Abstract
Ovarian cancer is commonly diagnosed at an advanced stage, with disease involving the upper abdomen. The finding of enlarged cardiophrenic lymph nodes (CPLNs) on pre-operative imaging often indicates the presence of malignant spread to the mediastinum. Surgical resection of CPLN through a transdiaphragmatic approach can help to achieve cytoreduction to no gross residual. A retrospective chart review was conducted on all patients who underwent transdiaphragmatic cardiophrenic lymph node resection from 8/1/11 through 2/1/15. All relevant pre-, intra-, and post-operative characteristics and findings were recorded. A brief description of the surgical technique is included for reference. Eleven patients were identified who had undergone transdiaphragmatic resection of cardiophrenic lymph nodes. Malignancy was identified in 18/21 (86%) of total lymph nodes submitted. The median number of post-operative days was 7. The overall post-operative morbidity associated with CPLN resection was low, with the most common finding being a small pleural effusion present on chest x-ray between POD# 3–5 (55%). Transdiaphragmatic CPLN resection is a feasible procedure with relatively minor short-term post-operative morbidities that can be used to achieve cytoreduction to no gross residual disease.
Keywords: Ovarian cancer, Cytoreductive surgery, Diaphragm resection, Cardiophrenic lymph nodes, Post-operative morbidity
Highlights
-
•
Pre-operative chest CT can help to identify enlarged cardiophrenic lymph nodes.
-
•
The technique of transdiaphragmatic cardiophrenic lymph node resection is described.
-
•
Resection of CPLNs aids in achieving cytoreduction to no gross residual disease.
-
•
The short-term morbidities associated with the procedure are relatively minor.
1. Introduction
Cancer of the ovary is a particularly insidious disease that is commonly diagnosed at an advanced stage. In 2015, the American Cancer Society estimated that 21,290 new cases were diagnosed and 14,180 women died of this disease (American Cancer Society, Inc. Cancer Facts, and Figures, 2015). One of the main reasons for such a high associated mortality is that approximately 65% of patients are initially diagnosed at International Federation of Gynecology and Obstetrics (FIGO) Stage III or IV (Pecorelli et al., 1998). Primary cytoreductive surgery to no gross residual (NGR) disease has consistently been found to be one of the most important prognostic indicators in patients with advanced stage ovarian cancer and remains the only factor under the control of the operating surgeon. Furthermore, statistically significant differences in recurrence and survival have been observed when residual tumor volume was reported as either microscopic (NGR), 0.1 to 5.0 cm, or > 5.0 cm (Winter et al., 2008).
Various surgical techniques have previously been described to achieve complete resection of diaphragmatic and CPLN related disease. Bashir et al. described a procedure for full thickness diaphragm resection which in addition to removing bulky diaphragm disease, allowed for access to the pleural and pericardial space (Bashir et al., 2010). In addition, our group recently published a surgical video displaying the technique of diaphragm resection and transdiaphragmatic resection of CPLNs (LaFargue and Bristow, 2015). Video-assisted thoracic surgery (VATS) has also been shown to be a successful modality for achieving resection of CPLNs (Lim et al., 2009).
The current case series was compiled to further define the pre-, intra-, and post-operative characteristics of patients undergoing transdiaphragmatic resection of CPLNs in advanced gynecologic cancer and delineate the associated short-term morbidities and time to adjuvant chemotherapy.
2. Methods
The study design was a retrospective review performed in the division of Gynecologic Oncology at the University of California Irvine Medical Center. All patients who underwent a transdiaphragmatic cardiophrenic lymph node resection from 8/1/11 through 2/1/15 as part of a primary or secondary cytoreductive surgery were selected for detailed review. Each identified subject had transdiaphragmatic CPLN resection performed in an effort to achieve cytoreduction to no gross residual disease. There were only two different physicians who were listed as the primary surgeon for all patients included in this report. All of the CPLN resection procedures were performed by the same gynecologic oncologist with the assistance of either the primary surgeon or a gynecologic oncology fellow. The necessity of prophylactic chest tube placement prior to closing the diaphragmatic defect was determined by the operating surgeon according to intra-operative findings and the overall patient status.
Individual subject data encompassing pre-, intra-, and post-operative characteristics were identified through electronic medical record review of inpatient and ambulatory records. Pre-operative data reviewed included: patient's age, initial CA-125, presence and size of CPLN on initial imaging, primary vs. recurrent cancer status, presumed stage, histology (if known), and whether neoadjuvant chemotherapy was given. Intra-operative characteristics were identified from operative notes and included: total operative time, estimated blood loss, amount of residual disease at conclusion, placement of prophylactic chest tube, and all ancillary procedures performed, including whether hyperthermic intraperitoneal chemotherapy (HIPEC) was performed at the conclusion of the operation. When an intra-operative frozen section was sent on the CPLN specimen, the initial diagnosis was recorded and included for analysis of congruence with final pathology. Pathology records were interrogated to determine the size and number of CPLNs obtained from each patient. Post-operative characteristics were identified through radiology and pathology reports as well as discharge summaries and included: final FIGO stage and histology, presence and size of pleural effusions or pneumothorax, all major complications, post-operative day of discharge, and time to adjuvant chemotherapy.
2.1. Surgical technique
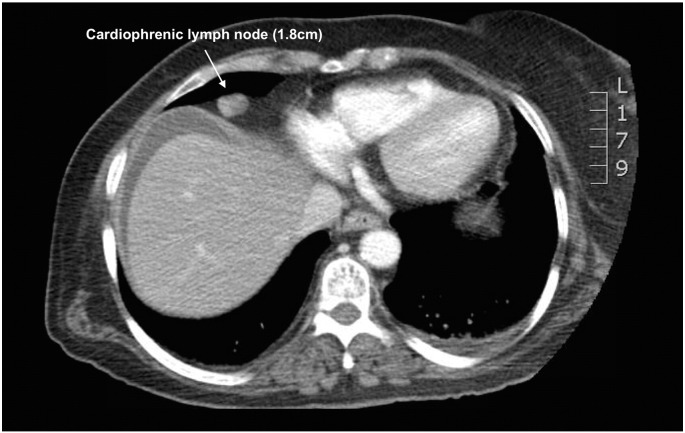
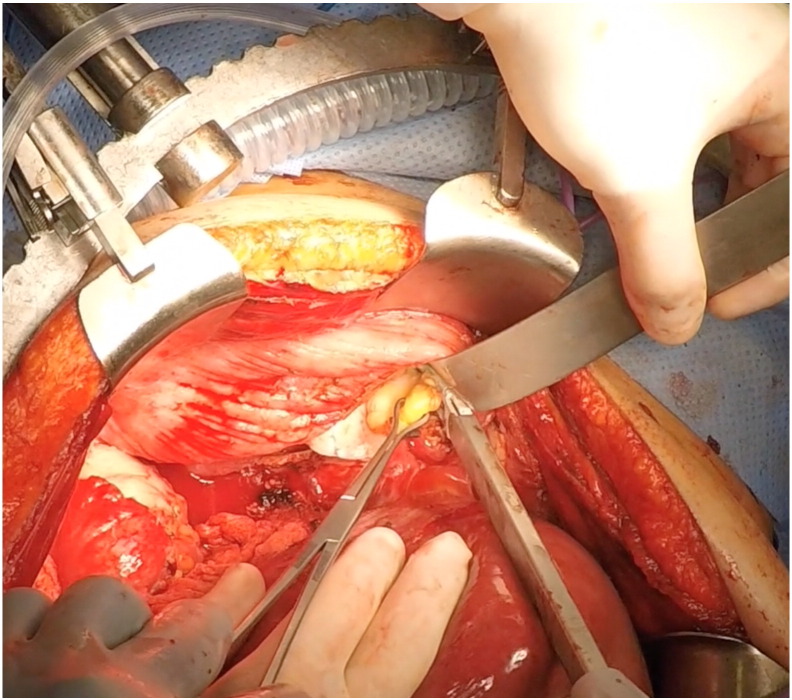
In most cases, enlarged cardiophrenic lymph nodes were first identified on pre-operative CT imaging (Fig. 1). For the patients in which pre-operative imaging did not show lymphadenopathy, the decision was made to proceed with CPLN resection based on an intraoperative assessment consisting of the degree of diaphragm involvement and palpation of the pericardial space. The surgical technique used to perform full-thickness diaphragm resection and CPLN resection has been previously described and illustrated (Bashir et al., 2010, LaFargue and Bristow, 2015). In brief, full exposure of the diaphragm peritoneum is achieved after mobilization the liver. The diaphragm is then incised using electrocautery, the pleural space is explored, and the cardiophrenic nodes are resected using a vessel sealing-cutting device (Fig. 2). A 15F, round Jackson Pratt (JP) drain is then used as an alternative to the standard chest tube and placed through the diaphragmatic defect into the pleural cavity under direct vision and brought out through a separate stab incision in the mid-axillary line above the seventh or eighth rib. The diaphragmatic defect is then closed using a non-absorbable suture (2–0 polypropylene in figure-of-eight stitches). The Jackson-Pratt drain is then connected to suction to evacuate the pneumothorax. If no chest tube is placed, prior to tying the last stitch, a fenestrated 16F Robinson catheter attached to wall suction is placed into the pleural cavity to remove the pneumothorax and withdrawn as the last knot is tied. The “bubble test” is then performed to evaluate for any air leak by filling the right upper quadrant with saline solution and delivering several large mechanical inspiratory breaths while inspecting the repair for extravasation of air bubbles. If present, air leaks are oversewn, the pneumothorax evacuated, and the test repeated.
Fig. 1.
Pre-operative CT scan demonstrating enlarged cardiophrenic lymph node in mediastinum.
Fig. 2.
Cardiophrenic fat pad is grasped with Babcock clamp and excised using vessel sealing-cutting device via diaphragmatic defect.
3. Results
3.1. Patient demographics
A total of 11 patients underwent transdiaphragmatic CPLN resection (10 with unilateral CPLN resection, and 1 patient with bilateral resection). Specific patient characteristics are displayed in Table 1. The mean age was 58 years (range 42–71 years), with FIGO stage IVB serous ovarian cancer being the most common stage, histology, and primary tumor site, respectively. One patient included in the study had clear cell uterine cancer. Enlarged cardiophrenic lymph nodes were identified in 7 patients on pre-operative imaging (64%), while the remainder was identified based on intra-operative suspicion.
Table 1.
Pre-operative characteristics of patients undergoing CPLN resection.
| Characteristics | N (11 total patients) | % | Mean | Range |
|---|---|---|---|---|
| Age | 58 | 42–71 | ||
| Pre-operative CA-125 | ||||
| < 200 | 2 | 18 | ||
| > 200 | 9 | 82 | ||
| Surgery | ||||
| Primary | 10 | 91 | ||
| Recurrent | 1 | 9 | ||
| Neoadjuvant chemotherapy | 2 | 20 | ||
| CPLN on pre-op CT | 7 | 64 | ||
| Cancer site | ||||
| Endometrial | 1 | 9 | ||
| Ovary | 8 | 73 | ||
| Peritoneal | 1 | 9 | ||
| Fallopian tube | 1 | 9 | ||
| Histology | ||||
| Serous | 8 | 73 | ||
| Mucinous/serous | 1 | 9 | ||
| Endometrioid | 1 | 9 | ||
| Clear cell | 1 | 9 | ||
| Stage | ||||
| IIIC | 1 | 10 | ||
| IVB | 9 | 90 |
3.2. Surgical outcomes
Table 2 displays the intra-operative and post-operative findings. The average operative time was 385 min (range 277–568 min), and the average estimated blood loss was 564 cm3 (range 200–900 cm3). Ten patients underwent optimal cytoreduction defined as < 1 cm (91%), while 7 of these patients had no gross residual disease (64%). A prophylactic Jackson-Pratt drain was placed in the pleural cavity, as an alternative chest tube, and brought out through a separate stab incision in the chest in 8 patients (73%).
Table 2.
Intra- and post-operative findings in patients undergoing transdiaphragmatic CPLN resection.
| Characteristics | N (11 total patients) | % | Mean | Range |
|---|---|---|---|---|
| Operative time | 385 min | 277–568 | ||
| Estimated blood loss | 564 cm3 | 200–900 | ||
| Residual disease | ||||
| No gross residual | 7 | 64 | ||
| < 1 cm | 3 | 27 | ||
| > 1 cm | 1 | 9 | ||
| Prophylactic chest tube placement | 8 | 73 | ||
| Ancillary procedures | ||||
| Modified radical hysterectomy | 8 | 73 | ||
| BSO | 9 | 82 | ||
| Omentectomy | 11 | 100 | ||
| Pelvic LND | 8 | 73 | ||
| Para-aortic LND | 8 | 73 | ||
| Recto-sigmoid colectomy | 8 | 73 | ||
| Small bowel resection | 1 | 9 | ||
| Ileocecal resection | 2 | 18 | ||
| Hepatic resection | 2 | 18 | ||
| Splenectomy | 4 | 36 | ||
| Appendectomy | 6 | 55 | ||
| Colectomy | 3 | 27 | ||
| Partial gastrectomy | 1 | 9 | ||
| Distal pancreatectomy | 2 | 18 | ||
| Diaphragm peritonectomy | 11 | 100 | ||
| Diaphragm resection | 10 | 91 | ||
| Pan-pelvic peritonectomy | 10 | 91 | ||
| Argon beam ablation | 5 | 45 | ||
| HIPEC | 6 | 55 | ||
| Median | Range | |||
| Post-op day of discharge | 7 | 4–37 | ||
| Days to adjuvant chemotherapy | Mean | |||
| HIPEC performed | 39 | 27–49 | ||
| HIPEC not performed | 38 | 28–53 | ||
| Post-operative morbidities | ||||
| Pleural effusions on POD 0–2 | ||||
| Small | 4 | 36 | ||
| Moderate | 3 | 27 | ||
| Pleural effusions on POD 3–5 | ||||
| Small | 6 | 55 | ||
| Moderate | 2 | 18 | ||
| Pneumothorax on POD 0–2 (small/apical) | 3 | 27 | ||
| Pneumothorax on POD 3–5 (small/apical) | 3 | 27 | ||
| Respiratory failure/reintubation | 1 | 9 | ||
| Tracheostomy | 1 | 9 | ||
| Post-operative pneumonia | 1 | 9 | ||
| Pulmonary embolism | 1 | 9 |
Intra-operative frozen section of the resected CPLNs was sent in 6 patients, with malignancy being identified in 5 of them. On final pathologic evaluation, the average number and size of CPLNs obtained per patient was 1.9 (range 1–4) and 1.6 cm (range 0.2–2.5 cm), respectively, with final diagnosis of malignancy confirmed in 10 of the 11 total patients. The total number of CPLNs resected from all patients was 21 with malignancy being confirmed in 18 (86%).
The median number of post-operative days was 7 (range 4–37). The overall post-operative morbidity associated with CPLN resection was low with the most common finding being a small pleural effusion present on chest x-ray between POD# 3 to 5 (55%). Other morbidities noted on POD# 0–5 were small and moderate pleural effusion as well as small apical pneumothorax. Major complications were rare (occurring in 1 patient only) and consisted of respiratory failure with need for re-intubation and eventual tracheostomy, which was not thought to be related to the CPLN resection. No patients required post-operative thoracentesis for effusion or pneumothorax. The average time to adjuvant chemotherapy was similar in patients who received HIPEC versus those who did not (39 and 38 days, respectively).
4. Discussion
Stage III or IV ovarian cancer by definition involves extrapelvic spread of tumor, however it often presents with tumor involvement of the diaphragm peritoneum and muscle, and has been reported to involve cardiophrenic lymph nodes. In a survey of gynecologic oncologists in 2001, 76.3% of respondents reported that bulky upper abdominal disease precluded complete cytoreductive surgery. In addition, only 24% of respondents reported performing diaphragm resection, and 30% stated they were not experienced with the procedure (Eisenkop and Spirtos, 2001).
The morbidity and mortality associated with such complex and invasive surgical procedures has been previously studied. The most common post-operative complications reported after undergoing diaphragmatic surgery (either peritonectomy or muscle resection) were pleural effusion (5–57%), pneumothorax (0–29%), and pneumonia (0–7%). Serious complications occurred rarely and included pleural effusion requiring thoracostomy or thoracentesis, need for re-operation, and death. Many of these studies however further stratified each complication based on grade/severity and post-operative day of occurrence, noting that the majority of complications occurred on POD# 1–2, were of low severity, and did not delay hospital discharge (Bashir et al., 2010, Chi et al., 2010, Tsolakidis et al., 2010, Sandadi et al., 2014).
Despite such a proactive approach to the surgical cytroreduction of advanced gynecologic cancer, the current small case series demonstrates that transdiaphragmatic CPLN resection is feasible and is accompanied by acceptable postoperative morbidity. When compared to the above reports of patients who underwent aggressive upper abdominal surgery (UAS) for ovarian cancer, the post-operative morbidities we identified did not differ significantly nor did the procedure result in any significant prolongation of postoperative hospital stay. Panici et al. reported that in a study of 120 patients who underwent UAS for ovarian cancer, the median hospital stay for patients with and without complication was 7 vs. 13 days, respectively (Benedetti Panici et al., 2015). In addition, Cliby et al. reported that diaphragm resection as part of cytoreductive surgery for ovarian cancer carries comparable risks to other radical debulking procedures (Cliby et al., 2004). The decision to proceed with extensive cytoreductive procedures must be made carefully and with the intent to achieve cytroreduction to no gross residual disease. Furthermore, multiple studies have demonstrated that patients who had optimal (< 1 cm) or microscopic residual disease had increased survival compared to those with suboptimal resection (Winter et al., 2008, Aletti et al., 2006, Eisenhauer et al., 2006). Given that the size of resected CPLNs in our study ranged from 0.2 to 2.5 cm, removal of mediastinal metastasis may make a significant difference in the ability to achieve optimal cytoreduction to < 1.0 cm.
The short-term morbidity of the patients included in our study was similar to previous reports of patients who underwent diaphragm resection or other aggressive upper abdominal surgery. As the majority of the CPLN specimens were positive for malignancy, the addition of this procedure contributed to achieving cytoreduction to NGR status. This suggests that careful attention should be paid to the lower mediastinum on pre-operative imaging of advanced gynecologic cancer patients — particularly when the diaphragm appears to be involved.
Currently, there is mixed data on the optimal time for initiation of adjuvant chemotherapy after primary cytoreductive surgery. One of the continually recognized prognostic factors for advanced ovarian cancer is residual disease at the conclusion of primary surgery. Aletti et al. reported that time to chemotherapy (TTC) was not a predictor of overall survival, and that concerns regarding the TTC interval should not be used to justify a more conservative approach to primary debulking surgery (Aletti et al., 2007). Although in our small case series TTC data was only available for 7 patients, we found that the addition of CPLN resection did not largely delay initiation of adjuvant chemotherapy, and in fact contributed to achieving R0 status as the majority of the CPLN specimens were positive for malignancy. Furthermore, even in the subset of our patients that had HIPEC performed during surgery, the initiation of adjuvant chemotherapy was only increased by one day. Again however, when considering the small number of patients included in our study, it is difficult to draw any statistical significance from these specific findings.
Some important limitations in our study must be noted before applying the described technique to routine clinical practice. First, we do not yet have recurrence or survival data on the patients included. Although the addition of the described procedure did not appear to pose any greater risk than other similarly aggressive surgical procedures, it is unclear as to whether it prolongs progression-free or overall survival. Second, the procedure described was performed by a single gynecologic oncologist at our institution. Although the majority of gynecologic oncologists receive adequate training during fellowship in the surgical management of ovarian cancer, few may feel comfortable performing such extensive and radical upper abdominal procedures. Third, despite our attempt to achieve cytoreduction to NGR in all patients, 4/11 patients had residual disease (three with < 1.0 cm, and one with > 1.0 cm). Although the resection of CPLNs certainly contributed to achieving optimal cytoreduction to < 1.0 cm in these three patients, an alternative approach would be to perform this procedure last after ensuring NGR in the remainder of the abdomen. Considering the impact of tumor biology and initial disease burden on PFS/OS, it may be prudent to ensure ability to achieve NGR prior to performing this specific procedure. Lastly, all patients had surgery performed at a major university-based hospital with physicians and staff that were highly experienced in managing potentially serious post-operative complications. Smaller hospitals with less experienced consulting physicians and ancillary staff may be reluctant to perform such aggressive surgical procedures in fear of potentially catastrophic events.
In conclusion, our small case series demonstrates the feasibility and short-term morbidities associated with transdiaphragmatic CPLN resection and describes an additional procedure used to achieve cytoreduction to NGR in patients with advanced gynecologic malignancies.
Conflict of interest statement
None of the authors have any potential conflicts of interest to declare.
References
- Aletti G.D., Dowdy S.C., Gostout B.S. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet. Gynecol. 2006;107(1):77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- Aletti G.D., Long H.J., Podratz K.C., Cliby W.A. Is time to chemotherapy a determinant of prognosis in advanced-stage ovarian cancer? Gynecol. Oncol. 2007;104(1):212–216. doi: 10.1016/j.ygyno.2006.07.045. [DOI] [PubMed] [Google Scholar]
- American Cancer Society, Inc. Cancer Facts & Figures 2015. Available at: www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf (Accessed on: May 25, 2015).
- Bashir S., Gerardi M.A., Giuntoli R.L., 2nd, Montes T.P., Bristow R.E. Surgical technique of diaphragm full-thickness resection and trans-diaphragmatic decompression of pneumothorax during cytoreductive surgery for ovarian cancer. Gynecol. Oncol. 2010;119(2):255–258. doi: 10.1016/j.ygyno.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Benedetti Panici P., Di Donato V., Fischetti M. Predictors of postoperative morbidity after cytoreduction for advanced ovarian cancer: analysis and management of complications in upper abdominal surgery. Gynecol. Oncol. 2015;137(3):406–411. doi: 10.1016/j.ygyno.2015.03.043. [DOI] [PubMed] [Google Scholar]
- Chi D.S., Zivanovic O., Levinson K.L. The incidence of major complications after the performance of extensive upper abdominal surgical procedures during primary cytoreduction of advanced ovarian, tubal, and peritoneal carcinomas. Gynecol. Oncol. 2010;119(1):38–42. doi: 10.1016/j.ygyno.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Cliby W., Dowdy S., Feitoza S.S., Gostout B.S., Podratz K.C. Diaphragm resection for ovarian cancer: technique and short-term complications. Gynecol. Oncol. 2004;94(3):655–660. doi: 10.1016/j.ygyno.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Eisenhauer E.L., Abu-Rustum N.R., Sonoda Y. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC–IV epithelial ovarian cancer. Gynecol. Oncol. 2006;103(3):1083–1090. doi: 10.1016/j.ygyno.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Eisenkop S.M., Spirtos N.M. What are the current surgical objectives, strategies, and technical capabilities of gynecologic oncologists treating advanced epithelial ovarian cancer? Gynecol. Oncol. 2001;82(3):489–497. doi: 10.1006/gyno.2001.6312. [DOI] [PubMed] [Google Scholar]
- LaFargue C.J., Bristow R.E. Transdiaphragmatic cardiophrenic lymph node resection for stage IV ovarian cancer. Gynecol. Oncol. 2015;138(3):762–763. doi: 10.1016/j.ygyno.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Lim M.C., Lee H.S., Jung D.C., Choi J.Y., Seo S.S., Park S.Y. Pathological diagnosis and cytoreduction of cardiophrenic lymph node and pleural metastasis in ovarian cancer patients using video-assisted thoracic surgery. Ann. Surg. Oncol. 2009;16(7):1990–1996. doi: 10.1245/s10434-009-0486-5. [DOI] [PubMed] [Google Scholar]
- Pecorelli S., Creasman W., Petterson F., Benedit J., Shepard J. FIGO annual reports on the results of treatment in gynaecological cancer. J. Epidemiol. Biostat. 1998;3:75–102. [Google Scholar]
- Sandadi S., Long K., Andikyan V. Postoperative outcomes among patients undergoing thoracostomy tube placement at time of diaphragm peritonectomy or resection during primary cytoreductive surgery for ovarian cancer. Gynecol. Oncol. 2014;132(2):299–302. doi: 10.1016/j.ygyno.2013.11.026. [DOI] [PubMed] [Google Scholar]
- Tsolakidis D., Amant F., Van Gorp T., Leunen K., Neven P., Vergote I. Diaphragmatic surgery during primary debulking in 89 patients with stage IIIB–IV epithelial ovarian cancer. Gynecol. Oncol. 2010;116(3):489–496. doi: 10.1016/j.ygyno.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Winter W.E., 3rd, Maxwell G.L., Tian C. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a gynecologic oncology group study. J. Clin. Oncol. 2008;26(1):83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]