Abstract
Neighborhood socioeconomic status (NSES) is associated with cognitive function, independently of individual demographic, health, and socioeconomic characteristics. However, research has been largely cross-sectional, and mechanisms of the association are unknown. In 1992–1993, Cardiovascular Health Study participants (n = 3,595; mean age = 74.8 years; 15.7% black) underwent cognitive testing and magnetic resonance imaging of white matter hyperintensities (WMH), and their addresses were geocoded. NSES was calculated using 1990 US Census data (block groups; 6 measures of wealth, education, and occupation). The Modified Mini-Mental State Examination (3MS) was used to assess general cognition, and the Digit Symbol Substitution Test (DSST) was used to assess speed of processing annually for 6 years. Associations of race-specific NSES tertiles with 3MS, DSST, and WMH were estimated using linear mixed-effects models accounting for geographic clustering, stratified by race, and adjusted for demographic, health, and individual socioeconomic status (education, income, lifetime occupational status) variables. In fully adjusted models, higher NSES was associated with higher 3MS scores in blacks (mean difference between highest and lowest NSES = 2.4 points; P = 0.004) and whites (mean difference = 0.7 points; P = 0.02) at baseline but not with changes in 3MS over time. NSES was marginally associated with DSST and was not associated with WMH. Adjustment for WMH did not attenuate NSES-3MS associations. Associations of NSES with cognition in late adulthood differ by race, are not explained by WMH, and are evident only at baseline.
Keywords: aging, cognitive function, health disparities, neighborhood socioeconomic status, white matter hyperintensities
Poor cognitive function increases in prevalence with older age and contributes to disability and loss of independence (1–3). Previous studies have demonstrated an association between neighborhood socioeconomic status (NSES) and cognitive function in adults across a range of ages and populations, independent of individual demographic and health characteristics (4–11). However, these studies have been almost exclusively cross-sectional, so it is unknown whether NSES is associated with declines in cognitive function. Further, racial differences in these associations and potential underlying mechanisms have not been well characterized.
NSES, as a compositional effect, is unlikely to have a direct influence on individual cognitive function. Rather, NSES may indicate lower access to and quality of institutional resources, increased levels of stress, and poorer norms regarding healthy behaviors or lesser ability to engage in healthy behaviors—such as physical activity, healthy diet, and not smoking—all of which affect cognition (5, 8, 12–14).
One way to clarify the potential mechanisms linking NSES and cognition is to understand the neural pathology underlying these associations. Small vessel disease (SVD) of the brain, visible upon magnetic resonance imaging (MRI) as white matter hyperintensities (WMH), is associated with lower cognitive function (15). Many risk factors for SVD (16) are associated with living in low–socioeconomic status (SES) neighborhoods through many of the same pathways described above. These include cardiovascular disease and vascular risk factors (17–20), diabetes (21, 22), and inflammation (20, 23). Therefore, low NSES could, for example, increase the likelihood of engaging in poorer health behaviors and confer greater risk of vascular disease, SVD, and poorer cognitive function.
Residential segregation in the United States has resulted in many blacks living in poorer neighborhoods relative to whites of similar socioeconomic status (24). As a result, factors related to NSES that influence cognitive function may be more prevalent for blacks than for whites. There is some evidence that NSES is more strongly related to cognitive function in minorities than in whites (8); however, most studies have included only persons of a single race/ethnicity or have not stratified analyses by race/ethnicity.
Using 6 years of longitudinal data from the Cardiovascular Health Study (CHS), we sought to determine whether NSES was associated with declines in the cognitive function of older adults, whether these associations differed by race, and whether these associations were explained by the presence of WMH. A measure of NSES previously constructed for the CHS has been found to be associated with greater subclinical cardiovascular disease (17), higher incidence of ischemic stroke (25), and higher cardiovascular mortality (26).
We hypothesized that lower NSES would be associated with poorer cognitive function, greater decline in cognitive function over time, and higher prevalence of WMH. We further hypothesized that associations would be stronger in blacks than in whites and that WMH would partially account for differences in cognitive function by NSES.
METHODS
Sample
The CHS is a population-based sample of 5,888 adults aged 65 years or older in 4 regions of the United States (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania). Initial recruitment in 1989–1990 was from a random sample of the Medicare eligibility lists and age-eligible household members living in census tracts close to the participating medical centers (27). A minority supplement was included in 1992–1993. Individuals were eligible to participate if they did not have cancer under active treatment, were not wheelchair users or bedbound, and did not plan to move out of the area within 3 years. The 1992–1993 study visit included an MRI scan and was the baseline for these analyses. Institutional review board approval was obtained from all participating locations, and all participants provided written informed consent at recruitment.
Participants were excluded from these analyses if they were missing data for any variable, except income or occupational status. Due to the low number of participants of other races, only whites and blacks were included. Of the 5,888 participants in CHS, 3,241 (55.0%) had complete data for these analyses. The primary reason for missing data was ineligibility (e.g., because of claustrophobia or metal implants) for or refusal of MRI (n = 2,258; 38.4%). In addition, 14 individuals were missing NSES data, 195 were missing cognitive data, 164 were missing covariate data, and 16 were of neither white nor black race. Persons with missing data were older, had lower incomes and educational attainment, and were more likely to have hypertension, heart disease, stroke, or diabetes; they also had a higher body mass index, a higher white matter grade, lower Digit Symbol Substitution Test (DSST) scores, and lower Modified Mini-Mental State Examination (3MS) scores than persons who were included (P < 0.002 for all).
Neighborhood SES
An NSES variable was previously constructed for CHS (26). Block groups, a subdivision of US census tracts, defined participant neighborhoods. A summary neighborhood score was constructed by summing z scores for 6 variables from the 1990 US Census that represented dimensions of wealth and income (median household income, median value of housing units, and percentage of households receiving interest, dividend, or net rental income), education (percentage of adults who completed high school, percentage of adults who completed college), and occupation (percentage of persons in managerial or professional specialty occupations). Higher scores represented higher NSES.
Cognitive function
Cognitive function was assessed annually through the 1998–1999 study visit, providing 6 years of follow-up, using the 3MS and the DSST. The 3MS measures global cognition; scores range from 0 to 100, with higher scores indicating better cognition (28). The DSST is a measure of processing speed, with higher scores indicating faster speed of processing (29).
Small vessel disease
Standardized sagittal T1-weighted spin-echo, axial spin-density/T2-weighted, and T1-weighted MRI images were acquired, and the images were interpreted by a neuroradiologist using a standardized protocol at a central reading center (30). WMH were graded according to an atlas of predefined visual standards (31) on a scale of 0–9 points, with higher scores indicating greater severity. Brain infarcts were defined as an area with abnormal signal intensity in a vascular distribution, lacking mass effect, hyperintense to gray matter on spin density and T2-weighted images, and 3 mm or larger in size. Primary analyses used a WMH grade of 3 or higher to define SVD (32).
MRI was conducted during the 1998–1999 visit using the same methods. Baseline grades for WMH were reread alongside a follow-up MRI scan with blinding as to the order of scans to minimize reader bias. Follow-up MRI scans were available for 1,793 participants.
Covariates
Demographic characteristics were self-reported, including age, sex, and race. Marital status was categorized as married, widowed, divorced or separated, or never married. Hypertension, diabetes, coronary heart disease (CHD), and stroke were self-reported physician diagnoses.
Individual-level socioeconomic characteristics included self-reported educational attainment, household income, and occupation type. Education was recorded by the number of years completed and categorized as less than high school, high school graduate, or beyond high school. Annual income in dollars was categorized as <12,000, 12,000–24,999, 25,000–34,999, and ≥35,000. To avoid loss of participants due to missing data for income, missing was included as a category in analyses; complete case analyses did not differ appreciably from those presented here. Usual lifetime occupation was categorized as: 1) professional/technical/managerial/administrative; 2) sales/clerical service; 3) craftsman/machine operator/laborer; 4) farming/forestry; 5) housewife; and 6) other or missing (26). Categories 3 and 4 were combined in these analyses.
Statistical analyses
Descriptive statistics of the sample are presented by race and tertile of NSES. Mean values are presented with standard deviations. For comparisons between race-specific NSES tertiles, we used analysis of variance for continuous variables and χ2 statistics for categorical ones.
There was a significant interaction between NSES score as a continuous variable and race (P for interaction < 0.001 for 3MS and P for interaction = 0.03 for DSST) and evidence of a nonlinear association between NSES as a continuous variable and cognitive function (P for quadratic term < 0.008 for both). Therefore, race-specific tertiles of NSES were used for all analyses (see Table 1).
Table 1.
Characteristics of a Sample of Community-Dwelling Adults Aged 65 Years or Older, by Race and Neighborhood Socioeconomic Status, Cardiovascular Health Study, United States, 1992–1993
| Characteristic | Race and Tertile |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White (n = 2,727) |
Black (n = 514) |
|||||||||||||
| Lowest Tertile (n = 917) |
Middle Tertile (n = 893) |
Highest Tertile (n = 917) |
P Value | Lowest Tertile (n = 176) |
Middle Tertile (n = 167) |
Highest Tertile (n = 171) |
P Value | |||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |||
| NSESa | −8.05, −0.82 | −0.83, 1.67 | 1.68, 13.9 | −8.94, −4.55 | −4.56, −2.62 | −2.63, 10.5 | ||||||||
| Age, years | 72.1 (4.7) | 71.8 (4.9) | 71.9 (4.8) | 0.6 | 73.4 (5.6) | 72.5 (5.4) | 71.8 (5.1) | 0.02 | ||||||
| Female sex | 57.5 | 59.8 | 53.7 | 0.03 | 67.1 | 65.9 | 56.1 | 0.07 | ||||||
| Marital status | 0.09 | 0.1 | ||||||||||||
| Married | 69.5 | 71.4 | 74.7 | 44.3 | 48.5 | 56.2 | ||||||||
| Widowed | 22.9 | 21.6 | 18.1 | 40.9 | 34.7 | 25.7 | ||||||||
| Divorced or separated | 3.5 | 3.7 | 2.6 | 12.5 | 12.6 | 14.6 | ||||||||
| Never married | 4.1 | 3.3 | 4.6 | 2.3 | 4.2 | 3.5 | ||||||||
| Education | <0.001 | <0.001 | ||||||||||||
| Did not complete high school | 35.6 | 21.3 | 9.8 | 47.2 | 37.1 | 26.9 | ||||||||
| High school graduate | 30.0 | 33.5 | 21.5 | 22.7 | 27.6 | 21.6 | ||||||||
| Beyond high school | 34.4 | 45.2 | 68.7 | 30.1 | 35.3 | 51.5 | ||||||||
| Annual income, dollars | <0.001 | <0.001 | ||||||||||||
| <12,000 | 26.0 | 17.5 | 8.1 | 53.4 | 48.5 | 29.8 | ||||||||
| 12,000–24,999 | 41.7 | 38.4 | 22.8 | 30.1 | 29.9 | 26.9 | ||||||||
| 25,000–34,999 | 14.5 | 18.9 | 14.5 | 4.5 | 9.0 | 19.3 | ||||||||
| ≥35,000 | 13.3 | 18.5 | 47.3 | 4.0 | 7.8 | 21.1 | ||||||||
| Missing | 4.5 | 6.7 | 7.3 | 8.0 | 4.8 | 2.9 | ||||||||
| Occupation | <0.001 | 0.004 | ||||||||||||
| Professional | 28.9 | 36.5 | 50.8 | 23.9 | 34.7 | 42.7 | ||||||||
| Service | 12.9 | 20.0 | 15.9 | 7.4 | 11.4 | 12.9 | ||||||||
| Labor | 22.8 | 13.6 | 7.4 | 29.0 | 25.1 | 18.1 | ||||||||
| Housewife | 24.6 | 22.1 | 20.1 | 17.0 | 10.8 | 9.9 | ||||||||
| Other or missing | 10.8 | 7.8 | 5.8 | 22.7 | 18.0 | 16.4 | ||||||||
| Hypertension | 0.02 | 0.4 | ||||||||||||
| Normal | 46.4 | 46.4 | 51.9 | 22.1 | 26.3 | 31.0 | ||||||||
| Borderline | 15.7 | 15.0 | 16.4 | 13.1 | 14.4 | 12.3 | ||||||||
| Hypertensive | 37.9 | 38.6 | 31.7 | 64.8 | 59.3 | 56.7 | ||||||||
| Diabetes | 9.7 | 9.9 | 6.7 | 0.02 | 17.6 | 15.6 | 22.8 | 0.2 | ||||||
| CHD | 22.9 | 18.4 | 18.7 | 0.03 | 23.9 | 16.8 | 19.3 | 0.2 | ||||||
| Stroke | 4.9 | 5.4 | 3.9 | 0.3 | 4.6 | 4.8 | 7.6 | 0.4 | ||||||
| Baseline WMH | 35.3 | 34.4 | 31.0 | 0.1 | 40.3 | 31.1 | 33.3 | 0.2 | ||||||
| Incident WMHb | 10.3 | 6.6 | 10.1 | 0.07 | 9.5 | 9.9 | 4.1 | 0.2 | ||||||
| 3MS score | 92.1 (6.8) | 92.1 (6.5) | 93.4 (5.6) | <0.001 | 82.6 (10.8) | 86.8 (8.2) | 88.0 (8.0) | <0.001 | ||||||
| Annual change in 3MS score | −0.86 (0.08) | −0.81 (0.08) | −0.64 (0.08) | 0.1 | −1.01 (0.19) | −0.69 (0.19) | −0.56 (0.18) | 0.2 | ||||||
| DSST score | 39.0 (13.1) | 40.6 (12.4) | 44.1 (12.1) | <0.001 | 26.1 (13.8) | 30.6 (11.6) | 32.6 (13.3) | <0.001 | ||||||
| Annual change in DSST score | −1.00 (0.05) | −0.92 (0.05) | −0.95 (0.05) | 0.5 | −0.36 (0.11) | −0.61 (0.11) | −0.42 (0.10) | 0.2 | ||||||
Abbreviations: CHD, coronary heart disease; DSST, digit symbol substitution test; 3MS, Modified Mini-Mental State Examination; NSES, neighborhood socioeconomic status; SD, standard deviation; WMH, white matter hyperintensities.
a NSES values represent minimum and maximum for each tertile.
b Among the 1,793 participants with follow-up MRI scans.
Analyses of the associations of NSES tertiles with cognitive function were conducted separately for 3MS and DSST as outcomes over 6 years of follow-up using mixed-effects models with robust standard errors. Data were too sparse to obtain reliable estimates from multilevel models (number of participants per block group: range, 1–205; median, 2) (33). Results, including 95% confidence intervals, are presented with and without adjustment for demographic factors, individual-level SES, and clinical diseases as described above. Differences in rate of change across race-specific tertiles of NSES were assessed by an interaction term between NSES and time. Time-squared and time-by-age interaction terms were included but were not significant in any of the analyses. We further adjusted the results for WMH to determine whether WMH might explain the association of NSES with cognitive function. Participants were censored from the models when they died (n = 592; 18.3%) or were otherwise lost to follow-up (n = 427; 13.2%). The majority of participants attended all 7 visits (n = 1,972; 60.9%), and the mean number of visits was 5.9 (standard deviation (SD), 1.8).
Terms for interaction between NSES and individual-level characteristics, including WMH, were assessed but are not included here because none were significant. Predicted cognitive scores for participants of average age (72 years) were plotted separately for blacks and whites. All analyses were completed using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina).
Sensitivity analyses were conducted defining SVD by infarcts rather than WMH, restricting analyses to persons who were stroke-free at baseline, using change in WMH instead of baseline WMH in longitudinal analyses, including participants who were missing MRI scans but had complete cognitive data (n = 4,445), and using quartiles of NSES instead of tertiles.
RESULTS
Neighborhood SES
Distributions of NSES by race are displayed in Figure 1. The median NSES score for white participants (n = 2,727) was 0.52, and the median NSES score for black participants (n = 514) was −3.69 (P < 0.001). White participants had higher baseline scores on both the 3MS (mean = 92.5 (SD, 6.4) for whites and mean = 85.8 (SD, 9.4) for blacks; P < 0.001) and the DSST (mean = 41.2 (SD, 12.7) for whites and mean = 29.7 (SD, 13.2) for blacks; P < 0.001). There were no racial differences in WMH (prevalence = 33.6% for whites vs. prevalence = 35.0% for blacks; P = 0.5).
Figure 1.
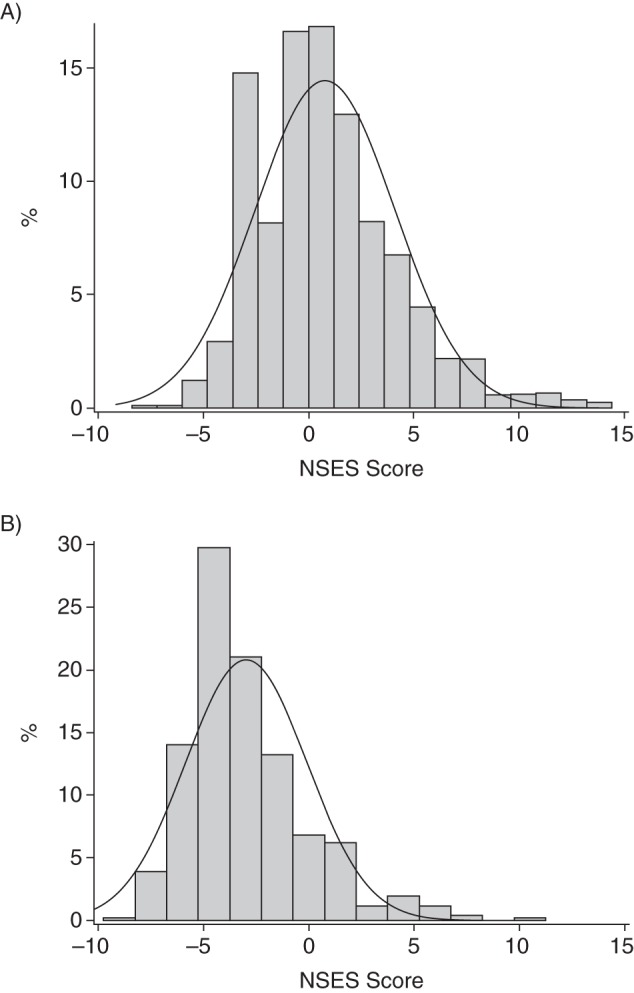
Distribution of neighborhood socioeconomic status (NSES) in a sample of (A) white (n = 2,727) and (B) black (n = 514) participants from the Cardiovascular Health Study, United States, 1992–1993.
Generally, similar patterns of associations between NSES and individual-level characteristics were observed for both races (Table 1). Men and those with higher education, higher income, and professional occupations were more likely to live in neighborhoods with higher NSES. Among black participants, older individuals were more likely to live in lower-NSES neighborhoods. For white participants only, hypertension, diabetes, and CHD were more common in lower-NSES neighborhoods.
White matter hyperintensities
WMH were significantly related to performance on both the 3MS and the DSST for both races (P < 0.001 for all). These associations remained significant after adjustment for age and sex. For both races, persons living in higher-SES neighborhoods performed better on both the 3MS and the DSST in unadjusted analyses (Table 1). There was no difference in prevalence of WMH by NSES (P ≥ 0.1).
Modified Mini-Mental State Examination
For white participants, being in either the middle or the lowest tertile of NSES was associated with lower 3MS scores at baseline compared with the highest tertile after adjustment for demographic, health, and individual SES characteristics (for lowest tertile, mean difference = −0.66 points, 95% confidence interval (CI): −1.24, −0.08). Further adjustment for the presence of WMH did not affect results (Table 2 and Figure 2; for lowest tertile, mean difference = −0.66 points, 95% CI: −1.24, −0.08). White participants in the middle and lowest tertiles of NSES had marginally significantly faster rates of decline in 3MS scores compared with those in the highest tertile (for lowest tertile, mean difference = −0.22 points, 95% CI: −0.43, −0.01). These results were not affected by adjustment for WMH (for lowest tertile, mean difference = −0.22 points, 95% CI: −0.43, −0.01). For comparison, the mean difference in 3MS scores per year of age at baseline in whites after adjustment was −0.30 points (95% CI: −0.34, −0.25). The annual rate of decline in 3MS scores per year of age in whites after adjustment was −0.15 points (95% CI: −0.16, −0.013).
Table 2.
Mean Difference in Modified Mini-Mental State Examination Scores by Neighborhood Socioeconomic Status from Mixed-Effects Models in Adults Aged 65 Years or Older, With and Without Adjustment for White Matter Hyperintensities, Cardiovascular Health Study, United States, 1992–1993
| Race and Tertile | Model 1a |
Model 2b |
Model 3c |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Rate of Change per Year |
Baseline |
Rate of Change per Year |
Baseline |
Rate of Change per Year |
|||||||
| Mean Difference | 95% CI | Mean Difference | 95% CI | Mean Difference | 95% CI | Mean Difference | 95% CI | Mean Difference | 95% CI | Mean Difference | 95% CI | |
| White (n = 2,727) | ||||||||||||
| Highest | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent |
| Middle | −1.51 | −2.06, −0.96 | −0.21 | −0.42, 0.01 | −0.62 | −1.15, −0.09 | −0.21 | −0.42, 0.003 | −0.62 | −1.15, −0.09 | −0.21 | −0.42, 0.003 |
| Lowest | −2.32 | −2.91, −1.74 | −0.22 | −0.43, −0.01 | −0.66 | −1.24, −0.08 | −0.22 | −0.43, −0.01 | −0.66 | −1.24, −0.08 | −0.22 | −0.43, −0.01 |
| Black (n = 514) | ||||||||||||
| Highest | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent |
| Middle | −1.14 | −2.90, 0.61 | −0.08 | −0.59, 0.44 | −0.03 | −1.61, 1.55 | −0.06 | −0.58, 0.46 | −0.05 | −1.64, 1.53 | −0.06 | −0.58, 0.46 |
| Lowest | −4.38 | −6.18, −2.58 | −0.32 | −0.83, 0.20 | −2.42 | −4.07, −0.76 | −0.33 | −0.85, 0.18 | −2.41 | −4.07, −0.76 | −0.34 | −0.85, 0.18 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; SES, socioeconomic status; WMH, white matter hyperintensities.
a Results were adjusted for age (centered), sex, marital status, hypertension, diabetes, CHD, and clinic location; the model included interaction terms for time squared and age × time.
b Results were adjusted for age (centered), sex, marital status, hypertension, diabetes, CHD, clinic location, and individual SES (education, income, and occupational history); the model included interaction terms for time squared and age × time.
c Results were adjusted for age (centered), sex, marital status, hypertension, diabetes, CHD, clinic location, individual SES, and WMH (high vs. low); the model included interaction terms for time squared and age × time.
Figure 2.
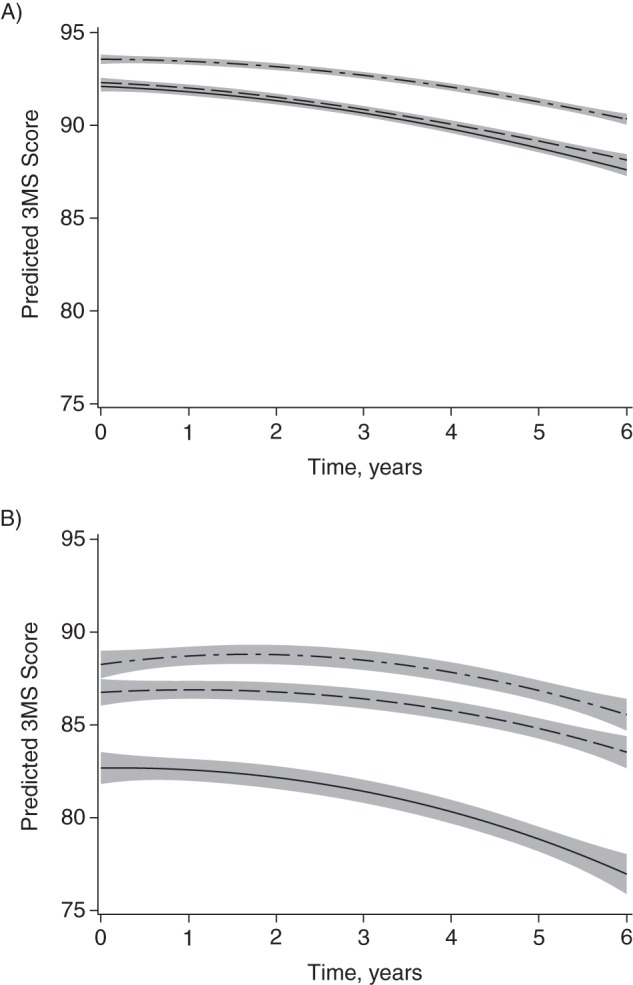
Predicted Modified Mini-Mental State Examination (3MS) score, by race and neighborhood socioeconomic status (SES), for (A) white and (B) black participants of average age (72 years), Cardiovascular Health Study, United States, 1992–1993. Top lines are for the highest tertile of neighborhood SES and bottom lines are for the lowest tertile. Adjusted for time squared, age, age × time, sex, marital status, hypertension, diabetes, coronary heart disease, clinic location, individual SES (education, income, and occupational history), and white matter hyperintensities. Shading indicates 95% confidence interval.
For black participants, differences in 3MS scores at baseline were observed only for the lowest tertile as compared with the highest tertile (mean difference = −2.42 points, 95% CI: −4.07, −0.76). Again, further adjustment for WMH did not affect results (Table 2 and Figure 2; mean difference = −2.41 points, 95% CI: −4.07, −0.76). There was no significant difference in the rate of decline of 3MS scores by NSES for black participants over 6 years of follow-up (Table 2). The mean difference in 3MS scores per year of age at baseline in black participants after adjustment was −0.42 points (95% CI: −0.54, −0.31), and the annual rate of decline in 3MS scores per year of age after adjustment was −0.09 points (95% CI: −0.13, −0.05).
Digit Symbol Substitution Test
For both races, there was a significant association between NSES and DSST scores after adjustment for demographic and health characteristics. However, after adjustment for individual-level SES, these results were attenuated in both races (Table 3). A marginally significant association between NSES and DSST score remained only between the highest and lowest tertiles of NSES for white participants (mean difference = −1.11 points, 95% CI: −2.23, 0.02; mean difference = −1.14 points, 95% CI: −2.26, −0.02 after adjustment for WMH). For comparison, the difference in DSST scores per year of age in white participants was −0.83 (95% CI: −0.91, −0.75). There was no difference observed for rate of change in DSST scores by NSES for either race after adjustment for health and demographic characteristics (Figure 3).
Table 3.
Mean Difference in Digit Symbol Substitution Test Scores by Neighborhood Socioeconomic Status from Mixed-Effects Models in Adults Aged 65 Years or Older, With and Without Adjustment for White Matter Hyperintensities, Cardiovascular Health Study, United States, 1992–1993
| Race and Tertile | Model 1a |
Model 2b |
Model 3c |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Rate of Change per Year |
Baseline |
Rate of Change per Year |
Baseline |
Rate of Change per Year |
|||||||
| Mean Difference | 95% CI | Mean Difference | 95% CI | Mean Difference | 95% CI | Mean Difference | 95% CI | Mean Difference | 95% CI | Mean Difference | 95% CI | |
| White (n = 2,727) | ||||||||||||
| Highest | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent |
| Middle | −2.68 | −3.77, −1.60 | −0.001 | −0.14, 0.14 | −0.93 | −1.97, 0.04 | −0.002 | −0.14, 0.14 | −0.96 | −1.99, 0.07 | −0.002 | −0.14, 0.14 |
| Lowest | −4.67 | −5.82, −3.52 | −0.06 | −0.20, 0.08 | −1.11 | −2.23, 0.02 | −0.06 | −0.20, 0.08 | −1.14 | −2.26, −0.02 | −0.06 | −0.20, 0.08 |
| Black (n = 514) | ||||||||||||
| Highest | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent | 0 | Referent |
| Middle | −2.07 | −4.62, 0.49 | −0.17 | −0.46, 0.12 | 0.17 | −2.06, 2.40 | −0.17 | −0.46, 0.12 | 0.01 | −2.21, 2.23 | −0.17 | −0.46, 0.12 |
| Lowest | −5.77 | −8.40, −3.15 | 0.11 | −0.18, 0.41 | −2.03 | −4.36, 0.30 | 0.11 | −0.18, 0.41 | −2.06 | −4.38, 0.26 | 0.12 | −0.18, 0.41 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; SES, socioeconomic status; WMH, white matter hyperintensities.
a Results were adjusted for age (centered), sex, marital status, hypertension, diabetes, CHD, and clinic location; the model included interaction terms for time squared and age × time.
b Results were adjusted for age (centered), sex, marital status, hypertension, diabetes, CHD, clinic location, and individual SES (education, income, and occupational history); the model included interaction terms for time squared and age × time.
c Results were adjusted for age (centered), sex, marital status, hypertension, diabetes, CHD, clinic location, individual SES, and WMH (high vs. low); the model included interaction terms for time squared and age × time.
Figure 3.
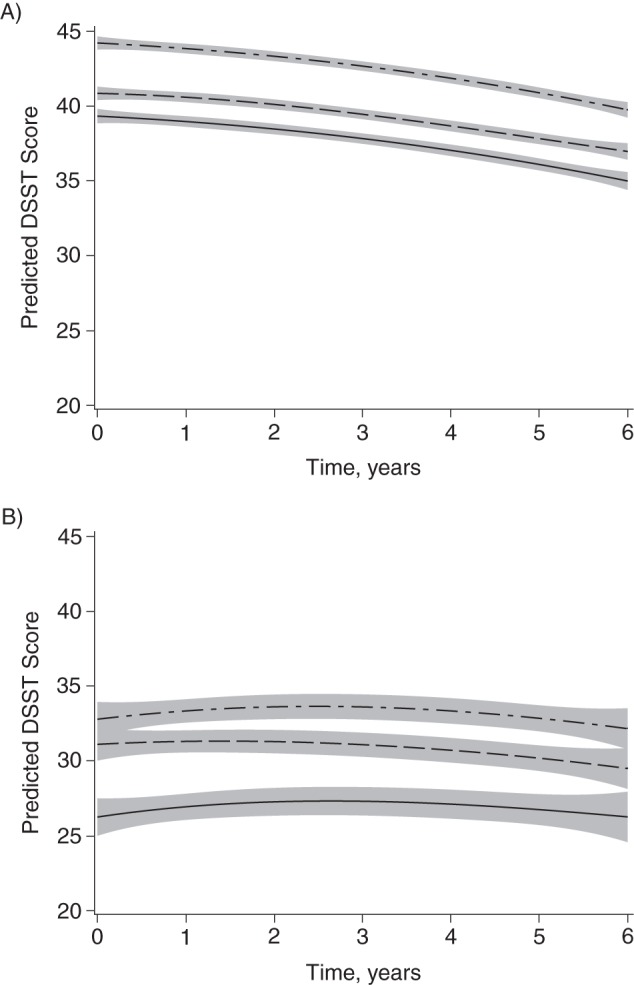
Predicted Digit Symbol Substitution Test (DSST) score by race and neighborhood socioeconomic status (SES) for (A) white and (B) black participants of average age (72 years), Cardiovascular Health Study, United States, 1992–1993. Top lines are for the highest tertile of neighborhood SES and bottom lines are for the lowest tertile. Adjusted for time squared, age, age × time, gender, sex, marital status, hypertension, diabetes, coronary heart disease, clinic location, individual SES (education, income, and occupational history), and white matter hyperintensities. Shading indicates 95% confidence interval.
Sensitivity analyses
Defining SVD by the presence of infarcts rather than WMH did not appreciably change the results. Also, results were not different when restricting analysis to those free of stroke or when additionally adjusting for incident WMH over 6 years for those with a follow-up MRI scan. Inclusion of all participants with complete data other than MRI scans also did not change the associations between NSES and cognitive function. Finally, using quartiles of NSES rather than tertiles did not qualitatively change the results.
DISCUSSION
We found that higher NSES was associated with higher 3MS scores, measuring general cognitive function, in both races at baseline but was not associated with differences in change of 3MS scores over 6 years. These associations were independent of individual demographic, health, and SES characteristics, including educational attainment. For white participants, these differences were relatively small; the difference in 3MS scores between the lowest and highest tertiles of NSES was equivalent to less than a year of age. In contrast, among black participants, the difference between the lowest and highest tertiles of NSES was equivalent to almost 6 years of age. Higher performance on the DSST, a measure of speed of processing, was marginally significantly associated with higher NSES at baseline in white participants only. There was no association between NSES and the presence of WMH and no attenuation of the associations between NSES and cognitive function with adjustment for WMH. Together, these results suggest that NSES may be related to differences in 3MS scores but that these differences are present prior to age 65 years and are not due to cerebral SVD.
Several previous studies have demonstrated cross-sectional associations between higher NSES and greater general cognitive function in middle-aged and older adults, even after adjustment for indicators of individual SES (4–6, 8–10). These findings were consistent across studies conducted in the United States (4, 5, 8, 10), the United Kingdom (6), and Singapore (9). Only 2 previous studies, both in Hispanic populations in the United States, have assessed this association using longitudinal cognitive assessments, with conflicting results (7, 11). In contrast to our findings, Sheffield et al. (7) reported that lower NSES was related to faster rates of decline in Mini-Mental State Examination scores over 5 years. However, consistent with our findings, Zeki et al. (11) found a cross-sectional association between lower NSES and lower 3MS scores but no association between NSES and change in 3MS score over 10 years. It is unclear why these results are conflicting given that these 2 studies were conducted in similar populations of comparable age (average ages of 73 and 71 years, respectively), at approximately the same time (baseline years of 1993–1994 and 1998–1999), using similar methods, although differences in geographic location may explain the discrepancies (11). Interestingly, findings from a study by Wilson et al. (34), who evaluated childhood NSES and late-life cognitive function, were also consistent with our findings; lower childhood NSES was associated with poorer cognitive function in late life at study baseline but not with greater declines in cognitive function over 6 years.
Low overlap in NSES by race made it difficult to assess racial differences in the association between NSES and cognitive function in the present study. However, we did observe an interaction between NSES as a continuous variable and race, suggesting that the association is stronger among blacks than among whites. Using a cross-sectional analysis in older women, Shih et al. (8) also found stronger associations between NSES and Mini-Mental State Examination scores in nonwhites compared with whites. Other studies either included persons of a single race or did not stratify by race. Some studies have found interactions between NSES and measures of individual SES (4, 10). We did not observe these same interactions within strata by race, but it is possible that between-race differences in individual SES could account for the stronger findings for black participants in our study. There are several other possible explanations for the observed differences by race. It is possible that these results indicate truly stronger influences of NSES on blacks compared with whites. They may also be due to the race-specific tertile cutoffs used; possibly there is a stronger nonlinearity of the association at lower NSES, regardless of race. Finally, these results may reflect stronger associations for persons with lower 3MS scores, as blacks had lower overall scores and greater variation in scores compared with whites. Studies with greater overlap in NSES and cognitive function by race are needed to determine which of these factors may be driving the observed results.
Our results suggest that the association between NSES and cognitive function cannot be attributed to differences in SVD, as measured by the presence of WMH or infarcts. It is possible that the cerebral mechanism is related to volumetric differences in specific gray matter regions, in neurotransmitter levels, or in more subtle white matter changes (35–37), which we were unable to assess in the present study. Our results were specific to the 3MS, which is used as a screening tool for dementia, and not to the DSST. Therefore, it may be that NSES is related either to dementia pathology or to resilience to dementia pathology. There is some evidence that individual-level SES does not protect against Alzheimer pathology but does confer some protection against pathology, resulting in later onset of clinical Alzheimer disease (38–41). These associations have not been explored in relation to NSES. Other proposed contributing mechanisms have included inflammatory pathways (35), cognitively stimulating resources in the neighborhood (5), cardiovascular factors (8), and health behaviors (8). Finally, it is possible that the observed differences are not biological but due to differences in literacy across NSES (42).
We did not have data on length of residence in the neighborhood to assess whether baseline NSES reflected a long-term exposure. Duration of exposure to low NSES may affect associations with health outcomes (43). Given that our results suggest a divergence in cognitive function by NSES earlier in life, which may even be related to early-life development (34), understanding the effects of lifetime NSES in relation to cognitive function may be particularly important. We were unable to account for factors pertaining to selection into particular neighborhoods beyond the measured confounders. It is possible that these associations could be explained by differential selection into neighborhoods. We also did not have reliable information on possible relocations after the baseline visit. We therefore assumed that participants who moved stayed within the same tertile of NSES or that change in NSES late in life did not affect cognition. We did not account for lifetime control of cardiovascular risk factors, such as blood pressure, which may influence late-life cognitive function. Inclusion of participants with complete data, particularly for MRI, resulted in a younger, healthier, higher-SES sample. However, sensitivity analyses that included individuals with complete data other than MRI scans resulted in findings similar to those reported here. Finally, the data used for these analyses were collected 2 decades ago. However, previously reported cross-sectional results span the years 1993–2012 and do not demonstrate any clear trend in associations between NSES and cognitive function with time (4–6, 8–10). In addition, NSES appears to be stable over decades, even during times of national economic transition (44). Despite limitations with the available data, these analyses were strengthened by being conducted in a large, population-based, biracial cohort with longitudinal measures of cognitive function in multiple domains.
The present study corroborated previous findings of cross-sectional associations between NSES and general cognitive function independent of individual SES, but we did not find NSES to be related to a faster decline in cognitive function in late life. This suggests that the relevant pathways are not related to differences in cerebral vascular pathology late in life. Studies carried out from a life-course perspective are needed to better understand the point at which differences emerge (37). A better understanding of when in the course of a lifetime NSES-related differences in cognition emerge may help to uncover the underlying pathways and mechanisms and point towards possible interventions. Also, NSES was more strongly related to cognitive function in black participants than in white participants. The difference in 3MS scores between the highest and lowest tertiles of NSES among black participants was equivalent to approximately 6 years of age, indicating that blacks living in low-SES neighborhoods may cross over a clinically relevant threshold of cognitive dysfunction several years earlier than their counterparts living in neighborhoods with higher SES. Finally, these results add to the growing body of research demonstrating the negative associations of poverty, at the level of both the individual and the neighborhood, with physical function, cognitive function, daily and social activities, health, and well-being.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania (Andrea L. Rosso, Caterina Rosano); Department of Medicine, Division of Geriatrics, University of California, San Francisco, San Francisco, California (Jason D. Flatt); Department of Mental Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Michelle C. Carlson); Center on Aging and Health, Johns Hopkins University, Baltimore, Maryland (Michelle C. Carlson); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Michelle C. Carlson); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Gina S. Lovasi); Division of General Internal Medicine and Health Services Research, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California (Arleen F. Brown); Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania (Karen A. Matthews); and Department of Psychology, Dietrich School of Arts and Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania (Peter J. Gianaros).
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grant HL080295 from the National Heart, Lung, and Blood Institute, with an additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by grant AG023629 from the National Institute on Aging. A full list of principal Cardiovascular Health Study (CHS) investigators and institutions can be found at https://chs-nhlbi.org/. Additional funding for this analysis came from the National Institute on Aging (training grant T32-AG-000181) and from the National Institutes of Health (grant KL2 TR000146).
Conflict of interest: none declared.
REFERENCES
- 1.Gill TM, Richardson ED, Tinetti ME. Evaluating the risk of dependence in activities of daily living among community-living older adults with mild to moderate cognitive impairment. J Gerontol A Biol Sci Med Sci. 1995;505:M235–M241. [DOI] [PubMed] [Google Scholar]
- 2.Katz MJ, Lipton RB, Hall CB et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;264:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park HL, O'Connell JE, Thomson RG. A systematic review of cognitive decline in the general elderly population. Int J Geriatr Psychiatry. 2003;1812:1121–1134. [DOI] [PubMed] [Google Scholar]
- 4.Aneshensel CS, Ko MJ, Chodosh J et al. The urban neighborhood and cognitive functioning in late middle age. J Health Soc Behav. 2011;522:163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke PJ, Ailshire JA, House JS et al. Cognitive function in the community setting: the neighbourhood as a source of ‘cognitive reserve’? J Epidemiol Community Health. 2012;668:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang IA, Llewellyn DJ, Langa KM et al. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2008;562:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheffield KM, Peek MK. Neighborhood context and cognitive decline in older Mexican Americans: results from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. Am J Epidemiol. 2009;1699:1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shih RA, Ghosh-Dastidar B, Margolis KL et al. Neighborhood socioeconomic status and cognitive function in women. Am J Public Health. 2011;1019:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wee LE, Yeo WX, Yang GR et al. Individual and area level socioeconomic status and its association with cognitive function and cognitive impairment (low MMSE) among community-dwelling elderly in Singapore. Dement Geriatr Cogn Dis Extra. 2012;21:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wight RG, Aneshensel CS, Miller-Martinez D et al. Urban neighborhood context, educational attainment, and cognitive function among older adults. Am J Epidemiol. 2006;16312:1071–1078. [DOI] [PubMed] [Google Scholar]
- 11.Zeki Al Hazzouri A, Haan MN, Osypuk T et al. Neighborhood socioeconomic context and cognitive decline among older Mexican Americans: results from the Sacramento Area Latino Study on Aging. Am J Epidemiol. 2011;1744:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diez Roux AV. Neighborhoods and health: where are we and were [sic] do we go from here? Rev Epidemiol Sante Publique. 2007;551:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianaros PJ, Hackman DA. Contributions of neuroscience to the study of socioeconomic health disparities. Psychosom Med. 2013;757:610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke PJ, Weuve J, Barnes L et al. Cognitive decline and the neighborhood environment. Ann Epidemiol. 2015;2511:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuller LH, Shemanski L, Manolio T et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;292:388–398. [DOI] [PubMed] [Google Scholar]
- 16.Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;598:818–826. [DOI] [PubMed] [Google Scholar]
- 17.Nordstrom CK, Diez Roux AV, Jackson SA et al. The association of personal and neighborhood socioeconomic indicators with subclinical cardiovascular disease in an elderly cohort. The Cardiovascular Health Study. Soc Sci Med. 2004;5910:2139–2147. [DOI] [PubMed] [Google Scholar]
- 18.Diez Roux AV, Merkin SS, Arnett D et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;3452:99–106. [DOI] [PubMed] [Google Scholar]
- 19.Schulz AJ, Mentz G, Lachance L et al. Associations between socioeconomic status and allostatic load: effects of neighborhood poverty and tests of mediating pathways. Am J Public Health. 2012;1029:1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bird CE, Seeman T, Escarce JJ et al. Neighbourhood socioeconomic status and biological ‘wear and tear’ in a nationally representative sample of US adults. J Epidemiol Community Health. 2010;6410:860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lysy Z, Booth GL, Shah BR et al. The impact of income on the incidence of diabetes: a population-based study. Diabetes Res Clin Pract. 2013;993:372–379. [DOI] [PubMed] [Google Scholar]
- 22.Menec VH, Shooshtari S, Nowicki S et al. Does the relationship between neighborhood socioeconomic status and health outcomes persist into very old age? A population-based study. J Aging Health. 2010;221:27–47. [DOI] [PubMed] [Google Scholar]
- 23.Petersen KL, Marsland AL, Flory J et al. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosom Med. 2008;706:646–652. [DOI] [PubMed] [Google Scholar]
- 24.Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff (Millwood). 2005;242:325–334. [DOI] [PubMed] [Google Scholar]
- 25.Brown AF, Liang LJ, Vassar SD et al. Neighborhood disadvantage and ischemic stroke: the Cardiovascular Health Study (CHS). Stroke. 2011;4212:3363–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diez Roux AV, Borrell LN, Haan M et al. Neighbourhood environments and mortality in an elderly cohort: results from the Cardiovascular Health Study. J Epidemiol Community Health. 2004;5811:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tell GS, Fried LP, Hermanson B et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;34:358–366. [DOI] [PubMed] [Google Scholar]
- 28.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;488:314–318. [PubMed] [Google Scholar]
- 29.Wechsler D. Manual for the Wechsler Adult Intelligence Scale–Revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 30.Longstreth WT Jr, Dulberg C, Manolio TA et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;3310:2376–2382. [DOI] [PubMed] [Google Scholar]
- 31.Yue NC, Arnold AM, Longstreth WT Jr et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the Cardiovascular Health Study. Radiology. 1997;2021:33–39. [DOI] [PubMed] [Google Scholar]
- 32.Rosano C, Kuller LH, Chung H et al. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;534:649–654. [DOI] [PubMed] [Google Scholar]
- 33.Clarke P. When can group level clustering be ignored? Multilevel models versus single-level models with sparse data. J Epidemiol Community Health. 2008;628:752–758. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RS, Scherr PA, Bienias JL et al. Socioeconomic characteristics of the community in childhood and cognition in old age. Exp Aging Res. 2005;314:393–407. [DOI] [PubMed] [Google Scholar]
- 35.Gianaros PJ, Marsland AL, Sheu LK et al. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex. 2013;239:2058–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gianaros PJ, Manuck SB. Neurobiological pathways linking socioeconomic position and health. Psychosom Med. 2010;725:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negash S, Wilson RS, Leurgans SE et al. Resilient brain aging: characterization of discordance between Alzheimer's disease pathology and cognition. Curr Alzheimer Res. 2013;108:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munoz DG, Ganapathy GR, Eliasziw M et al. Educational attainment and socioeconomic status of patients with autopsy-confirmed Alzheimer disease. Arch Neurol. 2000;571:85–89. [DOI] [PubMed] [Google Scholar]
- 40.Bennett DA, Schneider JA, Arvanitakis Z et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;6612:1837–1844. [DOI] [PubMed] [Google Scholar]
- 41.Bennett DA, Wilson RS, Schneider JA et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;6012:1909–1915. [DOI] [PubMed] [Google Scholar]
- 42.Dotson VM, Kitner-Triolo MH, Evans MK et al. Effects of race and socioeconomic status on the relative influence of education and literacy on cognitive functioning. J Int Neuropsychol Soc. 2009;154:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Do DP. The dynamics of income and neighborhood context for population health: do long-term measures of socioeconomic status explain more of the black/white health disparity than single-point-in-time measures? Soc Sci Med. 2009;688:1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geronimus AT, Bound J. Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. Am J Epidemiol. 1998;1485:475–486. [DOI] [PubMed] [Google Scholar]


