Abstract
Background and Purpose
Peak Aerobic capacity (V02 peak) is severely worsened after disabling stroke, having serious implications for function, metabolism and ongoing cardiovascular risk. Work from our lab and others has previously shown that modest improvements in VO2 peak are possible in stroke participants with aerobic exercise training. The purpose of the current investigation was to test the extent to which greater enhancements in VO2 peak after stroke are possible using a treadmill protocol with far greater emphasis on intensity progression compared to a protocol without such emphasis.
Methods
Using a randomized design we compared stroke survivors engaged in higher intensity treadmill training (HI-TM, 80% Heart Rate Reserve- HRR) with those undergoing lower intensity training (LO-TM, 50% HRR). Measured outcomes were change in VO2 peak, 6-minute walk distance (6MWD), 30-ft walk times (30WT) and 48-hr step counts (48SC). LO-TM participants trained for a longer period of time per session in an effort to approximately match workload/ caloric expenditure. Participants were randomized with stratification according to age and baseline walking capacity.
Results
HI-TM participants (N=18) had significantly greater gains in VO2 peak (+34%) than LO-TM participants (N=16) (+5%) across the 6 month intervention period (p=0.001, group × time interaction). Conversely, there was no statistical difference between groups in the changes observed for 6MWD, 30WT, or 48SC.
Conclusion
HI-TM is far more effective than LO-TM for improving VO2 peak after disabling stroke. The magnitude of relative improvement for HI-TM was double compared to previous reports from our laboratory with probable clinical significance for this population.
Keywords: stroke recovery, stroke rehabilitation, exercise training, oxygen consumption
INTRODUCTION
Stroke-related disability coincides with dramatic reductions in peak aerobic capacity (VO2 peak)1, 2, requiring exercise to complete exhaustion just to achieve the middle of the estimated range of oxygen consumption required for sustained activities of daily living (ADLs)3. This makes upper level ADLs difficult or impossible and renders lower level ADLs unsustainable for extended periods of time1. Beyond the functional consequences of reduced aerobic fitness after stroke, there are also metabolic and vascular event risk implications associated with decrements in VO2 peak4, 5. Hence, stroke rehabilitation strategies that specifically target this parameter should be developed and implemented. Small changes in VO2 peak likely carry far greater significance to stroke participants than to age-matched non-stroke individuals on the basis of where they stand relative to the range of oxygen required for ADLs1, the high prevalence of abnormal glucose metabolism after stroke6, 7, as well as the disproportionate cardiovascular risk in this population8, 9.
Previous investigations, including those from our own laboratory, have demonstrated that chronically disabled stroke patients retain the capacity to exercise at levels requisite for modest improvements in VO2 peak2, 4, 10–20. Relative VO2 peak gains from these studies (n=13) averaged almost 15%, widely varying according to a number of factors including training modality. Recently, our collaborators in Germany conducted the first experiment with what could legitimately be labeled a high-intensity treadmill protocol21, showing that greater gains in VO2 peak (+29%) are possible after stroke. Additionally, there have been other preliminary experiments undertaken to show the efficacy of higher-intensity exercise models in this population22–24. Despite the encouraging initial results, maximum adaptive capacity for this outcome in stroke remains unclear based upon the small amount of evidence stemming interventions that push training intensity limits. Further, it is unknown how training interventions with a strong intensity component compare to lower-intensity regimens when compared side-by-side in the same study after stroke.
The current investigation sought to better establish the true limits of VO2 peak adaptation in stroke with a treadmill training protocol focused predominantly on intensity/ velocity progression (HI-TM). Additionally, we make within study comparisons between HI-TM and a group who treadmill trained without intensity/ velocity progression (LO-TM). To keep the groups comparable in terms of total work, LO-TM was progressed by increasing training session duration with intensity held constant such that total work remained comparable between groups. Secondary analyses compared the effects of the two intervention strategies on other basic functional parameters (6MWD, 30WT, 48SC).
MATERIALS and METHODS
Subjects
Participants were recruited from the University of Maryland Medical System and the Baltimore VA Medical Center referral networks. Chronic hemiparetic stroke patients (>6 months) who had completed all conventional physical therapy were sought. Potential participants had mild to moderate hemiparetic gait and demonstrated preserved capacity for ambulation with an assistive device. All were sedentary with no prior participation in aerobic training programs. Baseline evaluation included a medical history and examination. This study was approved by the Institutional Review Board for research involving humans at the University of Maryland, Baltimore. Written informed consent was obtained from each participant.
VO2 peak Testing
A physician-supervised treadmill tolerance test at no incline was first performed to assess gait safety and to select walking velocity for subsequent peak exercise testing and treadmill training. Participants minimized handrail support, and a gait belt was worn for safety. For graded treadmill screening (cardiac stress test), required for all prior to participation, achieving adequate exercise intensities without signs of myocardial ischemia or other contraindications for regular exercise resulted in clearance for study entry with no additional monitoring. Following a rest interval of at least one week after screening to avoid the confounding effects of fatigue, treadmill testing with open circuit spirometry was conducted to measure VO2 peak. This was done using a previously described treadmill testing protocol for stroke survivors25. Peak aerobic testing was then repeated at the post-intervention time point.
6-minute Walk Distance (6MWD)
The 6-minute walk is a distance that is representative of community-based ADL tasks26. It may be a more sensitive floor walking outcome measure with exercise in stroke patients because it reflects added benefits of increased endurance. Participants used the same assistive device and/or orthoses used when walking across a parking lot. They were instructed to cover as much distance as they could over a flat 100 foot walking surface demarcated by traffic cones during the 6-minute time period.
30-ft walk time (30WT)
To gauge walking speed over shorter distance, we conducted standard 30-ft walk tests at both self-selected and fastest comfortable pace before and after training as previously described2. Standardized instructions and commands contributed to the validity of these short distance walking assessments.
Step Activity Monitoring
Step activity monitor (SAM) technology was used to quantify 48-hr ambulatory activity. We have previously reported that microprocessor-linked step activity monitors (SAM) provide valid and reliable quantitative measure of ambulatory activity recovery in stroke patients with a broad range of gait deficit severity27, 28. Participants had their total number of steps quantified over a 48-hr period before and after the training intervention period.
Randomization
Participants were randomized to HI-TM or LO-TM following baseline testing using a blocked allocation schema and a computer-based, pseudo-random number generator. Age and severity of deficits were considered in the randomization design. Specifically, separate blocked randomizations were performed according to age (<65 vs. ≥ 65 yrs.) and self-selected walking speed (< 0.44 m/sec vs. ≥ 0.44 m/sec), given the potential impact of these factors on rehabilitation outcomes.
Intervention Protocols (6 months)
Training programs were individualized based on each participant’s gait capacity and defined by peak heart rate (HR max) achieved during baseline TM test. Training started conservatively with a goal of 15 minutes total duration at 40–50% HRR determined according to the formula of Karvonen. Training target HR = % (HRmax − HRrest) + HRrest. HR max was defined as peak HR based on the 2 maximal exercise tests at baseline. Individuals unable to walk continuously would exercise intermittently for several minutes as tolerated, with interval rests, and advanced as tolerated with HR, blood pressure monitoring, and Borg Perceived Exertion (BPE) to assess subjective cardiopulmonary exercise tolerance. For those randomized to HI-TM, training velocity was advanced as tolerated by week 6 to a target intensity of 80–85% maximal HRR. Duration was similarly advanced by 5 minutes bi-weekly to a target of 30 minutes by week 6. Following week 6, the progressive training protocol continued with attempts at velocity increases on a weekly basis. Participants in both groups were encouraged to challenge themselves past the point of fatigue, but safety was always the highest priority. Vital signs were monitored before during and after each session with pre-established commencement and continuation criteria. Additionally, participants were queried regarding health status changes in a standardized way prior to each session and monitored carefully for signs of stress during the bout, with simultaneous encouragement for pushing forward when warranted. On rare occasions when exercise performance was hampered by minor health fluctuations or issues such as hydration status, training sessions were either temporarily suspended or completely discontinued depending on response to rest and water. To assess treatment fidelity, Exercise Physiology staff documented progression, with weekly reporting to the study meetings for review. Independent treatment fidelity monitoring was conducted to assure that training was being conducted according to specified guidelines. Safety during TM was assured with use of safety harnesses (BIODEX) in the non-weight bearing mode.
The LO-TM protocol provided matched exposure to staff, who aided participants in the performance of treadmill training with no emphasis on intensity progression, but rather a sole focus on increasing training session duration. The initial protocol was quite similar to that described above, but intensity was clamped at below 50% HRR, meaning no progression in treadmill training speed/ intensity across the 6 month training period, with only training session time gradually expanded to 50 minutes.
Data Analysis
Baseline values for age, height, weight, BMI, latency, VO2 peak, 6MWD, 30WT and 48SC Step Activity were compared between groups using an independent t-test. Categorical baseline variables (ratios) were compared between groups using Fisher’s Exact Test. Repeated measures ANOVA (2-factors, time × group) was used to predict values of outcome variables across time, assessing for significant 2-way interactions for changes in outcomes over 6 months. Baseline and repeated values are mean ± SE with a two-tailed p value of 0.05 required for significance. Within group changes were assessed for significance with a paired t-test.
RESULTS
Subjects
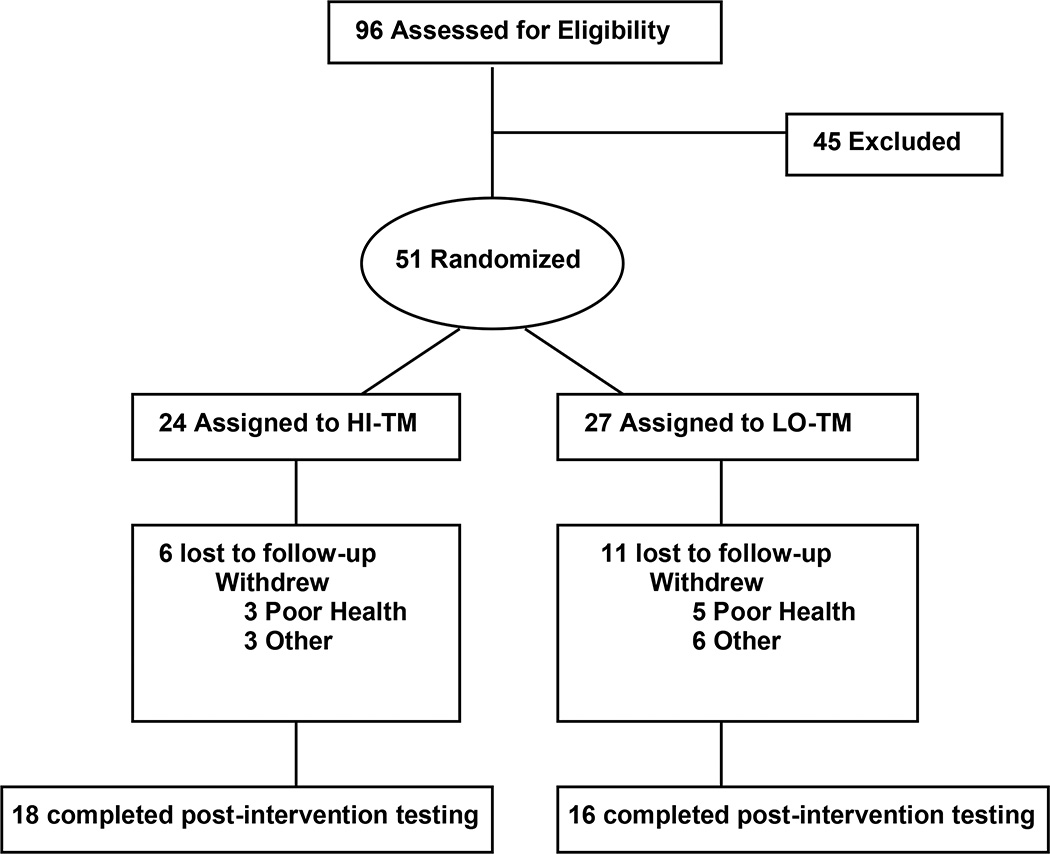
Of the 34 who completed, 18 were HI-TM and 16 were LO-TM. At baseline, there were no significant differences between groups for age, height, weight, or BMI (Table 1). Likewise, there were also no statistically significant baseline differences between groups for the primary outcome variable (V02 peak) or any of the secondary functional measures (6MWD, 30WT, 48SC) (Table 1). There was no significant difference between groups for racial mix, and both groups had similar percentages of participants requiring assistive devices for ambulation. Also, the ratio of males to females was the same for both groups. All participant physical and functional characteristics for both groups are summarized in Table 1. As depicted in figure 1, there were 6 lost to follow-up in the HI-TM group and 11 lost to follow-up in the LO-TM group. Dropouts in TM and CONTROL resulted from either medical reasons unrelated to study procedures or general compliance issues often resulting from a loss of interest. There were no serious adverse events resulting from either of the intervention protocols and both HI-TM and LO-TM were generally well-tolerated and adequately adhered to (>85% of sessions attended).
Table 1.
Participant characteristics by group
| Variable | HI-TM (N=18) | LO-TM (n=16) | P value |
|---|---|---|---|
| Age (yrs.) | 61 ± 1.6 | 63 ± 2.4 | 0.33 |
| Gender (M:F) | 10:8 | 11:5 | 0.49 |
| Race (W:NW) | 6:12 | 7:9 | 0.73 |
| Weight (kg) | 75.3 ± 4.1 | 76.7 ± 3.9 | 0.81 |
| Stroke Latency (months) | 41 ± 12 | 37 ± 14 | 0.84 |
| BMI kg/m2 | 25.7 ± 1.0 | 26.9 ± 1.2 | 0.45 |
| Ratio of assistive device use (Y:N) | 15:3 | 14:2 | 1.00 |
| Peak Aerobic Capacity (mL/kg/min) | 15.9 ± 1.7 | 16.6 ± 1.2 | 0.71 |
| Six-Minute Walk Distance (ft) | 780 ± 105 | 564 ± 73 (n=15) | 0.12 |
| Self-Selected 30ft Walking Time (sec) | 21.3 ± 3.4 | 24.0 ± 3.0 | 0.55 |
| Fastest Comfortable 30ft. Walking Time (sec) | 15.4 ± 2.1 (n=17) | 17.2 ± 2.2 (n=14) | 0.56 |
| 48-hour Step Counts | 5596 ± 980 | 5150 ± 679 (n=14) | 0.71 |
Mean ± SE (yrs.=years, kg=kilograms, cm= centimeters, BMI=Body Mass Index, mph=miles per hour, ml/kg/min = milliliters per kilogram per minute, TM=Treadmill, N=Number, P=Probability)
Figure 1.
Flow diagram
Effects of HI-TM vs. LO-TM on Peak Fitness (Figure 1)
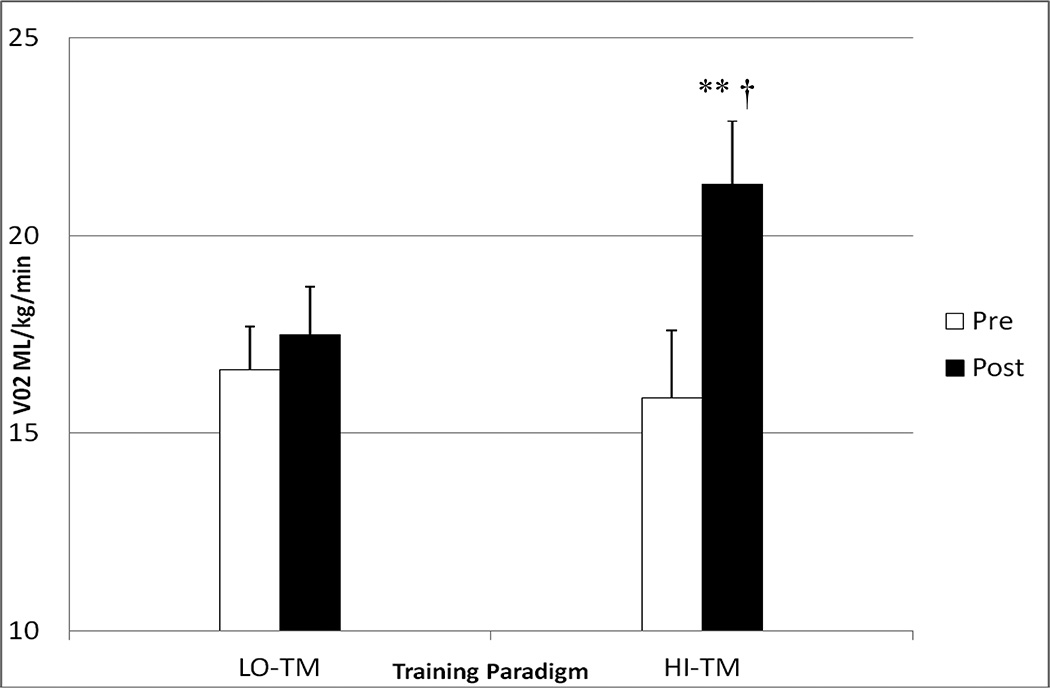
Two-way repeated measures analysis showed that HI-TM participants (N=18) had significantly greater gains in VO2 peak (15.9 ± 1.7 to 21.3 ± 1.6 ml/kg/min, mean ± SE, +34%) than LO-TM participants (N=16) (16.6 ± 1.1 to 17.5 ± 1.2, +5%) (Figure 2). Beyond the 2-way interaction, results showed that only the HI-TM participants had a significant within groups change over 6 months of treadmill training (p<0.001), with no significant within group change observed for LO-TM.
Figure 2.
Bar graph depicting change in VO2 peak (mls/kg/min) with training in HI-TM (n=18) vs. LO-TM (n=16). A significant time × group interaction (p<0.001, †) indicated that change in HI-TM was statistically significantly greater than change in LO-TM. ** denotes significant within group change for HI-TM (p<0.001).
Effects of HTM vs. LO-TM on 6MWT and 30WT (Table 2)
Table 2.
Basic functional measures before and after training in HI-TM and LO-TM
| Outcome/ Training Group | Pre-Training | Post-Training | Within Group P value |
Between Group P Value |
|---|---|---|---|---|
| 6MW HI-TM (ft) (n=18) | 780 ± 105 | 964 ± 131 | <0.001** | 0.22 |
| 6MW LO-TM (ft) (n=15) | 564 ± 73 | 668 ± 76 | 0.06 | |
| 30 ft. SSWT HI-TM (sec) (n=18) | 21.3 ± 3.4 | 20.9 ± 4.3 | 0.76 | 0.13 |
| 30 ft. SSWT LO-TM (sec) (n=16) | 24.0 ± 2.9 | 20.7 ± 2.6 | 0.03* | |
| 30 ft. FCWT HI-TM (sec) (n=16) | 15.4 ± 2.1 | 13.8 ± 2.1 | 0.001** | 0.81 |
| 30 ft. FCWT LO-TM (sec) (n=13) | 17.2 ± 2.2 | 15.9 ± 1.9 | 0.34 | |
| Activity HI-TM (total steps) (n=18) | 5596 ± 979 | 6968 ± 1064 | 0.07 | 0.11 |
| Activity LO-TM (total steps) (n=14) | 5150 ± 679 | 5044 ± 811 | 0.24 |
6MW= 6-minute walk distance, HI-TM= Higher intensity treadmill training, LO-TM =lower intensity treadmill training, SSWT= Self-selected walking time, sec = seconds, FCWT= fastest comfortable walking time, P value= probability value.
The changes in 6MWT recorded for HI-TM (+24%) and LO-TM (+18%) were not statistically different using 2×2 repeated measures ANOVA (p=0.22). However, within group analysis showed that only the HI-TM group achieved significant change (p<0.001) with LO-TM falling slightly short of significance (p=0.06). Similarly, neither of the 30WT measures (self-selected or fastest comfortable) showed time by group interactions over the 6 month intervention period (p= 0.13 and 0.81, respectively). In the case of self-selected walking time (SSWT), only the LO-TM group achieved within group significance (14%, p<0.05), with no change observed for HI-TM (2%, p=0.76), whereas the opposite was true for fastest comfortable walking speed (FCWS) with HI-TM (10%, p<0.01) but not LO-TM (7%, p=0.34) showing the within group effects.
Effects of HTM vs. LO-TM on 48-hr Step activity monitoring (Table 2)
As shown in Table 2, there was a statistically insignificant trend toward between group significance for 48-hr step count changes across the intervention period (p=0.11), but the study was underpowered to detect actual differences between groups. Those in the HI-TM group increased step counts by a mean 22% (p = 0.07, within group), while LO-TM participants demonstrated a statistically insignificant reduction in 48 hour step counts after the intervention period (2%, p = 0.24).
DISCUSSION
We directly compared stroke participants exposed to HI-TM vs. LO-TM in the same study. An important study feature was the approximate matching of total workloads across intensity groups. By having the LO-TM group train for a longer time per session than HI-TM participants, the groups were more comparable on the basis of total work or caloric expenditure. Results demonstrate clear superiority for HI-TM with respect to adaptation in VO2 peak, the primary outcome variable. The magnitude of VO2 peak gains observed with HI-TM undoubtedly carries clinical significance with respect to function, metabolism, and cardiovascular risk classification after stroke. In contrast to VO2, there were no between group differences shown for secondary functional outcome variables (6MWD, 30WT and 48SC), but this appears at least partially due to low statistical power, particularly in the case of 48-hr activity monitoring. We propose that the most important clinical implication associated with achieving much higher gains in VO2 peak in HI-TM vs. LO-TM relates to lower cardiometabolic risk secondary to gains that exceeded 1MET (Metabolic Equivalent, 3.5 mL/kg/min)29. Further, low cardiorespiratory fitness is now clearly established as a strong prospective predictor of all-cause mortality in both men and woman30. Thus, maintaining peak fitness at higher levels may improve health to the point of extending the life span according to seminal studies by Blair et. al.30, 31.
Clinical impacts of greater VO2 peak change in HI-TM may also stem from extending oxygen consuming capacity beyond the range of oxygen consumption required for ADLs. This would enable a person to sustain more challenging activities for a much longer period of time (an aspect of function not measured in the current study), while operating at a lower percentage of VO2 peak for any given level of activity post-training. Stroke survivors typically have VO2 peak levels somewhere in the middle of the oxygen consumption range required for ADLs, with serious implications for durable, community-based function1. These extraordinarily low levels of VO2 peak after stroke may prohibit higher level ADLs and limit sustainability of lower level ADLs32. A VO2 peak of 17.5 mls/kg/ min has been put forth as the upper limit of the ADL range3, 33, and our participants randomized to HI-TM far exceeded that number by the end of the 6-month period. Specifically, a mean VO2 peak level of 21.3 mL/kg/min following HI-TM training not only elevates our stroke participants to 85% of the lower end of the normal age-adjusted range (25 mL/kg/min), but also implies a greater capacity for sustainably conducting normal activities when necessary3. More work with hybrid interventions that combine HI-TM with other training models targeting both strength and quality of movement should be undertaken to determine whether these results indicate a true upper-limit or whether even greater adaptations in VO2 peak are possible in this population. Also, experimentation with interval training has shown some initial promise in this population22.
Deteriorating metabolic health is also a probable consequence of profoundly reduced peak fitness levels with disabling stroke6, 7. Our prior work shows an extremely high prevalence of abnormal glucose in chronic stroke7, and this virtual epidemic of diabetes and pre-diabetes appears to be related to VO2 peak changes given our previously published relationship between metabolic improvements and peak fitness gains following moderate-intensity treadmill exercise program4. Over the last decade, there have been major advances in our understanding of the effectiveness of exercise and lifestyle interventions to improve metabolic health and prevent progression to diabetes in high risk non-stroke populations34, with recent data showing a dose-intensity relationship between structured aerobic exercise and cardiometabolic health benefits in non-disabled populations35. Therefore, future research is warranted to quantify the broader metabolic impact of HI-TM after stroke.
Our relative gains for VO2 peak in HI-TM across training were slightly better than those produced by our German collaborators with a similar protocol (34% vs. 29%)21, and nearly double those observed in our own laboratory subsequent to the application of a more moderate treadmill training protocol (17%)2. Prior to the German study, the average relative gain in this parameter was 15% across 13 studies2, 4, 9–19, which although potentially meaningful taking into account the low starting point, does not compare to the unprecedented gains demonstrated in this study (+34%). The current study helps to clarify that it is not simply exposure to treadmill training in general, but rather fervent attention to intensity progression details that coveys the greatest weight when attempting to elevate the peak fitness status of those who are profoundly deconditioned secondary to neurologic disability. Further, the current results bolster support for the idea that stroke survivors with chronic disability retain the capacity to both tolerate an aggressive exercise stimulus and adapt at a level that may match or exceed other non-disabled populations in relative terms. Importantly, A 1 MET increase in peak fitness prospectively predicts a 28–51% reduction in fatal cardiac events in non-stroke men29. Thus, Our HI-TM data showing a >5 mL/kg/min absolute increase in VO2 peak (1.5 METS), has clinical ramifications and likely confers protection against the number one killer of stroke survivors in the chronic phase of recovery36.
The marked difference in VO2 peak gains between groups did not translate into statistical between group differences in gains for the other measured functional outcomes. However, there are some potential trends in these data that are worth noting. First, only the HI-TM group achieved within group significance for 6MWD with LO-TM falling slightly short of significance on this particular outcome. This may indicate that task-repetition alone is not optimally sufficient for promoting gains in this particular ambulatory measure. Interestingly, the gains for this outcome in HI-TM were no better than those previously resulting from our moderate intensity training protocols2. Testing walking capacity in a format that extends beyond a 6-minute test may be justified to yield a clearer picture of how the dramatic VO2 peak gains with HI-TM translates into sustainable community-based functional capacity. The 48-hour activity step activity testing did show a trend towards between group significance (P=0.11) with HI-TM trending towards higher step changes than LO-TM, but there is still a place for laboratory-based measures of sustained activity that aren’t partially confounded by either lifestyle or the attempts by participants to compensate for formalized exercise sessions by doing less in their outside daily lives. Finally, it was interesting and perhaps somewhat predictable that when analyzing within group changes, only HI-TM demonstrated significant improvements in fastest comfortable 30-ft walking times, while only LO-TM showed within group gains in self-selected short distance walking times. Larger studies should be undertaken with these and other functional outcomes to better understand how intensity of the treadmill training paradigm impacts the broader range of functional capacity in this unique population.
In summary, this small, randomized study demonstrated what added emphasis on treadmill training intensity progression can do in terms of elevating VO2 peak to an extent that far surpasses most of the studies conducted to date. Having a low-intensity comparator group ruled out the possibility that any part of these changes are governed by a learning effect, given the very small, statistically insignificant VO2 peak change recorded for the total-work matched LO-TM group. The study limitations included small sample size and broad heterogeneity in participant disability levels, mandating that results be interpreted cautiously. In addition, our high number of drop-out (35%) qualifies as a major limitation in terms of generalizability. Finally, resource constraints preventing assessor blinding in all cases was a potential confounder limiting interpretation of results.
Future studies should explore the full range of functional and cardiometabolic gains achievable with HI-TM, helping to better discern the full clinical implications for VO2 peak gains of this magnitude after stroke. Additionally, manipulating the HI-TM intervention with interval and hybrid approaches to training may help to disseminate whether even further enhancements in peak fitness adaptation are possible in this disabled patient population. Among the considerations going forward is how to distinguish neurological functional effects of more intensive training from non-specific improvements in cardiovascular fitness. Although this can’t be derived using a low intensity treadmill comparator group, as in the current study, it could be tested using a non-walking training program (e.g. stationary bicycles).
Acknowledgments
Sources of Funding:
Dr. Ivey was supported by 2 VA Merit Awards and an NIA- K01 AG19242. Dr. Stookey was supported by a VA Career Development Award (CDA-1), and Dr. Ryan was supported by a VA Career Scientist Research Award. The authors also wish to acknowledge support from the VA RR&D Maryland Exercise and Robotics Center of Excellence (MERCE) Department of Veterans Affairs and Veterans Affairs Medical Center, Baltimore Geriatric Research, Education and Clinical Center (GRECC), and the National Institute on Aging (NIA) Claude D. Pepper Older Americans Independence Center (P30-AG028747).
We thank all of our loyal stroke survivors for their commitment to regular testing and exercise training visits. We also acknowledge the dedication of our outstanding research staff and their commitment to participant safety, treatment fidelity and general satisfaction of participants.
Footnotes
Disclosure Statement:
There are no conflicts of interest to report.
REFERENCES
- 1.Ivey FM, Macko RF, Ryan AS, Hafer-Macko CE. Cardiovascular health and fitness after stroke. Topics in stroke rehabilitation. 2005;12:1–16. doi: 10.1310/GEEU-YRUY-VJ72-LEAR. [DOI] [PubMed] [Google Scholar]
- 2.Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: A randomized, controlled trial. Stroke; a journal of cerebral circulation. 2005;36:2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 3.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, et al. 2011 compendium of physical activities: A second update of codes and met values. Medicine and science in sports and exercise. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 4.Ivey FM, Ryan AS, Hafer-Macko CE, Goldberg AP, Macko RF. Treadmill aerobic training improves glucose tolerance and indices of insulin sensitivity in disabled stroke survivors: A preliminary report. Stroke; a journal of cerebral circulation. 2007;38:2752–2758. doi: 10.1161/STROKEAHA.107.490391. [DOI] [PubMed] [Google Scholar]
- 5.Ivey FM, Hafer-Macko CE, Ryan AS, Macko RF. Impaired leg vasodilatory function after stroke: Adaptations with treadmill exercise training. Stroke; a journal of cerebral circulation. 2010;41:2913–2917. doi: 10.1161/STROKEAHA.110.599977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kernan WN, Inzucchi SE, Viscoli CM, Brass LM, Bravata DM, Shulman GI, et al. Impaired insulin sensitivity among nondiabetic patients with a recent tia or ischemic stroke. Neurology. 2003;60:1447–1451. doi: 10.1212/01.wnl.0000063318.66140.a3. [DOI] [PubMed] [Google Scholar]
- 7.Ivey FM, Ryan AS, Hafer-Macko CE, Garrity BM, Sorkin JD, Goldberg AP, et al. High prevalence of abnormal glucose metabolism and poor sensitivity of fasting plasma glucose in the chronic phase of stroke. Cerebrovascular diseases. 2006;22:368–371. doi: 10.1159/000094853. [DOI] [PubMed] [Google Scholar]
- 8.Gordon NF, Gulanick M, Costa F, Fletcher G, Franklin BA, Roth EJ, et al. Physical activity and exercise recommendations for stroke survivors: An american heart association scientific statement from the council on clinical cardiology, subcommittee on exercise, cardiac rehabilitation, and prevention; the council on cardiovascular nursing; the council on nutrition, physical activity, and metabolism; and the stroke council. Circulation. 2004;109:2031–2041. doi: 10.1161/01.CIR.0000126280.65777.A4. [DOI] [PubMed] [Google Scholar]
- 9.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2014;45:2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 10.Potempa K, Lopez M, Braun LT, Szidon JP, Fogg L, Tincknell T. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke; a journal of cerebral circulation. 1995;26:101–105. doi: 10.1161/01.str.26.1.101. [DOI] [PubMed] [Google Scholar]
- 11.Rimmer JH, Riley B, Creviston T, Nicola T. Exercise training in a predominantly african-american group of stroke survivors. Medicine and science in sports and exercise. 2000;32:1990–1996. doi: 10.1097/00005768-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Duncan P, Studenski S, Richards L, Gollub S, Lai SM, Reker D, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke; a journal of cerebral circulation. 2003;34:2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 13.Chu KS, Eng JJ, Dawson AS, Harris JE, Ozkaplan A, Gylfadottir S. Water-based exercise for cardiovascular fitness in people with chronic stroke: A randomized controlled trial. Archives of physical medicine and rehabilitation. 2004;85:870–874. doi: 10.1016/j.apmr.2003.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang MY, Eng JJ, Dawson AS, McKay HA, Harris JE. A community-based fitness and mobility exercise program for older adults with chronic stroke: A randomized, controlled trial. Journal of the American Geriatrics Society. 2005;53:1667–1674. doi: 10.1111/j.1532-5415.2005.53521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: A randomized controlled trial. Stroke; a journal of cerebral circulation. 2008;39:3341–3350. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang A, Sibley KM, Thomas SG, Bayley MT, Richardson D, McIlroy WE, et al. Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters, and functional capacity in subacute stroke. Neurorehabilitation and neural repair. 2009;23:398–406. doi: 10.1177/1545968308326426. [DOI] [PubMed] [Google Scholar]
- 17.Rimmer JH, Rauworth AE, Wang EC, Nicola TL, Hill B. A preliminary study to examine the effects of aerobic and therapeutic (nonaerobic) exercise on cardiorespiratory fitness and coronary risk reduction in stroke survivors. Archives of physical medicine and rehabilitation. 2009;90:407–412. doi: 10.1016/j.apmr.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Letombe A, Cornille C, Delahaye H, Khaled A, Morice O, Tomaszewski A, et al. Early post-stroke physical conditioning in hemiplegic patients: A preliminary study. Annals of physical and rehabilitation medicine. 2010;53:632–642. doi: 10.1016/j.rehab.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Ivey FM, Ryan AS, Hafer-Macko CE, Macko RF. Improved cerebral vasomotor reactivity after exercise training in hemiparetic stroke survivors. Stroke; a journal of cerebral circulation. 2011;42:1994–2000. doi: 10.1161/STROKEAHA.110.607879. [DOI] [PubMed] [Google Scholar]
- 20.Gjellesvik TI, Brurok B, Hoff J, Torhaug T, Helgerud J. Effect of high aerobic intensity interval treadmill walking in people with chronic stroke: A pilot study with one year follow-up. Topics in stroke rehabilitation. 2012;19:353–360. doi: 10.1310/tsr1904-353. [DOI] [PubMed] [Google Scholar]
- 21.Globas C, Becker C, Cerny J, Lam JM, Lindemann U, Forrester LW, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: A randomized control trial. Neurorehabilitation and neural repair. 2012;26:85–95. doi: 10.1177/1545968311418675. [DOI] [PubMed] [Google Scholar]
- 22.Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. High-intensity interval training in stroke rehabilitation. Topics in stroke rehabilitation. 2013;20:317–330. doi: 10.1310/tsr2004-317. [DOI] [PubMed] [Google Scholar]
- 23.Outermans JC, van Peppen RP, Wittink H, Takken T, Kwakkel G. Effects of a high-intensity task-oriented training on gait performance early after stroke: A pilot study. Clinical rehabilitation. 2010;24:979–987. doi: 10.1177/0269215509360647. [DOI] [PubMed] [Google Scholar]
- 24.Lau KW, Mak MK. Speed-dependent treadmill training is effective to improve gait and balance performance in patients with sub-acute stroke. Journal of rehabilitation medicine : official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2011;43:709–713. doi: 10.2340/16501977-0838. [DOI] [PubMed] [Google Scholar]
- 25.Macko RF, Katzel LI, Yataco A, Tretter LD, DeSouza CA, Dengel DR, et al. Low-velocity graded treadmill stress testing in hemiparetic stroke patients. Stroke; a journal of cerebral circulation. 1997;28:988–992. doi: 10.1161/01.str.28.5.988. [DOI] [PubMed] [Google Scholar]
- 26.Enright PL. The six-minute walk test. Respiratory care. 2003;48:783–785. [PubMed] [Google Scholar]
- 27.Shaughnessy M, Michael KM, Sorkin JD, Macko RF. Steps after stroke: Capturing ambulatory recovery. Stroke; a journal of cerebral circulation. 2005;36:1305–1307. doi: 10.1161/01.STR.0000166202.00669.d2. [DOI] [PubMed] [Google Scholar]
- 28.Haeuber E, Shaughnessy M, Forrester LW, Coleman KL, Macko RF. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Archives of physical medicine and rehabilitation. 2004;85:1997–2001. doi: 10.1016/j.apmr.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Laukkanen JA, Kurl S, Salonen R, Rauramaa R, Salonen JT. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: A prospective population-based cohort study. Eur Heart J. 2004;25:1428–1437. doi: 10.1016/j.ehj.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. Jama. 1996;276:205–210. [PubMed] [Google Scholar]
- 31.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. Jama. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 32.Ivey FM, Hafer-Macko CE, Macko RF. Exercise rehabilitation after stroke. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2006;3:439–450. doi: 10.1016/j.nurx.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and met intensities. Medicine and science in sports and exercise. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the da qing diabetes prevention study: A 23-year follow-up study. The lancet. Diabetes & endocrinology. 2014 doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 35.Balducci S, Sacchetti M, Haxhi J, Orlando G, D'Errico V, Fallucca S, et al. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes/metabolism research and reviews. 2014;30(Suppl 1):13–23. doi: 10.1002/dmrr.2514. [DOI] [PubMed] [Google Scholar]
- 36.Roth EJ. Heart disease in patients with stroke: Incidence, impact, and implications for rehabilitation. Part 1: Classification and prevalence. Archives of physical medicine and rehabilitation. 1993;74:752–760. doi: 10.1016/0003-9993(93)90038-c. [DOI] [PubMed] [Google Scholar]