Summary
Post‐kala‐azar dermal leishmaniasis (PKDL) is a chronic dermal complication that occurs usually after recovery from visceral leishmaniasis (VL). The disease manifests into macular, papular and/or nodular clinical types with mono‐ or polymorphic presentations. Here, we investigated differences in immunological response between these two distinct clinical forms in Indian PKDL patients. Peripheral blood mononuclear cells of PKDL and naive individuals were exposed in vitro to total soluble Leishmania antigen (TSLA). The proliferation index was evaluated using an enzyme‐linked immunosorbent assay (ELISA)‐based lymphoproliferative assay. Cytokines and granzyme B levels were determined by cytometric bead array. Parasite load in tissue biopsy samples of PKDL was quantified by quantitative polymerase chain reaction (qPCR). The proportion of different lymphoid subsets in peripheral blood and the activated T cell population were estimated using flow cytometry. The study demonstrated heightened cellular immune responses in the polymorphic PKDL group compared to the naive group. The polymorphic group showed significantly higher lymphoproliferation, increased cytokines and granzyme B levels upon TSLA stimulation, and a raised proportion of circulating natural killer (NK) T cells against naive controls. Furthermore, the polymorphic group showed a significantly elevated proportion of activated CD4+ and CD8+ T cells upon in‐vitro TSLA stimulation. Thus, the polymorphic variants showed pronounced cellular immunity while the monomorphic form demonstrated a comparatively lower cellular response. Additionally, the elevated level of both activated CD4+ and CD8+ T cells, coupled with high granzyme B secretion upon in‐vitro TSLA stimulation, indicated the role of cytotoxic cells in resistance to L. donovani infection in polymorphic PKDL.
Keywords: granzyme B, leishmania, monomorphic, polymorphic, post‐kala‐azar dermal leishmaniasis
Introduction
Leishmaniasis is a parasitic disease, largely affecting economically underprivileged people in developing countries, and poses a risk to more than 350 million people worldwide 1. It is caused by more than 20 species of the genus Leishmania 2, requiring an infected female sandfly for transmission. The disease manifests into different clinical forms, ranging from self‐healing cutaneous leishmaniasis (CL) to disfiguring mucosal lesions to the visceral form, visceral leishmaniasis (VL). VL, commonly known in India as kala‐azar, caused by L. donovani, is the most lethal form, which proves fatal if not attended to in time. It is characterized by irregular bouts of fever, weight loss, enlargement of spleen and liver and anaemia. The disease is highly endemic in the Indian subcontinent and in East Africa, and more than 90% of new cases have been reported to occur in six countries: India, Sudan, South Sudan, Brazil, Ethiopia and Bangladesh 3.
A dermal sequel of VL, known as post‐kala‐azar dermal leishmaniasis (PKDL), develops in 5–10% of apparently cured VL individuals in the Indian subcontinent compared to 50–60% in Sudan 4, 5, 6, 7. It is characterized by different clinical complications such as nodular, macular or maculopapular dermal lesions. In the Indian subcontinent, patients with PKDL, based on their clinical presentations, can be categorized into two clinical subgroups: monomorphic PKDL, where the patients primarily have a single clinical lesion type as either hypopigmented macules, papule type and polymorphic PKDL where, in addition to macules, the patients also present with nodules and/or papules 8. Polymorphic PKDL is predominant, and represents 45–85% of Indian PKDL cases 9, 10. As transmission of VL in the Indian subcontinent is anthroponotic, PKDL patients are considered a reservoir of VL, which is an added concern in the context of sustainable elimination of VL.
Immunological factors predisposing patients with VL to PKDL remain poorly understood, and the underlying pathology is often attributed to parasite‐specific cellular immunity 5. In Sudan, PKDL cases invariably respond to Leishmania antigen 11, whereas cellular immune responses in Indian PKDL are not well characterized. Additionally, cellular responses in Indian PKDL have always been analysed as a single entity, and studies on the immunological differences between mono‐ and polymorphic forms of PKDL are poorly explored 12, 13, 14.
The current study aimed to examine the Leishmania‐specific cell‐mediated immune response in polymorphic and monomorphic forms of PKDL, measuring lymphoproliferation, cytokines and granzyme B levels and T lymphocyte activation following in‐vitro stimulation of peripheral blood mononuclear cells (PBMCs) with total soluble Leishmania antigen (TSLA). To characterize generalized cellular immunity we analysed the phenotype and proportion of the different lymphoid subsets [T helper, T cytotoxic, natural killer (NK), NK T and B cells] in peripheral blood of each individual in the study groups. Taken together, the study provides a precise understanding of immunopathological differences between the two distinct clinical forms prevalent in Indian PKDL.
Materials and methods
Study subjects
Patients with clinical diagnoses of PKDL (n = 19), admitted between January 2009 and April 2012 to the Department of Dermatology, Safdarjung Hospital, New Delhi, were mainly residents of Bihar, India. All patients were examined clinically for the identification of characteristic lesions such as macular, papular, nodular or mixed/polymorphic forms. They were subjected initially to the rapid rK39 immunochromatographic strip test, and positive cases were examined further to confirm PKDL through visualization of Leishmania amastigotes by direct microscopy in skin tissue/slit aspirates samples and/or a quantitative polymerase chain reaction (qPCR) test for Leishmania DNA, as reported previously 15, 16, 17. Of a total of 19 PKDL cases, all parameters under investigation were investigated for 16 cases. In three of 13 polymorphic cases, the sample was sufficient for only two studies, i.e. cell phenotyping and parasite load, and remaining studies could not be undertaken. Blood samples of healthy controls (n = 20), negative to the rK39 strip test and qPCR for Leishmania DNA, were also included in this study. All individuals in the study were seronegative for HIV and more than 16 years of age. Clinical features of the study groups are described in Table 1.
Table 1.
Clinical features of the study groups
| Features | PKDL (n = 19) | Naive (n = 20) |
|---|---|---|
| Age, years | ||
| Mean ± s.d. | 28·52 ± 11·24 | 26·55 ± 4·74 |
| Median (range) | 25 (16–55) | 27·5 (18–34) |
| Sex ratio, M : F | 16 : 3 | 19 : 1 |
| Lesion types | ||
| Polymorphic | 13 | n.a. |
| Monomorphic | 6 | n.a. |
| History of VL, % (proportion) | 73·68% (14/19) | n.a. |
| Time lapse between VL treatment and onset of PKDL, years | ||
| Mean ± s.d. | 9·35 ± 7·96 | n.a. |
| Median (range) | 9 (0·5–28) | n.a. |
PKDL = post‐kala‐azar dermal leishmaniasis; n.a. = not applicable; VL = visceral leishmaniasis; s.d. = standard deviation.
Categories of PKDL based on clinical presentation
Cases of PKDL were categorized into two groups based on clinical presentation: (1) monomorphic PKDL (n = 6): cases presenting with a single type of lesion, either hypopigmented macular (n = 5) or papular (n = 1); and (2) mixed/polymorphic PKDL (n = 13): cases with at least two different types of lesions of macular, papular and nodular types.
DNA isolation
Tissue biopsies were obtained from PKDL patients lesions and collected in NET buffer [150 mM NaCl, 15 mM Tris‐HCl (pH 8·3) and 1 mM ethylenediamine tetraacetic acid (EDTA)]. Tissue was first homogenized and subsequently DNA extraction was performed by using QIAamp DNA tissue mini kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. DNA was eluted in 100 µl distilled water. All samples were processed on the same day and DNA was stored at −80°C until use.
Real‐time PCR assay
SYBR Green I‐based real‐time PCR using kDNA‐based primers as described previously 16 was applied for accurate quantification of the target sequence in the study. Briefly, 10 µl of the PCR reaction was used, consisting of 1× SYBR Green I PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 5 pmol forward primer, 5 pmol reverse primer and 1 µl volume of DNA from the tissue samples. PCR cycling parameters were 50°C for 2 min, 95°C for 10 min and 40 cycles at 95°C for 15 s and 60°C for 1 min. A threshold cycle value (Ct) was calculated for each sample by determining the point at which the fluorescence exceeded the threshold limit. Each real‐time PCR reaction was carried out in triplicate. The blood samples of 20 healthy individuals from non‐endemic areas were taken for qPCR assay.
Preparation of Leishmania antigen
Total soluble Leishmania antigen (TSLA) was prepared as described previously 18. Briefly, promastigotes of L. donovani (MHOM/IN/80/DD8) in the stationary phase were harvested and lysed with solution containing 50 mM Tris/5 mM EDTA/HCl, pH 7. After three freeze/thaw cycles, the samples were given three pulses of 20 s at 40 W with a sonicator at 1‐min intervals. The supernatant was collected and protein estimated using the Bradford method. TSLA was stored at −80°C until use.
Lymphoproliferative assay
Lymphoproliferative assay was performed as described previously 18. Briefly, PBMCs were isolated from the heparinized blood samples of PKDL and naive individuals by density gradient sedimentation. PBMCs at a density of 1 × 106 cells/ml were cultured with TSLA (10 µg/ml) or phytohaemagglutinin M‐form (PHA‐M) (10 µg/ml) for 120 h in a humidified 37°C/5% CO2 incubator. At 104–106 h incubation, 20 µl 5‐bromo‐2'‐deoxyuridine (BrdU) labelling solution was added and samples reincubated for another 16–18 h. Lymphoproliferation was evaluated using the enzyme‐linked immunosorbent assay (ELISA) method. The proliferation index (PI) was calculated as the ratio of optical density (OD) of stimulated and unstimulated cultures for each sample.
Cytokines and granzyme B analysis
Quantitative analysis of cytokines and granzyme B in the culture supernatant was carried out as described previously 18. Briefly, PBMCs of PKDL and naive individuals were cultured in the presence of TSLA for 120 h, as described above. The culture supernatants were collected and stored at −80°C until use. The cytokine levels [interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α and interleukin (IL)−10] and granzyme B were determined using BD cytometric bead array (CBA) flex sets and measuring fluorescence on a BD FACSCaliburTM (BD Biosciences, Haryana, India) flow cytometry using BD CellQuestTM Pro software. The data were analysed using flow cytometric analysis program (FCAP) ArrayTM software. The detection sensitivity for IFN‐γ, TNF‐α, IL‐10 and granzyme B was 1·8, 1·2, 0·13 and 4 pg/ml, respectively.
Cell phenotyping of activated T cell populations
Estimation of activated CD4+ or CD8+ T cells was performed as described previously 18. Briefly, freshly isolated PBMCs (106/ml) from six PKDL with polymorphic lesions and six naive individuals from individuals recruited for the study were cultured in the presence of TSLA for 120 h at 37°C. Following incubation, the cells were harvested and surface‐stained with fluorochrome‐conjugated antibodies to CD3‐fluorescein isothiocyanate (FITC), CD4‐peridinin chlorophyll (PerCP)‐cyanin 5·5 (Cy5º5), CD8‐PerCP‐Cy5·5 and CD69‐allophycocyanin (APC), along with appropriate isotype controls for 30 min at 4°C. After washing, the cells were resuspended in 500 µl staining buffer. Samples were acquired and analysed on a BD FACSCaliburTM using BD CellQuestTM Pro software on at least 10 000 lymphocytes (R1) events. Analysis gates were set on lymphocytes using forward‐ and side‐scatter properties and the frequencies of activated CD4+ and CD8+ T cells were acquired on CD3+ T cells. A cell viability test using 7‐aminoactinomycin D (7‐AAD) sample staining was performed in separate tubes to assess the overall viability of analysed cells.
Cell phenotyping of cells from lysed whole blood
To characterize generalized cellular immunity we analysed the phenotype and proportion of the different lymphoid subsets (T helper, T cytotoxic, NK, NK T and B cells) in the peripheral blood of each individual in the study groups; 0·1 ml of well‐mixed anti‐coagulated whole blood was surface‐stained with appropriate fluorochrome‐conjugated antibodies along with their corresponding isotype controls (BD Biosciences). Three sets of sample tubes were prepared, one for T cell (CD3‐PerCP, CD8‐PE, CD4‐FITC) and the others for NK, NK T cells (CD45‐PerCP, CD3‐FITC, CD16 + 56‐PE) and B cells (CD45‐PerCP, CD3‐FITC, CD19‐PE). After incubation for 30 min at 4°C in the dark, 2 ml of diluted (1×) cell lysing solution was added to each tube, vortexed and incubated at room temperature for 12 min; 2 ml of washing solution was added to each tube. The tubes were centrifuged at 200 g for 5 min and supernatant was carefully aspirated out. The cell pellets were resuspended in 500 μl wash buffer and vortexed. Samples were acquired and analysed using BD FACSCaliburTM using BD CellQuestTM Pro software.
Ethics statement
The study was approved and carried out according to the guidelines of the Ethical Committee, VMMC and Safdarjung Hospital, New Delhi. All patients provided written informed consent for the collection of samples and subsequent analysis.
Statistical analysis
Results are represented as mean ± standard error (s.e.). Data were analysed using GraphPad Prism 5·0 (GraphPad Software, San Diego, CA, USA). Statistical significance was determined by non‐parametric Mann–Whitney U‐test between two groups and by Kruskal–Wallis test, followed by the post‐hoc Dunn's multiple comparison test for more than two groups. Correlation was calculated by Spearman's rank correlation test. The statistical tests were two‐tailed and P values < 0·05 were considered significant.
Results
Lymphoproliferative response to L. donovani TSLA
Cellular response was analysed in the PKDL (n = 16) and naive groups (n = 19) in terms of lymphoproliferative response in vitro to TSLA, using PHA‐M as a positive control. All cases showed high proliferation with PHA‐M [naive PI mean ± standard error (s.e.), 9·873 ± 0·775; PKDL, 9·949 ± 0·98]. In response to TSLA stimulation, the group mean of monomorphic PKDL (PI mean ± s.e., 2·165 ± 0·76) was found comparable to the naive group (PI mean ± s.e., 1·157 ± 0·073) (Fig. 1). However, the polymorphic PKDL response group was significantly high (PI mean ± s.e., 4·605 ± 1·554, P < 0·01) compared to the naive group.
Figure 1.

Lymphoproliferative response to total soluble Leishmania antigen (TSLA) in post‐kala‐azar dermal leishmaniasis (PKDL). Peripheral blood lymphocytes from individuals with PKDL (monomorphic, n = 6; polymorphic, n = 10) and naive (n = 16) groups were cultured in presence of TSLA (10 µg/ml) for 120 h and lymphoproliferation was measured by 5‐bromo‐2'‐deoxyuridine (BrdU) incorporation for the last 12–14 h using the BiotrakTM cell proliferation enzyme‐linked immunosorbent assay (ELISA) system. Data were analysed between groups by the non‐parametric Kruskal–Wallis test followed by the post‐hoc Dunn's multiple comparison test. The horizontal lines indicate mean value. *P < 0·05 is considered statistically significant.
Cytokine profile upon TSLA stimulation
PBMCs from all the study groups were examined for cytokine profile in response to TSLA (Fig. 2a–c). The cell‐mediated immune (CMI) response (IFN‐γ and TNF‐α) in the polymorphic groups was found significantly higher compared to the naive group. The polymorphic (mean ± s.e., 1461 pg/ml ± 622) group showed a significantly high (P < 0·001) IFN‐γ level compared to the naive group (mean ± s.e., 2·104 pg/ml ± 0·65), while the mean of the monomorphic PKDL group (mean ± s.e., 48·33 pg/ml ± 13·97) was found comparable to the naive group. Similarly, significantly high TNF‐α production was observed in response to TSLA stimulation in polymorphic (mean ± s.e., 75·99 pg/ml ± 37·15) group compared to the naive group (mean ± s.e., 1·05 pg/ml ± 0·48, P < 0·001), while the group mean of monomorphic PKDL (mean ± s.e., 6·428 pg/ml ± 2·23) was found comparable to the naive group. For IL‐10 cytokine, the measured values for polymorphic (mean ± s.e., 4·08 pg/ml ± 1·432) were low, and comparable to the naive (mean ± s.e., 0·786 pg/ml ± 0·393) or monomorphic PKDL groups (mean ± s.e., 0·433 pg/ml ± 0·4).
Figure 2.
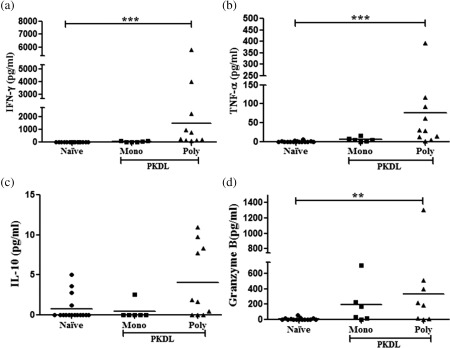
In‐vitro Leishmania‐specific cellular immune response in post‐kala‐azar dermal leishmaniasis (PKDL). Peripheral blood mononuclear cells (PBMCs) were cultured in the presence of total soluble Leishmania antigen (TSLA) (10 µg/ml) for 120 h. (a–c) Cytokines [interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α and interleukin (IL)−10] were measured in the culture supernatant of PKDL (monomorphic, n = 6; polymorphic, n = 10) and naive (n = 16) groups and (d) granzyme B were analysed in the culture supernatant of (monomorphic, n = 6; polymorphic, n = 8) and naive (n = 15) groups using cytometric bead array (CBA). Data were analysed between groups by the non‐parametric Kruskal–Wallis test followed by the post‐hoc Dunn's multiple comparison test. The horizontal lines indicate mean values. *P < 0·05; **P < 0·01; ***P < 0·001.
Granzyme B production in response to TSLA stimulation
Granzyme B, a serine proteinase expressed by the cytotoxic lymphocytes such as CD8+, CD4+ T cells and regulatory T cells (Treg), induces apoptosis of target cells 19, 20. Here, the granzyme B level upon in‐vitro stimulation of PBMCs with TSLA was estimated in culture supernatant by CBA assay. The granzyme B level of the polymorphic group (mean ± s.e., 331·8 pg/ml ± 154·6, P < 0·05) was found significantly high compared to the naive group (mean ± s.e., 9·499 pg/ml ± 4·12), while the group mean of monomorphic PKDL (mean ± s.e., 192·4 pg/ml ± 108·6) was found comparable to the naive group (Fig. 2d).
Parasite load in PKDL tissues
Leishmania DNA was detected in lesion tissue of all 19 patients with PKDL. All PKDL cases were positive for qPCR, with a mean value of parasite load of 5280 ± 1864 parasites/µg tissue DNA and range of 10–24 805 parasites/µg tissue DNA, while all naive controls (n = 20) were confirmed negative. Comparative assessment of parasite load in the two clinical forms of PKDL revealed a mean parasite load of 7582 ± 2492 parasites/µg tissue DNA in the polymorphic group (n = 13), which was significantly higher (P = 0·003) compared to the mean load of 293 ± 157 parasites/µg tissue DNA in the monomorphic group (n = 6). A scatter‐plot of the parasite load in different clinical forms of PKDL is shown in Fig. 3.
Figure 3.
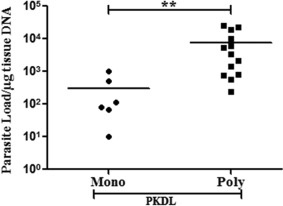
Parasite load in post‐kala‐azar dermal leishmaniasis (PKDL) lesion tissues. The parasite load (parasites/µg tissue DNA) was determined in PKDL (monomorphic, n = 6; polymorphic, n = 13) by real‐time polymerase chain reaction (PCR). Statistical significance was determined by non‐parametric Mann–Whitney test between polymorphic and monomorphic forms of dermal lesions in active PKDL cases. The horizontal line represents the mean parasite load. **P < 0·01.
Correlation of immune response with parasite load
Secretion of IFN‐γ, TNF‐α, IL‐10 and granzyme B upon TSLA stimulation was analysed in the PKDL group (n = 16) with respect to the parasite load. The levels of IFN‐γ and TNF‐α were significantly higher in those cases where the parasite load was high and were found significantly correlated (IFN‐γ, r s = 0·697, P = 0·002 and TNF‐α, r s = 0·629, P = 0·009) (Fig. 4). No such correlation with parasite load was observed for IL‐10 and granzyme B.
Figure 4.
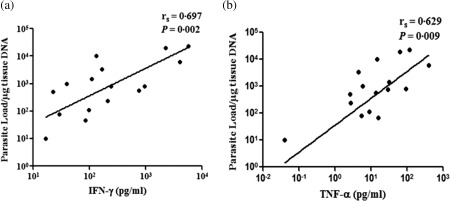
Comparative assessment of T helper type 1 (Th1) cytokines and parasite load in post‐kala‐azar dermal leishmaniasis (PKDL). The levels of interferon (IFN)‐γ and tumour necrosis factor (TNF)‐α in culture supernatant upon total soluble Leishmania antigen (TSLA) stimulation of peripheral blood mononuclear cells (PBMCs) from patients with PKDL (n = 16) were measured by cytometric bead array (CBA) and parasite load in tissue lesions was determined by quantitative polymerase chain reaction (qPCR). Correlation was calculated by Spearman's rank correlation test. Diagonal line represents the best‐fitting line.
Determination of activated T cell population
Using CD69 as an activation marker 21, we investigated the percentage of activated CD4+ and CD8+ T cell populations upon in‐vitro TSLA stimulation in the polymorphic PKDL and naive groups. Both CD4+ and CD8+ T cells showed pronounced activation, with a significantly higher percentage of the CD8+CD69+ T cell population in the polymorphic PKDL group (mean ± s.e., 4·348 ± 0·35, P = 0·002) compared to the naive group (mean ± s.e., 0·553 ± 0·234). There was also a significantly higher percentage of CD4+CD69+ T cells (mean ± s.e., 1·56 ± 0·133, P = 0·002) compared to the naive group (mean ± s.e., 0·451 ± 0·093) (Fig. 5). The values of unstimulated cells were subtracted from TSLA‐stimulated cells. A cell viability test using 7‐AAD sample staining confirmed that the gated lymphocytes were > 99% viable for both the PKDL and naive groups.
Figure 5.
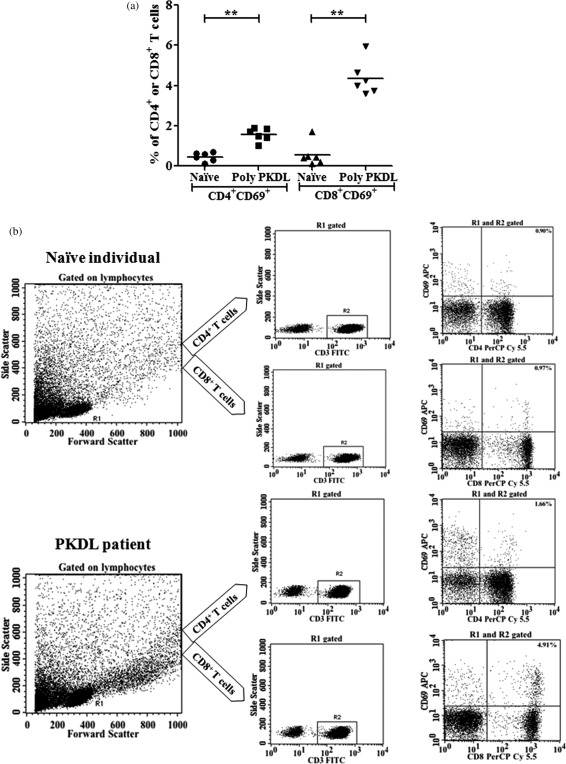
Determination of the activated T cell population in post‐kala‐azar dermal leishmaniasis (PKDL). (a) The percentage of total soluble Leishmania antigen (TSLA)‐activated CD4+ and CD8+ T cells in polymorphic PKDL and naive groups. Peripheral blood mononuclear cells (PBMCs) from the polymorphic PKDL (n = 6) and naive (n = 6) groups were incubated with TSLA (10 µg/ml) for 120 h at 37°C. Analysis gates were set for lymphocytes using forward‐ and side‐scatter properties and the frequencies of activated CD4+ and CD8+ cells were acquired on CD3+ T cells. The values of unstimulated cells were subtracted from TSLA‐stimulated cells. (b) The data show a representative cytofluorimetric protocol analysis in one of the PKDL individuals. Data were analysed using the non‐parametric Mann–Whitney U‐test between groups. Horizontal lines indicate mean values. **P < 0·01.
Lymphocyte profile in PKDL
Assessment based on the surface marker of the gated lymphocytes from lysed whole blood showed no differences between the polymorphic and monomorphic groups compared to the naive group in terms of the percentage of cells expressing CD4, CD8, CD16 + 56 or CD19, or any changes in the CD4+ and CD8+ T cell ratio. However, the mean percentage of CD3+ T cells was found significantly high in the polymorphic group (mean ± s.e., 64·78 ± 2·48, P < 0·01) compared to the naive group (mean ± s.e., 52·70 ± 2·24), whereas the mean for the monomorphic group was comparable to the naive group (P > 0·05). A similar result was obtained for NK T cells, where the polymorphic group (mean ± s.e., 9·48 ± 0·91, P < 0·05) showed a significantly high proportion compared to the naive group (mean ± s.e., 6·98 ± 0·24), whereas the mean of the monomorphic group (mean ± s.e., 7 ± 0·92) was comparable to the naive group (P > 0·05) (Fig. 6).
Figure 6.
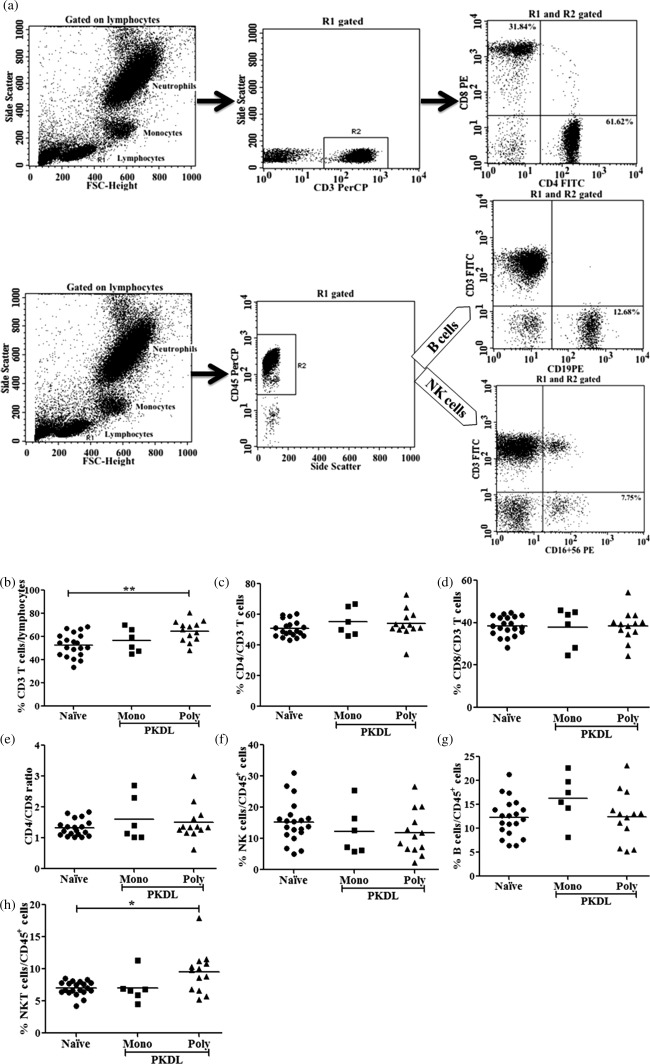
Flow cytometry analysis of lymphocyte subsets in post‐kala‐azar dermal leishmaniasis (PKDL). Whole blood samples from 19 PKDL cases (monomorphic, n = 6; polymorphic, n = 13) and 20 naive samples were surface‐stained with appropriate antibodies, as mentioned in the Material and methods section, and analysed using flow cytometry. (a) Cytofluorimetric analysis showing one representative PKDL individual. Lymphocytes (R1) were gated on the basis of forward‐ and side‐scatter and CD3+ (b) and CD45+ cells were gated on lymphocytes (R1). (c) CD4+ and (d) CD8+ cells were gated on CD3+ cells with (e) ratio of CD4 and CD8 T cells, whereas (f) natural killer (NK) (g) B and (h) NK T cells were gated on CD45+ cells. Horizontal lines indicate mean values. *P < 0·05; **P < 0·01.
Discussion
We initially evaluated the Leishmania‐specific cellular immune response by measuring lymphoproliferation upon in‐vitro TSLA stimulation in monomorphic and polymorphic forms of PKDL in comparison with the naive group. The study revealed significantly higher lymphoproliferation in the PKDL group with polymorphic lesions compared to the unexposed (naive group). Conversely, the PKDL group with monomorphic lesions showed lower levels of lymphoproliferation compared to the polymorphic PKDL group. The positive lymphoproliferation in polymorphic PKDL could be due to higher circulating Leishmania‐specific memory T cells responsible for the significantly pronounced cellular responses. In our previous study 18 similar observations were made with cured VL individuals, although patients with VL failed to show lymphoproliferation. Previous studies on Indian PKDL have documented the antigen‐specific CMI responses with varied results, possibly due to variation in the relative proportion of mono‐ and polymorphic variants in their study groups. Haldar et al. reported lymphoproliferation in two‐thirds of PKDL cases 12, whereas other studies recorded the absence of lymphoproliferation compared to healthy controls 13, 14, 22.
The cytokine‐level milieu has an important role in regulating an appropriate immune response against parasitic diseases. IFN‐γ, a Th1 cytokine, has a key role in controlling intracellular parasites, as demonstrated by the extreme susceptibility of IFN‐γ‐deficient mouse strains to Leishmania infection 23. It is secreted by primarily CD4+ Th1 and CD8+ T lymphocytes and NK cells in response to IL‐12 signalling. The mechanism of action of IFN‐γ is most clearly evident against the Leishmania parasite, which resides in macrophages that are activated readily by this cytokine 24, 25. The cytokine primarily restricts parasite growth by induction of inducible nitric oxide synthase (iNOS) and promoting NO production 26. Another Th1 cytokine, TNF‐α, that exerts cytotoxic effects on pathogens, has been implicated in VL pathogenesis 27.
Upon TSLA stimulation of PBMCs, the cytokine profiles corroborate our lymphoproliferation results. The PKDL group with polymorphic lesions showed higher secretion of Th1 cytokines (IFN‐γ and TNF‐α) upon TSLA stimulation in contrast to that observed for the monomorphic group. This finding is in line with our previous study with healed VL individuals exhibiting similar cellular immunity upon TSLA stimulation 18. Furthermore, the level of Th1 cytokines (IFN‐γ and TNF‐α) was higher in cases with a high parasite load for the PKDL group and showed moderate positive correlation between levels of Th1 cytokines (IFN‐γ and TNF‐α) and parasite load. IL‐10 is an anti‐inflammatory cytokine that facilitates the survival of intracellular Leishmania parasites by inhibiting NO‐mediated killing by its counter‐regulatory role against IL‐12 and IFN‐γ 28. The study demonstrated no significant difference in IL‐10 level between the different study groups, which could be due either to low detection for this cytokine or the dispersion of results. Previously it was shown that patients with PKDL in response to L. donovani antigens elicited pronounced IL‐10 secretion 14 and showed a 9·6‐fold rise in the percentage of IL‐10‐expressing CD8+ T lymphocytes 29. Additionally, Ganguly et al. showed raised levels of IFN‐γ and IL‐10 in the serum of polymorphic PKDL cases compared to the naive or monomorphic groups 29. The reported peripheral cytokine levels corroborated with enhanced immunoglobulin (Ig)G1 and IgG3 levels in polymorphic form, known to be driven by IL‐10 30, 31.
One of the most striking features of T cells is the lysis of cells expressing specific antigens. Most cells with cytotoxic activity are CD8+ T cells in adaptive immunity and have two major mechanisms of cytotoxicity: the granule exocytosis and death receptors (Fas/FasL) pathway 32, 33. The granule exocytosis pathway involves the production of perforin by CTL, a molecule that can insert into the membrane of target cells and facilitate cell lysis. This pathway is mediated by a number of enzymes produced by activated CTLs, known as granzymes. Of these, granzyme B is the most important effector molecule for triggering apoptosis in the target cell 34. Increased granzyme B levels have been associated with protection in human CL caused by L. major or L. mexicana 33, 35; however, CD8+ granzyme B+ T cells have been implicated to mediate tissue injury in CL caused by L. braziliensis 36, 37. Currently, only limited studies on parasite‐specific cell‐mediated cytotoxicity in VL, CL or mucocutaneous leishmaniasis are available, while no study has been documented so far for PKDL. To our knowledge, this is the first report of a Leishmania antigen‐induced granzyme B response in PKDL. Here, we evaluated granzyme B levels to investigate whether individuals with PKDL develop a cytotoxic immune response upon re‐exposure. We demonstrated significantly higher granzyme B secretion upon TSLA stimulation in the polymorphic PKDL group compared to the naive group, suggesting the presence of cytotoxic activity against reinfection to Leishmania in PKDL. Previously, we demonstrated the presence of cytotoxic activity in response to L. donovani infection in the healed VL individuals 18. In the present study, we observed a significantly high percentage of both CD8+CD69+ and CD4+CD69+ T cells in the PKDL group. This is similar to the observation in individuals with VL history 18 but distinct from cured CL individuals, where a high level of CD4+CD69+ T cells was demonstrated to be responsible for the cellular immunity to L. major infection 38. Taken together, the elevated levels of both activated CD4+ and CD8+ T cells, coupled with high secretion of granzyme B upon in‐vitro TSLA stimulation, suggests the role of cytotoxic activity in providing resistance against L. donovani infection in PKDL.
The characterization of cellular immunity based on the phenotype and proportion of the different peripheral blood lymphoid subsets was investigated to understand generalized cellular responses. Information pertaining to the immunophenotype profile of peripheral blood in Indian PKDL is limited, primarily because it occurs in a small subset of individuals recovered from VL. A previous study on PKDL cases from India reported a significant increase of CD3+CD8+ lymphocytes in peripheral blood at both pre‐ and post‐treatment stages 29. The present study demonstrated no major difference in proportion of circulating CD3+CD8+ T cells in PKDL compared to the naive group, an observation in line with an earlier study on Indian PKDL 39. However, the raised proportion of CD3+ T cells evident in polymorphic form could be due to the high percentage of NK T cells. NK T cells regulate both innate and adaptive immunity, and may have either effector or suppressive/regulatory functions in infectious diseases 40, 41. L. donovani is known to skew the CD56+ NK T cell response in human VL 42, but in PKDL its role is unknown and needs investigation.
Conclusion
In conclusion, the present study is an attempt to understand the differences in cellular immunological response upon in‐vitro stimulation with TSLA from L. donovani in two distinct clinical forms of Indian PKDL. The polymorphic variants of PKDL showed pronounced cellular immunity, while the monomorphic form demonstrated a comparatively lower cellular response. The phenotype and proportion of the different peripheral blood lymphoid subsets in whole blood were within normal limit, except for NK T cells. Furthermore, the significantly elevated levels of both activated CD4+ and CD8+ T cells and granzyme B in polymorphic cases indicated the role of cytotoxic cells in resistance to L. donovani infection in PKDL.
Disclosure
The authors declare no competing interests.
Author contributions
H. K., R. B.‐G., P. S., J.‐L. L. and the RAPSODI Consortium conceived and designed the experiments. H. K., K. A. and D. K. D. performed the experiments. H. K. and P. S. analysed the data. P. S., V. R., R. B.‐G., E. P., J.‐L. L. and G. P. contributed reagents/materials/analysis tools. H. K., S. K. and P. S. wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
H. K. is the recipient of Senior Research Fellowship from the Indian Council of Medical Research (ICMR), Government of India, registered for PhD at the Department of Biological Sciences, Birla Institute of Technology and Science, Pilani, India. This work received financial assistance from the European Commission FP7 project, RAPSODI. We are grateful to Mr. T. A. Nagarajuna and Dr Vinay Gupta of BD Biosciences, India for helpful discussions.
References
- 1. World Health Organization (WHO). Control of the leishmaniases. World Health Organ Tech Rep Ser 2010; 949:104. [PubMed] [Google Scholar]
- 2. Ashford RW. The leishmaniases as emerging and reemerging zoonoses. Int J Parasitol 2000; 30:1269–81. [DOI] [PubMed] [Google Scholar]
- 3. Alvar J, Vélez ID, Bern C et al Leishmaniasis worldwide and global estimates of its incidence. PLOS ONE 2012; 7:e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zijlstra EE, Khalil EA, Kager PA, El‐Hassan AM. Post‐kala‐azar dermal leishmaniasis in the Sudan: clinical presentation and differential diagnosis. Br J Dermatol 2000; 143:136–43. [DOI] [PubMed] [Google Scholar]
- 5. Ramesh V, Singh R, Salotra P. Short communication: post‐kala‐azar dermal leishmaniasis – an appraisal. Trop Med Int Health 2007; 12:848–51. [DOI] [PubMed] [Google Scholar]
- 6. Mondal D, Nasrin KN, Huda MM et al Enhanced case detection and improved diagnosis of PKDL in a kala‐azar‐endemic area of Bangladesh. PLOS Negl Trop Dis 2010; 4:e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahman KM, Islam S, Rahman MW et al Increasing incidence of post‐kala‐azar dermal leishmaniasis in a population‐based study in Bangladesh. Clin Infect Dis 2010; 50:73–6. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization (WHO) . Post‐kala‐azar dermal leishmaniasis: a manual for case management and control. Report of a WHO consultative meeting, Kolkata, India, 2–3 July 2012.
- 9. Ganguly S, Das NK, Barbhuiya JN, Chatterjee M. Post‐kala‐azar dermal leishmaniasis – an overview. Int J Dermatol 2010; 49:921–31. [DOI] [PubMed] [Google Scholar]
- 10. Singh A, Ramesh V, Ramam M. Histopathological characteristics of post kala‐azar dermal leishmaniasis: a series of 88 patients. Indian J Dermatol Venereol Leprol 2015; 81:29–34. [DOI] [PubMed] [Google Scholar]
- 11. Ismail A, El Hassan AM, Kemp K et al Immunopathology of post kala‐azar dermal leishmaniasis (PKDL): T‐cell phenotypes and cytokine profile. J Pathol 1999; 189:615–22. [DOI] [PubMed] [Google Scholar]
- 12. Haldar JP, Ghose S, Saha KC, Ghose AC. Cell‐mediated immune response in Indian kala‐azar and post‐kala‐azar dermal leishmaniasis. Infect Immun 1983; 42:702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neogy AB, Nandy A, Ghosh Dastidar B, Chowdhury AB. Modulation of the cell‐mediated immune response in kala‐azar and post‐kala‐azar dermal leishmaniasis in relation to chemotherapy. Ann Trop Med Parasitol 1988; 82:27–34. [DOI] [PubMed] [Google Scholar]
- 14. Saha S, Mondal S, Ravindran R et al IL‐10‐ and TGF‐beta‐mediated susceptibility in kala‐azar and post‐kala‐azar dermal leishmaniasis: the significance of amphotericin B in the control of Leishmania donovani infection in India. J Immunol 2007; 179:5592–603. [DOI] [PubMed] [Google Scholar]
- 15. Salotra P, Sreenivas G, Pogue GP et al Development of a species‐specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala‐azar and post‐kala‐azar dermal leishmaniasis. J Clin Microbiol 2001; 39:849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verma S, Kumar R, Katara GK et al Quantification of parasite load in clinical samples of leishmaniasis patients: Il‐10 level correlates with parasite load in visceral leishmaniasis. PLOS ONE 2010; 5:e10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramesh V, Kaushal H, Mishra AK, Singh R, Salotra P. Clinico‐epidemiological analysis of post kala‐azar dermal leishmaniasis (PKDL) cases in India over last two decades: a hospital based retrospective study. BMC Public Health 2015; 15:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaushal H, Bras‐Gonçalves R, Negi NS, Lemesre J‐L, Papierok G, Salotra P. Role of CD8+ T cells in protection against Leishmania donovani infection in healed Visceral Leishmaniasis individuals. BMC Infect Dis 2014; 14:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 2004; 104:2840–8. [DOI] [PubMed] [Google Scholar]
- 20. Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arvå E, Andersson B. Kinetics of cytokine release and expression of lymphocyte cell‐surface activation markers after in vitro stimulation of human peripheral blood mononuclear cells with Streptococcus pneumoniae . Scand J Immunol 1999; 49:237–43. [DOI] [PubMed] [Google Scholar]
- 22. Ganguly S, Mukhopadhyay D, Das NK et al Enhanced lesional Foxp3 expression and peripheral anergic lymphocytes indicate a role for regulatory T cells in Indian post‐kala‐azar dermal leishmaniasis. J Invest Dermatol 2010; 130:1013–22. [DOI] [PubMed] [Google Scholar]
- 23. Belosevic M, Finbloom DS, Van Der Meide PH, Slayter MV, Nacy CA. Administration of monoclonal anti‐IFN‐gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major . J Immunol 1989; 143:266–74. [PubMed] [Google Scholar]
- 24. Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling – does it mean JAK‐STAT? Cytokine Growth Factor Rev 2008; 19:383–94. [DOI] [PubMed] [Google Scholar]
- 25. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sacks D, Noben‐Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2002; 2:845–58. [DOI] [PubMed] [Google Scholar]
- 27. Peruhype‐Magalhães V, Martins‐Filho OA, Prata A et al Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferon‐γ and interleukin‐10 and low frequency of tumour necrosis factor‐α+ monocytes are hallmarks of active human visceral Leishmaniasis due to Leishmania chagasi infection. Clin Exp Immunol 2006; 146:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vouldoukis I, Bécherel PA, Riveros‐Moreno V et al Interleukin‐10 and interleukin‐4 inhibit intracellular killing of Leishmania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur J Immunol 1997; 27:860–5. [DOI] [PubMed] [Google Scholar]
- 29. Ganguly S, Das NK, Panja M et al Increased levels of interleukin‐10 and IgG3 are hallmarks of Indian post‐kala‐azar dermal leishmaniasis. J Infect Dis 2008; 197:1762–71. [DOI] [PubMed] [Google Scholar]
- 30. Brière F, Servet‐Delprat C, Bridon JM, Saint‐Remy JM, Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med 1994; 179:757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujieda S, Saxon A, Zhang K. Direct evidence that gamma 1 and gamma 3 switching in human B cells is interleukin‐10 dependent. Mol Immunol 1996; 33:1335–43. [DOI] [PubMed] [Google Scholar]
- 32. Kägi D, Vignaux F, Ledermann B et al Fas and perforin pathways as major mechanisms of T cell‐mediated cytotoxicity. Science 1994; 265:528–30. [DOI] [PubMed] [Google Scholar]
- 33. Bousoffara T, Louzir H, Ben Salah A, Dellagi K. Analysis of granzyme B activity as a surrogate marker of Leishmania specific cell mediated cytotoxicity in zoonotic cutaneous leishmaniasis. J Infect Dis 2004; 189:1265–73. [DOI] [PubMed] [Google Scholar]
- 34. Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T‐cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 1994; 370:650–2. [DOI] [PubMed] [Google Scholar]
- 35. Hernández‐Ruiz J, Salaiza‐Suazo N, Carrada G et al CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis 2010; 4:e871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santos CDS, Boaventura V, Ribeiro Cardoso C et al CD8(+) granzyme B(+)‐mediated tissue injury vs. CD4(+)IFNγ(+)‐mediated parasite killing in human cutaneous leishmaniasis. J Invest Dermatol 2013; 133:1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cardoso TM, Machado Á, Costa DL et al Protective and pathological functions of CD8+ T cells in Leishmania braziliensis infection. Infect Immun 2015; 83:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chamakh‐Ayari R, Bras‐Gonçalves R, Bahi‐Jaber N et al In vitro evaluation of a soluble Leishmania promastigote surface antigen as a potential vaccine candidate against human leishmaniasis. PLOS ONE 2014; 9:e92708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ghosh MK, Nandy A, Addy M, Maitra TK, Ghose AC. Subpopulations of T lymphocytes in the peripheral blood, dermal lesions and lymph nodes of post kala‐azar dermal leishmaniasis patients. Scand J Immunol 1995; 41:11–7. [DOI] [PubMed] [Google Scholar]
- 40. Patterson S, Chaidos A, Neville DCA et al Human invariant NKT cells display alloreactivity instructed by invariant TCR‐CD1d interaction and killer Ig receptors. J Immunol 2008; 181:3268–76. [DOI] [PubMed] [Google Scholar]
- 41. Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol 2010; 11:197–206. [DOI] [PubMed] [Google Scholar]
- 42. Kumari S, Jamal F, Shivam P et al Leishmania donovani skews the CD56(+) natural killer T cell response during human visceral leishmaniasis. Cytokine 2015; 73:53–60. [DOI] [PubMed] [Google Scholar]


