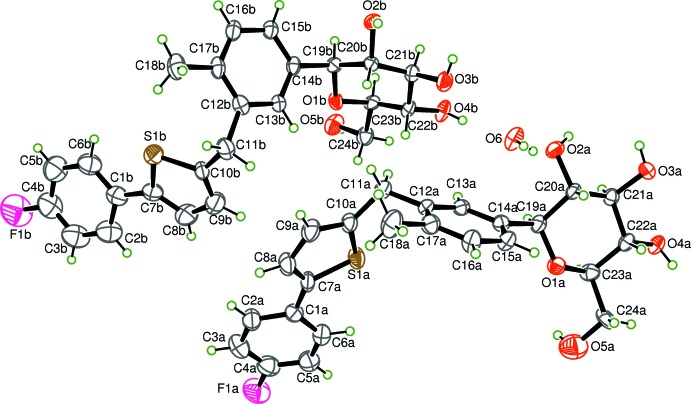
In canagliflozin hemihydrate, the hydropyran ring exhibits a chair conformation in both canagliflozin molecules. In the crystal, the canagliflozin molecules and lattice water molecules are connected via O—H⋯O hydrogen bonds into a three-dimensional supramolecular architecture.
Keywords: crystal structure, canagliflozin, hydrogen bonding
Abstract
There are two canagliflozin molecules (A and B) and one water molecule in the asymmetric unit of the title compound, C24H25FO5S·0.5H2O [systematic name: (2S,3R,4R,5S,6R)-2-(3-{[5-(4-fluorophenyl)thiophen-2-yl]methyl}-4-methylphenyl)-6-(hydroxymethyl)-3,4,5,6-tetrahydro-2H-pyran-3,4,5-triol hemihydrate]. The dihedral angles between the methylbenzene and thiophene rings are 115.7 (4) and 111.7 (4)°, while the dihedral angles between the fluorobenzene and thiophene rings are 24.2 (6) and 20.5 (9)° in molecules A and B, respectively. The hydropyran ring exhibits a chair conformation in both canagliflozin molecules. In the crystal, the canagliflozin molecules and lattice water molecules are connected via O—H⋯O hydrogen bonds into a three-dimensional supramolecular architecture.
Chemical context
Canagliflozin is a member of a new class of anti-diabetic drugs which are used to improve glycemic control of diabetics (Cefalu et al., 2013 ▸). The crystalline forms of canagliflozin have been reported (Mitsubishi et al., 2013 ▸; Ahmed et al., 2013 ▸; Chen et al., 2013 ▸), we report here the single-crystal structure of the title compound.
Structural commentary
The title compound crystallizes with two independent canagliflozin molecules and one water molecule in the asymmetric unit (Fig. 1 ▸). The water molecule links the two canagliflozin molecules (A and B) via two O—H⋯O hydrogen bonds (Table 1 ▸).
Figure 1.
The molecular structure of the title compound, (I), showing the atom-labeling scheme and displacement ellipsoids at the 40% probability level. H atoms are shown as small circles of arbitrary radii.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2A—H2A1⋯O2B i | 0.82 | 2.42 | 2.841 (4) | 113 |
| O3A—H3A1⋯O2B i | 0.82 | 2.17 | 2.951 (4) | 158 |
| O4A—H4A⋯O5B ii | 0.82 | 1.98 | 2.756 (5) | 157 |
| O2B—H2B1⋯O4A iii | 0.82 | 1.85 | 2.672 (4) | 179 |
| O3B—H3B1⋯O4B i | 0.82 | 1.99 | 2.797 (4) | 168 |
| O4B—H4B⋯O6 | 0.82 | 1.93 | 2.749 (5) | 172 |
| O5B—H5B1⋯O3B iv | 0.82 | 2.31 | 3.015 (5) | 144 |
| O6—H61⋯O2A | 0.82 | 2.23 | 3.031 (5) | 166 |
| O6—H62⋯O3A v | 0.83 | 2.30 | 3.058 (5) | 153 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  .
.
The conformations of the two canagliflozin molecules are somewhat different with regard to the orientation of the central benzene ring (C12–C17) with respect to the thiophene ring, as indicated by torsion angles C9A—C10A—C11A—C12A = 113.3 (6)° in molecule A and C9B—C10B—C11B—C12B = 108.0 (6)° in molecule B. The conformational difference is also shown by the angle C10—C11—C12, which is 115.7 (4)° in molecule A and 111.7 (4)° in molecule B. The terminal aromatic rings (C1–C6) are inclined to the thiophene rings, forming dihedral angles of 24.2 (6) and 20.5 (9)° in molecules A and B, respectively. The tetrahydropyran rings exhibit a distorted chair conformation in both molecules A and B.
Supramolecular features
In the crystal, O3B—H3B1⋯O4B
i, O2B–H2B1⋯O4A
iii, and O5B—H5B1⋯O3B
iv [symmetry code: (i) x −  , −y +
, −y +  , −z + 1; (iii) x, y + 1, z; (iv) x + 1, y, z] link canagliflozin molecules, generating a ring of graph-set motif
, −z + 1; (iii) x, y + 1, z; (iv) x + 1, y, z] link canagliflozin molecules, generating a ring of graph-set motif  (9). The presence of the water molecules results in the formation of zigzag chains mediated by alternating O4B—H4B⋯O6, O6—H61⋯O2A and O4A—H4A⋯O5B
ii [symmetry code: (ii) x − 1, y − 1, z] hydrogen bonds propagating along the a axis; the chains are stacked along the c axis by further hydrogen-bonding interactions, O3A—H3A1⋯O2B
i and O2A–-H2A1⋯O2B
i (Fig. 2 ▸).
(9). The presence of the water molecules results in the formation of zigzag chains mediated by alternating O4B—H4B⋯O6, O6—H61⋯O2A and O4A—H4A⋯O5B
ii [symmetry code: (ii) x − 1, y − 1, z] hydrogen bonds propagating along the a axis; the chains are stacked along the c axis by further hydrogen-bonding interactions, O3A—H3A1⋯O2B
i and O2A–-H2A1⋯O2B
i (Fig. 2 ▸).
Figure 2.
Part of the crystal packing of the title compound, showing the extensive intermolecular hydrogen-bonding interactions (dashed lines). H atoms not involved in hydrogen bonding have been omitted for clarity.
Synthesis and crystallization
The crude product was supplied by Zhejiang Huadong Pharmaceutical Co., Ltd. It was recrystallized from methanol solution, giving colorless crystals suitable for X-ray diffraction.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All H atoms were placed in calculated positions with C—H = 0.93–0.98 Å and O—H = 0.82 Å and included in the refinement using a riding model, with U iso(H) = 1.2U eq or 1.5U eq(carrier atom).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | 2C24H25FO5S·H2O |
| M r | 907.02 |
| Crystal system, space group | Orthorhombic, P212121 |
| Temperature (K) | 296 |
| a, b, c (Å) | 8.4259 (4), 11.4264 (7), 45.706 (2) |
| V (Å3) | 4400.4 (4) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.19 |
| Crystal size (mm) | 0.48 × 0.28 × 0.26 |
| Data collection | |
| Diffractometer | Rigaku R-AXIS RAPID |
| Absorption correction | Multi-scan (ABSCOR; Higashi, 1995 ▸) |
| T min, T max | 0.914, 0.952 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 43211, 9958, 5079 |
| R int | 0.145 |
| (sin θ/λ)max (Å−1) | 0.649 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.080, 0.147, 1.00 |
| No. of reflections | 9958 |
| No. of parameters | 575 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.38, −0.29 |
| Absolute structure | Flack (1983 ▸), 3246 Friedel pairs |
| Absolute structure parameter | 0.13 (11) |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989016006769/xu5886sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016006769/xu5886Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016006769/xu5886Isup3.cml
CCDC reference: 1475516
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
This project was supported by the Analysis and Measurement Foundation of Zhejiang Province, China (grant No. 2014 C37055).
supplementary crystallographic information
Crystal data
| 2C24H25FO5S·H2O | F(000) = 1912 |
| Mr = 907.02 | Dx = 1.369 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 23292 reflections |
| a = 8.4259 (4) Å | θ = 3.0–27.4° |
| b = 11.4264 (7) Å | µ = 0.19 mm−1 |
| c = 45.706 (2) Å | T = 296 K |
| V = 4400.4 (4) Å3 | Needle, colorless |
| Z = 4 | 0.48 × 0.28 × 0.26 mm |
Data collection
| Rigaku R-AXIS RAPID diffractometer | 9958 independent reflections |
| Radiation source: rotating anode | 5079 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.145 |
| Detector resolution: 10.00 pixels mm-1 | θmax = 27.5°, θmin = 3.0° |
| ω scans | h = −10→10 |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | k = −14→14 |
| Tmin = 0.914, Tmax = 0.952 | l = −59→59 |
| 43211 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.080 | H-atom parameters constrained |
| wR(F2) = 0.147 | w = 1/[σ2(Fo2) + (0.0408P)2 + 2.8647P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.00 | (Δ/σ)max < 0.001 |
| 9958 reflections | Δρmax = 0.38 e Å−3 |
| 575 parameters | Δρmin = −0.29 e Å−3 |
| 0 restraints | Absolute structure: Flack (1983), 3246 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Absolute structure parameter: 0.13 (11) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1A | 0.8230 (6) | 0.7018 (5) | 0.29229 (10) | 0.0474 (13) | |
| C2A | 0.9098 (7) | 0.7759 (5) | 0.27372 (11) | 0.0595 (16) | |
| H2A | 0.8808 | 0.8542 | 0.2721 | 0.071* | |
| C3A | 1.0373 (7) | 0.7347 (6) | 0.25788 (12) | 0.0692 (17) | |
| H3A | 1.0956 | 0.7843 | 0.2458 | 0.083* | |
| C4A | 1.0755 (7) | 0.6195 (7) | 0.26042 (13) | 0.0676 (18) | |
| C5A | 0.9969 (7) | 0.5431 (5) | 0.27796 (12) | 0.0652 (16) | |
| H5A | 1.0277 | 0.4651 | 0.2793 | 0.078* | |
| C6A | 0.8699 (7) | 0.5855 (5) | 0.29361 (11) | 0.0601 (15) | |
| H6A | 0.8134 | 0.5343 | 0.3055 | 0.072* | |
| C7A | 0.6928 (6) | 0.7483 (5) | 0.31027 (9) | 0.0451 (12) | |
| C8A | 0.6052 (6) | 0.8464 (5) | 0.30734 (11) | 0.0554 (15) | |
| H8A | 0.6133 | 0.8964 | 0.2914 | 0.066* | |
| C9A | 0.4994 (6) | 0.8657 (5) | 0.33098 (12) | 0.0557 (15) | |
| H9A | 0.4306 | 0.9293 | 0.3318 | 0.067* | |
| C10A | 0.5076 (6) | 0.7834 (4) | 0.35224 (10) | 0.0421 (12) | |
| C11A | 0.4200 (6) | 0.7753 (4) | 0.38050 (10) | 0.0477 (13) | |
| H11A | 0.3621 | 0.8478 | 0.3834 | 0.057* | |
| H11B | 0.4969 | 0.7688 | 0.3962 | 0.057* | |
| C12A | 0.3033 (5) | 0.6737 (4) | 0.38309 (9) | 0.0369 (11) | |
| C13A | 0.3383 (5) | 0.5781 (4) | 0.40050 (9) | 0.0343 (11) | |
| H13A | 0.4332 | 0.5779 | 0.4109 | 0.041* | |
| C14A | 0.2385 (5) | 0.4834 (4) | 0.40311 (9) | 0.0369 (11) | |
| C15A | 0.0979 (5) | 0.4846 (4) | 0.38708 (10) | 0.0404 (12) | |
| H15A | 0.0283 | 0.4216 | 0.3883 | 0.049* | |
| C16A | 0.0611 (6) | 0.5791 (5) | 0.36942 (10) | 0.0482 (13) | |
| H16A | −0.0332 | 0.5784 | 0.3588 | 0.058* | |
| C17A | 0.1607 (6) | 0.6745 (5) | 0.36711 (10) | 0.0463 (12) | |
| C18A | 0.1135 (7) | 0.7778 (5) | 0.34833 (12) | 0.0690 (17) | |
| H18A | 0.0041 | 0.7703 | 0.3428 | 0.104* | |
| H18B | 0.1787 | 0.7798 | 0.3311 | 0.104* | |
| H18C | 0.1278 | 0.8489 | 0.3592 | 0.104* | |
| C19A | 0.2741 (5) | 0.3786 (4) | 0.42179 (9) | 0.0357 (11) | |
| H19A | 0.3865 | 0.3797 | 0.4273 | 0.043* | |
| C20A | 0.1737 (5) | 0.3719 (4) | 0.44938 (9) | 0.0326 (11) | |
| H20A | 0.0615 | 0.3757 | 0.4438 | 0.039* | |
| C21A | 0.2017 (5) | 0.2584 (4) | 0.46571 (8) | 0.0339 (10) | |
| H21A | 0.3096 | 0.2591 | 0.4737 | 0.041* | |
| C22A | 0.1845 (5) | 0.1543 (4) | 0.44575 (9) | 0.0340 (11) | |
| H22A | 0.0726 | 0.1443 | 0.4405 | 0.041* | |
| C23A | 0.2830 (6) | 0.1693 (4) | 0.41808 (9) | 0.0424 (12) | |
| H23A | 0.3968 | 0.1644 | 0.4223 | 0.051* | |
| C24A | 0.2307 (7) | 0.0731 (5) | 0.39562 (10) | 0.0588 (13) | |
| H24A | 0.2386 | −0.0039 | 0.4045 | 0.071* | |
| H24B | 0.1215 | 0.0858 | 0.3897 | 0.071* | |
| C1B | 1.2588 (6) | 1.2409 (5) | 0.30205 (11) | 0.0562 (14) | |
| C2B | 1.3509 (7) | 1.1863 (6) | 0.28096 (13) | 0.0768 (19) | |
| H2B | 1.3240 | 1.1110 | 0.2751 | 0.092* | |
| C3B | 1.4806 (7) | 1.2395 (8) | 0.26844 (14) | 0.085 (2) | |
| H3B | 1.5430 | 1.2007 | 0.2548 | 0.102* | |
| C4B | 1.5136 (8) | 1.3496 (8) | 0.27672 (16) | 0.085 (2) | |
| C5B | 1.4341 (8) | 1.4082 (7) | 0.29795 (16) | 0.086 (2) | |
| H5B | 1.4656 | 1.4824 | 0.3039 | 0.103* | |
| C6B | 1.3035 (7) | 1.3528 (6) | 0.31044 (13) | 0.0697 (17) | |
| H6B | 1.2451 | 1.3917 | 0.3247 | 0.084* | |
| C7B | 1.1148 (6) | 1.1843 (5) | 0.31376 (10) | 0.0499 (13) | |
| C8B | 1.0747 (7) | 1.0717 (6) | 0.31352 (12) | 0.0637 (16) | |
| H8B | 1.1397 | 1.0133 | 0.3060 | 0.076* | |
| C9B | 0.9242 (7) | 1.0495 (5) | 0.32585 (12) | 0.0627 (16) | |
| H9B | 0.8806 | 0.9750 | 0.3274 | 0.075* | |
| C10B | 0.8499 (6) | 1.1468 (5) | 0.33522 (9) | 0.0441 (13) | |
| C11B | 0.6910 (6) | 1.1544 (5) | 0.35001 (10) | 0.0532 (14) | |
| H11C | 0.6363 | 1.0801 | 0.3480 | 0.064* | |
| H11D | 0.6274 | 1.2139 | 0.3404 | 0.064* | |
| C12B | 0.7072 (5) | 1.1844 (5) | 0.38244 (9) | 0.0389 (12) | |
| C13B | 0.7355 (5) | 1.0932 (4) | 0.40164 (9) | 0.0355 (11) | |
| H13B | 0.7394 | 1.0170 | 0.3945 | 0.043* | |
| C14B | 0.7583 (5) | 1.1132 (4) | 0.43165 (9) | 0.0334 (11) | |
| C15B | 0.7535 (5) | 1.2272 (4) | 0.44152 (10) | 0.0373 (11) | |
| H15B | 0.7706 | 1.2431 | 0.4612 | 0.045* | |
| C16B | 0.7237 (5) | 1.3172 (4) | 0.42246 (10) | 0.0437 (12) | |
| H16B | 0.7195 | 1.3933 | 0.4296 | 0.052* | |
| C17B | 0.6997 (5) | 1.2985 (4) | 0.39290 (11) | 0.0411 (12) | |
| C18B | 0.6624 (6) | 1.4007 (5) | 0.37319 (11) | 0.0586 (15) | |
| H18D | 0.6825 | 1.4725 | 0.3835 | 0.088* | |
| H18E | 0.5528 | 1.3977 | 0.3675 | 0.088* | |
| H18F | 0.7281 | 1.3971 | 0.3561 | 0.088* | |
| C19B | 0.7807 (5) | 1.0121 (4) | 0.45231 (9) | 0.0330 (10) | |
| H19B | 0.8318 | 1.0399 | 0.4703 | 0.040* | |
| C20B | 0.6217 (5) | 0.9556 (4) | 0.45993 (9) | 0.0302 (10) | |
| H20B | 0.5595 | 0.9465 | 0.4420 | 0.036* | |
| C21B | 0.6378 (5) | 0.8365 (4) | 0.47464 (9) | 0.0353 (11) | |
| H21B | 0.6707 | 0.8489 | 0.4950 | 0.042* | |
| C22B | 0.7590 (5) | 0.7601 (4) | 0.46008 (9) | 0.0341 (10) | |
| H22B | 0.7200 | 0.7347 | 0.4409 | 0.041* | |
| C23B | 0.9139 (5) | 0.8280 (4) | 0.45649 (9) | 0.0335 (11) | |
| H23B | 0.9519 | 0.8539 | 0.4757 | 0.040* | |
| C24B | 1.0424 (5) | 0.7610 (4) | 0.44114 (10) | 0.0466 (12) | |
| H24C | 1.0593 | 0.6864 | 0.4508 | 0.056* | |
| H24D | 1.0119 | 0.7461 | 0.4210 | 0.056* | |
| F1A | 1.2033 (4) | 0.5781 (4) | 0.24514 (8) | 0.0935 (12) | |
| F1B | 1.6394 (5) | 1.4058 (5) | 0.26352 (10) | 0.1351 (18) | |
| O1A | 0.2438 (4) | 0.2775 (3) | 0.40429 (6) | 0.0421 (8) | |
| O2A | 0.2095 (4) | 0.4714 (3) | 0.46711 (6) | 0.0440 (8) | |
| H2A1 | 0.2400 | 0.4495 | 0.4832 | 0.066* | |
| O3A | 0.0913 (4) | 0.2435 (3) | 0.48929 (6) | 0.0441 (8) | |
| H3A1 | 0.0983 | 0.2991 | 0.5006 | 0.066* | |
| O4A | 0.2376 (4) | 0.0535 (3) | 0.46192 (7) | 0.0406 (8) | |
| H4A | 0.1973 | −0.0057 | 0.4550 | 0.061* | |
| O5A | 0.3311 (6) | 0.0803 (4) | 0.37124 (10) | 0.0913 (14) | |
| H5A1 | 0.3019 | 0.1340 | 0.3606 | 0.137* | |
| O1B | 0.8812 (3) | 0.9285 (3) | 0.43833 (6) | 0.0370 (7) | |
| O2B | 0.5393 (3) | 1.0343 (3) | 0.47925 (6) | 0.0371 (8) | |
| H2B1 | 0.4465 | 1.0401 | 0.4741 | 0.056* | |
| O3B | 0.4879 (4) | 0.7782 (3) | 0.47478 (7) | 0.0472 (8) | |
| H3B1 | 0.4316 | 0.8062 | 0.4876 | 0.071* | |
| O4B | 0.7951 (4) | 0.6600 (3) | 0.47784 (7) | 0.0463 (8) | |
| H4B | 0.7223 | 0.6126 | 0.4768 | 0.069* | |
| O5B | 1.1853 (4) | 0.8292 (3) | 0.44195 (8) | 0.0559 (9) | |
| H5B1 | 1.2601 | 0.7872 | 0.4466 | 0.084* | |
| O6 | 0.5619 (4) | 0.4920 (3) | 0.47940 (8) | 0.0602 (10) | |
| H61 | 0.4711 | 0.4818 | 0.4733 | 0.090* | |
| H62 | 0.5714 | 0.4204 | 0.4819 | 0.090* | |
| S1A | 0.64414 (16) | 0.67821 (12) | 0.34263 (3) | 0.0523 (4) | |
| S1B | 0.96635 (17) | 1.26679 (13) | 0.32902 (3) | 0.0598 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1A | 0.055 (3) | 0.047 (4) | 0.041 (3) | −0.002 (3) | −0.004 (3) | 0.002 (2) |
| C2A | 0.067 (4) | 0.057 (4) | 0.054 (3) | −0.010 (3) | −0.002 (3) | 0.010 (3) |
| C3A | 0.063 (4) | 0.085 (5) | 0.060 (4) | 0.000 (4) | 0.014 (3) | 0.011 (4) |
| C4A | 0.060 (4) | 0.083 (5) | 0.060 (4) | −0.005 (4) | 0.005 (3) | −0.011 (4) |
| C5A | 0.077 (4) | 0.057 (4) | 0.062 (4) | 0.005 (4) | 0.010 (4) | −0.002 (3) |
| C6A | 0.071 (4) | 0.049 (4) | 0.060 (3) | −0.009 (3) | 0.009 (3) | 0.006 (3) |
| C7A | 0.052 (3) | 0.039 (3) | 0.044 (3) | −0.014 (3) | 0.000 (2) | 0.013 (3) |
| C8A | 0.066 (4) | 0.045 (4) | 0.055 (3) | 0.006 (3) | 0.010 (3) | 0.017 (3) |
| C9A | 0.062 (4) | 0.035 (3) | 0.070 (4) | 0.007 (3) | 0.003 (3) | 0.009 (3) |
| C10A | 0.047 (3) | 0.032 (3) | 0.048 (3) | 0.005 (2) | 0.006 (2) | 0.008 (2) |
| C11A | 0.061 (3) | 0.030 (3) | 0.052 (3) | −0.002 (3) | 0.005 (3) | 0.000 (2) |
| C12A | 0.047 (3) | 0.025 (3) | 0.038 (2) | −0.004 (2) | 0.008 (2) | −0.005 (2) |
| C13A | 0.033 (3) | 0.035 (3) | 0.035 (2) | −0.001 (2) | 0.001 (2) | −0.004 (2) |
| C14A | 0.042 (3) | 0.033 (3) | 0.036 (2) | 0.002 (2) | 0.004 (2) | 0.000 (2) |
| C15A | 0.042 (3) | 0.031 (3) | 0.048 (3) | −0.003 (2) | −0.009 (2) | 0.003 (2) |
| C16A | 0.045 (3) | 0.047 (3) | 0.053 (3) | −0.004 (3) | −0.013 (3) | 0.010 (3) |
| C17A | 0.055 (3) | 0.042 (3) | 0.042 (3) | 0.002 (3) | −0.005 (3) | 0.005 (2) |
| C18A | 0.074 (4) | 0.050 (4) | 0.083 (4) | −0.001 (3) | −0.020 (3) | 0.021 (3) |
| C19A | 0.034 (3) | 0.029 (3) | 0.044 (3) | 0.000 (2) | 0.002 (2) | 0.002 (2) |
| C20A | 0.037 (3) | 0.023 (3) | 0.038 (2) | −0.002 (2) | 0.000 (2) | −0.001 (2) |
| C21A | 0.033 (2) | 0.033 (3) | 0.036 (2) | −0.003 (2) | −0.004 (2) | 0.003 (2) |
| C22A | 0.033 (2) | 0.024 (3) | 0.045 (3) | 0.002 (2) | −0.005 (2) | 0.002 (2) |
| C23A | 0.049 (3) | 0.037 (3) | 0.042 (3) | 0.010 (3) | −0.003 (2) | −0.002 (2) |
| C24A | 0.086 | 0.044 (3) | 0.046 (3) | 0.026 (3) | 0.038 (3) | 0.008 (3) |
| C1B | 0.058 (3) | 0.063 (4) | 0.047 (3) | 0.017 (3) | −0.003 (3) | 0.002 (3) |
| C2B | 0.062 (4) | 0.089 (5) | 0.079 (4) | 0.006 (4) | 0.001 (4) | −0.013 (4) |
| C3B | 0.056 (4) | 0.116 (7) | 0.082 (5) | 0.004 (5) | 0.012 (4) | −0.016 (5) |
| C4B | 0.051 (4) | 0.116 (7) | 0.088 (5) | −0.020 (4) | 0.008 (4) | 0.000 (5) |
| C5B | 0.070 (5) | 0.090 (6) | 0.098 (5) | −0.013 (4) | 0.001 (4) | −0.001 (5) |
| C6B | 0.064 (4) | 0.078 (5) | 0.068 (4) | 0.006 (4) | 0.009 (3) | −0.007 (3) |
| C7B | 0.048 (3) | 0.056 (4) | 0.046 (3) | 0.008 (3) | 0.003 (3) | −0.006 (3) |
| C8B | 0.062 (4) | 0.056 (4) | 0.073 (4) | 0.016 (3) | 0.002 (3) | −0.003 (3) |
| C9B | 0.075 (4) | 0.049 (4) | 0.064 (4) | −0.006 (3) | −0.012 (3) | 0.004 (3) |
| C10B | 0.058 (3) | 0.043 (3) | 0.032 (3) | −0.014 (3) | 0.000 (2) | 0.009 (2) |
| C11B | 0.057 (3) | 0.058 (4) | 0.045 (3) | −0.006 (3) | −0.002 (3) | 0.011 (3) |
| C12B | 0.031 (2) | 0.051 (3) | 0.035 (2) | −0.003 (2) | −0.004 (2) | 0.007 (2) |
| C13B | 0.040 (3) | 0.033 (3) | 0.034 (2) | −0.002 (2) | 0.002 (2) | 0.005 (2) |
| C14B | 0.028 (2) | 0.029 (3) | 0.043 (3) | 0.003 (2) | 0.003 (2) | 0.009 (2) |
| C15B | 0.036 (2) | 0.034 (3) | 0.042 (2) | 0.002 (2) | 0.004 (2) | 0.006 (2) |
| C16B | 0.040 (3) | 0.032 (3) | 0.059 (3) | 0.002 (2) | 0.013 (3) | 0.008 (3) |
| C17B | 0.033 (3) | 0.038 (3) | 0.052 (3) | 0.006 (2) | 0.007 (2) | 0.018 (2) |
| C18B | 0.055 (3) | 0.050 (4) | 0.070 (4) | 0.014 (3) | 0.005 (3) | 0.026 (3) |
| C19B | 0.037 (3) | 0.028 (3) | 0.034 (2) | 0.006 (2) | 0.001 (2) | 0.002 (2) |
| C20B | 0.030 (2) | 0.031 (3) | 0.029 (2) | 0.001 (2) | 0.000 (2) | −0.0001 (19) |
| C21B | 0.034 (2) | 0.034 (3) | 0.038 (2) | −0.005 (2) | 0.000 (2) | 0.004 (2) |
| C22B | 0.038 (3) | 0.023 (3) | 0.041 (2) | −0.002 (2) | −0.007 (2) | 0.005 (2) |
| C23B | 0.040 (3) | 0.022 (2) | 0.038 (2) | 0.011 (2) | 0.000 (2) | −0.001 (2) |
| C24B | 0.045 (3) | 0.038 (3) | 0.056 (3) | 0.004 (3) | 0.003 (2) | 0.006 (3) |
| F1A | 0.074 (2) | 0.115 (3) | 0.091 (2) | 0.010 (2) | 0.028 (2) | −0.011 (2) |
| F1B | 0.076 (3) | 0.183 (5) | 0.146 (4) | −0.037 (3) | 0.029 (3) | −0.007 (4) |
| O1A | 0.056 (2) | 0.0316 (19) | 0.0392 (16) | 0.0070 (17) | 0.0008 (15) | 0.0012 (15) |
| O2A | 0.061 (2) | 0.031 (2) | 0.0395 (17) | −0.0058 (17) | −0.0058 (17) | −0.0042 (15) |
| O3A | 0.057 (2) | 0.039 (2) | 0.0357 (17) | −0.0041 (18) | 0.0126 (15) | 0.0011 (16) |
| O4A | 0.045 (2) | 0.0237 (18) | 0.0527 (19) | −0.0026 (16) | −0.0045 (16) | 0.0017 (15) |
| O5A | 0.101 (4) | 0.085 (4) | 0.088 (3) | 0.020 (3) | 0.006 (3) | −0.006 (3) |
| O1B | 0.0411 (17) | 0.0312 (19) | 0.0388 (16) | 0.0069 (15) | 0.0085 (15) | 0.0103 (15) |
| O2B | 0.0341 (16) | 0.037 (2) | 0.0405 (17) | 0.0026 (15) | 0.0010 (15) | −0.0046 (15) |
| O3B | 0.0437 (19) | 0.040 (2) | 0.058 (2) | −0.0110 (17) | 0.0070 (16) | 0.0015 (16) |
| O4B | 0.047 (2) | 0.0264 (19) | 0.066 (2) | −0.0040 (16) | −0.0110 (18) | 0.0141 (17) |
| O5B | 0.042 (2) | 0.042 (2) | 0.084 (3) | 0.0068 (18) | 0.009 (2) | −0.002 (2) |
| O6 | 0.062 (2) | 0.041 (2) | 0.079 (3) | −0.0114 (19) | −0.011 (2) | 0.007 (2) |
| S1A | 0.0602 (8) | 0.0419 (8) | 0.0549 (8) | 0.0073 (7) | 0.0109 (7) | 0.0161 (7) |
| S1B | 0.0673 (9) | 0.0499 (10) | 0.0621 (8) | −0.0040 (8) | 0.0172 (7) | −0.0030 (7) |
Geometric parameters (Å, º)
| C1A—C6A | 1.387 (7) | C3B—C4B | 1.344 (9) |
| C1A—C2A | 1.405 (7) | C3B—H3B | 0.9300 |
| C1A—C7A | 1.470 (7) | C4B—C5B | 1.356 (9) |
| C2A—C3A | 1.378 (8) | C4B—F1B | 1.378 (7) |
| C2A—H2A | 0.9300 | C5B—C6B | 1.391 (8) |
| C3A—C4A | 1.360 (8) | C5B—H5B | 0.9300 |
| C3A—H3A | 0.9300 | C6B—H6B | 0.9300 |
| C4A—C5A | 1.358 (8) | C7B—C8B | 1.330 (8) |
| C4A—F1A | 1.368 (7) | C7B—S1B | 1.715 (5) |
| C5A—C6A | 1.376 (8) | C8B—C9B | 1.411 (8) |
| C5A—H5A | 0.9300 | C8B—H8B | 0.9300 |
| C6A—H6A | 0.9300 | C9B—C10B | 1.345 (7) |
| C7A—C8A | 1.348 (7) | C9B—H9B | 0.9300 |
| C7A—S1A | 1.731 (4) | C10B—C11B | 1.502 (7) |
| C8A—C9A | 1.418 (7) | C10B—S1B | 1.710 (5) |
| C8A—H8A | 0.9300 | C11B—C12B | 1.527 (6) |
| C9A—C10A | 1.354 (6) | C11B—H11C | 0.9700 |
| C9A—H9A | 0.9300 | C11B—H11D | 0.9700 |
| C10A—C11A | 1.491 (6) | C12B—C13B | 1.383 (6) |
| C10A—S1A | 1.721 (5) | C12B—C17B | 1.390 (6) |
| C11A—C12A | 1.526 (6) | C13B—C14B | 1.404 (6) |
| C11A—H11A | 0.9700 | C13B—H13B | 0.9300 |
| C11A—H11B | 0.9700 | C14B—C15B | 1.379 (6) |
| C12A—C13A | 1.383 (6) | C14B—C19B | 1.504 (6) |
| C12A—C17A | 1.407 (6) | C15B—C16B | 1.371 (6) |
| C13A—C14A | 1.375 (6) | C15B—H15B | 0.9300 |
| C13A—H13A | 0.9300 | C16B—C17B | 1.383 (6) |
| C14A—C15A | 1.394 (6) | C16B—H16B | 0.9300 |
| C14A—C19A | 1.501 (6) | C17B—C18B | 1.508 (6) |
| C15A—C16A | 1.383 (6) | C18B—H18D | 0.9600 |
| C15A—H15A | 0.9300 | C18B—H18E | 0.9600 |
| C16A—C17A | 1.379 (7) | C18B—H18F | 0.9600 |
| C16A—H16A | 0.9300 | C19B—O1B | 1.427 (5) |
| C17A—C18A | 1.513 (7) | C19B—C20B | 1.528 (6) |
| C18A—H18A | 0.9600 | C19B—H19B | 0.9800 |
| C18A—H18B | 0.9600 | C20B—O2B | 1.439 (5) |
| C18A—H18C | 0.9600 | C20B—C21B | 1.524 (6) |
| C19A—O1A | 1.428 (5) | C20B—H20B | 0.9800 |
| C19A—C20A | 1.521 (6) | C21B—O3B | 1.428 (5) |
| C19A—H19A | 0.9800 | C21B—C22B | 1.499 (6) |
| C20A—O2A | 1.428 (5) | C21B—H21B | 0.9800 |
| C20A—C21A | 1.515 (6) | C22B—O4B | 1.436 (5) |
| C20A—H20A | 0.9800 | C22B—C23B | 1.526 (6) |
| C21A—O3A | 1.434 (5) | C22B—H22B | 0.9800 |
| C21A—C22A | 1.506 (6) | C23B—O1B | 1.444 (5) |
| C21A—H21A | 0.9800 | C23B—C24B | 1.500 (6) |
| C22A—O4A | 1.439 (5) | C23B—H23B | 0.9800 |
| C22A—C23A | 1.522 (6) | C24B—O5B | 1.435 (5) |
| C22A—H22A | 0.9800 | C24B—H24C | 0.9700 |
| C23A—O1A | 1.427 (5) | C24B—H24D | 0.9700 |
| C23A—C24A | 1.567 (7) | O2A—H2A1 | 0.8200 |
| C23A—H23A | 0.9800 | O3A—H3A1 | 0.8200 |
| C24A—O5A | 1.401 (6) | O4A—H4A | 0.8200 |
| C24A—H24A | 0.9700 | O5A—H5A1 | 0.8200 |
| C24A—H24B | 0.9700 | O2B—H2B1 | 0.8200 |
| C1B—C2B | 1.386 (7) | O3B—H3B1 | 0.8200 |
| C1B—C6B | 1.387 (8) | O4B—H4B | 0.8200 |
| C1B—C7B | 1.476 (7) | O5B—H5B1 | 0.8200 |
| C2B—C3B | 1.375 (8) | O6—H61 | 0.8228 |
| C2B—H2B | 0.9300 | O6—H62 | 0.8292 |
| C6A—C1A—C2A | 117.1 (5) | C4B—C3B—H3B | 121.3 |
| C6A—C1A—C7A | 122.3 (5) | C2B—C3B—H3B | 121.3 |
| C2A—C1A—C7A | 120.5 (5) | C3B—C4B—C5B | 124.1 (7) |
| C3A—C2A—C1A | 121.1 (6) | C3B—C4B—F1B | 118.2 (7) |
| C3A—C2A—H2A | 119.4 | C5B—C4B—F1B | 117.7 (8) |
| C1A—C2A—H2A | 119.4 | C4B—C5B—C6B | 117.4 (7) |
| C4A—C3A—C2A | 118.0 (6) | C4B—C5B—H5B | 121.3 |
| C4A—C3A—H3A | 121.0 | C6B—C5B—H5B | 121.3 |
| C2A—C3A—H3A | 121.0 | C1B—C6B—C5B | 121.3 (6) |
| C5A—C4A—C3A | 123.9 (6) | C1B—C6B—H6B | 119.3 |
| C5A—C4A—F1A | 117.5 (6) | C5B—C6B—H6B | 119.3 |
| C3A—C4A—F1A | 118.5 (6) | C8B—C7B—C1B | 129.0 (5) |
| C4A—C5A—C6A | 117.4 (6) | C8B—C7B—S1B | 110.5 (4) |
| C4A—C5A—H5A | 121.3 | C1B—C7B—S1B | 120.4 (4) |
| C6A—C5A—H5A | 121.3 | C7B—C8B—C9B | 113.5 (6) |
| C5A—C6A—C1A | 122.4 (5) | C7B—C8B—H8B | 123.3 |
| C5A—C6A—H6A | 118.8 | C9B—C8B—H8B | 123.3 |
| C1A—C6A—H6A | 118.8 | C10B—C9B—C8B | 113.4 (5) |
| C8A—C7A—C1A | 130.8 (4) | C10B—C9B—H9B | 123.3 |
| C8A—C7A—S1A | 109.9 (4) | C8B—C9B—H9B | 123.3 |
| C1A—C7A—S1A | 119.1 (4) | C9B—C10B—C11B | 127.3 (5) |
| C7A—C8A—C9A | 113.5 (5) | C9B—C10B—S1B | 110.0 (4) |
| C7A—C8A—H8A | 123.3 | C11B—C10B—S1B | 122.6 (4) |
| C9A—C8A—H8A | 123.3 | C10B—C11B—C12B | 111.7 (4) |
| C10A—C9A—C8A | 114.0 (5) | C10B—C11B—H11C | 109.3 |
| C10A—C9A—H9A | 123.0 | C12B—C11B—H11C | 109.3 |
| C8A—C9A—H9A | 123.0 | C10B—C11B—H11D | 109.3 |
| C9A—C10A—C11A | 129.8 (5) | C12B—C11B—H11D | 109.3 |
| C9A—C10A—S1A | 109.6 (4) | H11C—C11B—H11D | 107.9 |
| C11A—C10A—S1A | 120.6 (3) | C13B—C12B—C17B | 119.8 (4) |
| C10A—C11A—C12A | 115.7 (4) | C13B—C12B—C11B | 117.5 (5) |
| C10A—C11A—H11A | 108.4 | C17B—C12B—C11B | 122.7 (4) |
| C12A—C11A—H11A | 108.4 | C12B—C13B—C14B | 121.4 (4) |
| C10A—C11A—H11B | 108.4 | C12B—C13B—H13B | 119.3 |
| C12A—C11A—H11B | 108.4 | C14B—C13B—H13B | 119.3 |
| H11A—C11A—H11B | 107.4 | C15B—C14B—C13B | 118.0 (4) |
| C13A—C12A—C17A | 119.1 (4) | C15B—C14B—C19B | 121.6 (4) |
| C13A—C12A—C11A | 120.6 (4) | C13B—C14B—C19B | 120.4 (4) |
| C17A—C12A—C11A | 120.3 (4) | C16B—C15B—C14B | 120.4 (4) |
| C14A—C13A—C12A | 122.8 (4) | C16B—C15B—H15B | 119.8 |
| C14A—C13A—H13A | 118.6 | C14B—C15B—H15B | 119.8 |
| C12A—C13A—H13A | 118.6 | C15B—C16B—C17B | 122.1 (5) |
| C13A—C14A—C15A | 117.8 (4) | C15B—C16B—H16B | 118.9 |
| C13A—C14A—C19A | 123.7 (4) | C17B—C16B—H16B | 118.9 |
| C15A—C14A—C19A | 118.5 (4) | C16B—C17B—C12B | 118.3 (4) |
| C16A—C15A—C14A | 120.3 (5) | C16B—C17B—C18B | 119.6 (5) |
| C16A—C15A—H15A | 119.8 | C12B—C17B—C18B | 122.0 (5) |
| C14A—C15A—H15A | 119.8 | C17B—C18B—H18D | 109.5 |
| C17A—C16A—C15A | 121.7 (5) | C17B—C18B—H18E | 109.5 |
| C17A—C16A—H16A | 119.2 | H18D—C18B—H18E | 109.5 |
| C15A—C16A—H16A | 119.2 | C17B—C18B—H18F | 109.5 |
| C16A—C17A—C12A | 118.4 (5) | H18D—C18B—H18F | 109.5 |
| C16A—C17A—C18A | 120.0 (4) | H18E—C18B—H18F | 109.5 |
| C12A—C17A—C18A | 121.6 (5) | O1B—C19B—C14B | 107.9 (3) |
| C17A—C18A—H18A | 109.5 | O1B—C19B—C20B | 109.9 (3) |
| C17A—C18A—H18B | 109.5 | C14B—C19B—C20B | 110.9 (4) |
| H18A—C18A—H18B | 109.5 | O1B—C19B—H19B | 109.4 |
| C17A—C18A—H18C | 109.5 | C14B—C19B—H19B | 109.4 |
| H18A—C18A—H18C | 109.5 | C20B—C19B—H19B | 109.4 |
| H18B—C18A—H18C | 109.5 | O2B—C20B—C21B | 109.3 (3) |
| O1A—C19A—C14A | 106.9 (3) | O2B—C20B—C19B | 107.4 (3) |
| O1A—C19A—C20A | 109.0 (3) | C21B—C20B—C19B | 113.5 (3) |
| C14A—C19A—C20A | 113.6 (4) | O2B—C20B—H20B | 108.8 |
| O1A—C19A—H19A | 109.1 | C21B—C20B—H20B | 108.8 |
| C14A—C19A—H19A | 109.1 | C19B—C20B—H20B | 108.8 |
| C20A—C19A—H19A | 109.1 | O3B—C21B—C22B | 109.5 (4) |
| O2A—C20A—C21A | 111.7 (3) | O3B—C21B—C20B | 109.8 (4) |
| O2A—C20A—C19A | 108.2 (3) | C22B—C21B—C20B | 112.6 (3) |
| C21A—C20A—C19A | 111.4 (4) | O3B—C21B—H21B | 108.3 |
| O2A—C20A—H20A | 108.5 | C22B—C21B—H21B | 108.3 |
| C21A—C20A—H20A | 108.5 | C20B—C21B—H21B | 108.3 |
| C19A—C20A—H20A | 108.5 | O4B—C22B—C21B | 110.9 (3) |
| O3A—C21A—C22A | 107.4 (4) | O4B—C22B—C23B | 106.5 (3) |
| O3A—C21A—C20A | 111.7 (4) | C21B—C22B—C23B | 109.5 (4) |
| C22A—C21A—C20A | 111.3 (3) | O4B—C22B—H22B | 109.9 |
| O3A—C21A—H21A | 108.8 | C21B—C22B—H22B | 109.9 |
| C22A—C21A—H21A | 108.8 | C23B—C22B—H22B | 109.9 |
| C20A—C21A—H21A | 108.8 | O1B—C23B—C24B | 105.9 (3) |
| O4A—C22A—C21A | 106.9 (3) | O1B—C23B—C22B | 107.6 (3) |
| O4A—C22A—C23A | 110.3 (4) | C24B—C23B—C22B | 114.1 (4) |
| C21A—C22A—C23A | 111.2 (4) | O1B—C23B—H23B | 109.7 |
| O4A—C22A—H22A | 109.5 | C24B—C23B—H23B | 109.7 |
| C21A—C22A—H22A | 109.5 | C22B—C23B—H23B | 109.7 |
| C23A—C22A—H22A | 109.5 | O5B—C24B—C23B | 108.5 (4) |
| O1A—C23A—C22A | 109.8 (4) | O5B—C24B—H24C | 110.0 |
| O1A—C23A—C24A | 104.7 (3) | C23B—C24B—H24C | 110.0 |
| C22A—C23A—C24A | 108.2 (4) | O5B—C24B—H24D | 110.0 |
| O1A—C23A—H23A | 111.3 | C23B—C24B—H24D | 110.0 |
| C22A—C23A—H23A | 111.3 | H24C—C24B—H24D | 108.4 |
| C24A—C23A—H23A | 111.3 | C23A—O1A—C19A | 114.3 (3) |
| O5A—C24A—C23A | 108.1 (5) | C20A—O2A—H2A1 | 109.5 |
| O5A—C24A—H24A | 110.1 | C21A—O3A—H3A1 | 109.5 |
| C23A—C24A—H24A | 110.1 | C22A—O4A—H4A | 109.5 |
| O5A—C24A—H24B | 110.1 | C24A—O5A—H5A1 | 109.5 |
| C23A—C24A—H24B | 110.1 | C19B—O1B—C23B | 112.8 (3) |
| H24A—C24A—H24B | 108.4 | C20B—O2B—H2B1 | 109.5 |
| C2B—C1B—C6B | 117.1 (6) | C21B—O3B—H3B1 | 109.5 |
| C2B—C1B—C7B | 121.0 (6) | C22B—O4B—H4B | 109.5 |
| C6B—C1B—C7B | 121.8 (5) | C24B—O5B—H5B1 | 109.5 |
| C3B—C2B—C1B | 122.4 (6) | H61—O6—H62 | 89.8 |
| C3B—C2B—H2B | 118.8 | C10A—S1A—C7A | 93.0 (2) |
| C1B—C2B—H2B | 118.8 | C10B—S1B—C7B | 92.6 (3) |
| C4B—C3B—C2B | 117.5 (6) | ||
| C6A—C1A—C2A—C3A | −0.9 (8) | C7B—C1B—C6B—C5B | −176.1 (5) |
| C7A—C1A—C2A—C3A | 176.6 (5) | C4B—C5B—C6B—C1B | 1.6 (10) |
| C1A—C2A—C3A—C4A | 0.9 (8) | C2B—C1B—C7B—C8B | 20.5 (9) |
| C2A—C3A—C4A—C5A | −1.0 (10) | C6B—C1B—C7B—C8B | −162.9 (6) |
| C2A—C3A—C4A—F1A | −179.0 (5) | C2B—C1B—C7B—S1B | −156.6 (4) |
| C3A—C4A—C5A—C6A | 1.1 (9) | C6B—C1B—C7B—S1B | 20.1 (7) |
| F1A—C4A—C5A—C6A | 179.1 (5) | C1B—C7B—C8B—C9B | −177.8 (5) |
| C4A—C5A—C6A—C1A | −1.0 (9) | S1B—C7B—C8B—C9B | −0.5 (6) |
| C2A—C1A—C6A—C5A | 0.9 (8) | C7B—C8B—C9B—C10B | 0.6 (7) |
| C7A—C1A—C6A—C5A | −176.5 (5) | C8B—C9B—C10B—C11B | −178.3 (5) |
| C6A—C1A—C7A—C8A | −161.5 (6) | C8B—C9B—C10B—S1B | −0.3 (6) |
| C2A—C1A—C7A—C8A | 21.2 (8) | C9B—C10B—C11B—C12B | 108.0 (6) |
| C6A—C1A—C7A—S1A | 24.2 (6) | S1B—C10B—C11B—C12B | −69.8 (5) |
| C2A—C1A—C7A—S1A | −153.1 (4) | C10B—C11B—C12B—C13B | −84.3 (5) |
| C1A—C7A—C8A—C9A | −174.5 (5) | C10B—C11B—C12B—C17B | 93.7 (6) |
| S1A—C7A—C8A—C9A | 0.2 (6) | C17B—C12B—C13B—C14B | −0.6 (7) |
| C7A—C8A—C9A—C10A | 0.9 (7) | C11B—C12B—C13B—C14B | 177.4 (4) |
| C8A—C9A—C10A—C11A | 178.1 (5) | C12B—C13B—C14B—C15B | −0.7 (6) |
| C8A—C9A—C10A—S1A | −1.5 (6) | C12B—C13B—C14B—C19B | 177.0 (4) |
| C9A—C10A—C11A—C12A | 113.3 (6) | C13B—C14B—C15B—C16B | 1.4 (6) |
| S1A—C10A—C11A—C12A | −67.1 (5) | C19B—C14B—C15B—C16B | −176.2 (4) |
| C10A—C11A—C12A—C13A | 106.6 (5) | C14B—C15B—C16B—C17B | −0.9 (7) |
| C10A—C11A—C12A—C17A | −71.5 (6) | C15B—C16B—C17B—C12B | −0.4 (7) |
| C17A—C12A—C13A—C14A | −0.6 (7) | C15B—C16B—C17B—C18B | 178.1 (4) |
| C11A—C12A—C13A—C14A | −178.7 (4) | C13B—C12B—C17B—C16B | 1.1 (7) |
| C12A—C13A—C14A—C15A | 0.9 (7) | C11B—C12B—C17B—C16B | −176.8 (4) |
| C12A—C13A—C14A—C19A | 179.8 (4) | C13B—C12B—C17B—C18B | −177.3 (4) |
| C13A—C14A—C15A—C16A | −0.5 (7) | C11B—C12B—C17B—C18B | 4.7 (7) |
| C19A—C14A—C15A—C16A | −179.5 (4) | C15B—C14B—C19B—O1B | −141.6 (4) |
| C14A—C15A—C16A—C17A | −0.2 (8) | C13B—C14B—C19B—O1B | 40.9 (5) |
| C15A—C16A—C17A—C12A | 0.5 (8) | C15B—C14B—C19B—C20B | 98.1 (5) |
| C15A—C16A—C17A—C18A | −178.0 (5) | C13B—C14B—C19B—C20B | −79.5 (5) |
| C13A—C12A—C17A—C16A | −0.1 (7) | O1B—C19B—C20B—O2B | 167.9 (3) |
| C11A—C12A—C17A—C16A | 178.0 (4) | C14B—C19B—C20B—O2B | −72.9 (4) |
| C13A—C12A—C17A—C18A | 178.3 (4) | O1B—C19B—C20B—C21B | 46.9 (5) |
| C11A—C12A—C17A—C18A | −3.6 (7) | C14B—C19B—C20B—C21B | 166.2 (4) |
| C13A—C14A—C19A—O1A | −132.3 (4) | O2B—C20B—C21B—O3B | 73.6 (4) |
| C15A—C14A—C19A—O1A | 46.7 (5) | C19B—C20B—C21B—O3B | −166.5 (3) |
| C13A—C14A—C19A—C20A | 107.5 (5) | O2B—C20B—C21B—C22B | −164.1 (3) |
| C15A—C14A—C19A—C20A | −73.6 (5) | C19B—C20B—C21B—C22B | −44.2 (5) |
| O1A—C19A—C20A—O2A | 178.0 (3) | O3B—C21B—C22B—O4B | −69.7 (4) |
| C14A—C19A—C20A—O2A | −62.9 (5) | C20B—C21B—C22B—O4B | 167.8 (3) |
| O1A—C19A—C20A—C21A | 54.8 (5) | O3B—C21B—C22B—C23B | 173.0 (3) |
| C14A—C19A—C20A—C21A | 173.9 (4) | C20B—C21B—C22B—C23B | 50.5 (5) |
| O2A—C20A—C21A—O3A | 67.4 (5) | O4B—C22B—C23B—O1B | 179.2 (3) |
| C19A—C20A—C21A—O3A | −171.4 (3) | C21B—C22B—C23B—O1B | −60.8 (4) |
| O2A—C20A—C21A—C22A | −172.5 (4) | O4B—C22B—C23B—C24B | 62.0 (5) |
| C19A—C20A—C21A—C22A | −51.4 (5) | C21B—C22B—C23B—C24B | −178.0 (4) |
| O3A—C21A—C22A—O4A | −66.4 (4) | O1B—C23B—C24B—O5B | 67.8 (4) |
| C20A—C21A—C22A—O4A | 171.0 (4) | C22B—C23B—C24B—O5B | −174.0 (4) |
| O3A—C21A—C22A—C23A | 173.2 (3) | C22A—C23A—O1A—C19A | 61.0 (5) |
| C20A—C21A—C22A—C23A | 50.5 (5) | C24A—C23A—O1A—C19A | 176.9 (4) |
| O4A—C22A—C23A—O1A | −172.3 (3) | C14A—C19A—O1A—C23A | 175.6 (4) |
| C21A—C22A—C23A—O1A | −53.9 (5) | C20A—C19A—O1A—C23A | −61.2 (5) |
| O4A—C22A—C23A—C24A | 74.0 (4) | C14B—C19B—O1B—C23B | 178.8 (3) |
| C21A—C22A—C23A—C24A | −167.6 (4) | C20B—C19B—O1B—C23B | −60.1 (4) |
| O1A—C23A—C24A—O5A | 68.2 (5) | C24B—C23B—O1B—C19B | −169.8 (3) |
| C22A—C23A—C24A—O5A | −174.7 (4) | C22B—C23B—O1B—C19B | 67.7 (4) |
| C6B—C1B—C2B—C3B | −0.5 (9) | C9A—C10A—S1A—C7A | 1.4 (4) |
| C7B—C1B—C2B—C3B | 176.3 (5) | C11A—C10A—S1A—C7A | −178.3 (4) |
| C1B—C2B—C3B—C4B | −2.0 (10) | C8A—C7A—S1A—C10A | −0.9 (4) |
| C2B—C3B—C4B—C5B | 4.7 (11) | C1A—C7A—S1A—C10A | 174.5 (4) |
| C2B—C3B—C4B—F1B | −177.5 (6) | C9B—C10B—S1B—C7B | 0.0 (4) |
| C3B—C4B—C5B—C6B | −4.5 (11) | C11B—C10B—S1B—C7B | 178.1 (4) |
| F1B—C4B—C5B—C6B | 177.7 (6) | C8B—C7B—S1B—C10B | 0.3 (4) |
| C2B—C1B—C6B—C5B | 0.7 (8) | C1B—C7B—S1B—C10B | 177.8 (4) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2A—H2A1···O2Bi | 0.82 | 2.42 | 2.841 (4) | 113 |
| O3A—H3A1···O2Bi | 0.82 | 2.17 | 2.951 (4) | 158 |
| O4A—H4A···O5Bii | 0.82 | 1.98 | 2.756 (5) | 157 |
| O2B—H2B1···O4Aiii | 0.82 | 1.85 | 2.672 (4) | 179 |
| O3B—H3B1···O4Bi | 0.82 | 1.99 | 2.797 (4) | 168 |
| O4B—H4B···O6 | 0.82 | 1.93 | 2.749 (5) | 172 |
| O5B—H5B1···O3Biv | 0.82 | 2.31 | 3.015 (5) | 144 |
| O6—H61···O2A | 0.82 | 2.23 | 3.031 (5) | 166 |
| O6—H62···O3Av | 0.83 | 2.30 | 3.058 (5) | 153 |
Symmetry codes: (i) x−1/2, −y+3/2, −z+1; (ii) x−1, y−1, z; (iii) x, y+1, z; (iv) x+1, y, z; (v) x+1/2, −y+1/2, −z+1.
References
- Ahmed, F. A., Maureen, C., Steven, M., Lorraine, S., Kenneth, M. W., Fan, Z., Sumihiro, N., Mitsuya, H. & Yuichi, K. (2013). US Patent 2009/0233874 A1.
- Brandenburg, K. & Putz, H. (2005). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Cefalu, W. T., Leiter, L. A., Yoon, K. H., Arias, P., Niskanen, L., Xie, J., Balis, D. A., Canovatchel, W. & Meininger, G. (2013). Lancet, 382, 941–950. [DOI] [PubMed]
- Chen, M.-H., Zhang, Y.-F., Zhao, Y. & Zhang, X.-Y. (2013). Chin. Patent CN103588762A.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Higashi, T. (1995). ABSCORM. Rigaku Corporation, Tokyo, Japan.
- Mitsubishi, T., Nomura, S. & Kawanishi, A. (2013). World Patent WO2008069327A1.
- Rigaku (2006). PROCESS-AUTO. Rigaku Corporation, Tokyo, Japan.
- Rigaku. (2007). CrystalStructure. Rigaku Americas, The Woodlands, Texas, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2056989016006769/xu5886sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016006769/xu5886Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016006769/xu5886Isup3.cml
CCDC reference: 1475516
Additional supporting information: crystallographic information; 3D view; checkCIF report