Abstract
Background
It has been reported that three common loci, SstI, C-482T, and T-455C, in the apolipoprotein C3 (APOC3) gene might be associated with an increased risk of coronary heart disease (CHD). Considering the inconsistent results and ethnicity variations, we performed a systematic meta-analysis to evaluate the association between three single nucleotide polymorphisms (SNPs) and the risk of CHD.
Methods
We searched HuGE Navigator and PubMed databases to screen for the related literature published before 25 September, 2015. Two independent reviewers extracted the data and assessed the study quality. A random-effect model was used to pool the effect size.
Results
A total of 29 studies met inclusion criteria. Nineteen studies, including 11,186 subjects relative to SstI, five studies comprising 3727 subjects relative to C-482T, and nine studies with 6753 subjects relative to T-455C were included in the final analysis. A significant increase in CHD risk was observed in the SstI polymorphism (S2 versus S1: odds ratio [OR] = 1.30, 95% confidence interval [CI] 1.10–1.55. There was also a significant increasing trend of CHD risk in the T-455C polymorphism (C versus T: OR = 1.28, 95% CI 1.16–1.41. However, no associations between C-482T and CHD risk were found in this meta-analysis.
Conclusions
The pooled evidence suggests that two SNPs (SstI and T-455C) are associated with an increased risk of CHD. However, because of the limited sample size and heterogeneity, further large-scale and well-designed studies are needed to validate our findings.
Keywords: Apolipoprotein C3, Coronary heart disease, Meta-analysis
1. Introduction
Coronary heart disease (CHD) is a complex condition caused by genetic and environmental factors (Sayols-Baixeras et al., 2014). Evidence obtained from observational studies suggests that hypertriglyceridemia is a prevalent risk factor for CHD (Abdel-Maksoud and Hokanson, 2002). However, because of the interactive effects of triglyceride (TG) with other blood lipid components, especially with an inverse association between TG and high-density lipoprotein cholesterol (HDL-C), whether high levels of TG have a direct repercussion on the development of CHD, or simply represent a risk biomarker, has remained a controversial issue for a long time. TG is mostly transported in very-low-density lipoprotein (VLDL), chylomicrons (CM), and remnants of their metabolism (Nakajima et al., 2011), which are commonly referred to as triglyceride-rich lipoproteins (TRLs). Genetic association studies demonstrate that apolipoprotein C3 (APOC3) gene mutations, resulting in high circulating levels, may lead to decreased catabolism of TRLs, and have been associated with hypertriglyceridemia and CHD progression (Jorgensen et al., 2014, Tg, Hdl Working Group of the Exome Sequencing Project NHL,).
ApoC3 is a 79-amino-acid glycoprotein and a major component of circulating TRLs that plays a key regulative role in lipoprotein metabolism (Bruns et al., 1984). Indeed, apoC3 impairs the lipolysis of TRLs by inhibiting lipoprotein lipase (Brown and Baginsky, 1972, Havel et al., 1973, Ekman and Nilsson-Ehle, 1975) and the hepatic uptake of TRLs by remnant receptors (Shelburne et al., 1980, Windler et al., 1980, Quarfordt et al., 1982). A few genetic studies suggest that the single nucleotide polymorphisms (SNPs) in the APOC3 gene may have implications for hypertriglyceridemia (Talmud and Humphries, 1997) and susceptibility to CHD (Ooi et al., 2008). Carriers of the SstI polymorphisms have higher apoC3 and TG levels (Song et al., 2015). Whether this gene variant confers an increased CHD risk is still unclear. Homozygotes for the C-482T and T-455C variants are resistant to insulin-mediated down-regulation of APOC3 gene transcription, which results in high TG levels (Li et al., 1995). However, there are inconsistent results on the relationship of these two polymorphisms and an increased risk of CHD.
Considering that most studies investigating the potential association between APOC3 gene variants and the risk of CHD have been conducted on diverse ethnic populations with limited sample size, we conducted a meta-analysis to systematically estimate the association of three polymorphisms in APOC3 gene with the risk of CHD.
2. Methods
2.1. Search strategy
A systematic search was performed in HuGE Navigator and PubMed databases. Two independent reviewers screened the literature published before 25 September, 2015, using Mesh terms “apolipoprotein C-III and coronary artery disease” or free index terms “[APOC3 or apolipoprotein C3] and [cardiovascular disease or coronary heart disease or myocardial infarction or ischemic vascular disease]”. Language was not a restrictive condition. According to the titles and abstracts of publications, duplications in the two electronic databases were identified and discussed as a decision for eliminating from the list of searched results.
2.2. Inclusion and exclusion criteria
Studies were eligible for inclusion if they met the following criteria: (1) the subject for the association of SstI, C-482T, or T-455C polymorphisms with CHD risk; (2) case–control study; (3) essential information on genotype or allele frequencies to estimate the odds ratios (ORs) and with 95% confidence intervals (CIs). Exclusion criteria included: (1) pedigree or family-based studies; (2) clinical trials; (3) animal studies; (4) editorials, comments, reviews, or short articles; (5) scarce or insufficient information on genotype or allele frequencies. Two reviewers independently assessed the original articles according to inclusion and exclusion criteria. Discrepancies were resolved via discussion in the review team.
2.3. Data extraction
Two independent reviewers extracted the original data using a standardized and consistent method. The following information were collected from each study: name of first author, year of publication, country of origin, ethnicity, available demographic characteristics of study population, genotyping methods, diagnostic criteria of CHD, numbers with each genotype or allele in cases and controls for SstI, C-482T, or T-455C polymorphisms. Any discordance was settled by discussion to reach a consensus.
2.4. Methodological quality assessment
We applied an adjusted methodological tool based on The Newcastle–Ottawa Scale (NOS) to assess the qualities of eligible studies, and the scale for methodological quality assessment was shown in a Data in Brief article (Li et al., 2016). The assessment form included seven following items: ascertainment of CHD, representativeness of cases, source of controls, definition of controls, quality control of genotyping methods, Hardy–Weinberg equilibrium (HWE) in the control group, and sample size. Total quality scores ranged between 0 and 10 and were calculated by two independent reviewers, with a higher score representing a better quality. Disagreements were resolved by discussion.
2.5. Statistical analysis
HWE deviation for each eligible study was evaluated using the Chi-square test in control groups. To estimate the extent of the association of SstI, C-482T, or T-455C SNPs in the APOC3 gene with the susceptibility of CHD, the allelic model (SstI: S2 versus S1; C-482T: T versus C; T-455C: C versus T), heterozygote model (SstI: S1S2 versus S1S1; C-482T: CT versus CC; T-455C: TC versus TT), homozygote model (SstI: S2S2 versus S1S1; C-482T: TT versus CC; T-455C: CC versus TT), dominant model (SstI: S2S2 + S1S2 versus S1S1; C-482T: TT + CT versus CC; T-455C: CC + TC versus TT), as well as recessive model (SstI: S2S2 versus S1S2 + S1S1; C-482T: TT versus CT + CC; T-455C: CC versus TC + TT) were applied to measure pooled ORs and 95% CIs, respectively. The statistical significance level was determined by the Z-test with a two-sided P value less than 0.05.
Heterogeneity between studies was assessed by I2 statistic and its statistical significance level was checked by Chi-square-based Q test. The random-effect model based DerSimonian–Laird method was applied to pool the data from different studies irrespective of heterogeneity. Meta-regression was performed to detect the source of heterogeneity.
Cumulative meta-analyses considering to the ascending date of publication or sample size were applied to identify the dynamic trends of summary estimate with evolutional time or incremental sample size. Moreover, sensitivity analysis was conducted to estimate the influence of a single study on the pooled effect size of combined studies by removing one study each time.
Potential publication bias was detected with Begg's funnel plot and Egger's regression test. An asymmetric funnel plot and a P value of Egger's test less than 0.05 was considered a significant publication bias. All statistical analysis was implemented with STATA 12.0 and RevMan 5.1 software.
3. Results
3.1. Characteristics of eligible studies
We searched HuGE Navigator and PubMed databases for 453 publications in accordance with the index strategy, of which 53 were obtained as full-text literature and screened for eligibility in the review. A total of 29 case–control studies met inclusion criteria, among which 19 studies including 11,186 subjects relative to SstI, five studies comprising 3727 subjects relative to C-482T, and nine studies with 6753 subjects relative to T-455C were included in the final analysis. Details of the study selection flow are documented in Fig. 1. There are 21 studies performing genotyping using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), four studies with sequence-specific oligonucleotide probes (PCR-SOP), and four studies using TaqMan assay. Most of the studies were in agreement with HWE regarding genotyping distribution in control groups, except for three. Additionally, we applied a methodological scale to assess the qualities of inclusion studies, the lowest score was 5 and the highest score was 8, based on a total of a 10-point system. The characteristics and the quality scores for each study were shown in a Data in Brief article (Li et al., 2016).
Fig. 1.
Study selection flow diagram.
3.2. Meta-analysis for relationship of APOC3 gene variants with CHD risk
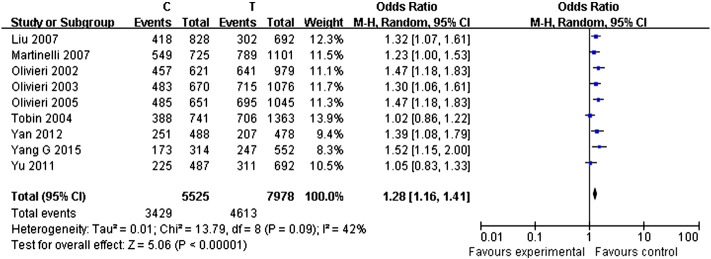
Pooled effect of polymorphisms from SstI, C-482T, and T-455C in the APOC3 gene on CHD risk were considered and computed under different modes of inheritance. Random-effect models were applied to compute pooled effect sizes (ORs and 95% CIs) irrespective of heterogeneity between studies. A significant increased risk of CHD with SstI polymorphism was observed in allele model S2 versus S1 (OR = 1.30, 95% CI: 1.10–1.55, P = 0.002, I2 = 77%, Fig. 2); heterozygote comparison S1S2 versus S1S1 (OR = 1.21, 95% CI: 1.02–1.44, P = 0.03, I2 = 65%) (Li et al., 2016); homozygote comparison S2S2 versus S1S1 (OR = 1.48, 95% CI: 1.09–2.03, P = 0.01, I2 = 38%) (Li et al., 2016); dominant model S1S2/S2S2 versus S1S1 (OR = 1.27, 95% CI: 1.06–1.53, P = 0.01, I2 = 72%) (Li et al., 2016); recessive model S2S2 versus S1S2/S1S1 (OR = 1.43, 95% CI: 1.09–1.87, P = 0.01, I2 = 27%) (Li et al., 2016). There was also a significant increase in the trend of CHD risk from T-455C polymorphism in allele model C versus T (OR = 1.28, 95% CI: 1.16–1.41, P < 0.00001, I2 = 42%, Fig. 3); heterozygote comparison TC versus TT (OR = 1.19, 95% CI: 1.07–1.33, P = 0.002, I2 = 0%) (Li et al., 2016); homozygote model CC versus TT (OR = 1.77, 95% CI: 1.35–2.31, P < 0.0001, I2 = 63%) (Li et al., 2016); dominant model TC/CC versus TT (OR = 1.30, 95% CI: 1.17–1.44, P < 0.00001, I2 = 0%) (Li et al., 2016); recessive model CC versus TC/TT (OR = 1.57, 95% CI: 1.22–2.01, P = 0.0004, I2 = 66%) (Li et al., 2016). However, no associations between C-482T and CHD risk were found in allele, heterozygote, or homozygote comparisons, with either dominant or recessive models (Fig. 4) (Li et al., 2016).
Fig. 2.
Forest plot of allele comparison of SstI polymorphism (S2 versus S1).
Fig. 3.
Forest plot of allele comparison of T-455C polymorphism (C versus T).
Fig. 4.
Forest plot of allele comparison of C-482T polymorphism (T versus C).
3.3. Heterogeneity
We conducted a meta-regression after adjustment for publication year, ethnicity, sex, genotyping method, and sample size to detect the source of heterogeneity. Considering that four studies on the SstI polymorphism and a single study on the C-482T polymorphism were restricted to male subjects, we categorized covariate sex into male or both sexes. In addition, all covariates information can be obtained directly from the literature except for the sample size indirectly computed by STATA software. Meta regression results in the allele model revealed that sex (P = 0.048) rather than publication year (P = 0.156), ethnicity (P = 0.502), genotyping method (P = 0.755), or sample size (P = 0.349), contributed to the source of heterogeneity. While executing the Monte Carlo validation with univariable 5000 permutations, covariates of sex (P = 0.130) became not statistically significant. Similar results have been observed using the heterozygote or homozygote comparisons, the dominant or recessive models. In contrast, no significant covariates were found to contribute to the heterogeneity of studies from T-455C or C-482T polymorphisms.
3.4. Sensitivity analysis and cumulative meta-analysis
The sensitivity analysis showed that no individual study affected summary ORs. A cumulative meta-analysis was performed by sorting by publication date or sample size, and the effect size estimate remained a stable trend.
3.5. Publication bias
No publication bias for the association between the T-455C polymorphism and CHD risk was identified by Begg's funnel plots (allelic model: PT-455C = 0.466; dominant model: PT-455C = 0.466; recessive model: PT-455C = 0.754) or Egger's regression tests (allelic model: PT-455C = 0.845; dominant model: PT-455C = 0.257; recessive model: PT-455C = 0.788). Similarly, no publication bias for the association between the SstI polymorphism and CHD risk were detected by Begg's funnel plots (allelic model: PSstI = 0.208; dominant model: PSstI = 0.050; recessive model: PSstI = 0.443) or Egger's regression tests with allelic model (PSstI = 0.475) or dominant model (PSstI = 0.269) except for the recessive model (PSstI = 0.003). Symmetrical funnel plots were obtained in all genetic models (data not shown). However, no funnel plot or Egger's test was performed for the association between the C-482T polymorphism and CHD risk based on the limited numbers of included studies.
4. Discussion
This meta-analysis shows that two polymorphisms, SstI and T-455C, in the APOC3 gene are associated with CHD risk.
The human APOC3 gene is located in the APOA1/C3/A4/A5 gene cluster on the chromosome 11q23, and is expressed in the liver and intestine (Lai et al., 2005). Plasma apoC3 is an essential constituent of TRL particles, including CM and VLDL, and to a lesser extent of HDL (Nakajima et al., 2011). Previous research reported that apoC3 contributed to the development of CHD via several possible mechanisms as follows: firstly, in vitro studies found that apoC3 could delay the hydrolysis of TG by inhibiting both lipoprotein lipase (LPL) (Brown and Baginsky, 1972, Havel et al., 1973, Ekman and Nilsson-Ehle, 1975) and hepatic lipase (HL) (Kinnunen and Ehnolm, 1976) activity. Secondly, liver perfusion studies reported that apoC3 inhibited the elimination of CM and VLDL by hepatocytes (Shelburne et al., 1980, Windler et al., 1980, Quarfordt et al., 1982). Thirdly, apoC3 has also been shown to stimulate VLDL synthesis in cultured cells (Sundaram et al., 2010), playing therefore a key role in regulating VLDL output by the liver. Finally, apoC3 was also shown to antagonizes the action of apoE (Mendivil et al., 2013), by inhibiting VLDL binding to cellular receptors, reducing the clearance of TRLs, and promoting the formation of dense LDL (Mendivil et al., 2010, Zheng et al., 2010). In addition, there is also some evidence suggesting that apoC3 directly contributes to atherosclerotic changes by enhancing monocyte adhesion to endothelial cells (Kawakami et al., 2006).
The SstI polymorphism in the 3′ untranslated region (UTR) of the APOC3 gene was first described in 1983 (Rees et al., 1983). It is caused by the substitution of a cytosine to guanosine on position 3238, which results in the generation of two separate alleles, S1 and S2. The SstI site is unlikely to be functionally significant because it is located in the gene's UTR. It was therefore proposed to act as a marker for a functional mutation elsewhere on the gene locus. There are substantial differences in frequency of the S2 allele among different ethical groups. However, subtle discrepancies in minor allele frequencies (MAFs) of two sites, T-455C and C-482T, were observed in different ethnicities between the included studies in the present meta-analysis. The sequences between T-455C and C-482T in the promoter region of the APOC3 gene have shown a strong homology to a negative insulin-responsive element (IRE), and the presence of the mutant sequences reduced the inhibitory effect of the hormone (Li et al., 1995), but the association of these two polymorphisms with CHD susceptibility has not been established yet. So far, the SstI, T-455C, and C-482T polymorphisms are the most concerned and have been the main focus of research on APOC3 gene.
Among all 29 inclusion studies, three showed a deviation from HWE in population control. The divergence from HWE may be a sign of genotyping errors (Xu et al., 2002, Hosking et al., 2004, Salanti et al., 2005). However, the statistical power of detection minor genotyping errors by testing for HWE divergence was low (McCarthy et al., 2008), and the presence of HWE was generally not altered by the introduction of genotyping errors (Zou and Donner, 2006). Moreover, the analysis suggested that exclusion of HWE-violating studies may have resulted in loss of statistical significance of some postulated gene and disease associations. The adjustment for the magnitude of deviation from the model may also have the same consequence for other gene and disease associations (Trikalinos et al., 2006). Based on the aforementioned consideration, we did not eliminate these three studies from the meta-analysis to avoid selection bias.
Previous studies showed that variations in the APOC3 gene region might generate sex-specific effects (Kessling et al., 1992, Espino-Montoro et al., 2003, Coban et al., 2012). Meta-regression was performed after adjustment for publication year, ethnicity, sex, genotyping methods, and sample size to detect the source of heterogeneity. The results suggested that sex was one of the possible factors of heterogeneity despite the loss of statistical significance after Monte Carlo validation. In contrast, other factors were not considered as the cause of heterogeneity. Nonetheless, the existence of heterogeneity between studies is an inevitable limitation of this meta-analysis. Furthermore, case–control studies which are a common design for current available genetic reports and the relatively small numbers of eligible studies on the C-482T polymorphism influencing CHD were additional limitations of the present meta-analysis.
This study was independently completed from conception to completion, including analysis and writing. The literature search strategy differs from previous study (Zhang et al., 2016): Firstly, we performed a systematic databases search in PubMed and HuGE Navigator, which provide access to a continuously updated knowledge base in human genome epidemiology, including information on population prevalence of genetic variants and gene-disease associations. Secondly, the search terms used in the current study differs from the previous study. Thirdly, the literature search cutoff was 25 September, 2015, which includes more recent reports than previous analyses. Based on the above variations, the final references included in our study are significantly distinct. Some literatures have been included in the present study, which were not included in previous study. The current study had not overthrown previous findings, but more studies will be needed to validate the conclusions with increasing new data.
5. Conclusions
Our results suggested that carriers of the APOC3 allele mutation SstI and T-455C polymorphisms have an increased risk of CHD development. However, the association between C-482T polymorphism and CHD susceptibility has not been established, and needs to be validated in future studies.
List of abbreviations
- APOC3
apolipoprotein C3
- CHD
coronary heart disease
- SNPs
single nucleotide polymorphisms
- TG
triglyceride
- HDL-C
high-density lipoprotein cholesterol
- VLDL
very-low-density lipoprotein
- CM
chylomicrons
- TRLs
triglyceride-rich lipoproteins
- NOS
Newcastle–Ottawa Scale
- HWE
Hardy–Weinberg equilibrium
- PCR
Polymerase Chain Reaction
- PCR-RFLP
PCR-Restriction Fragment Length Polymorphism
- PCR-SOP
PCR-sequence-specific oligonucleotide probes
- ORs
odds ratios
- CIs
confidence intervals
- LPL
lipoprotein lipase
- HL
hepatic lipase
- UTR
untranslated region
- MAFs
minor allele frequencies
- IRE
insulin-responsive element
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YL conceived of the study and participated in its design. CL and JG carried out the study searches and collected the data. YL performed statistical analyses and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Beijing Natural Science Foundation (7123208).
References
- Abdel-Maksoud M.F., Hokanson J.E. The complex role of triglycerides in cardiovascular disease. Semin. Vasc. Med. 2002;2:325–333. doi: 10.1055/s-2002-35403. [DOI] [PubMed] [Google Scholar]
- Brown W.V., Baginsky M.L. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem. Biophys. Res. Commun. 1972;46:375–382. doi: 10.1016/s0006-291x(72)80149-9. [DOI] [PubMed] [Google Scholar]
- Bruns G.A., Karathanasis S.K., Breslow J.L. Human apolipoprotein A-I–C-III gene complex is located on chromosome 11. Arteriosclerosis. 1984;4:97–102. doi: 10.1161/01.atv.4.2.97. [DOI] [PubMed] [Google Scholar]
- Coban N., Onat A., Guclu-Geyik F., Komurcu-Bayrak E., Sansoy V., Hergenc G., Can G., Erginel-Unaltuna N. Gender- and obesity-specific effect of apolipoprotein C3 gene (APOC3) − 482C > T polymorphism on triglyceride concentration in Turkish adults. Clin. Chem. Lab. Med.: CCLM/FESCC. 2012;50:285–292. doi: 10.1515/CCLM.2011.747. [DOI] [PubMed] [Google Scholar]
- Ekman R., Nilsson-Ehle P. Effects of apolipoproteins on lipoprotein lipase activity of human adipose tissue. Clin. Chim. Acta. 1975;63:29–35. doi: 10.1016/0009-8981(75)90374-5. [DOI] [PubMed] [Google Scholar]
- Espino-Montoro A., Barrios-Artillo M., Lopez-Chozas J.M., Cayuela A., Stiefel P., Villar J. Influence of polymorphism (RFLP-sstI) at the apolipoprotein C-III gene locus on the lipoprotein metabolism and insulin resistance in essential hypertensive patients. Interaction between gender and genetic polymorphism. Nutr. Metab. Cardiovasc. Dis. 2003;13:194–201. doi: 10.1016/s0939-4753(03)80011-x. [DOI] [PubMed] [Google Scholar]
- Havel R.J., Fielding C.J., Olivecrona T., Shore V.G., Fielding P.E., Egelrud T. Cofactor activity of protein components of human very low density lipoproteins in the hydrolysis of triglycerides by lipoproteins lipase from different sources. Biochemistry. 1973;12:1828–1833. doi: 10.1021/bi00733a026. [DOI] [PubMed] [Google Scholar]
- Hosking L., Lumsden S., Lewis K., Yeo A., McCarthy L., Bansal A., Riley J., Purvis I., Xu C.F. Detection of genotyping errors by Hardy–Weinberg equilibrium testing. Eur. J. Hum. Genet. 2004;12:395–399. doi: 10.1038/sj.ejhg.5201164. [DOI] [PubMed] [Google Scholar]
- Jorgensen A.B., Frikke-Schmidt R., Nordestgaard B.G., Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- Kawakami A., Aikawa M., Libby P., Alcaide P., Luscinskas F.W., Sacks F.M. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation. 2006;113:691–700. doi: 10.1161/CIRCULATIONAHA.105.591743. [DOI] [PubMed] [Google Scholar]
- Kessling A., Ouellette S., Bouffard O., Chamberland A., Betard C., Selinger E., Xhignesse M., Lussier-Cacan S., Davignon J. Patterns of association between genetic variability in apolipoprotein (apo) B, apo AI-CIII-AIV, and cholesterol ester transfer protein gene regions and quantitative variation in lipid and lipoprotein traits: influence of gender and exogenous hormones. Am. J. Hum. Genet. 1992;50:92–106. [PMC free article] [PubMed] [Google Scholar]
- Kinnunen P.K., Ehnolm C. Effect of serum and C-apoproteins from very low density lipoproteins on human postheparin plasma hepatic lipase. FEBS Lett. 1976;65:354–357. doi: 10.1016/0014-5793(76)80145-7. [DOI] [PubMed] [Google Scholar]
- Lai C.Q., Parnell L.D., Ordovas J.M. The APOA1/C3/A4/A5 gene cluster, lipid metabolism and cardiovascular disease risk. Curr. Opin. Lipidol. 2005;16:153–166. doi: 10.1097/01.mol.0000162320.54795.68. [DOI] [PubMed] [Google Scholar]
- Li W.W., Dammerman M.M., Smith J.D., Metzger S., Breslow J.L., Leff T. Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J. Clin. Invest. 1995;96:2601–2605. doi: 10.1172/JCI118324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li C., Gao J. Data in support of the association between apolipoprotein C3 gene variants and the risk of coronary heart disease. Data Brief. 2016 doi: 10.1016/j.mgene.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M.I., Abecasis G.R., Cardon L.R., Goldstein D.B., Little J., Ioannidis J.P., Hirschhorn J.N. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- Mendivil C.O., Zheng C., Furtado J., Lel J., Sacks F.M. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler. Thromb. Vasc. Biol. 2010;30:239–245. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendivil C.O., Rimm E.B., Furtado J., Sacks F.M. Apolipoprotein E in VLDL and LDL with apolipoprotein C-III is associated with a lower risk of coronary heart disease. J. Am. Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Nakano T., Tokita Y., Nagamine T., Inazu A., Kobayashi J., Mabuchi H., Stanhope K.L., Havel P.J., Okazaki M. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin. Chim. Acta. 2011;412:1306–1318. doi: 10.1016/j.cca.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi E.M., Barrett P.H., Chan D.C., Watts G.F. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin. Sci. 2008;114:611–624. doi: 10.1042/CS20070308. [DOI] [PubMed] [Google Scholar]
- Quarfordt S.H., Michalopoulos G., Schirmer B. The effect of human C apolipoproteins on the in vitro hepatic metabolism of triglyceride emulsions in the rat. J. Biol. Chem. 1982;257:14642–14647. [PubMed] [Google Scholar]
- Rees A., Shoulders C.C., Stocks J., Galton D.J., Baralle F.E. DNA polymorphism adjacent to human apoprotein A-1 gene: relation to hypertriglyceridaemia. Lancet. 1983;1:444–446. doi: 10.1016/s0140-6736(83)91440-x. [DOI] [PubMed] [Google Scholar]
- Salanti G., Amountza G., Ntzani E.E., Ioannidis J.P. Hardy–Weinberg equilibrium in genetic association studies: an empirical evaluation of reporting, deviations, and power. Eur. J. Hum. Genet. 2005;13:840–848. doi: 10.1038/sj.ejhg.5201410. [DOI] [PubMed] [Google Scholar]
- Sayols-Baixeras S., Lluis-Ganella C., Lucas G., Elosua R. Pathogenesis of coronary artery disease: focus on genetic risk factors and identification of genetic variants. Appl. Clin. Genet. 2014;7:15–32. doi: 10.2147/TACG.S35301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne F., Hanks J., Meyers W., Quarfordt S. Effect of apoproteins on hepatic uptake of triglyceride emulsions in the rat. J. Clin. Invest. 1980;65:652–658. doi: 10.1172/JCI109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Zhu L., Richa M., Li P., Yang Y., Li S. Associations of the APOC3 rs5128 polymorphism with plasma APOC3 and lipid levels: a meta-analysis. Lipids Health Dis. 2015;14:32. doi: 10.1186/s12944-015-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M., Zhong S., Bou Khalil M., Links P.H., Zhao Y., Iqbal J., Hussain M.M., Parks R.J., Wang Y., Yao Z. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J. Lipid Res. 2010;51:150–161. doi: 10.1194/jlr.M900346-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmud P.J., Humphries S.E. Apolipoprotein C-III gene variation and dyslipidaemia. Curr. Opin. Lipidol. 1997;8:154–158. doi: 10.1097/00041433-199706000-00005. [DOI] [PubMed] [Google Scholar]
- Tg, Hdl Working Group of the Exome Sequencing Project NHL, Blood I, Crosby J., GM P., PL A., DR C., NO S., LA L., Y L. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Trikalinos T.A., Salanti G., Khoury M.J., Ioannidis J.P. Impact of violations and deviations in Hardy–Weinberg equilibrium on postulated gene-disease associations. Am. J. Epidemiol. 2006;163:300–309. doi: 10.1093/aje/kwj046. [DOI] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R.J. Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J. Biol. Chem. 1980;255:8303–8307. [PubMed] [Google Scholar]
- Xu J., Turner A., Little J., Bleecker E.R., Meyers D.A. Positive results in association studies are associated with departure from Hardy–Weinberg equilibrium: hint for genotyping error? Hum. Genet. 2002;111:573–574. doi: 10.1007/s00439-002-0819-y. [DOI] [PubMed] [Google Scholar]
- Zhang J.Z., Xie X., Ma Y.T., Zheng Y.Y., Yang Y.N., Li X.M., Fu Z.Y., Dai C.F., Zhang M.M., Yin G.T. Association between apolipoprotein C-III gene polymorphisms and coronary heart disease: A meta-analysis. Aging and Dis. 2016;7:36–44. doi: 10.14336/AD.2015.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Khoo C., Furtado J., Sacks F.M. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 2010;121:1722–1734. doi: 10.1161/CIRCULATIONAHA.109.875807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G.Y., Donner A. The merits of testing Hardy–Weinberg equilibrium in the analysis of unmatched case–control data: a cautionary note. Ann. Hum. Genet. 2006;70:923–933. doi: 10.1111/j.1469-1809.2006.00267.x. [DOI] [PubMed] [Google Scholar]