Summary
CD26/DPP4 (dipeptidyl peptidase 4/DP4/DPPIV) is a surface T cell activation antigen and has been shown to have DPP4 enzymatic activity, cleaving‐off amino‐terminal dipeptides with either L‐proline or L‐alanine at the penultimate position. It plays a major role in glucose metabolism by N‐terminal truncation and inactivation of the incretins glucagon‐like peptide‐1 (GLP) and gastric inhibitory protein (GIP). In 2006, DPP4 inhibitors have been introduced to clinics and have been demonstrated to efficiently enhance the endogenous insulin secretion via prolongation of the half‐life of GLP‐1 and GIP in patients. However, a large number of studies demonstrate clearly that CD26/DPP4 also plays an integral role in the immune system, particularly in T cell activation. Therefore, inhibition of DPP4 might represent a double‐edged sword. Apart from the metabolic benefit, the associated immunological effects of long term DPP4 inhibition on regulatory processes such as T cell homeostasis, maturation and activation are not understood fully at this stage. The current data point to an important role for CD26/DPP4 in maintaining lymphocyte composition and function, T cell activation and co‐stimulation, memory T cell generation and thymic emigration patterns during immune‐senescence. In rodents, critical immune changes occur at baseline levels as well as after in‐vitro and in‐vivo challenge. In patients receiving DPP4 inhibitors, evidence of immunological side effects also became apparent. The scope of this review is to recapitulate the role of CD26/DPP4 in the immune system regarding its pharmacological inhibition and T cell‐dependent immune regulation.
Keywords: autoimmunity, B cell, cell activation, chemokines, T cells
Structure and characterization of CD26
Originally described 50 years ago 1, the lymphocyte cell surface protein CD26 possess a dipeptidyl peptidase‐4 (DPP4) activity. It cleaves dipeptides from the N‐termini of oligopeptides and smaller peptides with proline or alanine at the penultimate position, as illustrated in Fig. 1b [International Union of Biochemistry and Molecular Biology (IUBMB) Enzyme Nomenclature EC 3.4.14.5].
Figure 1.
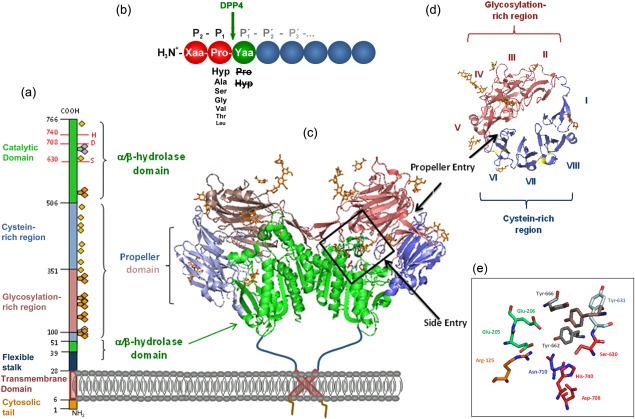
Primary and quaternary structure of human dipeptidyl peptidase 4 (DPP4), based on Protein Data Bank: 1W1I. (a) Primary structure of DPP4 subunit, consisting of an intracellular tail (aa 1–6), transmembrane region (aa 7–28), flexible stalk (aa 29–39), glycosylated region (aa 101–350), cysteine‐rich region (aa 55–100, 351–497), and catalytic region (aa 506–766).  , N‐glycosylation;
, N‐glycosylation;  , potential unoccupied N‐glycosylation;
, potential unoccupied N‐glycosylation;  , cysteine residues involved in S‐bridges; red numbers and letters indicate the catalytic triad. (b) Substrate specificity of DPP4. Xaa and Yaa indicate any amino acid. Decreasing font of amino acid at P1 position represents declining rate of hydrolysis. Amino acids crossed out must not occupy P1′. Arrow indicates site of cleavage. (c) quaternary structure of homodimeric human recombinant DPP4 as determined by Weihofen et al., 2004, showing the α/β‐hydrolase domain (aa 39–51 and 506–766) in green and propeller domain (aa 55–497) with the glycosylation‐rich subdomain (red) and the cystein‐rich subdomain (blue). (d) Propeller domain viewed from the top, illustrating the eight propeller blades designated with roman numbers and two subdomains. (e) Active site zoomed in, depicting the residues involved in catalysis, catalytic triad Ser630, Asp708, His740 are shown in red, Tyr547 responsible for oxyanion hole in brown, Tyr662 and Tyr666 forming the hydrophobic pocket in grey, Arg125 and Asn710, contributing to an electrostatic sink in orange and blue, respectively, and Glu205 and Glu206 ensuring N‐terminal anchoring in pale green. S–S bridges are illustrated in yellow and carbohydrates in orange. Structures were drawn with PyMOLTM 2008 DeLano Scientific LLC, using Protein Data Base: 1W1I 7.
, cysteine residues involved in S‐bridges; red numbers and letters indicate the catalytic triad. (b) Substrate specificity of DPP4. Xaa and Yaa indicate any amino acid. Decreasing font of amino acid at P1 position represents declining rate of hydrolysis. Amino acids crossed out must not occupy P1′. Arrow indicates site of cleavage. (c) quaternary structure of homodimeric human recombinant DPP4 as determined by Weihofen et al., 2004, showing the α/β‐hydrolase domain (aa 39–51 and 506–766) in green and propeller domain (aa 55–497) with the glycosylation‐rich subdomain (red) and the cystein‐rich subdomain (blue). (d) Propeller domain viewed from the top, illustrating the eight propeller blades designated with roman numbers and two subdomains. (e) Active site zoomed in, depicting the residues involved in catalysis, catalytic triad Ser630, Asp708, His740 are shown in red, Tyr547 responsible for oxyanion hole in brown, Tyr662 and Tyr666 forming the hydrophobic pocket in grey, Arg125 and Asn710, contributing to an electrostatic sink in orange and blue, respectively, and Glu205 and Glu206 ensuring N‐terminal anchoring in pale green. S–S bridges are illustrated in yellow and carbohydrates in orange. Structures were drawn with PyMOLTM 2008 DeLano Scientific LLC, using Protein Data Base: 1W1I 7.
CD26/DPP4 is a homodimer and an integral type II glycoprotein anchored to the membrane by its signal peptide. The primary structure consists of a short six amino acid cytoplasmic tail, a 22 amino acid transmembrane, a 738 amino acid extracellular portion comprised of a flexible stalk, glycosylation‐rich region, cysteine‐rich region and catalytic region with the catalytic triad Ser630, Asp708 and His740 (Fig. 1e). Recent studies have revealed that the transmembrane region contributes to enzyme activity and quaternary structure by dimerization 2. The crystal structure of human CD26/DPP4 has been elucidated to reveal two domains: an eight‐bladed propeller and an α/β‐hydrolase domain. The propeller is open and consists of two subdomains made up of blades II–V and VI–VIII for the glycosylation‐rich and cysteine‐rich regions, respectively (Fig. 1d). Most monoclonal anti‐CD26/DPP4 antibodies, as well as adenosine deaminase (ADA) and caveolin‐1, bind to the glycosylation‐rich domain of human CD26/DPP4, whereas collagen, fibronectin, plasminogen and streptokinase bind to the cysteine‐rich region (Fig. 1a) 3, 4, 5. There are two openings: a side opening and a propeller tunnel (Fig. 1a) 6, 7. The DPP4 substrate neuropeptide Y (NPY) was found to enter DPP4 at the side opening 8.
Post‐translational modification
Glycosylation‐based heterogeneity
Carbohydrates contribute approximately 18–25% of the total molecular weight, and human DPP4 contains nine potential N‐glycosylation sites 4. Analysis of oligosaccharides revealed extensive heterogeneity composed of one high mannose type and several mono‐, bi‐, tri‐ and tetra‐antennary complex types of N‐glycans 9, 10. Thus, DPP4 is comprised of several isoforms differing in sialylation and being dependent upon species, tissue, epitope and differentiation status 11, 12. While co‐translational core N‐glycosylation is responsible for the folding and stability of DPP4 13, 14, 15, N‐terminal sialylation appears to play a more (patho‐)physiological role (summarized in Fig. 2).
Figure 2.
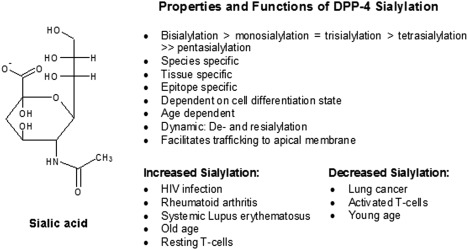
Structure, properties and functions of dipeptidyl peptidase 4 (DPP4) sialylation 10, 11, 12, 16, 17, 18, 20.
Resting T cells were determined to be more sialylated than activated cells 16. Hypersialylation has been associated with HIV‐infection, rheumatoid arthritis, systemic lupus erythematosus and ageing 16, 17, whereas decreased sialylation has been observed in lung cancer 18. The process of sialylation seems to be dynamic, as de‐ and re‐sialylation has been detected in rat hepatocytes 19, 20. Furthermore, trafficking of DPP4 to the apical surfaces has been shown to be influenced greatly by terminal sialylation 21, 22.
Tyrosine phosphorylation
Tyrosine phosphorylation of DPP4 has been described recently in association with cellular c‐Scr, HIV‐Tat and mannose 6‐phosphate binding 23, 24, 25.
Soluble CD26/DPP4 (sCD26)
CD26/DPP4 exists in a soluble form, thought to be shed from the membrane into plasma, which still maintains its enzymatic activity (for review see 26, 27). Recently, the bone marrow – but not the kidney – could be determined as one of the sources of soluble serum CD26/DPP4 by transplantation studies in DPP4‐deficient rats 28. Standard concentrations of serum and cerebrospinal fluid (CSF) levels for healthy children and adults have been assessed 4, 26, 27, 29, 30. The alterations of human DPP4 activity in the serum and CD26/DPP4 expression in numerous diseases will be discussed in more detail below and are summarized in Table 3.
Table 3.
Summary of altered CD26/dipeptidyl peptidase 4 (DPP4) activity and expression in human sera 4, 5, 26, 27, 30, 91
| Disease | Serum CD26/DPP4 | Serum CD26/DPP4 activity | Remarks | |
|---|---|---|---|---|
| Healthy | Male* | ⇑ | ||
| Female* | ⇓ | |||
| Age* | ⇑ | |||
| Psychological diseases | Major depression | ⇓ | ⇓ | ⇓ ADA activity |
| Schizophrenia | ⇑ | |||
| Anxiety | ⇓ | |||
| Stress | ⇓ | |||
| Anorexia nervosa | ⇑ | ⇓ T+CD26/CD25 cells | ||
| Bulimia | ⇑ | ⇓ T+CD26/CD25 cells | ||
| Alcoholism | ⇓ | |||
| Autoimmune diseases | Rheumathoid arthritis | ⇓ | ⇓ |
⇑ sDPP‐2 activity, ⇑ sCD30 ⇑ SynoviocytesCD26 |
| Lupus erythematosus | ⇓ | ⇓ | ⇑ DPP‐2 ⇓ PBMCCD26 | |
| Sjögren syndrome | ⇓ | ⇓ | ⇑ DPP‐2 in leucocytes | |
| Psoriaris | ⇓ | ⇑ ADA | ||
| Scleroderma | ⇓ | |||
| ANCA‐associated vasculitis | ⇓ | ⇑ sCD30 ⇑ IL‐10 | ||
| Coeliac disease | ≈ | ⇑ intestinal CD26 | ||
| Allergic asthmatics | ⇑ | ⇓ | ⇑ TCD26/CD4 cell, ⇑ iNK ⇑ eosinophils | |
| Diabetes type 1 | ⇑ | ≈ | ⇓ TCD26/CD4 ⇓ TCD26/CD8 | |
| Inflammatory/infectious diseases | Pancreatitis | ≈ | ||
| Gastric ulcer | ⇓ | |||
| Acute hepatitis | ⇑ | ⇑ | ||
| Chronic hepatitis | ⇓ | ⇑ | ||
| Crohn's disease | ⇓ | ⇑ DPP4 ⇑ FAP enterocyes | ||
| HIV | ≈ | ⇓ | ⇑ ADA, ⇓ TCD26 cell | |
| Sepsis | ⇓ | |||
| Metabolic/cardiovascular | Diabetes type 2 | ⇓ | ||
| Hypertension | ⇑ | |||
| Cirrhosis | ⇑ | |||
| Osteoporosis | ⇑ | |||
| Cancer/tumour | Gastric cancer | ≈ | ⇓ | |
| Bile duct cancer | ⇑ | |||
| Colorectum | ⇓ | ⇓ | ||
| Pancreatic cancer | ⇓ | |||
| Oral sqamous cell carcinoma | ⇓ | ⇓ | ||
| Hepatocellular carcinoma | ⇑ | |||
| Multiple myeloma | ⇓ | |||
| Hodgkin's disease | ⇓ | |||
| Lymphosarcoma | ⇓ |
*In healthy subjects, males show a higher baseline activity of CD26/DPP4 compared to females. In males as well in females DPPV activity is higher in older individuals compared to younger ones.
Gene
The gene structures of human and mouse DPP4 show great homology, with some minor variation in gene and exon size 31, 32. In humans, the gene is located on chromosome 2q24.3, spans 81.8 kb and contains 26 exons. The nucleotides encoding the sequence around the active site serine (Gly‐X‐Ser‐X‐Gly) are split between exons 21 and 22. Similarly, the exons of the catalytic triad are 22 for Ser, 24 for Asp and 26 for His 31. In F344/DuCrj(DPP4neg) rats, among other point mutations, a G to A transition at nucleotide 1897 in the Dpp4 cDNA sequence leads to a substitution of Gly633 to Arg in the catalytic centre of the enzyme (Gly629–Trp–Ser–Tyr–Gly633) 33 and a retention of the mutant protein in endoplasmatic reticulum largely abrogating expression of the mutant CD26/DPP4 protein 34, 35. The Ser631 is the active serine of rat DPP4 and the same point mutations were reconfirmed in otherwise independent substrains of F344 rats 36, 37, and were also used to generate DPP4‐deficient congenic DA.F344‐Dpp4m/SvH rats 38.
DPP4 contains neither a TATAA nor a CCAAT box as a promoter, but has a C‐ and G‐rich region containing several consensus binding sites for transcriptional factors 39, 40. The expression is regulated at RNA level and is organ‐specific 41, 42, 43, 44. Within an organ, it is dependent upon cell type, differentiation state and activation state. Several cytokines are known to regulate DPP4 expression in a cell‐type‐specific manner such as interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α and lipopolysaccharide (LPS) in human umbilical vein endothelial cells (HUVEC) 42, 45, 46, 47, 48, 49. In some tumours, binding of the transcription factors is enhanced by certain cytokines also modifying the expression of CD26/DPP4 50.
Expression of CD26/DPP4
CD26/DPP4 is expressed ubiquitously in many tissues – endothelia and epithelia – including but not limited to kidney, liver, lung, intestine and, interestingly, also on immune cells (e.g. T cells, activated B, activated natural killer (NK) cells and myeloid cells) 31, 35, 41, 51, 52, 53, 54, 55.
T cells
CD26/DPP4 is expressed on only a fraction of resting T cells, mainly CD4+CD45RO+ memory T cells, but is up‐regulated strongly following T cell activation 54. Detailed expression patterns have been recently reviewed elsewhere 56. Altogether, up to 70% of peripheral blood lymphocytes can express detectable CD26/DPP4 protein levels 55. Importantly, CD26/DPP4 has been described as a negative selection marker for human regulatory T cells (Tregs) 57, 58. In contrast, human T helper type 17 (Th17) cells showed very high expression of enzymatically active CD26/DPP4 59. Recently, mucosal‐associated invariant T cells (MAITs) have also been shown to express high levels of CD26/DPP4 in humans 60.
NK cells
NK cells usually express only low amounts of CD26/DPP4, but surface expression increases significantly up to 30% after interleukin (IL)‐2 stimulation as well as IL‐12 or IL‐15 stimulation 61, 62, 63. A functional aspect of this up‐regulated expression of CD26/DPP4 on NK cells might be an increased CD16‐dependent lysis. This may be caused by the mediation of protein tyrosine phosphorylation and an involvement of CD26/DPP4 in the production of cytokines by NK cells 35, 64, 65. In a model of lung metastasis, NK cell cytotoxicity against tumour (MADB106) cells proved to be diminished in a CD26/DPP4‐deficient F344 rat substrain. Additionally, the absolute capacity of single NK cells to lyse tumour target cells is reduced in a congenic rat model, suggesting that CD26/DPP4 enzymatic activity sustains NK cytotoxicity 35, 38. NK cells exert their cytotoxicity via secretory lysosomes, and CD26/DPP4 was identified on the membrane of secretory lysosomes in NK cells by proteomic analysis 66, 67. Concerning the NK cell maturation, the percentage of NK cells in DPP4‐deficient animals was increased significantly, while total leucocyte numbers were decreased in a congenic DPP4‐deficient rat model, as well as in knock‐out mice 38, 68.
B cells
Upon activation, up to 50% of human B cells express CD26/DPP4 53. Specific suppression of DPP4 activity reduces the B cell activation and synthesis of DNA in a dose‐dependent manner 53, 69. In mice, an impaired immunoglobulin isotype switching of B cells in CD26‐deficient mice became apparent in one study 68, while another could not show any differences 70. Another in‐vitro study showed no effect of CD26/DPP4 deficiency on B cells in rats expressing a truncated CD26 molecule lacking the DPP4 activity 71. However, monitoring the long‐term effect of DPP4 deficiency in vivo, we found B cell numbers to be decreased markedly in later life 72. One of the best substrates of DPP4, neuropeptide Y (NPY), has been shown to mobilize B1‐like B cells selectively 73. Hence, a pharmacologically induced lack of DPP4 function may, indirectly, modulate ‘stress‐induced’ B cell redistribution and composition of B cell reservoirs. In humans, CD26/DPP4 is currently under investigation as a possible prognostic marker in B cell carcinoma 74.
Myeloid cells
CD26/DPP4 was shown to be chemorepellent for human and murine neutrophils, whereas DPP4 truncation affected recruitment of eosinophils via its substrate eotaxin (CCL11) 38. CD26/DPP4 has also been shown to be expressed on dendritic cells 75, 76, 77 and, in rodents, on monocytes/macrophages 78. In rats, DPP4 could be shown in Küpffer and microglia cells, respectively, with DPP4 being expressed in lysosomes and increased upon activation 79, 80, 81. Data on the role of CD26/DPP4 on monocytes/macrophages in humans are scarce. Nevertheless, a special interest arises from the fact that long‐term DPP4 inhibition influences atherosclerosis positively by inhibiting inflammation mediated by myeloid cells 82. The detailed involvement of CD26/DPP4 in atherosclerosis has been reviewed recently elsewhere 56.
Substrates of DPP4
Many gastrointestinal hormones, growth factors, neuropeptides and chemokines share either the X‐Pro or ‐Ala motif at their N‐terminus and have been shown to be cleaved by DPP4, as summarized in Table 1 (for a review, see 4). Substrates of DPP4 are involved in neuroendocrine system, nociception, metabolism/nutrition, cardiovascular functions, immune regulation such as chemotaxis, and in infection (Table 1; Fig. 3) 4. Structure–activity relationships have shown that truncation by DPP4 either results in modulation of receptor selectivity with different physiological responses such as in NPY or ablation of receptor selectivity with additional but lower physiological outputs, such as in substance P, or inactivation towards receptor response such as in glucagon‐like peptide 1 (GLP‐1), pituitary adenylate cyclase‐activating polypeptide (PACAP), eotaxin and stromal‐derived factor (SDF)‐α 4, 56, 83. However, the regulation of chemokines with regard to immune response and receptor selectivity is extremely diverse (for a review, see 84). After truncation, most DPP4 substrates, being devoid of the X‐proline N‐terminal dipeptide, are degraded more rapidly by additional peptidases 85. This is the case for substrates such as substance P being degraded further by aminopeptidase N, or GLP‐1 being degraded by neprilysin 85, 86. Intriguingly, many cytokines also contain an X‐Pro N‐terminal motif, but DPP4 could only truncate their fragments 87.
Table 1.
Selection of known dipeptidyl peptidase 4 (DPP4) substrates 4.
| Peptide | N‐terminus | # Amino acids | Selectivity‡ | Physiological effect | |
|---|---|---|---|---|---|
| Inactivation/Alteration in vivo and in vitro | Pancreatic polypeptides: | ||||
| Peptide YY | YP↓IKPE… | 36 | + (++)* | M/N | |
| Neuropeptide Y | YP↓SKPD… | 36 | +++ | Ne, No, C, Im | |
| Chemokines: | |||||
| SDF‐1α | KP↓VSLS… | 68 | ++++ | Im | |
| MDC | GP↓YG↓AN | 69 | ++++ (++)† | Im | |
| I‐TAC | FP↓MFKR… | 73 | +++ | Im | |
| IP‐10 | VP↓LSRT… | 77 | ++ | Im | |
| Mig | TP↓VVRK… | 10 | ++ | Im | |
| RANTES | SP↓YSSD… | 68 | + | Im | |
| Eotaxin | GP↓ASVP… | 74 | + | Im | |
| LD78β | AP↓LAAD… | 70 | + | Imm | |
| PACAP/glucagon family: | |||||
| GLP‐1 | HA↓EG↓TF… | 30 | ++ (+++)* | M/N | |
| GIP | YA↓EGTF… | 42 | ++ | M/N | |
| PACAP38/PACAP27 | HS↓EG↓IF… | 38/27 | ++ (+)†/+ (+)† | M/N, Ne | |
| Glucagon | HS↓QGTF… | 29 | ++ | M/N | |
| GLP‐2 | HA↓DG↓SF… | 33 | + | M/N | |
| Neuropeptides/Peptides: | |||||
| Substance P | RP↓KP↓Q… | 11 | ++ (++)† | No, Ne, C, Im | |
| Endomorphin‐2 | YP↓WF‐NH2 | 4 | + | No | |
| GRP | VP↓LP↓AG… | 27 | +++ (+++)† | M/N | |
| Procalcitonin | AP↓FRSA… | 116 | n.d. | Inf | |
| Inactivation/Alteration shown in vitro only | Chemokines: | ||||
| SDF‐1β | KP↓VSLS… | 72 | n.d. | Im | |
| PACAP/Glucagon family: | |||||
| GHRH44/GHRH29‐NH2 | YA↓DAIF… | 44/29 | +++ | Ne | |
| Oxyntomodulin | HS↓QGTF… | 37 | ++ | Ne | |
| PHM | HA↓DGVF… | 27 | ++ | Ne | |
| VIP | HS↓DA↓VF… | 59 | + (+)† | Ne, M/N | |
| Secretin | HS↓DGTF… | 27 | + | M/N | |
| Neuropeptides/peptides: | |||||
| BNP‐32 | SP↓KMVQG… | 32 | ++ | C | |
| IGF‐I | GP↓ETLCGA… | 105 | + | Ne, M/N | |
| Haemorphin‐7 | LV↓VYPW… | 10 | ++ | C | |
| β‐casomorphin | YP↓FVEPI | 7 | ++ | Ne, M/N | |
| Endomorphin‐1 | YP↓FF‐NH2 | 4 | + | No | |
| Enterostatin | VP↓DP↓R | 5 | + | M/N | |
| Tyr‐MIF‐1 | YP↓LG‐NH2 | 4 | + | No | |
| Morphiceptin | YP↓FP‐NH2 | 4 | n.d. | No | |
| Kentsin | TP↓RK | 4 | n.d. | No | |
| Vasostatin‐1 (chromogranin A1–76) | LP↓VNSPM… | 76 | +++ | C | |
| SR‐17 (chromogranin B586–602) | SA↓EFPDFY… | 17 | + | C | |
| Pro‐colipase | VP↓DP↓R… | 101 | + | M/N | |
| CLIP | RP↓V… | 22 | + | Ne | |
| Trypsinogen pro‐peptide | FP↓T… | 8 | + | M/N | |
| Trypsinogen (pig) | FP↓T… | 231 | + | M/N | |
| Prolactin (sheep) | TP↓V… | 198 | + | Ne, M/N | |
| Aprotinin (bovine) | RP↓D… | 58 | + | Trypsin inhibitor | |
| Chorionic gonadotrophin | AP↓D… | 243 | + | Ne | |
| Promellitin | AP↓EP↓EP↓ | 50 | n.d. | Bee venom | |
| Chemokine: | |||||
| GCP‐2 | GP↓VS… | 75 | n.d. | Im | |
*Different values obtained by various laboratories. †Selectivity of second cleavage of the same substrate. ‡ In‐vitro values. kcat/KM values: + = 0 – 1 × 105 M−1 s−1, ++ = 1 – 10 × 105 M−1 +++ = 10 – 30 × 105 M−1 s−1, ++++ = 30 – 50 × 105 M−1 s−1. Ne = neuroendocrine; No = nociception; M/N = metabolic/nutrition; C = cardiovascular; Im = immunology; Inf = infection; n.d. = not determined.
Figure 3.
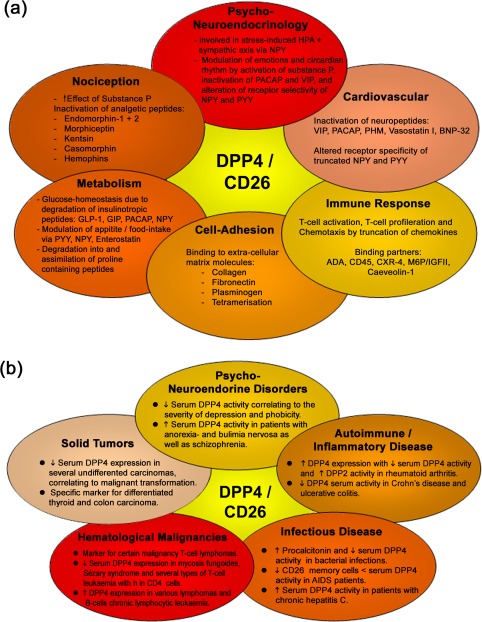
Physiological and pathological processes influenced by dipeptidyl peptidase 4 (DPP4) 4, 92, 93. (a) Summary of physiological roles of DPP4; (b) pathophysiological role of DPP4 with either altered expression and/or activity.
Binding partners
Several molecules have been shown to bind to DPP4, thereby triggering various physiological responses and modulation immune responsiveness 4. These can be subdivided into four categories: immune regulation, cell adhesion, cell–cell communication and peptide transport (Table 2).
Table 2.
Summary of molecules known to associate with dipeptidyl peptidase 4 (DPP4).
| Binding partner | Binding site | Function | Refs | |
|---|---|---|---|---|
| Immunology | ADA |
α1 and α2 of ADA bind to DPP4 via loop A between blades IV and V, and loop B between β 3 and β 4 of blade V, respectively. Glycosylation of DPP4‐Asn‐229 involved, as observed in crystal structure Ternary complex between A2BR‐ADA of dentritic APC to lymphocytic CD26. ADA binding only in higher mammalian and species‐dependent: human > porcine ≠ rat ≠ mouse |
Binding of extracellular ADA to AB2 receptor on dentritic APC cells and CD26 on T cells to form a ternary complex, resulting in: co‐stimulation of T cells, T cell proliferation, T cell protection | 7, 193 24 |
| CD45 | Binding of DPP4 at the intracellular PTP2 domain of CD45 causes recruitment of both enzymes on lipid rafts | Signal transduction resulting in phosphorylation of Erk1/2TCR‐zeta, ZAP70 by p56lck | 191 | |
| M6P | Carbohydrate moiety of DPP4 | Induces association of M6P/IGFRII and DPP4 | 190 | |
| Caveolin‐1 | Binding of caveolin‐1 on APC cells to soluble CD26 at aa 201–210 and Ser630 leading to: T cell proliferation + ⇑ CD26 on T cells ⇒ binding of CARMA‐1 on cytoplasmic tail of CD26 ⇒ phosphorylation of caveolin‐1 ⇒ dissociation of Tollip and IRAK‐1 ⇒ phosphorylation of IRAK‐1 ⇒ activation of NF‐κB ⇒ ⇑ CD86 | Causes up‐regulation of CD86 on TT‐loaded dentritic monocytes, thus leading to the association of APC with CD28 on T cells and subsequently to T cell activation |
189
114 112 |
|
| CARMA1(CARD11) | Binding of CARMA‐1 on cytoplasmic tail of CD26 ⇒ recruitment of CARMA‐1, CD26, Bcl10 and IkappaB kinase complex to lipid rafts ⇒ signal transduction | Leading to activation of ZAP70, PLC, MAPK, phosphatyl inositol and ⇑ IL‐2 |
112
112 |
|
| M6P/IGFRII | Needs M6P bound on DPP4 | T cell activation, internalization of DPP4, transendothelial migration by binding of lymphocytes to endothelial DPP4 |
25
190 |
|
| CXCR4 receptor | ? | Reduction of chemoattraction, co‐internalization in presence of SDF‐α, formation of invadopodia in presence of SDF‐α and gp120 | 194 | |
| Tromoxane A2 receptor | ? |
Natural DPP4 inhibitor, T cell suppression |
195 | |
| HIV‐TAT |
2 binding sites, sialic acid moiety and active site of DPP4 Crystal structures shows P2 and P1 of Tat1–9 bind to S1 and S2 of DPP4, respectively |
HIV‐entry, inhibitor of DPP4 due to reverse binding at the active site | 16, 24 | |
| HIV‐gp120 | Cysteine‐rich region, HIV‐gp 120 interacts via its C3 region with DPP4 on lymphocytes | HIV‐entry and subsequent apoptosis; inhibits ADA binding to DPP4 in presence of CXCR4, although binding site distinct to ADA | 194 | |
| Cell adhesion/cell‐ cell communication | Collagen | Cysteine‐rich region between aa 238 and 495 | Extracellular adhesion? Cancer? Metastasis? | 196 |
| Fibronectin (FN) | Cysteine‐rich region of DPP4 between aa 469–479 via aa LTSRPA motif (FN) | Fibronectin‐mediated spreading of fibroblasts, lung metastasis, dissociates in presence of soluble DPP4 | 197 | |
|
Plasminogen receptor (PgR) Plasminogen/ Plasmin (Pg/Pl) |
Cysteine‐rich region of DPP4 close to ADA binding site, sialic acid carbohydrate moieties of plasminogen binds to Pg‐receptor/DPP4 complex (aa 313–319)/αIIbβ3 and urinary/tissue plasminogen activator uPA/tPA. Activated plasmin (Pl) changes conformation and binds to DPP4. Quintary complex abolished by angiostatin binding to DPP4 | ⇑ Ca2+ response in synovial fibroblasts, activation of synovial fibroblasts, signal transduction in prostate cancer cells resulting in ⇑ MMP 9. Quintary complex of ADA, Pg 2, DPP4 and urinary plasminogen activator (uPA/tPA) and PgR ⇒ ⇑ Pg 2 to plasmin |
198
199 5 |
|
| Streptokinase (SK) | Cysteine‐rich region of DPP4 only from rheumatoid synovial fibroblasts via aa LTSRPA motif (SK) | Ca2+ response in synovial fibroblasts, ⇑ DPP4 autoantibodies, SK bound to DPP4 hydrolysis FN |
188
198 |
|
| Vitronectin | Sialic acid moiety of DPP4 | Extracellular adhesion? Metastasis? Complement system? Coagulation? | 16 | |
| Glypican 3 | Both glycosylated and unglycosylated glypican 3 bind to DPP4 | Natural DPP4 inhibitor. Binding of soluble glypican 3 to CD26 ⇓ cell‐proliferation and induces apoptosis | 200 | |
| FAP | Heteromeric complex | Heteromeric complex on invadopodia causing metastasis, tumor invasion, angiogenesis, wound healing and fibroblast migration | 201 | |
| DPP4 | Blades IV of each subunit align to form an eight‐stranded antiparallel sheet, possibly Asn229 involved | Tetramerization, cell‐adhesion, cell–cell communication?, chemotaxis? | 202 | |
| Peptide transport | Na+/H+ exchanger isoform NH3 | ? |
Peptide transporter on microvilli membrane of renal proximal tubule, reabsorption of dipeptides with proline. In prostate cancer, association of DPP4, Pg 2 and NH3 results in Ca2+ signal cascade and in intracellular pH ⇓ tumour cell‐proliferation + invasiveness |
203
204 |
Physiological role of DPP4
DPP4 has been described as a ‘moonlighting’ protein due to its multiple functions. DPP4 exerts its physiological roles either via its enzymatic activity by regulating many peptides or via its interactions with a variety of binding partners 88. It is involved in processes such as nutrition, nociception, cell‐adhesion, psychoneuroendocrine regulation, immune response and cardiovascular adaptation, as reviewed recently 4, 5, 27, 88, 89, 90, 91, 92 and summarized in Fig. 3a.
Function of CD26/DPP4 in the immune system
T cell development
Bone marrow‐derived T progenitor cells undergo maturation in the thymus 93. The vast majority of cells in the thymus express CD26/DPP4 and, therefore, it is thought to be a thymic maturation marker in rodents as well as humans 55, 94. CD26/DPP4 has been described as a mediator of lymphocyte migration through the thymus. It is down‐regulated on cells that undergo apoptosis and up‐regulated on maturing thymocytes, reaching the highest level of CD26/DPP4 expression in mature CD4 or CD8 single‐positive T cells within the thymus 94, 95, 96. Findings are conflicted in the periphery, but describe the expression of CD26/DPP4 favourably as a characteristic of memory T cells, with CD26/DPP4 bright cells responding maximally to recall antigens 97, 98, 99, 100. CD26/DPP4 has shown the ability to act as a non‐integrin receptor, being able to bind fibronectin and collagen 101, 102. Another study indicated that CD26/DPP4 acts as an endogenous inhibitor of T cell motility regulated by a cascade of interacting cell surface molecules 103. Proper adhesion is of great importance: first for progenitor cells entering the thymus; secondly, for thymocytes trafficking from cortex to medulla during their maturation; and thirdly, egressing as mature T cells 93. Apparently, CD26/DPP4‐associated enzymatic activity is controlled ontogenetically during T cell maturation and may be involved in thymic deletion of emerging clones 95, 96. However, the precise functional role of CD26/DPP4 expressed on maturing thymocytes remains unclear.
The thymus undergoes an age‐dependent involution but remains active up to a high age, playing a central role in replenishing the peripheral T cell pool 104. Impairment of CD26/DPP4 function under long‐term conditions had a remarkable effect on T cell subpopulations in a Fischer‐344 (F344) rat model. In CD26/DPP4‐deficient F344 rats the CD4+ T cell pool showed decreased numbers of memory T cells, as well as rat tracheal epithelial (RTE) and increased numbers of naive T cells instead 72. Also, thymus architecture appears to be altered in this model of chronic genetic CD26/DPP4‐deficiency. Again, in CD26/DPP4‐deficient mice, the percentage of CD4+ T cells is lower among the splenic lymphocyte population 68. In another congenic CD26/DPP4 rat model, the overall number of leucocytes proved to be decreased in CD26/DPP4‐deficient animals 38. Similar observations were made in humans, as (reversible) dose‐dependent decreases in absolute lymphocyte numbers were observed in patients receiving DPP4‐inhibitors 105. One case of severe leucopenia associated with DPP4 inhibition has been reported, but causality has not been proven 106. In contrast, a current meta‐analysis, including 16 papers with randomized trials comparing DDP4 inhibitors in addition to sulphonylurea, could not identify a significantly increased risk of this potential side effect 107.
T cell stimulation
Early in‐vitro studies showed that DPP4 inhibition decreases the induction and activation of cytokines controlling human T lymphocyte proliferation 108. DPP4 inhibition on mitogen‐stimulated thymocytes and splenocytes inhibited DNA synthesis as well as production of IL‐2, IL‐6 and IL‐10, and increased secretion of the regulatory cytokine transforming growth factor (TGF)‐β1 109. In congenic rats, the T cell proliferative response of CD26/DPP4‐deficient rats upon stimulation with anti‐T cell receptor (TCR) antibodies was decreased fivefold in vitro 38. In the past, there has been a controversial debate as to what extent CD26/DPP4 and its catalytic region are important for T cell co‐stimulation 110, 111. Recent in‐vitro findings now demonstrate that CD26/DPP4 is able to trigger direct T cell activation and proliferation directly via (=CARD11) CARMA1‐mediated nuclear factor (NF)‐κB activation in T cells 112. Additionally, CD26/DPP4 on T cells interacts directly with antigen‐presenting cells (APCs) via caveolin‐1. Upon linkage, Tollip and interleukin‐1 receptor‐associated kinase 1 (IRAK‐1) disengage from caveolin‐1 leading to subsequent IRAK‐1 phosphorylation 113. As illustrated in Fig. 4, this results in an up‐regulation of the co‐stimulatory molecule CD86, which enhances the bond of the immunological synapse 113. One the other side of the immunological synapse, blocking CD26/DPP4‐mediated T cell co‐stimulation with soluble caveolin‐1‐immunoglobulin (Ig) fusion protein induces anergy in CD4+ T cells 114. A recent study demonstrates that CD26‐mediated co‐stimulation of CD8+ T cells is enhanced compared to that obtained through CD28‐mediated co‐stimulation 115. The clinical relevance of these findings remains to be determined, as one study showed intact T cell‐dependent immune responses to antigenic challenge after specific DPP4‐inhibition and in CD26–/– mice 70. However, the clinical use of DPP4‐inhibitors could prove to be critical, as the catalytic center of CD26/DPP4 is part of the linking site required for co‐stimulation 113. Besides co‐stimulation, direct anti‐inflammatory mechanisms of DPP4 inhibitors are discussed 116. Yazbeck et al. propose a model of conformational change in the intracellular domain after binding an inhibitor to the catalytic center of CD26/DPP4. Subsequently, T cell proliferation and production of proinflammatory cytokines are suppressed 116, 117, 118.
Figure 4.
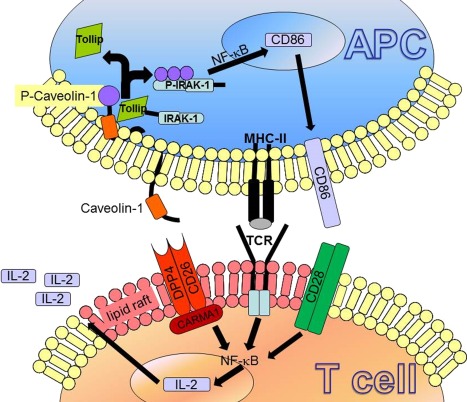
A model of CD26 interacting with caveolin‐1 resulting in T cell co‐stimulation and activation as proposed by Ohnuma et al. 188: after antigen uptake via caveolae by antigen‐presenting cells (APCs), caveolin‐1 is exposed on the cell surface and aggregates in the immunological synaps in lipid rafts. Consequently, caveolin‐1 binds to CD26 and is phosphorylated, leading dissociation of interleukin (IL)−1 receptor‐associated kinase 1 (IRAK‐1) and Tollip. This lead to activation of nuclear factor (NF)‐κB and results in CD86 up‐regulation, supporting the immunological synapse and thus T cell co‐stimulation. In T cells, after caveolin‐1 to CD26 binding, (CARD11) CARMA1 is recruited to the cytosolic portion of CD26. Activation of NF‐κB lead to T cell proliferation and IL‐2 production.
Figure 5.
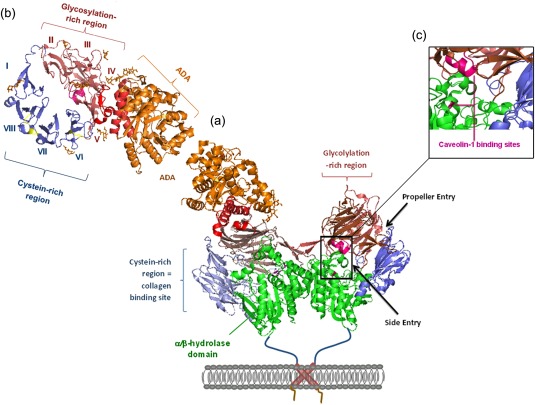
Crystal structure of human dipeptidyl peptidase 4 (DPP4) and bovine adenosine deaminase (ADA) obtained from Protein Data Bank: 1W1I. (a) DPP4 crystal structure associated with bovine ADA at its glycosylation‐rich region of the propeller domain. (b) Top view of propeller domain, showing ADA binding site at bladea 4 and 5 as well as ADA interactions with carbohydrates of N229. (c) Caveolin‐1 binding site at aa 201–210 and Ser630 7.
Involvement of CD26/DPP4 in pathology
Due to its ubiquitous distribution and involvement in various physiological processes, a great number of pathological conditions are associated with either altered DPP4 expression and/or activity correlating with the severity of the respective condition. These can be subdivided into at least five categories, as illustrated in Fig. 4b: psychoneuroendocrine disorders, autoimmune and inflammatory diseases, infectious diseases, haematological malignancies, as well as solid tumors 4, 91, 92. However, to the best of our knowledge, DPP4 expression or activity is not used routinely for diagnostic purposes in the clinic. Nevertheless, altered CD26/DPP4 activities or concentrations in serum have been associated with various pathogenic conditions involving psychological, autoimmune, inflammatory, infectious, metabolic and cardiovascular disorders, as well as tumor and cancer, as summarized in Table 3. Although, previously, several DPP4‐like enzymes were described to contribute to the overall DPP4‐like activity in serum such as attractin and β‐DPP IV, it is now generally accepted that CD26/DPP4 constitute more than 90% of the overall DPP4‐like activity in serum and plasma 119, 120.
Role of CD26/DPP4 and its inhibition in human diseases and their animal model
CD26/DPP4 has been linked to a number of diseases as summarized in Table 3, including but not limited to asthma, multiple sclerosis, arthritis and inflammatory bowel disease.
Table 4.
Involvement of CD26/dipeptidyl peptidase 4 (DPP4) in T helper type 1 (Th1) and Th2 responses 3, 17, 49, 67, 81, 88, 112, 116, 188, 189, 190, 191, 192.
| Th1 | Th2 | |
|---|---|---|
| CD26 expression |
High expression on CD26bright memory T cell subset Binding of caveolin‐1 on dentritic APC cells to CD26 on Th1 cells, results in binding of CARMA‐1 cytoplasmic tail of CD26 ⇒ recruitment of CARMA‐1, CD26, Bcl10 and IkappaB kinase complex to lipid rafts ⇒ signal transduction ⇑ up‐regulation of CD26 expression upon induction of Th1 response |
Slight up‐regulation of CD26 expression |
| Subcellular compartmentation |
Association of CD26 to lipid rafts Binding of CD26 to CD45R0+ on lipid rafts, resulting in signal transduction; followed by disassembly of CD45R0 from lipid rafts |
Binding of CD26 to M6P/IGFIIR ⇒ internalization + T cell activation Association of CD26 + CXCR4 ⇒ internalization |
|
Chemokine DPP4 substrates |
CXCR3 → IP‐10 CCR1 → RANTES1–68 > RANTES3–68 CCR5 → RANTES3–68 > RANTES 1‐68 |
CXCR4 → SDF‐1α1–68 > SDF‐1α3–68
CCR3 → eotaxin1–74 > eotaxin3–74 CCR4 → MDC1–67 ≠ MDC3–67 CCR1 → RANTES1–68 > RANTES3–68 CCR3 → RANTES3–68 > RANTES 1–68 |
|
Neuropeptide/peptide DPP4 substrates |
NPY released from SNS involved in inflammatory response of macrophages, NK and T cells GLP‐2 involved in Crohn's disease |
Mast‐cells: substanceP → allergy + asthma NPY released from SNS involved in inflammatory response of PMN and B cells |
| Inflammatory response |
Soluble DPP4 found in secretory lysosomes of TC cells Membrane‐bound DPP4 in secretory lysosomes of NK cells ⇑ DPP4 on activated macrophages truncate NPY → loss of Y1‐R binding → ⇓ IL‐1β and IL‐6 release ⇑ DPP4 on activated microglia |
T cell recruitment in asthma is DPP4‐dependent |
| Site of inflammation |
Arthritis: ⇑ DPP4 on activated synoviocytes + CD26 internalized via caveolae ⇑ hypersialylation of DPP4 → ⇓ sDPP4 activity → ⇑ SDF‐α → ⇑ inflammation ⇑ DPP4 on endothelial cells via IFN‐γ, TNF‐α and LPS stimulation Crohn's disease: ⇑ DPP4 in enterocytes → ⇓ GLP‐2 ⇑ DPP4 on activated microglia + astrocytes after ischaemia |
Asthma: ⇑ DPP4 in lung parenchyma Binding of CD26 to M6P/IGFIIR → transendothelial migration of lymphocytes |
| Diseases associated with DPP4/CD26 |
Rheumatoid arthritis Multiple sclerosis Truncation of RANTES by DPP4 → ⇑ protection against HIV entry Ischaemia |
Truncation of SDF‐1α by DPP4 → ⇓ protection against HIV entry CD26+ T cells involved in the pathogenesis of asthma correlating to IgE titre of antigen |
Asthma
Allergic asthma is one of the most common diseases, with its prevalence having increased dramatically in developed countries during the last two decades 121. Its pathogenesis involves a complex series of reactions within the airways that is associated with allergen‐specific airway hyper‐responsiveness and inflammation, which can be studied in animal models 122. The expression of CD26/DPP4 in the bronchi was described first by the group of van der Velden, showing a localization of CD26/DPP4 in serosal glands, blood vessels and on T cells 123, but there were no differences between asthmatics and healthy controls for the expression of CD26/DPP4 in the lamina propria determined by biopsies. However, investigating the effects of airway inflammation in rats, we found a significant increase of DPP4 enzymatic activity in the lung parenchyma. Also, strong immunohistochemical staining and high mRNA levels were detected in bronchial epithelium and trachea 124. Furthermore, the expression of the soluble form of CD26/DPP4, in the blood as well as on T cells, increased in patients suffering from asthma 125. Conflicting results arise from a mouse study indicating an enhanced ovalbumin‐induced airway inflammation in CD26/DPP4‐deficient mice 126.
Does CD26/DPP4 play a role in the pathogenesis of asthma or allergic‐like airway inflammation? Using a model of ovalbumin‐induced airway inflammation in rats, we found a CD26/DPP4‐dependent T cell recruitment to the lungs, with reduced signs of inflammation in CD26/DPP4‐deficient rats 127. These results were confirmed additionally by a significant reduction of the airway‐specific recruitment of T cells to bronchi and lung parenchyma in rats genetically lacking expression of CD26/DPP4. This site‐specific recruitment appeared and was mediated by chemokines, rather than nerve–T cell interactions 128. Furthermore, the amount of T cells expressing CD26/DPP4 was increased, and correlated with the severity of airway inflammation 129. To address further the questions of the role of T cells expressing CD26/DPP4 in airway inflammation, we have transferred labelled T cells from CD26‐expressing or CD26/DPP4‐deficient F344 rat donors and subsequently cross‐transferred to recipients of the other substrain 130. Here, we found significantly more T cells in CD26/DPP4‐deficient recipient lungs, regardless of the origin of the transferred T cells 130. Additionally, CD26‐deficient rats exhibited a significantly increased influx of Tregs into the lungs in vivo and increased IL‐10 production of draining lymph node cells in vitro 131.
These findings demonstrate a negative regulatory role of the bronchus‐associated lymphatic tissue (BALT)‐specific expression of CD26/DPP4 in T cell adhesion during an asthma‐like inflammation. However, first data concerning studies targeting CD26/DPP4 by a pharmacological treatment regimens show differential effects depending on the route, dose and time of the application 132. Additionally, inhibition of CD26/DPP4 enhances CCL11/eotaxin‐mediated recruitment of eosinophils in vivo 133.
Multiple sclerosis/EAE
Multiple sclerosis (MS) and its corresponding animal model of experimental autoimmune encephaolomyelitis (EAE) are chronic inflammatory autoimmune diseases affecting the central nervous system (CNS) 134. Patients suffering from MS exhibit increased numbers of CD26+ T cells, also showing higher expression levels of CD26/DPP4, which correlate with disease activity 135, 136. Compelling evidence has demonstrated that besides myelin specific T helper 1 (Th1) cells, IL‐17‐producing CD4+ cells (Th17) are major contributors to the pathogenesis of autoimmune inflammation 137. In line with these findings, human Th17 cells have been shown to express high amounts of enzymatically active CD26/DPP4 59. Pharmacological inhibition of DPP4 decreased incidence, onset of symptoms and overall disease severity in EAE significantly, while neither acting as generally immunosuppressive nor eliminating encephalitogenic T cells, and not inhibiting T cell priming 138. In humans, inhibitors of CD26/DPP4 suppress activation of MBP‐specific CD4+ T cell clones 139. Demonstrating the limitations of disease models and/or selectivity of pharmacological intervention, CD26–/– mice demonstrate a higher disease severity compared to wild‐type (WT) controls, which the authors explained by a functional deregulation of Th1 immunity because of a reduced TGF‐β production 117. A possible involvement of other members of the DPP4 family or the encephalopathic role of Th17 cells has not been addressed at this point. Later, it has be shown conclusively that the combined suppression of DPP4 and aminopeptidase N (APN) results in decreased T cell‐specific IL‐17 production and thus disease amelioration 140.
Arthritis
Rheumatoid arthritis is a chronic, systemic inflammatory disease with progressive destruction of articular cartilage 141. A number of studies show decreased levels of DPP4 activity in subjects suffering from this disease 142. Furthermore, the expression of CD26/DPP4 on joint‐infiltrating T cells has also been shown to be decreased 143. Lower serum DPP4 activity in rheumatoid arthritis is caused by hypersialylation and DPP4 autoantibodies, as illustrated in Fig. 2 17. The involvement of CD26/DPP4 in arthritis has been reviewed recently, involving glycosylation and DPP4 autoantibodies on one hand and SDF‐α on the other hand 17, 144, 145, 146. Additionally, one study summarizes three cases of DPP4 inhibitor‐induced polyarthritis 147.
Again, CD26–/– mice showed a markedly increased severity of disease due to lower DPP4 activity in synovial fluids, resulting in increased levels of SDF‐α [145].
Inflammatory bowel disease (IBD)
IBD, with is two major forms Crohn's disease and ulcerative colitis, is characterized by chronic, remittent or progressive inflammatory processes in the gastrointestinal tract 148. T cells from patients with IBD have higher levels of CD26/DPP4 expression, while levels of circulating CD26/DPP4 are decreased 149, 150. This parallels the findings of colitis models in mice 151. In one study, CD26–/– mice show a greater disease severity 152. In another study, the acute phase of colitis, loss of body mass and disease activity in CD26–/– mice was less intensive than in the controls, while no pronounced histopathological differences could be found 151. Interestingly, lack of CD26/DPP4 led to a twofold increase in the number of macrophages during the acute phase of disease, while an increased influx of dendritic cells became apparent in controls 151. Another study focused on the gut–brain axis and the altered receptor specificity of neuropeptide Y after DPP4‐mediated cleavage, finding that CD26/DPP4 deficiency affects the neuroimmune response at systemic and local levels during colitis development and resolution in mice 153. Furthermore, higher familial adenomatous polyposis (FAP) levels were detected in patients with Crohn's disease 154.
Again, the pharmacological inhibition of DPP4 by two different inhibitors reduced disease activity significantly in Crohn's disease, due to increased levels of GLP‐2 155, 156. These findings suggest a pathophysiological role of CD26/DPP4 in the nature of immune responses activated during Crohn's disease.
Others
CD26/DPP4 appears to play a role in a number of other diseases (see Table 2). In atopic dermatitis, CD26/DPP4 expression was up‐regulated in the skin biopsies of patients compared with healthy controls, as well as in both models of contact hypersensitivity 157. In psoriasis, reduced expression of CD26/DPP4 on CD8+ T cells has been observed 158. In atherosclerosis, inhibition of DPP4 exerts anti‐atherosclerotic effects and reduces inflammation via inhibition of monocyte activation/chemotaxis 82.
Clinical use of DPP4 inhibitors
DPP4 has been identified as a therapeutic target for T2DM due to its ability to cleave and inactivate insulinotrophic incretins such as GIP and GLP‐1 159. These incretins are released upon glucose intake and enhance the insulin secretion with a half‐life of a few minutes, strictly dependent upon DPP4‐like enzymatic activity. Furthermore, incretins exhibit positive effects on pancreatic β cells in the islets, including stimulation of growth and replenishing insulin stores by stimulation gene transcription. Once released, GIP and GLP are degraded rapidly by DPP4 and thus the inhibition of DPP4 prolongs GIP/GLP half‐life and insulinotrophic effect 159, 160. After the first DPP4 inhibitor sitagliptin (Januvia®) had been approved by the Food and Drug Administration (FDA) in 2006 [European Medicines Agency (EMA), 2007], numerous functionally related drugs, commonly called gliptins, were released 161. Currently, there are nine DPP4 inhibitors commercially available on the market, with sitagliptin Januvia® (Merck & Co., Inc., Kenilworth, NJ, USA), saxagliptin Onglyza® (Bristol Myers Squibb, New York, NY, USA), linagliptin Tradjenta™ (Böhringer Ingelheim, Ingelheim, Germany) and alogliptin Nesina® (Takeda Pharmaceuticals, London, UK) being approved by the FDA. Sitagliptin, vildagliptin Galvus® (Norvatis, Basel, Switzerland), saxagliptin and linagliptin were approved by the EMA; and anagliptin Suiny® (Sanwa Kagaku Kenkyusho Company Ltd and Kowa Company Ltd, Nagoya, Japan), teneligliptin Tenelia® (Mitsubishi Tanabe Pharma and Daiichi Sankyo, Dusseldorf, Germany), trelagliptin Zafatek® (Takeda Pharmaceuticals) and omarigliptin Marizev® (Merck & Co., Inc.) being approved in Japan. All of them are administered orally and taken daily, except for omarigliptin, which has weekly doses. To date, 125 meta‐analyses have been reported in PubMed, focusing on the efficacy and drug safety of DPP4 inhibitors, as well as its effects on comorbidities such as renal impairment and cardiovascular outcome 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173. So far more than 500 clinical trials have been performed throughout the world, covering all ethnic population groups, and aproximately 250 further trials are currently ongoing (www.clinicaltrials.gov; 31 January 2016). Generally, DPP4 inhibitors reduce DPP4 activity at approximately 70–90% of baseline and also lower the haemoglobin A1c (HbA1c) 0.74%. All DPP4 inhibitors are excreted via the renal route except for linagliptin, which is eliminated via the biliary route 174.
Although demonstrating an overall favourable adverse side‐effect profile, meta‐analysis showed that infections (most common: upper respiratory tract infection and urinary tract infection) increased significantly after DPP4 inhibitor treatment 160, 161, 162, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173. Other side effects may include pancreatitis, headache, nausea, angioedema, hypersensitivity and skin reactions, as well as severe joint pain 160, 161, 162, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173. In response to a report of precancerous changes in transplanted pancreases of donors treated with the DPP IV inhibitor sitagliptin, the FDA and the EMA each undertook independent reviews of all clinical and preclinical data related to DPP4 inhibitors. These reviews revealed no association of DDP4 inhibition with pancreatic cancer 175, 176. Currently, gastrointestinal, cutaneous and mucosal side effects, atherosclerosis and cancer are also of special interest and have initiated extensive, ongoing research 165. When considering the more recent findings, DPP4 inhibitors might be considered to represent even more of a double‐edged sword. Apart from the metabolic benefit, the associated immunological effects induced by long‐term DPP4 inhibition, in particular on T cells, are not understood fully at this stage. Further post‐marketing surveillance will hopefully elucidate the potential risks of this class of drugs for immunological side effects.
Almost all anti‐diabetic DPP4 inhibitors were designed to exhibit a long half‐life, with ‘one pill a day’ facilitating both patients’ compliance and marketing. The short‐acting PSN‐9301 appears to be the only exception 177. A once‐daily application is convenient from a patient viewpoint. However, long‐acting inhibitors of DPP4 might compete with other natural substrates of DPP4 and their associated physiological functions, such as surfactant protein (SP) in rhinosinusitis and angioedema, SDF‐α in arthritis and NPY/PYY, as well as substance P in blood pressure 80, 178, 179. Recently, the FDA revised its prescribing information to include case reports on acute pancreatitis as well as polyarthritis in patients using sitagliptin 146, 180. Further case reports describe contradicting effects of sitagliptin in psoriasis: as sitagliptin was observed, on one hand, to trigger psoriasis, it was also claimed to ameliorate the disease on the other hand 181, 182. Interestingly, investigating NPY hydrolysis in serum and blood 80, a novel C‐terminal truncation of NPY by an angiotensin‐converting‐enzyme (ACE)‐like enzyme was detected. This finding strongly suggests a potential interaction within current drug treatments that use anti‐diabetic DPP4 inhibitors and anti‐hypertensive ACE inhibitors in combination, causing potentiated NPY‐induced hypertension and vasoconstriction. A suspected increase of vasocontrictive NPY1–36 after treatment with anti‐diabetic DPP4 inhibitor may be compensated by the C‐terminal inactivation of NPY mediated by ACE, but fails if ACE is also blocked 80. This hypothesis has been substantiated by physiological animal studies, using spontaneously hypertensive rats (SHR) and normotensive Wistar‐Kyoto (WKY), respectively 183. Intriguingly, when treating SHR and WKY rats with either the pan‐DPP inhibitor P32/98 alone or in combination with captopril, only SHR developed hypertension after combined therapy. This suggests a genetic background involving nephropathic hypertension similar to the human metabolic syndrome 184. However, Y1‐R antagonists ablated the hypertensive effects of combined treatment with DPP4 and ACE inhibitors, supporting the involvement of either NPY or PYY 183. Similar findings have been observed with substance P and ACE inhibitors 178. This is of pharmacological significance, as hypertension is a frequent co‐morbidity with diabetes. In recent reports, the development of hypertension was associated with the combined application of the anti‐diabetic compound sitagliptin and anti‐hypertensive drug enalapril in patients suffering from metabolic syndrome 185, 186. Because the anti‐diabetic effects of DPP4 inhibition is only required upon glucose challenge, the development of short‐acting and highly specific DPP4 inhibitors might minimize side effects due to off‐target inhibition.
Conclusion
The introduction of DPP4 inhibitors into clinics aimed to enhance the endogenous insulin secretion in diabetes mellitus type 2 via elevated levels of glucagon, such as GLP‐1 and GIP. At present, the majority of findings for non‐diabetes effects mediated by DPP4 inhibitor treatment in patients are indicative of largely beneficial secondary effects. Nevertheless, the application of these new compounds might represent a double‐edged sword: apart from the metabolic benefit, the associated immunological effects of long‐term DPP4 inhibition on regulatory processes such as T cell maturation and activation are not understood fully at this stage. Several Phase III trials of new DPP4 inhibitors are currently ongoing. These trials, along with postmarketing surveillance data, will hopefully increase our knowledge about the long‐term efficacy and safety of DPP4 inhibitor therapy. The scope of these studies should be focused not only on the current questions of incretin action in the cardiovascular system, pancreatitis and cancer, but also on (long‐term) immunological parameters such as infections, T cell development and immune homeostasis.
Disclosure
The authors declare no disclosures.
Acknowledgement
The authors thank Anika Dreier for formatting Fig. 4.
References
- 1. Hopsu‐Havu VK, Glenner GG. A new dipeptide naphthylamidase hydrolyzing glycyl‐prolyl‐beta‐naphthylamide. Histochemie 1966; 7:197–201. [DOI] [PubMed] [Google Scholar]
- 2. Chung K‐M, Cheng J‐H, Suen C‐S et al The dimeric transmembrane domain of prolyl dipeptidase DPP‐IV contributes to its quaternary structure and enzymatic activities. Protein Sci 2010; 19:1627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Meester I, Korom S, Van Damme J, Scharpé S. CD26, let it cut or cut it down. Immunol Today 1999; 20:367–75. [DOI] [PubMed] [Google Scholar]
- 4. Lambeir A‐M, Durinx C, Scharpé S, De Meester I. Dipeptidyl‐peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 2003; 40:209–94. [DOI] [PubMed] [Google Scholar]
- 5. Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci (Lond) 2005; 108:277–92. [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen HB, Branner S, Wiberg FC, Wagtmann N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat Struct Biol 2003; 10:19–25. [DOI] [PubMed] [Google Scholar]
- 7. Weihofen WA, Liu J, Reutter W, Saenger W, Fan H. Crystal structure of CD26/dipeptidyl‐peptidase IV in complex with adenosine deaminase reveals a highly amphiphilic interface. J Biol Chem 2004; 279:43330–5. [DOI] [PubMed] [Google Scholar]
- 8. Aertgeerts K. Crystal structure of human dipeptidyl peptidase IV in complex with a decapeptide reveals details on substrate specificity and tetrahedral intermediate formation. Protein Sci 2004; 13:412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamashita K, Tachibana Y, Matsuda Y, Katunuma N, Kochibe N, Kobata A. Comparative studies of the sugar chains of aminopeptidase N and dipeptidylpeptidase IV purified from rat kidney brush‐border membrane. Biochemistry 1988; 27:5565–73.] [DOI] [PubMed] [Google Scholar]
- 10. Stehling P, Grams S, Nuck R, Grunow D, Reutter W, Gohlke M. In vivo modulation of the acidic N‐glycans from rat liver dipeptidyl peptidase IV by N‐propanoyl‐D‐mannosamine. Biochem Biophys Res Commun 1999; 263:76–80. [DOI] [PubMed] [Google Scholar]
- 11. Kähne T, Kröning H, Thiel U, Ulmer AJ, Flad HD, Ansorge S. Alterations in structure and cellular localization of molecular forms of DP IV/CD26 during T cell activation. Cell Immunol 1996; 170:63–70. [DOI] [PubMed] [Google Scholar]
- 12. Schmauser B, Kilian C, Reutter W, Tauber R. Sialoforms of dipeptidylpeptidase IV from rat kidney and liver. Glycobiology 1999; 9:1295–305. [DOI] [PubMed] [Google Scholar]
- 13. Fan H, Meng W, Kilian C, Grams S, Reutter W. Domain‐specific N‐glycosylation of the membrane glycoprotein dipeptidylpeptidase IV (CD26) influences its subcellular trafficking, biological stability, enzyme activity and protein folding. Eur J Biochem 1997; 246:243–51. [DOI] [PubMed] [Google Scholar]
- 14. Loch N, Tauber R, Becker A, Hartel‐Schenk S, Reutter W. Biosynthesis and metabolism of dipeptidylpeptidase IV in primary cultured rat hepatocytes and Morris hepatoma 7777 cells. Eur J Biochem 1992; 210:161–8. [DOI] [PubMed] [Google Scholar]
- 15. Erickson RH, Suzuki Y, Sedlmayer A, Kim YS. Biosynthesis and degradation of altered immature forms of intestinal dipeptidyl peptidase IV in a rat strain lacking the enzyme. J Biol Chem 1992; 267:21623–9. [PubMed] [Google Scholar]
- 16. Smith RE, Talhouk JW, Brown EE, Edgar SE. The significance of hypersialylation of dipeptidyl peptidase IV (CD26) in the inhibition of its activity by Tat and other cationic peptides. CD26: a subverted adhesion molecule for HIV peptide binding. AIDS Res Hum Retroviruses 1998; 14:851–68. [DOI] [PubMed] [Google Scholar]
- 17. Cuchacovich M, Gatica H, Pizzo SV, Gonzalez‐Gronow M. Characterization of human serum dipeptidyl peptidase IV (CD26) and analysis of its autoantibodies in patients with rheumatoid arthritis and other autoimmune diseases. Clin Exp Rheumatol 2001; 19:673–80. [PubMed] [Google Scholar]
- 18. Sedo A, Krepela E, Kasafírek E, Kraml J, Kadlecová L. Dipeptidyl peptidase IV in the human lung and spinocellular lung cancer. Physiol Res 1991; 40:359–62.] [PubMed] [Google Scholar]
- 19. Volk BA, Kreisel W, Köttgen E, Gerok W, Reutter W. Heterogeneous turnover of terminal and core sugars within the carbohydrate chain of dipeptidylaminopeptidase IV isolated from rat liver plasma membrane. FEBS Lett 1983; 163:150–2. [DOI] [PubMed] [Google Scholar]
- 20. Kreisel W, Hildebrandt H, Mössner W, Tauber R, Reutter W. Oligosaccharide reprocessing and recycling of a cell surface glycoprotein in cultured rat hepatocytes. Biol Chem Hoppe Seyler 1993; 374:255–63. [DOI] [PubMed] [Google Scholar]
- 21. Slimane TA, Lenoir C, Sapin C, Maurice M, Trugnan G. Apical secretion and sialylation of soluble dipeptidyl peptidase IV are two related events. Exp Cell Res 2000; 258:184–94. [DOI] [PubMed] [Google Scholar]
- 22. Delacour D, Gouyer V, Leteurtre E et al 1‐benzyl‐2‐acetamido‐2‐deoxy‐alpha‐D‐galactopyranoside blocks the apical biosynthetic pathway in polarized HT‐29 cells. J Biol Chem 2003; 278:37799–809. [DOI] [PubMed] [Google Scholar]
- 23. Bilodeau N, Fiset A, Poirier GG et al Insulin‐dependent phosphorylation of DPP IV in liver. Evidence for a role of compartmentalized c‐Src. FEBS J 2006; 273:992–1003. [DOI] [PubMed] [Google Scholar]
- 24. Fan H, Tansi FL, Weihofen WA et al Molecular mechanism and structural basis of interactions of dipeptidyl peptidase IV with adenosine deaminase and human immunodeficiency virus type‐1 transcription transactivator. Eur J Cell Biol 2012; 91:265–73. [DOI] [PubMed] [Google Scholar]
- 25. Ikushima H, Munakata Y, Ishii T et al Internalization of CD26 by mannose 6‐phosphate/insulin‐like growth factor II receptor contributes to T cell activation. Proc Natl Acad Sci USA 2000; 97:8439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cordero OJ, Salgado FJ, Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother 2009; 58:1723–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol 2001; 54:249–64. [DOI] [PubMed] [Google Scholar]
- 28. Wang Z, Grigo C, Steinbeck J, von Hörsten S, Amann K, Daniel C. Soluble DPP4 originates in part from bone marrow cells and not from the kidney. Peptides 2014; 57:109–17. [DOI] [PubMed] [Google Scholar]
- 29. Delezuch W, Marttinen P, Kokki H et al Serum and CSF soluble CD26 and CD30 concentrations in healthy pediatric surgical outpatients. Tissue Antigens 2012; 80:368–75. [DOI] [PubMed] [Google Scholar]
- 30. Durinx C, Neels H, Van der Auwera JC, Naelaerts K, Scharpe S, De Meester I. Reference values for plasma dipeptidyl‐peptidase IV activity and their association with other laboratory parameters. Clin Chem Lab Med 2001; 39:155–9. [DOI] [PubMed] [Google Scholar]
- 31. Abbott CA, Baker E, Sutherland GR, McCaughan GW. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics 1994; 40:331–8. [DOI] [PubMed] [Google Scholar]
- 32. Bernard AM, Mattei MG, Pierres M, Marguet D. Structure of the mouse dipeptidyl peptidase IV (CD26) gene. Biochemistry 1994; 33:15204–14. [DOI] [PubMed] [Google Scholar]
- 33. Karl T, Chwalisz WT, Wedekind D et al Localization, transmission, spontaneous mutations, and variation of function of the Dpp4 (dipeptidyl‐peptidase IV; CD26) gene in rats. Regul Pept 2003; 115:81–90. [DOI] [PubMed] [Google Scholar]
- 34. Frerker N, von Horsten S, Raber KA, Krahn M, Naim HYAM. A single mutation at amino acid 359 of dipeptidyl peptidase IV (CD26) causes a transport block in the endoplasmic reticulum and cis‐Golgi compartment. Eur J Cell Biol 2005; 84:123. 15819395 [Google Scholar]
- 35. Shingu K, Helfritz A, Zielinska‐Skowronek M et al CD26 expression determines lung metastasis in mutant F344 rats: involvement of NK cell function and soluble CD26. Cancer Immunol Immunother 2003; 52:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karl T, Hoffmann T, Pabst R, von Hörsten S. Extreme reduction of dipeptidyl peptidase IV activity in F344 rat substrains is associated with various behavioral differences. Physiol Behav 2003; 80:123–34. [DOI] [PubMed] [Google Scholar]
- 37. Karl T, Hoffmann T, Pabst R, von Hörsten S. Behavioral effects of neuropeptide Y in F344 rat substrains with a reduced dipeptidyl‐peptidase IV activity. Pharmacol Biochem Behav 2003; 75:869–79. [DOI] [PubMed] [Google Scholar]
- 38. Frerker N, Raber K, Bode F et al Phenotyping of congenic dipeptidyl peptidase 4 (DP4) deficient Dark Agouti (DA) rats suggests involvement of DP4 in neuro‐, endocrine, and immune functions. Clin Chem Lab Med 2009; 47:275–87. [DOI] [PubMed] [Google Scholar]
- 39. Qvist H, Sjöström H, Norén O. The TATA‐less, GC‐rich porcine dipeptidylpeptidase IV (DPPIV) promoter shows bidirectional activity. Biol Chem 1998; 379:75–81. [PubMed] [Google Scholar]
- 40. Böhm SK, Gum JR, Erickson RH, Hicks JW, Kim YS. Human dipeptidyl peptidase IV gene promoter: tissue‐specific regulation from a TATA‐less GC‐rich sequence characteristic of a housekeeping gene promoter. Biochem J 1995; 311:835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hong WJ, Petell JK, Swank D, Sanford J, Hixson DC, Doyle D. Expression of dipeptidyl peptidase IV in rat tissues is mainly regulated at the mRNA levels. Exp Cell Res 1989; 182:256–66. [DOI] [PubMed] [Google Scholar]
- 42. Erickson RH, Gum JR, Lotterman CD, Hicks JW, Lai RS, Kim YS. Regulation of the gene for human dipeptidyl peptidase IV by hepatocyte nuclear factor 1 alpha. Biochem J 1999; 338:91–7. [PMC free article] [PubMed] [Google Scholar]
- 43. Hildebrandt M, Reutter W, Gitlin JD. Tissue‐specific regulation of dipeptidyl peptidase IV expression during development. Biochem J 1991; 277:331–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Darmoul D, Voisin T, Couvineau A et al Regional expression of epithelial dipeptidyl peptidase IV in the human intestines. Biochem Biophys Res Commun 1994; 203:1224–9. [DOI] [PubMed] [Google Scholar]
- 45. Riemann D, Kehlen A, Langner J. Stimulation of the expression and the enzyme activity of aminopeptidase N/CD13 and dipeptidylpeptidase IV/CD26 on human renal cell carcinoma cells and renal tubular epithelial cells by T cell‐derived cytokines, such as IL‐4 and IL‐13. Clin Exp Immunol 1995; 100:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fujiwara H, Fukuoka M, Yasuda K et al Cytokines stimulate dipeptidyl peptidase‐IV expression on human luteinizing granulosa cells. J Clin Endocrinol Metab 1994; 79:1007–11. [DOI] [PubMed] [Google Scholar]
- 47. Kehlen A, Göhring B, Langner J, Riemann D. Regulation of the expression of aminopeptidase A, aminopeptidase N/CD13 and dipeptidylpeptidase IV/CD26 in renal carcinoma cells and renal tubular epithelial cells by cytokines and cAMP‐increasing mediators. Clin Exp Immunol 1998; 111:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cordero OJ, Salgado FJ, Viñuela JE, Nogueira M. Interleukin‐12 enhances CD26 expression and dipeptidyl peptidase IV function on human activated lymphocytes. Immunobiology 1997; 197:522–33. [DOI] [PubMed] [Google Scholar]
- 49. Silva AP, Cavadas C, Baïsse‐Agushi B, Spertini O, Brunner HR, Grouzmann E. NPY, NPY receptors, and DPP IV activity are modulated by LPS, TNF‐alpha and IFN‐gamma in HUVEC. Regul Pept 2003; 116:71–9. [DOI] [PubMed] [Google Scholar]
- 50. Bauvois B, Djavaheri‐Mergny M, Rouillard D, Dumont J, Wietzerbin J. Regulation of CD26/DPPIV gene expression by interferons and retinoic acid in tumor B cells. Oncogene 2000; 19:265–72. [DOI] [PubMed] [Google Scholar]
- 51. Gutschmidt S, Gossrau R. A quantitative histochemical study of dipeptidylpeptidase IV (DPP IV). Histochemistry 1981; 73:285–304. [DOI] [PubMed] [Google Scholar]
- 52. Dikov A, Dimitrova M, Krieg R, Halbhuber KJ. New fluorescent method for the histochemical detection of dipeptidyl peptidase IV using glycyl‐l‐prolyl‐2‐anthraquinonyl hydrazide as substrate. Cell Mol Biol (Noisy‐Le‐Grand) 2004; 50: Online: OL553–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15555420 [PubMed] [Google Scholar]
- 53. Bühling F, Junker U, Reinhold D, Neubert K, Jäger L, Ansorge S. Functional role of CD26 on human B lymphocytes. Immunol Lett 1995; 45:47–51. [DOI] [PubMed] [Google Scholar]
- 54. Tanaka T, Camerini D, Seed B et al Cloning and functional expression of the T cell activation antigen CD26. J Immunol 1992; 149:481–6. [PubMed] [Google Scholar]
- 55. Gorrell MD, Wickson J, McCaughan GW. Expression of the rat CD26 antigen (dipeptidyl peptidase IV) on subpopulations of rat lymphocytes. Cell Immunol 1991; 134:205–15. [DOI] [PubMed] [Google Scholar]
- 56. Waumans Y, Baerts L, Kehoe K, Lambeir A‐M, De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front Immunol 2015; 6:387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salgado FJ, Pérez‐Díaz A, Villanueva NM, Lamas O, Arias P, Nogueira M. CD26: a negative selection marker for human Treg cells. Cytometry A 2012; 81:843–55. [DOI] [PubMed] [Google Scholar]
- 58. Garcia Santana CA, Tung JW, Gulnik S. Human Treg cells are characterized by low/negative CD6 expression. Cytometry A 2014; 85:901–8. [DOI] [PubMed] [Google Scholar]
- 59. Bengsch B, Seigel B, Flecken T, Wolanski J, Blum HE, Thimme R. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26). J Immunol 2012; 188:5438–47. [DOI] [PubMed] [Google Scholar]
- 60. Sharma PK, Wong EB, Napier RJ et al High expression of CD26 accurately identifies human bacteria‐reactive MR1‐restricted MAIT cells. Immunology 2015; 145:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bühling F, Kunz D, Reinhold D et al Expression and functional role of dipeptidyl peptidase IV (CD26) on human natural killer cells. Nat Immun 1994; 13:270–9. [PubMed] [Google Scholar]
- 62. Biuling F, Tonevitskiĭ AG, Kiuster U, Anzorge S. Study of dipeptidyl peptidase IV as a surface marker of human natural killer cells. Biull Eksp Biol Med 1990; 110:411–3. [PubMed] [Google Scholar]
- 63. Yamabe T, Takakura K, Sugie K et al Induction of the 2B9 antigen/dipeptidyl peptidase IV/CD26 on human natural killer cells by IL‐2, IL‐12 or IL‐15. Immunology 1997; 91:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bühling F, Reinhold D, Lendeckel U, Faust J, Neubert K, Ansorge S. CD26 is involved in regulation of cytokine production in natural killer cells. Adv Exp Med Biol 1997; 421:141–7. [DOI] [PubMed] [Google Scholar]
- 65. Madueño JA, Muñoz E, Blazquez V, Gonzalez R, Aparicio P, Peña J. The CD26 antigen is coupled to protein tyrosine phosphorylation and implicated in CD16‐mediated lysis in natural killer cells. Scand J Immunol 1993; 37:425–9. [DOI] [PubMed] [Google Scholar]
- 66. Topham NJ, Hewitt EW. Natural killer cell cytotoxicity: how do they pull the trigger? Immunology 2009; 128:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Casey TM, Meade JL, Hewitt EW. Organelle proteomics: identification of the exocytic machinery associated with the natural killer cell secretory lysosome. Mol Cell Proteomics 2007; 6:767–80. [DOI] [PubMed] [Google Scholar]
- 68. Yan S, Marguet D, Dobers J, Reutter W, Fan H. Deficiency of CD26 results in a change of cytokine and immunoglobulin secretion after stimulation by pokeweed mitogen. Eur J Immunol 2003; 33:1519–27. [DOI] [PubMed] [Google Scholar]
- 69. Micouin A, Bauvois B. Expression of dipeptidylpeptidase IV (DPP IV/CD26) activity on human myeloid and B lineage cells, and cell growth suppression by the inhibition of DPP IV activity. Adv Exp Med Biol 1997; 421:201–5. [DOI] [PubMed] [Google Scholar]
- 70. Vora KA, Porter G, Peng R et al Genetic ablation or pharmacological blockade of dipeptidyl peptidase IV does not impact T cell‐dependent immune responses. BMC Immunol 2009; 10:19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Coburn MC, Hixson DC, Reichner JS. In vitro immune responsiveness of rats lacking active dipeptidylpeptidase IV. Cell Immunol 1994; 158:269–80. [DOI] [PubMed] [Google Scholar]
- 72. Klemann C, Schade J, Pabst R et al CD26/dipeptidyl peptidase 4‐deficiency alters thymic emigration patterns and leukcocyte subsets in F344‐rats age‐dependently. Clin Exp Immunol 2009; 155:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bedoui S, Kuhlmann S, Nave H, Drube J, Pabst R, von Hörsten S. Differential effects of neuropeptide Y (NPY) on leukocyte subsets in the blood: mobilization of B‐1‐like B‐lymphocytes and activated monocytes. J Neuroimmunol 2001; 117:125–32. [DOI] [PubMed] [Google Scholar]
- 74. Cro L, Morabito F, Zucal N et al CD26 expression in mature B‐cell neoplasia: its possible role as a new prognostic marker in B‐CLL. Hematol Oncol 2009; 27:140–7. [DOI] [PubMed] [Google Scholar]
- 75. Zhong J, Rao X, Deiuliis J et al A potential role for dendritic cell/macrophage‐expressing DPP4 in obesity‐induced visceral inflammation. Diabetes 2013; 62:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gliddon DR, Howard CJ. CD26 is expressed on a restricted subpopulation of dendritic cells in vivo. Eur J Immunol 2002; 32:1472–81. [DOI] [PubMed] [Google Scholar]
- 77. Epardaud M, Bonneau M, Payot F et al Enrichment for a CD26hi SIRP– subset in lymph dendritic cells from the upper aero‐digestive tract. J Leukoc Biol 2004; 76:553–61. [DOI] [PubMed] [Google Scholar]
- 78. Ellingsen T, Hornung N, Møller BK, Hjelm‐Poulsen J, Stengaard‐Pedersen K. In active chronic rheumatoid arthritis, dipeptidyl peptidase IV density is increased on monocytes and CD4(+) T lymphocytes. Scand J Immunol 2007; 66:451–7. [DOI] [PubMed] [Google Scholar]
- 79. Fukui Y, Yamamoto A, Kyoden T, Kato K, Tashiro Y. Quantitative immunogold localization of dipeptidyl peptidase IV (DPP IV) in rat liver cells. Cell Struct Funct 1990; 15:117–25. [DOI] [PubMed] [Google Scholar]
- 80. Wagner L, Wolf R, Zeitschel U et al Proteolytic degradation of neuropeptide Y (NPY) from head to toe: identification of novel NPY‐cleaving peptidases and potential drug interactions in CNS and Periphery. J Neurochem 2015; 135:1019–37. [DOI] [PubMed] [Google Scholar]
- 81. Röhnert P, Schmidt W, Emmerlich P et al Dipeptidyl peptidase IV, aminopeptidase N and DPIV/APN‐like proteases in cerebral ischemia. J Neuroinflammation 2012; 9:44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shah Z, Kampfrath T, Deiuliis J et al Long‐term dipeptidyl‐peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011; 124:2338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Severini C, Improta G, Falconieri‐Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev 2002; 54:285–322. [DOI] [PubMed] [Google Scholar]
- 84. Mortier A, Gouwy M, Van Damme J, Proost P, Struyf S. CD26/dipeptidylpeptidase IV‐chemokine interactions: double‐edged regulation of inflammation and tumor biology. J Leukoc Biol 2016; doi:10.1189/jlb.3MR0915-401R PMID: 26744452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP‐1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23:443–52. [DOI] [PubMed] [Google Scholar]
- 86. Grouzmann E, Monod M, Landis B et al Loss of dipeptidylpeptidase IV activity in chronic rhinosinusitis contributes to the neurogenic inflammation induced by substance P in the nasal mucosa. FASEB J 2002; 16:1132–4. [DOI] [PubMed] [Google Scholar]
- 87. Hoffmann T, Faust J, Neubert K, Ansorge S. Dipeptidyl peptidase IV (CD 26) and aminopeptidase N (CD 13) catalyzed hydrolysis of cytokines and peptides with N‐terminal cytokine sequences. FEBS Lett 1993; 336:61–4. [DOI] [PubMed] [Google Scholar]
- 88. Boonacker E, Van Noorden CJF. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol 2003; 82:53–73. [DOI] [PubMed] [Google Scholar]
- 89. Mentlein R. Dipeptidyl‐peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul Pept 1999; 85:9–24. [DOI] [PubMed] [Google Scholar]
- 90. Dang NH, Morimoto C. CD26: an expanding role in immune regulation and cancer. Histol Histopathol 2002; 17:1213–26. [DOI] [PubMed] [Google Scholar]
- 91. Hildebrandt M, Reutter W, Arck P, Rose M, Klapp BF. A guardian angel: the involvement of dipeptidyl peptidase IV in psychoneuroendocrine function, nutrition and immune defence. Clin Sci (Lond) 2000; 99:93–104. [PubMed] [Google Scholar]
- 92. Demuth H‐U, McIntosh CHS, Pederson RA. Type 2 diabetes–therapy with dipeptidyl peptidase IV inhibitors. Biochim Biophys Acta 2005; 1751:33–44. [DOI] [PubMed] [Google Scholar]
- 93. Misslitz A, Bernhardt G, Förster R. Trafficking on serpentines: molecular insight on how maturating T cells find their winding paths in the thymus. Immunol Rev 2006; 209:115–28. [DOI] [PubMed] [Google Scholar]
- 94. Dang NH, Torimoto Y, Shimamura K et al 1F7 (CD26): a marker of thymic maturation involved in the differential regulation of the CD3 and CD2 pathways of human thymocyte activation. J Immunol 1991; 147:2825–32. [PubMed] [Google Scholar]
- 95. Ruiz P, Zacharievich N, Hao L, Viciana AL, Shenkin M. Human thymocyte dipeptidyl peptidase IV (CD26) activity is altered with stage of ontogeny. Clin Immunol Immunopathol 1998; 88:156–68. [DOI] [PubMed] [Google Scholar]
- 96. Savino W, Villa‐Verde DM, Lannes‐Vieira J. Extracellular matrix proteins in intrathymic T‐cell migration and differentiation? Immunol Today 1993; 14:158–61. [DOI] [PubMed] [Google Scholar]
- 97. Cordero OJ, Yang C‐P, Bell EB. On the role of CD26 in CD4 memory T cells. Immunobiology 2007; 212:85–94. [DOI] [PubMed] [Google Scholar]
- 98. Ibegbu CC, Xu Y‐X, Fillos D, Radziewicz H, Grakoui A, Kourtis AP. Differential expression of CD26 on virus‐specific CD8(+) T cells during active, latent and resolved infection. Immunology 2009; 126:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Morimoto C, Schlossman SF. The structure and function of CD26 in the T‐cell immune response. Immunol Rev 1998; 161:55–70. [DOI] [PubMed] [Google Scholar]
- 100. Krakauer M, Sorensen PS, Sellebjerg F. CD4(+) memory T cells with high CD26 surface expression are enriched for Th1 markers and correlate with clinical severity of multiple sclerosis. J Neuroimmunol 2006; 181:157–64. [DOI] [PubMed] [Google Scholar]
- 101. Piazza GA, Callanan HM, Mowery J, Hixson DC. Evidence for a role of dipeptidyl peptidase IV in fibronectin‐mediated interactions of hepatocytes with extracellular matrix. Biochem J 1989; 262:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hanski C, Huhle T, Gossrau R, Reutter W. Direct evidence for the binding of rat liver DPP IV to collagen in vitro. Exp Cell Res 1988; 178:64–72. [DOI] [PubMed] [Google Scholar]
- 103. Liu Z, Christensson M, Forslöw A, De Meester I, Sundqvist K‐G. A CD26‐controlled cell surface cascade for regulation of T cell motility and chemokine signals. J Immunol 2009; 183:3616–24. [DOI] [PubMed] [Google Scholar]
- 104. Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci USA 2006; 103:8447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rosenstock J, Sankoh S, List JF. Glucose‐lowering activity of the dipeptidyl peptidase‐4 inhibitor saxagliptin in drug‐naive patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:376–86. [DOI] [PubMed] [Google Scholar]
- 106. Pitocco D, Giubilato S, Martini F et al Combined atherogenic effects of celiac disease and type 1 diabetes mellitus. Atherosclerosis 2011; 217:531–5. [DOI] [PubMed] [Google Scholar]
- 107. Mishriky BM, Cummings DM, Tanenberg RJ. The efficacy and safety of DPP4 inhibitors compared to sulfonylureas as add‐on therapy to metformin in patients with Type 2 diabetes: a systematic review and meta‐analysis. Diabetes Res Clin Pract 2015; 109:378–88. [DOI] [PubMed] [Google Scholar]
- 108. Schön E, Demuth HU, Eichmann E et al Dipeptidyl peptidase IV in human T lymphocytes. Impaired induction of interleukin 2 and gamma interferon due to specific inhibition of dipeptidyl peptidase IV. Scand J Immunol 1989; 29:127–32. [DOI] [PubMed] [Google Scholar]
- 109. Reinhold D, Bank U, Bühling F et al Inhibitors of dipeptidyl peptidase IV (DP IV, CD26) induces secretion of transforming growth factor‐beta 1 (TGF‐beta 1) in stimulated mouse splenocytes and thymocytes. Immunol Lett 1997; 58:29–35. [DOI] [PubMed] [Google Scholar]
- 110. Steeg C, Hartwig U, Fleischer B. Unchanged signaling capacity of mutant CD26/dipeptidylpeptidase IV molecules devoid of enzymatic activity. Cell Immunol 1995; 164:311–5. [DOI] [PubMed] [Google Scholar]
- 111. Tanaka T, Kameoka J, Yaron A, Schlossman SF, Morimoto C. The costimulatory activity of the CD26 antigen requires dipeptidyl peptidase IV enzymatic activity. Proc Natl Acad Sci USA 1993; 90:4586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ohnuma K, Uchiyama M, Yamochi T et al Caveolin‐1 triggers T‐cell activation via CD26 in association with CARMA1. J Biol Chem 2007; 282:10117–31. [DOI] [PubMed] [Google Scholar]
- 113. Ohnuma K, Inoue H, Uchiyama M et al T‐cell activation via CD26 and caveolin‐1 in rheumatoid synovium. Mod Rheumatol 2006; 16:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ohnuma K, Uchiyama M, Hatano R et al Blockade of CD26‐mediated T cell costimulation with soluble caveolin‐1‐Ig fusion protein induces anergy in CD4+T cells. Biochem Biophys Res Commun 2009; 386:327–32. [DOI] [PubMed] [Google Scholar]
- 115. Hatano R, Ohnuma K, Yamamoto J, Dang NH, Morimoto C. CD26‐mediated co‐stimulation in human CD8(+) T cells provokes effector function via pro‐inflammatory cytokine production. Immunology 2013; 138:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci 2009; 30:600–7. [DOI] [PubMed] [Google Scholar]
- 117. Preller V, Gerber A, Wrenger S et al TGF‐beta1‐mediated control of central nervous system inflammation and autoimmunity through the inhibitory receptor CD26. J Immunol 2007; 178:4632–40. [DOI] [PubMed] [Google Scholar]
- 118. Fan H, Yan S, Stehling S, Marguet D, Schuppaw D, Reutter W. Dipeptidyl peptidase IV/CD26 in T cell activation, cytokine secretion and immunoglobulin production. Adv Exp Med Biol 2003; 524:165–74. [DOI] [PubMed] [Google Scholar]
- 119. Durinx C, Lambeir AM, Bosmans E et al Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is responsible for the release of X‐Pro dipeptides. Eur J Biochem 2000; 267:5608–13. [DOI] [PubMed] [Google Scholar]
- 120. Friedrich D, Hoffmann T, Bär J et al Does human attractin have DP4 activity? Biol Chem 2007; 388:155–62. [DOI] [PubMed] [Google Scholar]
- 121. Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol 2002; 3:715–20. [DOI] [PubMed] [Google Scholar]
- 122. Holmes AM, Solari R, Holgate ST. Animal models of asthma: value, limitations and opportunities for alternative approaches. Drug Discov Today 2011; 16:659–70. [DOI] [PubMed] [Google Scholar]
- 123. van der Velden VH, Wierenga‐Wolf AF, Adriaansen‐Soeting PW et al Expression of aminopeptidase N and dipeptidyl peptidase IV in the healthy and asthmatic bronchus. Clin Exp Allergy 1998; 28:110–20. [DOI] [PubMed] [Google Scholar]
- 124. Schade J, Stephan M, Schmiedl A et al Regulation of expression and function of dipeptidyl peptidase 4 (DP4), DP8/9, and DP10 in allergic responses of the lung in rats. J Histochem Cytochem 2008; 56:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lun SWM, Wong CK, Ko FWS, Hui DSC, Lam CWK. Increased expression of plasma and CD4+ T lymphocyte costimulatory molecule CD26 in adult patients with allergic asthma. J Clin Immunol 2007; 27:430–7. [DOI] [PubMed] [Google Scholar]
- 126. Yan S, Gessner R, Dietel C, Schmiedek U, Fan H. Enhanced ovalbumin‐induced airway inflammation in CD26‐/‐ mice. Eur J Immunol 2012; 42:533–40. doi:10.1002/eji.201041038. [DOI] [PubMed] [Google Scholar]
- 127. Kruschinski C, Skripuletz T, Bedoui S et al CD26 (dipeptidyl‐peptidase IV)‐dependent recruitment of T cells in a rat asthma model. Clin Exp Immunol 2005; 139:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schade J, Schmiedl A, Kehlen A et al Airway‐specific recruitment of T cells is reduced in a CD26‐deficient F344 rat substrain. Clin Exp Immunol 2009; 158:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Skripuletz T, Schmiedl A, Schade J et al Dose‐dependent recruitment of CD25+ and CD26+ T cells in a novel F344 rat model of asthma. Am J Physiol Lung Cell Mol Physiol 2007; 292:L1564–71. [DOI] [PubMed] [Google Scholar]
- 130. Schade J, Schmiedl A, Stephan M, Pabst R, von Hörsten S. Transferred T cells preferentially adhere in the BALT of CD26‐deficient recipient lungs during asthma. Immunobiology 2010; 215:321–31. [DOI] [PubMed] [Google Scholar]
- 131. Schmiedl A, Krainski J, Schwichtenhövel F et al Reduced airway inflammation in CD26/DPP4‐deficient F344 rats is associated with altered recruitment patterns of regulatory T cells and expression of pulmonary surfactant proteins. Clin Exp Allergy 2010; 40:1794–808. [DOI] [PubMed] [Google Scholar]
- 132. Stephan M, Suhling H, Schade J et al Effects of dipeptidyl peptidase‐4 inhibition in an animal model of experimental asthma: a matter of dose, route, and time. Physiol Rep 2013; 1:e00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Forssmann U, Stoetzer C, Stephan M et al Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxin‐mediated recruitment of eosinophils in vivo . J Immunol 2008; 181:1120–7. [DOI] [PubMed] [Google Scholar]
- 134. Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol 2005; 23:683–747. [DOI] [PubMed] [Google Scholar]
- 135. Reinhold D, Biton A, Pieper S et al Dipeptidyl peptidase IV (DP IV, CD26) and aminopeptidase N (APN, CD13) as regulators of T cell function and targets of immunotherapy in CNS inflammation. Int Immunopharmacol 2006; 6:1935–42. [DOI] [PubMed] [Google Scholar]
- 136. Constantinescu CS, Kamoun M, Dotti M, Farber RE, Galetta SL, Rostami A. A longitudinal study of the T cell activation marker CD26 in chronic progressive multiple sclerosis. J Neurol Sci 1995; 130:178–82. [DOI] [PubMed] [Google Scholar]
- 137. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL‐17 and Th17 cells. Annu Rev Immunol 2009; 27:485–517. [DOI] [PubMed] [Google Scholar]
- 138. Steinbrecher A, Reinhold D, Quigley L et al Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up‐regulates TGF‐beta 1 secretion in vivo . J Immunol 2001; 166:2041–8. [DOI] [PubMed] [Google Scholar]
- 139. Reinhold D, Hemmer B, Gran B et al Inhibitors of dipeptidyl peptidase IV/CD26 suppress activation of human MBP‐specific CD4+ T cell clones. J Neuroimmunol 1998; 87:203–9. [DOI] [PubMed] [Google Scholar]
- 140. Reinhold D, Bank U, Täger M et al DP IV/CD26, APN/CD13 and related enzymes as regulators of T cell immunity: implications for experimental encephalomyelitis and multiple sclerosis. Front Biosci 2008; 13:2356–63. [DOI] [PubMed] [Google Scholar]
- 141. Ohnuma K, Hosono O, Dang NHNH Morimoto C. Dipeptidyl peptidase in autoimmune pathophysiology. Adv Clin Chem 2011; 53:51. [DOI] [PubMed] [Google Scholar]
- 142. Ulusoy H, Kamanli A, Ilhan N et al Serum levels of soluble CD26 and CD30 and their clinical significance in patients with rheumatoid arthritis. Rheumatol Int 2011; 32:3857–62. [DOI] [PubMed] [Google Scholar]
- 143. Sromova L, Mareckova H, Sedova L, Balaziova E, Sedo A. Dipeptidyl peptidase‐IV in synovial fluid and in synovial fluid mononuclear cells of patients with rheumatoid arthritis. Clin Chim Acta 2010; 411:1046–50. [DOI] [PubMed] [Google Scholar]
- 144. Wagner L, Klemann C, Stephan M, von Hörsten S. Unravelling the immunological roles of dipeptidyl peptidase 4 (DPP4) activity and/or structure homolog (DASH) proteins. Clin Exp Immunol 2015; doi:10.1111/cei.12757. PMID: 26671446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Busso N, Wagtmann N, Herling C et al Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol 2005; 166:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Saito T, Ohnuma K, Suzuki H et al Polyarthropathy in type 2 diabetes patients treated with DPP4 inhibitors. Diabetes Res Clin Pract 2013; 102:e8–12. [DOI] [PubMed] [Google Scholar]
- 147. Crickx E, Marroun I, Veyrie C et al DPP4 inhibitor‐induced polyarthritis: a report of three cases. Rheumatol Int 2014; 34:291–2. [DOI] [PubMed] [Google Scholar]
- 148. Abbott CA, Yazbeck R, Geier MS, Demuth H‐U, Howarth GS. Dipeptidyl peptidases and inflammatory bowel disease. Adv Exp Med Biol 2006; 575:155–62. [DOI] [PubMed] [Google Scholar]
- 149. Hildebrandt M, Rose M, Rüter J, Salama A, Mönnikes H, Klapp BF. Dipeptidyl peptidase IV (DP IV, CD26) in patients with inflammatory bowel disease. Scand J Gastroenterol 2001; 36:1067–72. [DOI] [PubMed] [Google Scholar]
- 150. Xiao Q, Boushey RP, Cino M, Drucker DJ, Brubaker PL. Circulating levels of glucagon‐like peptide‐2 in human subjects with inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol 2000; 278:R1057–63. [DOI] [PubMed] [Google Scholar]
- 151. Detel D, Pugel EP, Pucar LB, Buljevic S, Varljen J. Development and resolution of colitis in mice with target deletion of dipeptidyl peptidase IV. Exp Physiol 2012; 97:486–96. [DOI] [PubMed] [Google Scholar]
- 152. Geier MS, Tenikoff D, Yazbeck R, McCaughan GW, Abbott CA, Howarth GS. Development and resolution of experimental colitis in mice with targeted deletion of dipeptidyl peptidase IV. J Cell Physiol 2005; 204:687–92. [DOI] [PubMed] [Google Scholar]
- 153. Baticic L, Detel D, Kucic N, Buljevic S, Pugel EP, Varljen J. Neuroimmunomodulative properties of dipeptidyl peptidase IV/CD26 in a TNBS‐induced model of colitis in mice. J Cell Biochem 2011; 112:3322–33. [DOI] [PubMed] [Google Scholar]
- 154. Rovedatti L, Di Sabatino A, Knowles CH et al Fibroblast activation protein expression in Crohn's disease strictures. Inflamm Bowel Dis 2011; 17:1251–3. [DOI] [PubMed] [Google Scholar]
- 155. Bank U, Bohr URM, Reinhold D et al Inflammatory bowel diseases: multiple benefits from therapy with dipeptidyl‐ and alanyl‐aminopeptidase inhibitors. Front Biosci 2008; 13:3699–713. [DOI] [PubMed] [Google Scholar]
- 156. Yazbeck R, Howarth GS, Geier MS, Demuth H‐U, Abbott CA. Inhibiting dipeptidyl peptidase activity partially ameliorates colitis in mice. Front Biosci 2008; 13:6850–8. [DOI] [PubMed] [Google Scholar]
- 157. Tasic T, Bäumer W, Schmiedl A et al Dipeptidyl peptidase IV (DPP4) deficiency increases Th1‐driven allergic contact dermatitis. Clin Exp Allergy 2011; 41:1098–107. [DOI] [PubMed] [Google Scholar]
- 158. Bock O, Kreiselmeyer I, Mrowietz U. Expression of dipeptidyl‐peptidase IV (CD26) on CD8+ T cells is significantly decreased in patients with psoriasis vulgaris and atopic dermatitis. Exp Dermatol 2001; 10:414–9. [DOI] [PubMed] [Google Scholar]
- 159. Pederson RA, White HA, Schlenzig D, Pauly RP, McIntosh CH, Demuth HU. Improved glucose tolerance in Zucker fatty rats by oral administration of the dipeptidyl peptidase IV inhibitor isoleucine thiazolidide. Diabetes 1998; 47:1253–8. [DOI] [PubMed] [Google Scholar]
- 160. Scheen AJ. DPP‐4 inhibitors in the management of type 2 diabetes: a critical review of head‐to‐head trials. Diabetes Metab 2011; doi:10.1016/j.diabet.2011.11.001. PMID: 22197148. [DOI] [PubMed] [Google Scholar]
- 161. Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase‐4 inhibitors: similarities and differences. Drugs 2011; 71:1441–67. [DOI] [PubMed] [Google Scholar]
- 162. Schuetz CA, Ong SH, Blüher M. Clinical trial simulation methods for estimating the impact of DPP‐4 inhibitors on cardiovascular disease. Clinicoecon Outcomes Res 2015; 7:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Tricco AC, Antony J, Khan PA et al Safety and effectiveness of dipeptidyl peptidase‐4 inhibitors versus intermediate‐acting insulin or placebo for patients with type 2 diabetes failing two oral antihyperglycaemic agents: a systematic review and network meta‐analysis. BMJ Open 2014; 4:e005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Cheng D, Fei Y, Liu Y et al Efficacy and safety of dipeptidyl peptidase‐4 inhibitors in type 2 diabetes mellitus patients with moderate to severe renal impairment: a systematic review and meta‐analysis. PLoS One 2014; 9:e111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Matteucci E, Giampietro O. Dipeptidyl peptidase‐4 inhibition: linking chemical properties to clinical safety. Curr Med Chem 2011; 18:4753–60. [DOI] [PubMed] [Google Scholar]
- 166. Matteucci E, Giampietro O. Dipeptidyl peptidase‐4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem 2009; 16:2943–51. [DOI] [PubMed] [Google Scholar]
- 167. Willemen MJ, Mantel‐Teeuwisse AK, Straus SM, Meyboom RH, Egberts TC, Leufkens HG. Use of dipeptidyl peptidase‐4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care 2011; 34:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Richter B, Bandeira‐Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase‐4 (DPP‐4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008; CD006739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ahrén B, Hughes TE. Inhibition of dipeptidyl peptidase‐4 augments insulin secretion in response to exogenously administered glucagon‐like peptide‐1, glucose‐dependent insulinotropic polypeptide, pituitary adenylate cyclase‐activating polypeptide, and gastrin‐releasing peptid. Endocrinology 2005; 146:2055–9. [DOI] [PubMed] [Google Scholar]
- 170. White J. Efficacy and safety of incretin based therapies: clinical trial data. J Am Pharm Assoc 2003; 49 (Suppl. 1):S30–40. [DOI] [PubMed] [Google Scholar]
- 171. Barnett A. DPP‐4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract 2006; 60:1454–70. [DOI] [PubMed] [Google Scholar]
- 172. Neumiller JJ. Differential chemistry (structure), mechanism of action, and pharmacology of GLP‐1 receptor agonists and DPP‐4 inhibitors. J Am Pharm Assoc 2009; 49 (Suppl. 1):S16–29. [DOI] [PubMed] [Google Scholar]
- 173. Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin‐based therapies–review of the scientific evidence. J Clin Endocrinol Metab 2011; 96:2027–31. [DOI] [PubMed] [Google Scholar]
- 174. Röhrborn D, Wronkowitz N, Eckel J. DPP4 in diabetes. Front Immunol 2015; 6:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Butler AE, Campbell‐Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon‐producing neuroendocrine tumors. Diabetes 2013; 62:2595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Egan AG, Blind E, Dunder K et al Pancreatic safety of incretin‐based drugs – FDA and EMA assessment. N Engl J Med 2014; 370:794–7. [DOI] [PubMed] [Google Scholar]
- 177. Epstein BJ. Drug evaluation: PSN‐9301, a short‐acting inhibitor of dipeptidyl peptidase IV. Curr Opin Investig Drugs 2007; 8:331–7. [PubMed] [Google Scholar]
- 178. Devin JK, Pretorius M, Nian H, Yu C, Billings FT, Brown NJ. Substance P increases sympathetic activity during combined angiotensin‐converting enzyme and dipeptidyl peptidase‐4 inhibition. Hypertension 2014; 63:951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Grouzmann E, Buclin T. Is dipeptidylpeptidase IV the missing link in angiotensin‐converting enzyme inhibitor–induced angioedema? Hypertension 2008; 51:45–7. [DOI] [PubMed] [Google Scholar]
- 180. Engel SS, Williams‐Herman DE, Golm GT et al Sitagliptin: review of preclinical and clinical data regarding incidence of pancreatitis. Int J Clin Pract 2010; 64:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Mas‐Vidal A, Santos‐Juanes J, Esteve‐Martinez A, Caminal‐Montero L, Coto‐Segura P. Psoriasiform eruption triggered by a dipeptidyl peptidase IV inhibitor. Australas J Dermatol 2012; 53:70–2. [DOI] [PubMed] [Google Scholar]
- 182. Nishioka T, Shinohara M, Tanimoto N, Kumagai C, Hashimoto K. Sitagliptin, a dipeptidyl peptidase‐IV inhibitor, improves psoriasis. Dermatology 2012; 224:20–1. PMID: 22056790. [DOI] [PubMed] [Google Scholar]
- 183. Jackson EK, Dubinion JH, Mi Z. Effects of dipeptidyl peptidase iv inhibition on arterial blood pressure. Clin Exp Pharmacol Physiol 2008; 35:29–34. [DOI] [PubMed] [Google Scholar]
- 184. Hultström M. Development of structural kidney damage in spontaneously hypertensive rats. J Hypertens 2012; 30:1087–91. [DOI] [PubMed] [Google Scholar]
- 185. Jackson EK. Dipeptidyl peptidase IV inhibition alters the hemodynamic response to angiotensin‐converting enzyme inhibition in humans with the metabolic syndrome. Hypertension 2010; 56:581–3. [DOI] [PubMed] [Google Scholar]
- 186. Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase‐IV inhibition and angiotensin‐converting enzyme inhibition in humans. Hypertension 2010; 56:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol 2008; 29:295–301. [DOI] [PubMed] [Google Scholar]
- 188. Gonzalez‐Gronow M, Weber MR, Gawdi G, Pizzo SV. Dipeptidyl peptidase IV (CD26) is a receptor for streptokinase on rheumatoid synovial fibroblasts. Fibrinolysis Proteol 1998; 12:129–35. [Google Scholar]
- 189. Ohnuma K, Yamochi T, Hosono O, Morimoto C. CD26 T cells in the pathogenesis of asthma. Clin Exp Immunol 2005; 139:13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Ikushima H, Munakata Y, Iwata S et al Soluble CD26/dipeptidyl peptidase IV enhances transendothelial migration via its interaction with mannose 6‐phosphate/insulin‐like growth factor II receptor. Cell Immunol 2002; 215:106–10. [DOI] [PubMed] [Google Scholar]
- 191. Ishii T, Ohnuma K, Murakami A et al CD26‐mediated signaling for T cell activation occurs in lipid rafts through its association with CD45RO. Proc Natl Acad Sci USA 2001; 98:12138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Schmidt H, Gelhaus C, Nebendahl M et al Effector granules in human T lymphocytes: the luminal proteome of secretory lysosomes from human T cells. Cell Commun Signal 2011; 9:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science 1993; 261:466–9. [DOI] [PubMed] [Google Scholar]
- 194. Herrera C, Morimoto C, Blanco J et al Comodulation of CXCR4 and CD26 in human lymphocytes. J Biol Chem 2001; 276:19532–9. [DOI] [PubMed] [Google Scholar]
- 195. Wrenger S, Faust J, Mrestani‐Klaus C et al Down‐regulation of T cell activation following inhibition of dipeptidyl peptidase IV/CD26 by the N‐terminal part of the thromboxane A2 receptor. J Biol Chem 2000; 275:22180–6. [DOI] [PubMed] [Google Scholar]
- 196. Löster K, Zeilinger K, Schuppan D, Reutter W. The cysteine‐rich region of dipeptidyl peptidase IV (CD 26) is the collagen‐binding site. Biochem Biophys Res Commun 1995; 217:341–8. [DOI] [PubMed] [Google Scholar]
- 197. Cheng H‐C, Abdel‐Ghany M, Pauli BU. A novel consensus motif in fibronectin mediates dipeptidyl peptidase IV adhesion and metastasis. J Biol Chem 2003; 278:24600–7. [DOI] [PubMed] [Google Scholar]
- 198. Cuchacovich M, Gatica H, Vial P, Yovanovich J, Pizzo SV, Gonzalez‐Gronow M. Streptokinase promotes development of dipeptidyl peptidase IV (CD26) autoantibodies after fibrinolytic therapy in myocardial infarction patients. Clin Diagn Lab Immunol 2002; 9:1253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Gonzalez‐Gronow M, Hershfield MS, Arredondo‐Vega FX, Pizzo SV. Cell surface adenosine deaminase binds and stimulates plasminogen activation on 1‐LN human prostate cancer cells. J Biol Chem 2004; 279:20993–8. [DOI] [PubMed] [Google Scholar]
- 200. Davoodi J, Kelly J, Gendron NH, MacKenzie AE. The Simpson–Golabi–Behmel syndrome causative glypican‐3, binds to and inhibits the dipeptidyl peptidase activity of CD26. Proteomics 2007; 7:2300–10. [DOI] [PubMed] [Google Scholar]
- 201. Chen W‐T, Kelly T, Ghersi G. DPPIV, seprase, and related serine peptidases in multiple cellular functions. Curr Top Dev Biol 2003; 54:207–32. [DOI] [PubMed] [Google Scholar]
- 202. Engel M, Hoffmann T, Wagner L et al The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc Natl Acad Sci USA 2003; 100:5063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Girardi ACC, Knauf F, Demuth H‐U, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 2004; 287:C1238–45. [DOI] [PubMed] [Google Scholar]
- 204. Gonzalez‐Gronow M, Misra UK, Gawdi G, Pizzo SV. Association of plasminogen with dipeptidyl peptidase IV and Na+/H+ exchanger isoform NHE3 regulates invasion of human 1‐LN prostate tumor cells. J Biol Chem 2005; 280:27173–8. [DOI] [PubMed] [Google Scholar]


