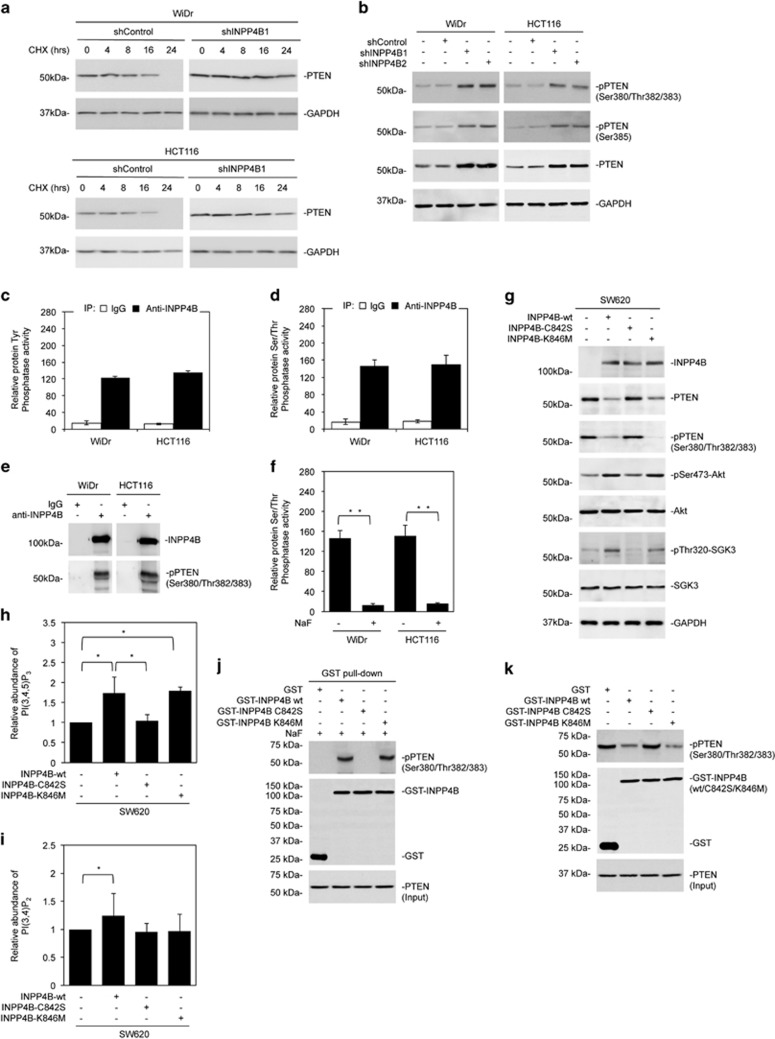
Figure 6.
Downregulation of PTEN by INPP4B is mediated by its protein phosphatase activity. (a) WiDr and HCT116 cells stably transduced with the control shRNA (shControl) or INPP4B shRNA (shINPP4B1) were treated with cycloheximide (CHX) (100 μg/ml) for the indicated periods. Whole-cell lysates were subjected to western blotting analysis of PTEN and GAPDH (as a loading control). Data are representative of three individual experiments. (b) Whole-cell lysates from WiDr and HCT116 cells stably transduced with shControl or two individual INPP4B shRNAs (shINPP4B1 and shINPP4B2) were subjected to western blotting analysis of phosphorylated PTEN (pSer380/Thr382/383 and pSer385), PTEN and GAPDH (as a loading control). Data are representative of three individual experiments. (c) Whole-cell lysates from WiDr and HCT116 cells were subjected to immunoprecipitation with a rabbit antibody against INPP4B or rabbit IgG. Precipitated INPP4B was then subjected to analysis of INPP4B protein Tyrosine phosphatase activity using a Tyrosine phosphatase assay system. The relative INPP4B protein Tyrosine phosphatase activity in cells precipitated with rabbit IgG was arbitrarily designated as 1. Data are represented as mean±s.e.m. of three individual experiments. (d) Whole-cell lysates from WiDr and HCT116 cells were subjected to immunoprecipitation with a rabbit antibody against INPP4B or rabbit IgG. Precipitated INPP4B was then subjected to analysis of INPP4B protein Serine/Threonine phosphatase activity using a Serine/Threonine phosphatase assay system. The relative INPP4B protein Serine/Threonine phosphatase activity in cells precipitated with rabbit IgG was arbitrarily designated as 1. Data are represented as mean±s.e.m. of three individual experiments. (e) Whole-cell lysates from WiDr and HCT116 cells were subjected to immunoprecipitation with a rabbit antibody against INPP4B or rabbit IgG. The resulting precipitates were subjected to western blotting analysis of INPP4B and phosphorylated PTEN (pSer380/Thr382/383). Data are representative of three individual experiments. (f) Whole-cell lysates from WiDr and HCT116 cells were subjected to immunoprecipitation with a rabbit antibody against INPP4B in the absence or presence of NaF (50 mM). The resulting precipitates were then subjected to analysis of INPP4B protein Serine/Threonine phosphatase activity using a Serine/Threonine phosphatase assay system. Data are represented as mean±s.e.m. of three individual experiments. **P<0.01, Student's t-test. (g) Whole-cell lysates from SW620 cells stably transduced with the wild-type INPP4B cDNA (INPP4B-wt) or mutant INPP4B cDNAs (INPP4B-C842S or INPP4B-K846M) cloned into the pCDH vector were subjected to western blotting analysis of INPP4B, PTEN, phosphorylated PTEN (pSer380/Thr382/383), PTEN, phosphorylated Akt (pSer473-Akt), Akt, phosphorylated SGK3 (pThr320-SGK3), SGK3 and GAPDH (as a loading control). Data are representative of three individual experiments. (h) The relative abundance of PI(3,4,5)P3 in SW620 cells transduced with the wild-type INPP4B cDNA (INPP4B-wt) or mutant INPP4B cDNAs (INPP4B-C842S or INPP4B-K846M) cloned into the pCDH vector was measured by using ELISA in lipid extractions of whole cells. Data are represented as mean±s.e.m. of three individual experiments. *P<0.05, Student's t-test. (i) The relative abundance of PI(3,4)P2 in SW620 cells transduced with the wild-type INPP4B cDNA (INPP4B-wt) or mutant INPP4B cDNAs (INPP4B-C842S or INPP4B-K846M) cloned into the pCDH vector was measured by using ELISA in lipid extractions of whole cells. Data are represented as mean±s.e.m. of three individual experiments. *P<0.05, Student's t-test. (j) Whole-cell lysates from HCT116 cells with INPP4B stably knocked down were subjected to GST pull-down using GST-INPP4B, GST-INPP4B-C842S, GST-INPP4B-K846M or GST as bait in the presence of NaF (10 mm). The resulting proteins were then subjected to western blotting analysis of phosphorylated PTEN (pSer380/Thr382/383), GST and PTEN (input control). Data are representative of three individual experiments. (k) Whole-cell lysates from HCT116 cells with INPP4B stably knocked down were incubated with purified GST-INPP4B, GST-INPP4B-C842S, GST-INPP4B-K846M or GST protein for 16 h. Samples were then subjected to western blotting analysis of phosphorylated PTEN (pSer380/Thr382/383), GST and PTEN (input control). Data are representative of three individual experiments.