Abstract
The most consistently reported risk indicators for the male genital anomalies cryptorchidism and hypospadias are prematurity and low birth weight. Placental dysfunction has been hypothesized as a possible underlying cause, and an association between placental weight at birth and hypospadias has been indicated. In a population-based cohort of 388,422 Danish singleton boys born alive (1997–2008), we studied the association between placental weight and cryptorchidism and hypospadias. Missing data were handled with multiple imputation, and we estimated hazard ratios by means of Cox regression models. During follow-up, 1,713 boys were diagnosed with hypospadias and 6,878 with cryptorchidism (3,624 underwent corrective surgery). We observed an association between low placental weight and risk of both genital anomalies. Boys with a placental weight in the lowest decile (<10%) had higher risks of both cryptorchidism (hazard ratio = 1.52, 95% confidence interval: 1.31, 1.76) and hypospadias (hazard ratio = 1.97, 95% confidence interval: 1.59, 2.45) than boys in the reference decile (50.0–59.9%). In conclusion, we found higher risks of both genital malformations in boys born with a low placental weight. The relationship seemed stronger for hypospadias than for cryptorchidism. Taken together, our data support a role for placental dysfunction in the etiology of these anomalies.
Keywords: congenital abnormalities, cryptorchidism, hypospadias, placenta, pregnancy, risk factors
Cryptorchidism and hypospadias are frequent genital anomalies. Many potential risk indicators have been studied, but the etiology of these anomalies remains largely unknown. A growing body of literature has underlined associations with early delivery and low birth weight (1–4), presumably reflecting an association with fetal growth restriction. This has led to suggestions that there are shared risk factors for fetal growth restriction and these genital malformations (3–5), and we hypothesize that first-trimester placental dysfunction constitutes at least part of these common factors. During this period, Leydig cell testosterone biosynthesis in the fetal testis is stimulated by human chorionic gonadotropin from the placenta (6, 7). Insufficient androgen production may adversely affect masculinization, urethral fusion, and testicular descent (8–10), and an intricate interplay between early placental function and fetal genital development is plausible.
It has been suggested that the weight of the placenta at birth or the ratio between placental weight and birth weight is related to placental function (11–13). Consequently, these measures have received considerable attention regarding fetal origins of perinatal, child, and adult morbidity (12, 14, 15). A few small studies have shown lower placental weight among boys with hypospadias (16–20), whereas a potential association with cryptorchidism has not been revealed.
In this large registry-based study, we investigated the associations between placental weight at birth and the occurrence of cryptorchidism and hypospadias in a nationwide population-based Danish cohort of 388,422 singleton boys.
METHODS
Study population
We identified all singleton boys born alive in Denmark between January 1, 1997, and December 31, 2008 (n = 388,422) from the Danish Civil Registration System (21). The system assigns a unique personal identifier to all residents of Denmark upon birth or immigration and enables individual-level data linkage with other Danish registries. Pregnancy and birth-related data were available from the Danish Medical Birth Registry (22), which is based on mandatory reporting by the attending midwife or physician shortly after delivery (both in-hospital and at-home deliveries). Placental weight at birth (in grams) has been recorded since 1997; immediately after delivery of the placenta, it is examined systematically by the midwife and placed in a plastic bag, which is subsequently weighted using a digital weight scale. All anthropometric measurements, including placental weight, are recorded on standard forms as part of routine clinical practice.
We obtained information on diagnoses of congenital anomalies, including cryptorchidism and hypospadias, as well as corrective surgical procedures, from the Danish National Patient Registry, which holds information on all in- and outpatient medical contacts in Denmark (23, 24). Boys with a diagnosis of cryptorchidism (International Classification of Diseases, Tenth Revision (ICD-10), codes DQ53, DQ531, DQ531A, DQ532, DQ532A, and DQ539) that persisted until the time of surgery (corrective orchiopexy; Nordic Classification of Surgical Procedures codes KKFH00, KKFH01, and KKFH10) were considered cryptorchid cases. Boys with a diagnosis of hypospadias (ICD-10 codes DQ540, DQ541, DQ542, DQ548, and DQ549) were defined as hypospadias cases. We excluded boys diagnosed with other congenital anomalies or genetic syndromes (ICD-10 codes Q00–Q99) during follow-up (January 1, 1997–October 21, 2009), as our focus was on nonsyndromic cases of cryptorchidism and hypospadias.
Statistical analyses
Missing information
In total, 95% of the participants had complete information on exposure, outcome, and covariates used in the main analyses. For all participants, the outcome variables were completely recorded. Information on placental weight was missing for 4.6% of participants, and information on other covariates was missing for 0%–1.5% of participants. Information on maternal cigarette smoking, used in a secondary analysis, was available for the years 1997–2007 and was missing among 4.3% of the participants during this period. Based on the assumption that data were missing at random, we applied the multiple imputation method (25), including only subjects with recorded information on at least 1 of the variables birth weight, placental weight, and gestational age. We performed multiple imputation using chained equations. The following variables were included in the model: placental weight (g), birth weight (g), gestational age (weeks), cryptorchidism (yes/no), hypospadias (yes/no), parity (continuous), maternal age (years), and birth year (1997–2008; continuous), and 15 complete data sets were created.
Directed acyclic graphs
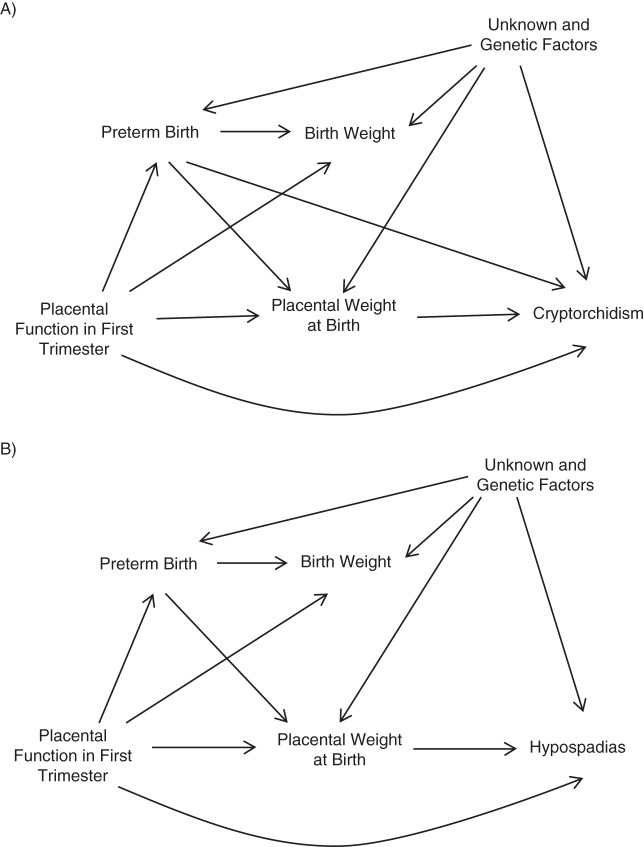
We defined the causal framework of the associations studied using directed acyclic graphs (26, 27). Figure 1 presents the graphs visualizing our hypotheses on the interrelations between placental weight and cryptorchidism (A) and hypospadias (B).
Figure 1.
Directed acyclic graphs for the hypothesized causal structure of associations between placental weight at birth and cryptorchidism (A) and hypospadias (B).
We reasoned that placental function during the first trimester of pregnancy may affect placental weight at birth, duration of gestation, and birth weight. There is consistent evidence that preterm birth and low birth weight are strong risk indicators for both cryptorchidism and hypospadias. However, the fusion of the urethral folds takes place during gestational weeks 8–14; therefore, duration of gestation is not in itself causally related to hypospadias. On the contrary, the origin of cryptorchidism could lie in either early or late gestation. Testicular descent is a complex 2-stage process; the transabdominal phase occurs between weeks 8 and 15 of gestation, and around weeks 25–28, the testes descend through the inguinal canal (transinguinal phase) and may not be located in the scrotum until weeks 35–40 of gestation (28). Therefore, it is possible and perhaps even likely that gestational age may act as a confounder in the association between placental weight and cryptorchidism. Naturally, birth weight per se is not causally related to either cryptorchidism or hypospadias and thus is not a potential confounder. We therefore found no justification for adjusting our results for birth weight.
Data analyses
We detected extreme outlying values of placental weight by truncating the sample at the mean value for gestational age ± 5 standard deviations (weeks 22–45). This was done with placental weight, birth weight, and gestational age on a log scale for a better model fit. The outlying values (n = 277) were subsequently imputed by use of multiple imputation.
We used Cox proportional hazards regression to account for variations in follow-up time, as several cases were diagnosed not at birth but during infancy (29). We estimated adjusted hazard ratios and 95% confidence intervals for cryptorchidism and hypospadias according to deciles of placental weight using the percentiles 50.0–59.9% as the reference category. For cryptorchidism, we further assessed gestational-age-specific z scores for placental weight, as placental weight varies by duration of gestation at delivery. These were calculated using means and standard deviations of placental weight from our study population.
The boys' age (in days) was used as the time scale. Follow-up started at birth, and the boys were followed until first diagnosis, death, emigration, or the end of follow-up (October 21, 2009), whichever came first. We adjusted for maternal age (years; continuous) and calendar year at birth (continuous), the latter to account for possible systematic changes in the prevalence of the congenital anomalies over time. Because the data set included siblings, confidence intervals were based on robust standard errors to account for maternal clustering.
In secondary analyses, we 1) adjusted for maternal cigarette smoking (yes/no), because maternal cigarette smoking in early pregnancy is a possible confounder; 2) assessed the risk of cryptorchidism using all diagnosed cases, because diagnosed boys who do not receive surgery may represent true cases with spontaneous testicular descent; 3) checked the robustness of the results from different multiple imputation models; and 4) repeated the analyses in the subset of complete cases (96%).
The study protocol was approved by the Danish Data Protection Agency. The statistical analyses were performed in STATA 13 (StataCorp LP, College Station, Texas).
RESULTS
Between January 1, 1997, and December 31, 2008, a total of 388,422 singleton boys were born alive in Denmark. We excluded 22,764 boys (5.9%) with other congenital anomalies and 3,141 boys (0.9%) who did not fulfill our criteria for performing multiple imputations. Thus, a total of 362,517 boys constituted the final study population. During follow-up, 6,878 boys were diagnosed with cryptorchidism, among whom 3,624 had corrective surgery (10.0 per 1,000 births). A total of 1,713 hypospadias cases (4.7 per 1,000 births) were diagnosed during follow-up.
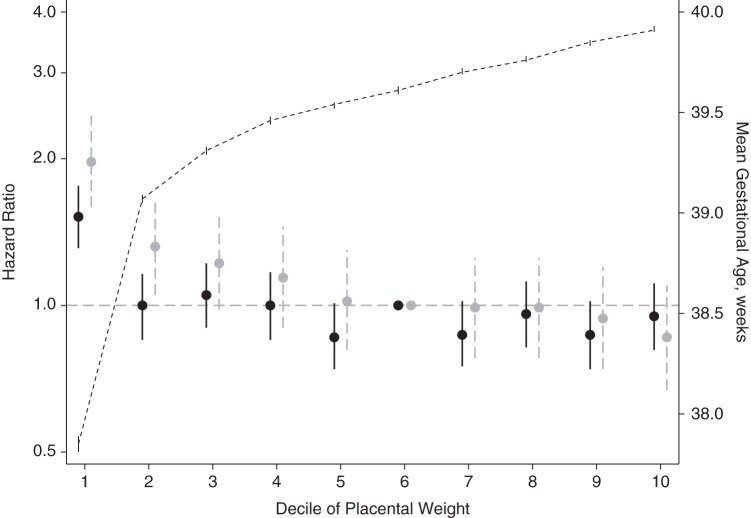
Low placental weight at birth was associated with higher risks of being diagnosed with both genital anomalies (Table 1 and Figure 2). We observed an inverse association between low placental weight and risk of hypospadias up to the reference decile (50.0–59.9%) of placental weight. Boys in the lowest decile of placental weight had the highest risk of hypospadias (adjusted hazard ratio (aHR) = 1.97, 95% confidence interval (CI): 1.59, 2.45) compared with boys in the percentiles 50.0–59.9%. There was a tendency toward a lower risk in the highest decile (aHR = 0.86, 95% CI: 0.67, 1.10) in comparison with the reference group. For cryptorchidism, risk was increased only for boys with the lowest placental weights (aHR = 1.52, 95% CI: 1.31, 1.76).
Table 1.
Associations Between Placental Weight and Genital Anomalies Among 362,517 Danish Singleton Boys, 1997–2008
| Percentile of Placental Weight | Distribution of Participants, % | Cryptorchidism |
Hypospadias |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases, % | Crude HR | aHRa | 95% CI | Cases, % | Crude HR | aHRa | 95% CI | ||
| <10.0 | 8.8 | 13.3 | 1.57 | 1.52 | 1.31, 1.76 | 15.2 | 1.98 | 1.97 | 1.59, 2.45 |
| 10.0–19.9 | 9.7 | 9.7 | 1.03 | 1.00 | 0.85, 1.16 | 11.3 | 1.33 | 1.32 | 1.05, 1.65 |
| 20.0–29.9 | 10.9 | 11.5 | 1.08 | 1.05 | 0.90, 1.22 | 11.8 | 1.24 | 1.22 | 0.98, 1.53 |
| 30.0–39.9 | 8.3 | 8.5 | 1.03 | 1.00 | 0.85, 1.17 | 8.5 | 1.14 | 1.14 | 0.90, 1.45 |
| 40.0–49.9 | 11.7 | 10.1 | 0.88 | 0.86 | 0.74, 1.01 | 10.6 | 1.04 | 1.02 | 0.81, 1.30 |
| 50.0–59.9 | 8.6 | 8.5 | 1.00 | 1.00 | Referent | 7.6 | 1.00 | 1.00 | Referent |
| 60.0–69.9 | 11.1 | 9.8 | 0.89 | 0.87 | 0.75, 1.02 | 9.7 | 1.00 | 0.99 | 0.78, 1.25 |
| 70.0–79.9 | 10.9 | 10.4 | 0.98 | 0.96 | 0.82, 1.12 | 9.5 | 1.00 | 0.99 | 0.78, 1.25 |
| 80.0–89.9 | 9.4 | 8.2 | 0.89 | 0.87 | 0.74, 1.02 | 7.8 | 0.95 | 0.94 | 0.74, 1.20 |
| ≥90.0 | 10.6 | 10.0 | 0.96 | 0.95 | 0.81, 1.11 | 8.0 | 0.85 | 0.86 | 0.67, 1.10 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio.
a Adjusted for calendar year at birth (continuous) and maternal age (years; continuous).
Figure 2.
Hazard ratios for cryptorchidism (black) and hypospadias (gray) among singleton boys born alive according to deciles of placental weight at birth, Denmark, 1997–2008. The short-dashed line presents the mean gestational age (z-axis) according to deciles of placental weight at birth. Vertical lines represent 95% confidence intervals.
In Figure 2, the estimates are graphically presented accompanied by the mean gestational age in each placental weight decile. Boys in the lowest decile of placental weight had a mean gestational age of 37.85 weeks (95% CI: 37.81, 37.89), as compared with 39.54 weeks (95% CI: 39.52, 39.55) in the percentiles 50.0–59.9%. Because gestational age could confound the association with cryptorchidism, we further assessed the risk of cryptorchidism according to gestational-age-specific placental weight z score. The estimates were attenuated, but the higher risk of genital malformations in the lowest decile persisted (see Web Table 1, available at http://aje.oxfordjournals.org/).
Further adjustment for maternal cigarette smoking did not change the results (Web Table 2). In the analysis including all boys diagnosed with cryptorchidism, we observed similar (if somewhat attenuated) risk estimates as those seen in the main analyses including only cryptorchidism cases verified by surgical correction (Web Table 3).
We assessed the validity of the analyses based on multiple imputations, both by fitting alternative imputation models and by performing complete-case analyses. Both approaches produced results similar to those presented (data not shown).
DISCUSSION
This large population-based study showed a higher risk of genital anomalies among boys born with a low placental weight. Previously stated hypotheses of shared risk factors for fetal growth restriction and genital anomalies (3–5) prompted us to study the influence of placental weight on cryptorchidism and hypospadias. Under the assumption that placental weight at birth reflects placental function, we aimed to address the following question: Is poor placental function part of the “shared risk factors” for fetal growth restriction and genital malformations? Our results corroborated those from previous reports of a lower placental weight in boys born with hypospadias, and we extended this association to cryptorchidism (16–20). The previous studies were all small, and to our knowledge the present study was the first large registry-based study on this topic. We observed a clear relationship between low placental weight and hypospadias, but for cryptorchidism the association was weaker and persisted only for the smallest 10% of placentas. Taken together, our data suggest that placental dysfunction is involved in the etiology of genital anomalies, especially for hypospadias.
The interrelations between placental weight, birth weight, gestational age, and the genital malformations studied are complex. Placental weight at birth increases with duration of gestation, but not necessarily in a linear manner. Further, placental weight measured at birth may not be representative of placental weight for fetuses still in utero, and both gestational age and the weights of the placenta and the child are influenced by several unknown and unmeasured factors. Thus, the limited understanding of the true relationships between placental function, gestational age, and placental weight at birth makes decisions for statistical modeling complicated. Based on the causal framework presented in the directed acyclic graphs (Figure 1) and the widely accepted theory that adjustment for birth weight or gestational age often introduces collider-stratification bias (30–32), we chose a simple and direct focus on the association between placental weight and genital anomalies, without adjustment for gestational age or birth weight. In our secondary analysis of placental weight z scores for gestational age and risk of cryptorchidism, we could have introduced bias from unmeasured factors that act on both the risk of preterm birth and the risk of cryptorchidism, but the extent of possible bias is difficult to assess. Adjustment for gestational age, however, provided similar estimates (Web Table 1), suggesting that unknown factors determining gestational age were not strong confounding factors.
A major strength of our study was the large population-based cohort design, without selective participation or attrition during follow-up. The Danish health registries we used have all been validated and have good coverage (22–24). Reporting to the Danish Medical Birth Registry is routinely performed by midwives and is mandatory by law for both in-hospital and at-home deliveries (22). Although we do expect some variability in the weighing of placentae, we find it unlikely that this would be systematically associated with case status, except perhaps for the most severe cases of hypospadias. Registration of placental weight took place prior to diagnosis of genital anomalies, making any misclassification of placental weight most likely nondifferential (30). We obtained data on genital anomalies from the Danish National Patient Registry. Data on the genital malformations studied are considered valid. Hypospadias diagnoses have not yet been formally validated; however, Pedersen et al. (33) assessed the completeness of diagnosis among 43 hypospadias cases in Denmark and found only 3 cases to be misclassified. To diminish the potential for misclassification of cryptorchidism due to incorrect case registration, we based the case ascertainment on diagnosis of cryptorchidism combined with registration of corrective surgical treatment (orchiopexy), a strategy shown to increase the positive predictive value to 99% (34). Consequently, only very limited misclassification of the genital anomalies would be expected. We cannot reach conclusions on associations between placental weight and very mild types of genital malformations, because they may not have come to clinical attention.
We had the ability to adjust for some potentially confounding factors. In the main analysis, we adjusted for calendar year at birth and maternal age. One could also reason that maternal cigarette smoking is a confounder of the placental weight–genital anomaly association; thus, we decided to adjust for maternal cigarette smoking in secondary analyses, and the results were essentially unchanged (Web Table 2). Due to the registry-based nature of the study, we lacked information on some potentially confounding factors and data on placental growth and function. Unquestionably, neonates with deviant placental weights at birth carry additional and heterogeneous risks reflecting the reason for placental insufficiency. These risks could also influence the occurrence of genital malformations, and ideally adjustment should be made. However, we excluded boys diagnosed with syndromes or chromosomal abnormalities during follow-up, which dealt with part of this potential problem. Unmeasured factors, such as circumstances at delivery and obstetrical complications, could possibly have influenced the procedure of placental weighing, as well as the actual weight recorded (35). However, because the etiological time window for genital anomalies is in early pregnancy, factors present at birth cannot confound the associations studied. Errors in the measurement of placental weights were most likely nondifferential and might have attenuated the associations.
A well-functioning placenta is essential for fetal growth and development. Through its production of human chorionic gonadotropin, it also plays a vital role in genital development and testicular descent (7). During the proposed “male programming window” in gestational weeks 8–14 (8–10), the formation of reproductive organs depends on sufficient androgen action. Leydig cell testosterone biosynthesis is stimulated by human chorionic gonadotropin from the placenta (6, 7). Thus, placental dysfunction at this stage of gestation may in turn lead to inadequate testosterone levels and interference with normal genital development. Studies investigating the role of placental hormones in the risk of genital anomalies are sparse. Lower levels of maternal human chorionic gonadotropin measured in gestational weeks 12–16 have been reported among cryptorchid boys as compared with normal boys (36). Correspondingly, high maternal α-fetoprotein levels, possibly reflecting placental dysfunction, have also been associated with an increased risk of cryptorchidism (37). This lends support to the hypothesis that placental hormonal dysfunction affects genital development, but it needs further investigation.
It also alludes to another important and ongoing debate: How well does the weight of the placenta at birth reflect its function during pregnancy? Placental hormones and placental weight at birth are correlated in animal studies (38), and very small placentas are known to be dysfunctional in humans (11, 39), with reduced capability of providing sufficient oxygen and nutrition to the fetus. On the other hand, the implications of a large placenta are uncertain. It is a crude measure that probably reflects multiple etiologies (40), and a large placenta could be a physiological compensatory mechanism in response to placental dysfunction (41). However, we observed no increased risk of these genital anomalies with a large placenta. Even if placental function is quantifiable by placental weight, placental weight may not capture placental function during the vulnerable time window wherein genital development and testicular descent occurs. Therefore, despite our findings, a naive causal interpretation of the crude association between placental weight and genital anomalies should be avoided, and the questions of whether placental dysfunction is involved, the strength of its influence, and whether alternative causes exist remain to be answered by future studies.
In summary, we corroborated the association between low placental weight and hypospadias, and we also observed a higher risk of cryptorchidism with low placental weight. Taken together, our data suggest that placental dysfunction is involved in the etiology of genital anomalies, and it may be a good candidate for at least part of the shared risk factors for fetal growth restriction and genital anomalies.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Section for Epidemiology, Department of Public Health, Faculty of Health, Aarhus University, Aarhus, Denmark (Linn Håkonsen Arendt, Cecilia Høst Ramlau-Hansen, Jørn Olsen); Perinatal Epidemiology Research Unit, Department of Pediatrics, Aarhus University Hospital, Aarhus, Denmark (Linn Håkonsen Arendt, Morten Søndergaard Lindhard, Tine Brink Henriksen); Epidemiology Branch, National Institute of Environmental Health Sciences, Durham, North Carolina (Allen J. Wilcox); and Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark (Jørn Olsen).
This work was supported by a grant from the Faculty of Health, Aarhus University (Aarhus, Denmark).
Conflict of interest: none declared.
REFERENCES
- 1.Jones ME, Swerdlow AJ, Griffith M et al. Prenatal risk factors for cryptorchidism: a record linkage study. Paediatr Perinat Epidemiol. 1998;124:383–396. [DOI] [PubMed] [Google Scholar]
- 2.Akre O, Lipworth L, Cnattingius S et al. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology. 1999;104:364–369. [PubMed] [Google Scholar]
- 3.Weidner IS, Møller H, Jensen TK et al. Risk factors for cryptorchidism and hypospadias. J Urol. 1999;1615:1606–1609. [PubMed] [Google Scholar]
- 4.Jensen MS, Wilcox AJ, Olsen J et al. Cryptorchidism and hypospadias in a cohort of 934,538 Danish boys: the role of birth weight, gestational age, body dimensions, and fetal growth. Am J Epidemiol. 2012;1759:917–925. [DOI] [PubMed] [Google Scholar]
- 5.Møller H, Weidner IS. Epidemiology of cryptorchidism and hypospadias. Epidemiology. 1999;104:352–354. [PubMed] [Google Scholar]
- 6.Clements JA, Reyes FI, Winter JS et al. Studies on human sexual development. III. Fetal pituitary and serum, and amniotic fluid concentrations of LH, CG, and FSH. J Clin Endocrinol Metab. 1976;421:9–19. [DOI] [PubMed] [Google Scholar]
- 7.Huhtaniemi IT, Korenbrot CC, Jaffe RB. HCG binding and stimulation of testosterone biosynthesis in the human fetal testis. J Clin Endocrinol Metab. 1977;445:963–967. [DOI] [PubMed] [Google Scholar]
- 8.Welsh M, Saunders PT, Fisken M et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;1184:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macleod DJ, Sharpe RM, Welsh M et al. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl. 2010;332:279–287. [DOI] [PubMed] [Google Scholar]
- 10.van den Driesche S, Kolovos P, Platts S et al. Inter-relationship between testicular dysgenesis and Leydig cell function in the masculinization programming window in the rat. PLoS One. 2012;71:e30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thame M, Osmond C, Bennett F et al. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004;586:894–900. [DOI] [PubMed] [Google Scholar]
- 12.Haavaldsen C, Samuelsen SO, Eskild A. Fetal death and placental weight/birthweight ratio: a population study. Acta Obstet Gynecol Scand. 2013;925:583–590. [DOI] [PubMed] [Google Scholar]
- 13.Strøm-Roum EM, Haavaldsen C, Tanbo TG et al. Placental weight relative to birthweight in pregnancies with maternal diabetes mellitus. Acta Obstet Gynecol Scand. 2013;927:783–789. [DOI] [PubMed] [Google Scholar]
- 14.Risnes KR, Romundstad PR, Nilsen TI et al. Placental weight relative to birth weight and long-term cardiovascular mortality: findings from a cohort of 31,307 men and women. Am J Epidemiol. 2009;1705:622–631. [DOI] [PubMed] [Google Scholar]
- 15.Hutcheon JA, McNamara H, Platt RW et al. Placental weight for gestational age and adverse perinatal outcomes. Obstet Gynecol. 2012;1196:1251–1258. [DOI] [PubMed] [Google Scholar]
- 16.Stoll C, Alembik Y, Roth MP et al. Genetic and environmental factors in hypospadias. J Med Genet. 1990;279:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatti JM, Kirsch AJ, Troyer WA et al. Increased incidence of hypospadias in small-for-gestational age infants in a neonatal intensive-care unit. BJU Int. 2001;876:548–550. [DOI] [PubMed] [Google Scholar]
- 18.Boisen KA, Chellakooty M, Schmidt IM et al. Hypospadias in a cohort of 1072 Danish newborn boys: prevalence and relationship to placental weight, anthropometrical measurements at birth, and reproductive hormone levels at three months of age. J Clin Endocrinol Metab. 2005;907:4041–4046. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto T, Suwa T, Kabe K et al. Placental insufficiency in early gestation is associated with hypospadias. J Pediatr Surg. 2008;432:358–361. [DOI] [PubMed] [Google Scholar]
- 20.Yinon Y, Kingdom JC, Proctor LK et al. Hypospadias in males with intrauterine growth restriction due to placental insufficiency: the placental role in the embryogenesis of male external genitalia. Am J Med Genet A. 2010;152A1:75–83. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22–25. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;453:320–323. [PubMed] [Google Scholar]
- 23.Andersen TF, Madsen M, Jørgensen J et al. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;463:263–268. [PubMed] [Google Scholar]
- 24.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 suppl):30–33. [DOI] [PubMed] [Google Scholar]
- 25.Sterne JA, White IR, Carlin JB et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;115:550–560. [DOI] [PubMed] [Google Scholar]
- 27.Hernán MA, Hernández-Díaz S, Werler MM et al. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;1552:176–184. [DOI] [PubMed] [Google Scholar]
- 28.Hutson JM, Nation T, Balic A et al. The role of the gubernaculum in the descent and undescent of the testis. Ther Adv Urol. 2009;12:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen MS, Olsen LH, Thulstrup AM et al. Age at cryptorchidism diagnosis and orchiopexy in Denmark: a population based study of 508,964 boys born from 1995 to 2009. J Urol. 2011;186(4 suppl):1595–1600. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 31.Hernández-Díaz S, Wilcox AJ, Schisterman EF et al. From causal diagrams to birth weight-specific curves of infant mortality. Eur J Epidemiol. 2008;233:163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;1749:1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen L, Skriver MV, Nørgaard M et al. Maternal use of loratadine during pregnancy and risk of hypospadias in offspring. Int J Med Sci. 2006;31:21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen MS, Snerum TM, Olsen LH et al. Accuracy of cryptorchidism diagnoses and corrective surgical treatment registration in the Danish National Patient Registry. J Urol. 2012;1884:1324–1329. [DOI] [PubMed] [Google Scholar]
- 35.Leary SD, Godfrey KM, Greenaway LJ et al. Contribution of the umbilical cord and membranes to untrimmed placental weight. Placenta. 2003;24(2-3):276–278. [DOI] [PubMed] [Google Scholar]
- 36.Chedane C, Puissant H, Weil D et al. Association between altered placental human chorionic gonadotrophin (hCG) production and the occurrence of cryptorchidism: a retrospective study. BMC Pediatr. 2014;14:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyd HA, Myrup C, Wohlfahrt J et al. Maternal serum alpha-fetoprotein level during pregnancy and isolated cryptorchidism in male offspring. Am J Epidemiol. 2006;1645:478–486. [DOI] [PubMed] [Google Scholar]
- 38.Hau J, Skovgaard Jensen HJ. Diagnosis and monitoring of pregnancy in mice: correlations between maternal weight, fetal and placental mass and the maternal serum levels of progesterone, pregnancy-associated murine protein-2 and alpha-fetoprotein. Lab Anim. 1987;214:306–310. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa J, Arakawa K, Nakamura M et al. Analysis of placental weight centiles is useful to estimate cause of fetal growth restriction. J Obstet Gynaecol Res. 2011;3711:1658–1665. [DOI] [PubMed] [Google Scholar]
- 40.McNamara H, Hutcheon JA, Platt RW et al. Risk factors for high and low placental weight. Paediatr Perinat Epidemiol. 2014;282:97–105. [DOI] [PubMed] [Google Scholar]
- 41.Haavaldsen C, Samuelsen SO, Eskild A. The association of maternal age with placental weight: a population-based study of 536,954 pregnancies. BJOG. 2011;11812:1470–1476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.