity in the prediction of intrauterine growth retardation in a low risk population. Br J Obstet Gynaecol. 1998;1055:536–540. [DOI] [PubMed] [Google Scholar]
- 24.Alkandari F, Ellahi A, Aucott L et al. Fetal ultrasound measurements and associations with postnatal outcomes in infancy and childhood: a systematic review of an emerging literature. J Epidemiol Community Health. 2015;691:41–48. [DOI] [PubMed] [Google Scholar]
- 25.Parker M, Rifas-Shiman SL, Oken E et al. Second trimester estimated fetal weight and fetal weight gain predict childhood obesity. J Pediatr. 2012;1615:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gishti O, Gaillard R, Manniesing R et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. J Clin Endocrinol Metab. 2014;997:2557–2566. [DOI] [PubMed] [Google Scholar]
- 27.Papageorghiou AT, Ohuma EO, Altman DG et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;3849946:869–879. [DOI] [PubMed] [Google Scholar]
- 28.Benton SJ, Hu Y, Xie F et al. Can placental growth factor in maternal circulation identify fetuses with placental intrauterine growth restriction? Am J Obstet Gynecol. 2012;2062:163.e1–163.e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


