The supramolecular structure of the title 1:1 co-crystal consists of (100) sheets linked by O—H⋯N and N—H⋯O hydrogen bonds.
Keywords: crystal structure; 5-aminoisophthalic acid; 5AIA; 1,2-bis(pyridin-4-yl)ethene (BE); co-crystal; hydrogen bonding
Abstract
In the title 1:1 co-crystal, C12H10N2·C8H7NO4, the bipyridine moiety shows whole-molecule disorder over two sets of sites in a 0.588 (3): 0.412 (3) ratio. In the crystal, the components form hydrogen-bonded sheets linked by N—H⋯O and O—H⋯N interactions, which stack along the a axis. A comparison to a related and previously published co-crystal of 5-amino-isophthalic acid and the shorter 4,4′-bipryidine is presented.
Chemical context
5-Amino-isophthalic acid (5AIA) is an emerging secondary building unit for a wide variety of metal–organic frameworks (MOFs). (Zeng et al., 2009 ▸; Wang et al., 2011 ▸; Cox et al., 2015 ▸) This compound is also a convenient precursor for the synthesis of azo-derivatized framework ligands, a key component in the rapidly evolving field of photochromic MOFs. (Brown et al., 2013 ▸; Castellanos et al., 2016 ▸; Walton et al., 2013 ▸; Patel et al., 2014 ▸). Similarly, 1,2-bis(pyridin-4-yl)ethene (BE) is also commonly used in MOF synthesis; however, it is routinely used in co-crystal engineering as well (Kongshaug & Fjellvag, 2003 ▸; MacGillivray et al., 2008 ▸; Desiraju, 1995 ▸) The 5AIA–BE co-crystal presented herein was produced as part of an undergraduate physical chemistry laboratory experiment developed by Jason Benedict.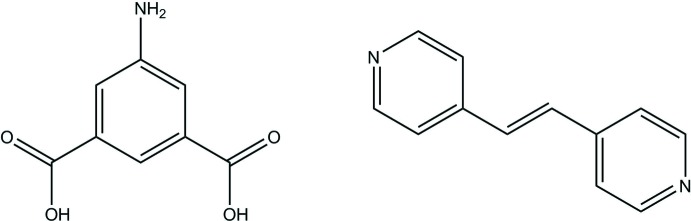
Recently, the co-crystal structure of 5AIA and 4,4′-bipyridine (BP), a shorter analogue of BE, was reported (Zhang et al., 2009 ▸). Unlike many MOFs in which different length linkers lead to isorecticular structures (Eddaoudi et al., 2002 ▸), the 5AIA–BP co-crystal exhibits several notable similarities and differences when compared to 5AIA–BE. As shown in Figs. 4, 5AIA forms hydrogen bonds with two 5AIA molecules and two BP molecules. The 5AIA–BP interactions and one of the 5AIA–5AIA interactions are similar to those found in 5AIA–BE. The remaining 5AIA–5AIA interaction in 5AIA–BP consists solely of an N(amine)–H⋯OH hydrogen bond, as opposed to the N(amine)—H⋯O=C interaction found in 5AIA–BP. Interestingly, this results in a total of five hydrogen bonds in the 5AIA–BP structure compared to the six hydrogen bonds observed in 5AIA–BE.
Structural commentary
The 5AIA–BE co-crystal crystallizes with one molecule of 5AIA and one molecule of BE in the asymmetric unit (Fig. 1 ▸). Both molecules are effectively planar in the solid state (r.m.s. deviation for 5AIA = 0.155 Å). The BE moiety shows whole molecule disorder over two sets of sites, consistent with a local C2 rotation about the long axis of the molecule. The occupancy of the major and minor components was refined to be 0.588 (3) and 0.412 (3), respectively.
Figure 1.
The asymmetric unit of the title compound, showing the numbering scheme. Displacement ellipsoids are shown at the 50% probability level.
Supramolecular features
In this structure, the 5AIA molecule forms hydrogen bonds to both itself and the BE moiety, forming extended sheets (Table 1 ▸ and Fig. 2 ▸). The 5AIA–5AIA interactions consist of N(amine)—H⋯O=C hydrogen bonds where each 5AIA makes two hydrogen bonds with two neighboring 5AIA molecules. The 5AIA–BE interaction consists of an O—H⋯N(pyridyl) hydrogen bond such that each 5AIA makes one hydrogen bond with two neighboring BE molecules. The sheets formed by these interactions stack along the the a axis to produce a layered structure (Fig. 3 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1A⋯O1i | 0.899 (17) | 2.062 (17) | 2.9540 (13) | 171.0 (15) |
| N1—H1B⋯O3ii | 0.894 (17) | 2.157 (17) | 3.0500 (13) | 178.6 (13) |
| O2—H2⋯N3iii | 0.989 (19) | 1.70 (2) | 2.688 (8) | 173.4 (18) |
| O2—H2⋯N3A iii | 0.989 (19) | 1.63 (2) | 2.619 (12) | 177 (2) |
| O4—H4⋯N2iv | 0.98 (2) | 1.72 (2) | 2.702 (7) | 173.2 (19) |
| O4—H4⋯N2A iv | 0.98 (2) | 1.59 (2) | 2.566 (11) | 175 (2) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Figure 2.
Diagram illustrating the hydrogen-bonding interactions present in the two-dimensional sheets found in the 5AIA–BE co-crystal.
Figure 3.
View down [001] showing the (100) sheets in the extended structure of the title compound.
Database survey
Recently, the co-crystal structure of 5AIA and 4,4′-bipyridine (BP), a shorter analogue of BE, was reported (Zhang et al., 2009 ▸). Unlike many MOFs in which different length linkers lead to isorecticular structures (Eddaoudi et al., 2002 ▸), the 5AIA–BP co-crystal exhibits several notable similarites and differences when compared to 5AIA–BE. As shown in Figs. 4 ▸, 5AIA forms hydrogen bonds with two 5AIA molecules and two BP molecules. The 5AIA–BP interactions and one of the 5AIA–5AIA interactions are similar to those found in 5AIA–BE. The remaining 5AIA–5AIA interaction in 5AIA–BP consists solely of an N(amine)—H⋯OH hydrogen bond, as opposed to the N(amine)—H⋯O=C interaction found in 5AIA–BP. Interestingly, this results in a total of five hydrogen bonds in the 5AIA–BP structure compared to the six hydrogen bonds observed in 5AIA–BE.
Figure 4.
Diagram illustrating the hydrogen bonding interactions present in the previously reported 5AIA–BP co-crystal.
Synthesis and crystallization
Solid BE (0.0119 g, 6.53 × 10−5 mol) and 5AIA (0.0109 g, 6.02 × 10−5 mol) were added to a 25 ml scintillation vial. To this was added approximately 15 ml of ethyl acetate followed by gentle heating. An additional 2 ml of methanol was added and all remaining solids dissolved. The loosely capped vial was then placed into a dark cabinet. After two weeks, yellow block-shaped crystals of the title compound suitable for single-crystal X-ray diffraction measurements were obtained.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. Heteroatom hydrogen atoms were located in difference electron-density maps and freely refined. Hydrogen atoms attached to carbon atoms were refined using riding models with C—H = 0.95 Å and U iso(H) = 1.2U eq(C). The BE was found to be disordered over two sets of sites in a 0.588 (3): 0.412 (3) ratio.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C12H10N2·C8H7NO4 |
| M r | 363.36 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 90 |
| a, b, c (Å) | 10.1614 (10), 12.0782 (12), 14.0537 (14) |
| β (°) | 95.027 (2) |
| V (Å3) | 1718.2 (3) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.10 |
| Crystal size (mm) | 0.22 × 0.2 × 0.18 |
| Data collection | |
| Diffractometer | Bruker SMART APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2014 ▸) |
| T min, T max | 0.683, 0.747 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 24372, 6546, 4519 |
| R int | 0.033 |
| (sin θ/λ)max (Å−1) | 0.771 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.047, 0.143, 1.02 |
| No. of reflections | 6546 |
| No. of parameters | 378 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.40, −0.24 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989016005259/hb7561sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016005259/hb7561Isup2.hkl
CCDC reference: 1471029
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
This material is based upon work supported by the National Science Foundation under grant No. DMR-1455039.
supplementary crystallographic information
Crystal data
| C12H10N2·C8H7NO4 | F(000) = 760 |
| Mr = 363.36 | Dx = 1.405 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.1614 (10) Å | Cell parameters from 428 reflections |
| b = 12.0782 (12) Å | θ = 2.8–22.0° |
| c = 14.0537 (14) Å | µ = 0.10 mm−1 |
| β = 95.027 (2)° | T = 90 K |
| V = 1718.2 (3) Å3 | Block, yellow |
| Z = 4 | 0.22 × 0.2 × 0.18 mm |
Data collection
| Bruker SMART APEXII CCD diffractometer | 6546 independent reflections |
| Radiation source: microfocus rotating anode, Incoatec Iµs | 4519 reflections with I > 2σ(I) |
| Mirror optics monochromator | Rint = 0.033 |
| Detector resolution: 7.9 pixels mm-1 | θmax = 33.2°, θmin = 2.2° |
| ω scans | h = −15→15 |
| Absorption correction: multi-scan (SADABS; Bruker, 2014) | k = −16→18 |
| Tmin = 0.683, Tmax = 0.747 | l = −19→21 |
| 24372 measured reflections |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.047 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.143 | w = 1/[σ2(Fo2) + (0.0727P)2 + 0.2884P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max < 0.001 |
| 6546 reflections | Δρmax = 0.40 e Å−3 |
| 378 parameters | Δρmin = −0.24 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1 | 0.21821 (10) | 0.91797 (6) | 0.42759 (6) | 0.0299 (2) | |
| O2 | 0.18838 (9) | 0.81890 (6) | 0.55840 (5) | 0.02654 (18) | |
| H2 | 0.1646 (19) | 0.8932 (16) | 0.5810 (13) | 0.058 (5)* | |
| O3 | 0.35542 (10) | 0.33662 (6) | 0.42624 (6) | 0.0309 (2) | |
| O4 | 0.35891 (9) | 0.43117 (6) | 0.56369 (5) | 0.02640 (18) | |
| H4 | 0.388 (2) | 0.3606 (16) | 0.5933 (14) | 0.064 (6)* | |
| N1 | 0.27113 (12) | 0.63283 (8) | 0.17186 (7) | 0.0291 (2) | |
| H1A | 0.2656 (17) | 0.5692 (14) | 0.1385 (12) | 0.043 (4)* | |
| H1B | 0.2351 (16) | 0.6925 (14) | 0.1424 (11) | 0.040 (4)* | |
| C1 | 0.27076 (11) | 0.62966 (8) | 0.26981 (7) | 0.02010 (19) | |
| C2 | 0.29909 (11) | 0.53166 (8) | 0.32097 (7) | 0.02032 (19) | |
| H2A | 0.3164 | 0.4657 | 0.2874 | 0.024* | |
| C3 | 0.30223 (10) | 0.52951 (8) | 0.42012 (7) | 0.01921 (19) | |
| C4 | 0.27368 (11) | 0.62462 (8) | 0.47130 (7) | 0.01990 (19) | |
| H4A | 0.2728 | 0.6226 | 0.5388 | 0.024* | |
| C5 | 0.24644 (10) | 0.72289 (8) | 0.42033 (7) | 0.01881 (19) | |
| C6 | 0.24657 (10) | 0.72561 (8) | 0.32144 (7) | 0.01947 (19) | |
| H6 | 0.2300 | 0.7935 | 0.2884 | 0.023* | |
| C7 | 0.21669 (11) | 0.82903 (8) | 0.46877 (7) | 0.0211 (2) | |
| C8 | 0.34070 (11) | 0.42315 (8) | 0.46985 (7) | 0.0215 (2) | |
| C18 | 0.89056 (13) | 0.89912 (9) | 0.43551 (9) | 0.0306 (3) | |
| H18 | 0.8831 | 0.9091 | 0.5019 | 0.037* | 0.588 (3) |
| H18A | 0.8865 | 0.9167 | 0.5011 | 0.037* | 0.412 (3) |
| N2 | 0.9309 (8) | 0.2578 (6) | 0.1571 (3) | 0.0206 (8) | 0.588 (3) |
| N3 | 0.8888 (10) | 0.9864 (5) | 0.3724 (9) | 0.0203 (11) | 0.588 (3) |
| C9 | 0.9283 (3) | 0.3491 (2) | 0.1046 (2) | 0.0259 (5) | 0.588 (3) |
| H9 | 0.9195 | 0.3426 | 0.0370 | 0.031* | 0.588 (3) |
| C10 | 0.9382 (2) | 0.45426 (16) | 0.1453 (2) | 0.0246 (4) | 0.588 (3) |
| H10 | 0.9393 | 0.5181 | 0.1058 | 0.029* | 0.588 (3) |
| C11 | 0.94634 (18) | 0.46501 (16) | 0.24375 (18) | 0.0202 (4) | 0.588 (3) |
| C12 | 0.9527 (2) | 0.36771 (18) | 0.29764 (18) | 0.0260 (5) | 0.588 (3) |
| H12 | 0.9624 | 0.3709 | 0.3654 | 0.031* | 0.588 (3) |
| C13 | 0.9446 (4) | 0.2666 (3) | 0.2511 (2) | 0.0240 (6) | 0.588 (3) |
| H13 | 0.9491 | 0.2008 | 0.2883 | 0.029* | 0.588 (3) |
| C14 | 0.9425 (2) | 0.57258 (15) | 0.29274 (13) | 0.0245 (5) | 0.588 (3) |
| H14 | 0.9538 | 0.5724 | 0.3606 | 0.029* | 0.588 (3) |
| C15 | 0.9246 (2) | 0.66976 (15) | 0.24941 (15) | 0.0241 (4) | 0.588 (3) |
| H15 | 0.9170 | 0.6692 | 0.1816 | 0.029* | 0.588 (3) |
| C16 | 0.9152 (3) | 0.7790 (2) | 0.2963 (2) | 0.0182 (5) | 0.588 (3) |
| C17 | 0.9045 (7) | 0.7916 (5) | 0.3934 (2) | 0.0242 (8) | 0.588 (3) |
| H17 | 0.9064 | 0.7278 | 0.4330 | 0.029* | 0.588 (3) |
| C19 | 0.9010 (9) | 0.9740 (6) | 0.2814 (5) | 0.0214 (8) | 0.588 (3) |
| H19 | 0.8997 | 1.0381 | 0.2422 | 0.026* | 0.588 (3) |
| C20 | 0.9153 (4) | 0.8733 (3) | 0.2402 (3) | 0.0244 (6) | 0.588 (3) |
| H20 | 0.9254 | 0.8678 | 0.1738 | 0.029* | 0.588 (3) |
| C17A | 0.9027 (10) | 0.7907 (7) | 0.4220 (4) | 0.0258 (10) | 0.412 (3) |
| H17A | 0.9026 | 0.7388 | 0.4728 | 0.031* | 0.412 (3) |
| C9A | 0.9486 (4) | 0.3433 (3) | 0.0717 (3) | 0.0219 (7) | 0.412 (3) |
| H9A | 0.9550 | 0.3259 | 0.0064 | 0.026* | 0.412 (3) |
| C20A | 0.9161 (6) | 0.8429 (4) | 0.2607 (4) | 0.0260 (11) | 0.412 (3) |
| H20A | 0.9275 | 0.8265 | 0.1958 | 0.031* | 0.412 (3) |
| N2A | 0.9319 (11) | 0.2579 (10) | 0.1328 (4) | 0.0202 (10) | 0.412 (3) |
| N3A | 0.8830 (15) | 0.9861 (9) | 0.3829 (13) | 0.025 (2) | 0.412 (3) |
| C19A | 0.8995 (15) | 0.9532 (9) | 0.2902 (9) | 0.034 (2) | 0.412 (3) |
| H19A | 0.8996 | 1.0094 | 0.2428 | 0.040* | 0.412 (3) |
| C10A | 0.9569 (3) | 0.4532 (2) | 0.0982 (3) | 0.0243 (6) | 0.412 (3) |
| H10A | 0.9663 | 0.5087 | 0.0514 | 0.029* | 0.412 (3) |
| C11A | 0.9515 (3) | 0.4826 (2) | 0.1934 (3) | 0.0195 (6) | 0.412 (3) |
| C12A | 0.9434 (3) | 0.3974 (3) | 0.2586 (3) | 0.0250 (6) | 0.412 (3) |
| H12A | 0.9448 | 0.4125 | 0.3250 | 0.030* | 0.412 (3) |
| C13A | 0.9330 (6) | 0.2880 (4) | 0.2245 (4) | 0.0293 (10) | 0.412 (3) |
| H13A | 0.9262 | 0.2309 | 0.2703 | 0.035* | 0.412 (3) |
| C14A | 0.9493 (3) | 0.6007 (2) | 0.21878 (19) | 0.0235 (6) | 0.412 (3) |
| H14A | 0.9676 | 0.6526 | 0.1709 | 0.028* | 0.412 (3) |
| C15A | 0.9237 (3) | 0.6405 (2) | 0.3033 (2) | 0.0226 (6) | 0.412 (3) |
| H15A | 0.9095 | 0.5884 | 0.3521 | 0.027* | 0.412 (3) |
| C16A | 0.9155 (4) | 0.7585 (4) | 0.3276 (3) | 0.0186 (7) | 0.412 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0508 (6) | 0.0126 (3) | 0.0286 (4) | 0.0008 (3) | 0.0170 (4) | −0.0002 (3) |
| O2 | 0.0459 (5) | 0.0155 (3) | 0.0195 (3) | 0.0059 (3) | 0.0096 (3) | −0.0016 (3) |
| O3 | 0.0510 (6) | 0.0143 (3) | 0.0270 (4) | 0.0041 (3) | 0.0012 (4) | −0.0046 (3) |
| O4 | 0.0438 (5) | 0.0149 (3) | 0.0201 (3) | 0.0063 (3) | 0.0006 (3) | −0.0003 (3) |
| N1 | 0.0510 (7) | 0.0180 (4) | 0.0186 (4) | 0.0067 (4) | 0.0058 (4) | −0.0018 (3) |
| C1 | 0.0246 (5) | 0.0175 (4) | 0.0187 (4) | 0.0010 (4) | 0.0046 (4) | −0.0022 (3) |
| C2 | 0.0255 (5) | 0.0148 (4) | 0.0210 (4) | 0.0017 (4) | 0.0039 (4) | −0.0038 (3) |
| C3 | 0.0242 (5) | 0.0130 (4) | 0.0206 (4) | 0.0003 (3) | 0.0030 (4) | −0.0012 (3) |
| C4 | 0.0269 (5) | 0.0142 (4) | 0.0191 (4) | 0.0002 (4) | 0.0047 (4) | −0.0016 (3) |
| C5 | 0.0235 (5) | 0.0130 (4) | 0.0206 (4) | −0.0001 (3) | 0.0056 (4) | −0.0026 (3) |
| C6 | 0.0236 (5) | 0.0140 (4) | 0.0213 (4) | 0.0010 (3) | 0.0050 (4) | −0.0006 (3) |
| C7 | 0.0285 (5) | 0.0142 (4) | 0.0214 (4) | 0.0004 (4) | 0.0069 (4) | −0.0025 (3) |
| C8 | 0.0285 (5) | 0.0145 (4) | 0.0217 (5) | −0.0002 (4) | 0.0025 (4) | −0.0023 (3) |
| C18 | 0.0394 (7) | 0.0178 (5) | 0.0360 (6) | 0.0017 (4) | 0.0118 (5) | −0.0001 (4) |
| N2 | 0.0261 (11) | 0.0147 (9) | 0.021 (2) | −0.0007 (7) | 0.0014 (18) | −0.0088 (18) |
| N3 | 0.0306 (19) | 0.0099 (16) | 0.022 (3) | 0.0050 (11) | 0.0112 (14) | −0.0001 (12) |
| C9 | 0.0351 (14) | 0.0194 (9) | 0.0238 (13) | −0.0024 (8) | 0.0066 (10) | −0.0027 (10) |
| C10 | 0.0400 (12) | 0.0158 (8) | 0.0182 (11) | −0.0012 (7) | 0.0044 (9) | −0.0006 (8) |
| C11 | 0.0232 (9) | 0.0158 (10) | 0.0219 (11) | −0.0012 (6) | 0.0037 (7) | −0.0024 (7) |
| C12 | 0.0388 (12) | 0.0170 (9) | 0.0225 (10) | 0.0016 (8) | 0.0038 (9) | −0.0012 (8) |
| C13 | 0.0341 (13) | 0.0160 (13) | 0.0225 (15) | 0.0007 (10) | 0.0055 (12) | −0.0034 (9) |
| C14 | 0.0348 (11) | 0.0173 (9) | 0.0218 (8) | 0.0012 (7) | 0.0053 (7) | −0.0064 (6) |
| C15 | 0.0325 (10) | 0.0176 (8) | 0.0223 (9) | −0.0004 (7) | 0.0021 (7) | −0.0056 (7) |
| C16 | 0.0226 (9) | 0.0109 (14) | 0.0211 (13) | 0.0001 (8) | 0.0021 (10) | 0.0004 (10) |
| C17 | 0.0346 (13) | 0.0133 (9) | 0.026 (2) | 0.0011 (8) | 0.0086 (18) | −0.0038 (17) |
| C19 | 0.0301 (15) | 0.0192 (19) | 0.0155 (15) | 0.0019 (14) | 0.0054 (11) | −0.0047 (15) |
| C20 | 0.0324 (12) | 0.0204 (16) | 0.0205 (14) | 0.0012 (12) | 0.0019 (10) | −0.0034 (10) |
| C17A | 0.0330 (18) | 0.0195 (15) | 0.027 (3) | 0.0003 (12) | 0.011 (3) | −0.005 (2) |
| C9A | 0.0281 (17) | 0.0136 (11) | 0.0247 (18) | −0.0015 (10) | 0.0065 (14) | −0.0010 (13) |
| C20A | 0.0378 (18) | 0.019 (3) | 0.021 (2) | 0.0028 (19) | 0.0022 (17) | −0.0085 (17) |
| N2A | 0.0234 (15) | 0.0201 (13) | 0.017 (3) | 0.0010 (10) | −0.001 (2) | −0.010 (2) |
| N3A | 0.026 (3) | 0.033 (4) | 0.016 (3) | −0.001 (2) | 0.008 (2) | −0.011 (2) |
| C19A | 0.039 (3) | 0.029 (4) | 0.032 (3) | −0.002 (3) | 0.0015 (19) | 0.006 (2) |
| C10A | 0.0320 (15) | 0.0166 (11) | 0.0243 (15) | −0.0019 (10) | 0.0035 (12) | −0.0024 (10) |
| C11A | 0.0250 (13) | 0.0163 (11) | 0.0170 (15) | −0.0012 (9) | 0.0008 (10) | −0.0038 (11) |
| C12A | 0.0386 (17) | 0.0155 (16) | 0.0209 (15) | 0.0010 (11) | 0.0017 (12) | −0.0020 (11) |
| C13A | 0.041 (2) | 0.019 (2) | 0.027 (3) | 0.0004 (15) | 0.0012 (19) | 0.0024 (15) |
| C14A | 0.0299 (14) | 0.0125 (10) | 0.0283 (13) | −0.0003 (9) | 0.0033 (10) | −0.0035 (9) |
| C15A | 0.0308 (14) | 0.0119 (12) | 0.0251 (14) | 0.0007 (9) | 0.0020 (10) | −0.0013 (9) |
| C16A | 0.0207 (13) | 0.0107 (14) | 0.024 (2) | 0.0002 (10) | 0.0012 (14) | 0.0033 (15) |
Geometric parameters (Å, º)
| O1—C7 | 1.2209 (12) | C12—H12 | 0.9500 |
| O2—H2 | 0.990 (19) | C12—C13 | 1.385 (4) |
| O2—C7 | 1.3222 (12) | C13—H13 | 0.9500 |
| O3—C8 | 1.2274 (12) | C14—H14 | 0.9500 |
| O4—H4 | 0.98 (2) | C14—C15 | 1.327 (3) |
| O4—C8 | 1.3191 (12) | C15—H15 | 0.9500 |
| N1—H1A | 0.899 (17) | C15—C16 | 1.482 (3) |
| N1—H1B | 0.894 (17) | C16—C17 | 1.387 (4) |
| N1—C1 | 1.3775 (13) | C16—C20 | 1.385 (3) |
| C1—C2 | 1.4017 (14) | C17—H17 | 0.9500 |
| C1—C6 | 1.4005 (13) | C19—H19 | 0.9500 |
| C2—H2A | 0.9500 | C19—C20 | 1.360 (7) |
| C2—C3 | 1.3912 (14) | C20—H20 | 0.9500 |
| C3—C4 | 1.3991 (13) | C17A—H17A | 0.9500 |
| C3—C8 | 1.4979 (14) | C17A—C16A | 1.399 (6) |
| C4—H4A | 0.9500 | C9A—H9A | 0.9500 |
| C4—C5 | 1.4014 (13) | C9A—N2A | 1.361 (10) |
| C5—C6 | 1.3902 (14) | C9A—C10A | 1.379 (4) |
| C5—C7 | 1.4948 (13) | C20A—H20A | 0.9500 |
| C6—H6 | 0.9500 | C20A—C19A | 1.410 (11) |
| C18—H18 | 0.9500 | C20A—C16A | 1.388 (5) |
| C18—H18A | 0.9500 | N2A—C13A | 1.338 (7) |
| C18—N3 | 1.376 (9) | N3A—C19A | 1.39 (2) |
| C18—C17 | 1.439 (5) | C19A—H19A | 0.9500 |
| C18—C17A | 1.331 (9) | C10A—H10A | 0.9500 |
| C18—N3A | 1.283 (13) | C10A—C11A | 1.390 (5) |
| N2—C9 | 1.326 (7) | C11A—C12A | 1.385 (4) |
| N2—C13 | 1.320 (5) | C11A—C14A | 1.472 (4) |
| N3—C19 | 1.305 (13) | C12A—H12A | 0.9500 |
| C9—H9 | 0.9500 | C12A—C13A | 1.406 (6) |
| C9—C10 | 1.392 (3) | C13A—H13A | 0.9500 |
| C10—H10 | 0.9500 | C14A—H14A | 0.9500 |
| C10—C11 | 1.386 (3) | C14A—C15A | 1.329 (4) |
| C11—C12 | 1.397 (3) | C15A—H15A | 0.9500 |
| C11—C14 | 1.472 (2) | C15A—C16A | 1.469 (5) |
| C7—O2—H2 | 107.5 (11) | C15—C14—C11 | 125.02 (18) |
| C8—O4—H4 | 111.7 (11) | C15—C14—H14 | 117.5 |
| H1A—N1—H1B | 116.4 (15) | C14—C15—H15 | 116.7 |
| C1—N1—H1A | 119.4 (11) | C14—C15—C16 | 126.5 (2) |
| C1—N1—H1B | 116.6 (10) | C16—C15—H15 | 116.7 |
| N1—C1—C2 | 121.17 (9) | C17—C16—C15 | 123.3 (3) |
| N1—C1—C6 | 120.72 (9) | C20—C16—C15 | 118.4 (3) |
| C6—C1—C2 | 118.07 (9) | C20—C16—C17 | 118.3 (4) |
| C1—C2—H2A | 119.5 | C18—C17—H17 | 119.2 |
| C3—C2—C1 | 121.01 (9) | C16—C17—C18 | 121.5 (4) |
| C3—C2—H2A | 119.5 | C16—C17—H17 | 119.2 |
| C2—C3—C4 | 120.80 (9) | N3—C19—H19 | 118.6 |
| C2—C3—C8 | 117.74 (8) | N3—C19—C20 | 122.9 (6) |
| C4—C3—C8 | 121.44 (9) | C20—C19—H19 | 118.6 |
| C3—C4—H4A | 120.9 | C16—C20—H20 | 120.4 |
| C3—C4—C5 | 118.24 (9) | C19—C20—C16 | 119.2 (4) |
| C5—C4—H4A | 120.9 | C19—C20—H20 | 120.4 |
| C4—C5—C7 | 122.17 (9) | C18—C17A—H17A | 122.4 |
| C6—C5—C4 | 120.90 (9) | C18—C17A—C16A | 115.3 (5) |
| C6—C5—C7 | 116.93 (8) | C16A—C17A—H17A | 122.4 |
| C1—C6—H6 | 119.5 | N2A—C9A—H9A | 117.7 |
| C5—C6—C1 | 120.93 (9) | N2A—C9A—C10A | 124.5 (5) |
| C5—C6—H6 | 119.5 | C10A—C9A—H9A | 117.7 |
| O1—C7—O2 | 123.09 (9) | C19A—C20A—H20A | 120.4 |
| O1—C7—C5 | 121.82 (9) | C16A—C20A—H20A | 120.4 |
| O2—C7—C5 | 115.09 (8) | C16A—C20A—C19A | 119.2 (6) |
| O3—C8—O4 | 123.31 (9) | C13A—N2A—C9A | 114.3 (9) |
| O3—C8—C3 | 122.38 (9) | C18—N3A—C19A | 107.5 (10) |
| O4—C8—C3 | 114.30 (8) | C20A—C19A—H19A | 117.5 |
| N3—C18—H18 | 122.5 | N3A—C19A—C20A | 125.0 (9) |
| N3—C18—C17 | 115.0 (5) | N3A—C19A—H19A | 117.5 |
| C17—C18—H18 | 122.5 | C9A—C10A—H10A | 120.1 |
| C17A—C18—H18A | 111.8 | C9A—C10A—C11A | 119.9 (3) |
| N3A—C18—H18A | 111.8 | C11A—C10A—H10A | 120.1 |
| N3A—C18—C17A | 136.5 (8) | C10A—C11A—C14A | 118.9 (3) |
| C13—N2—C9 | 119.0 (5) | C12A—C11A—C10A | 117.2 (2) |
| C19—N3—C18 | 123.1 (6) | C12A—C11A—C14A | 123.8 (3) |
| N2—C9—H9 | 118.9 | C11A—C12A—H12A | 120.7 |
| N2—C9—C10 | 122.3 (3) | C11A—C12A—C13A | 118.6 (3) |
| C10—C9—H9 | 118.9 | C13A—C12A—H12A | 120.7 |
| C9—C10—H10 | 120.3 | N2A—C13A—C12A | 125.3 (6) |
| C11—C10—C9 | 119.4 (2) | N2A—C13A—H13A | 117.4 |
| C11—C10—H10 | 120.3 | C12A—C13A—H13A | 117.4 |
| C10—C11—C12 | 117.31 (16) | C11A—C14A—H14A | 117.4 |
| C10—C11—C14 | 123.2 (2) | C15A—C14A—C11A | 125.2 (3) |
| C12—C11—C14 | 119.4 (2) | C15A—C14A—H14A | 117.4 |
| C11—C12—H12 | 120.4 | C14A—C15A—H15A | 117.3 |
| C13—C12—C11 | 119.2 (2) | C14A—C15A—C16A | 125.3 (3) |
| C13—C12—H12 | 120.4 | C16A—C15A—H15A | 117.3 |
| N2—C13—C12 | 122.7 (4) | C17A—C16A—C15A | 120.1 (5) |
| N2—C13—H13 | 118.6 | C20A—C16A—C17A | 116.4 (5) |
| C12—C13—H13 | 118.6 | C20A—C16A—C15A | 123.5 (4) |
| C11—C14—H14 | 117.5 | ||
| N1—C1—C2—C3 | 178.19 (10) | C11—C14—C15—C16 | 177.6 (2) |
| N1—C1—C6—C5 | −179.82 (10) | C12—C11—C14—C15 | −173.6 (2) |
| C1—C2—C3—C4 | 1.71 (16) | C13—N2—C9—C10 | −0.7 (9) |
| C1—C2—C3—C8 | −176.64 (10) | C14—C11—C12—C13 | 174.4 (3) |
| C2—C1—C6—C5 | −2.05 (16) | C14—C15—C16—C17 | −10.0 (5) |
| C2—C3—C4—C5 | −2.20 (16) | C14—C15—C16—C20 | 170.8 (3) |
| C2—C3—C8—O3 | −7.84 (17) | C15—C16—C17—C18 | −177.6 (3) |
| C2—C3—C8—O4 | 171.27 (10) | C15—C16—C20—C19 | 177.1 (5) |
| C3—C4—C5—C6 | 0.59 (16) | C17—C18—N3—C19 | −1.1 (12) |
| C3—C4—C5—C7 | −179.15 (10) | C17—C18—C17A—C16A | 1.4 (17) |
| C4—C3—C8—O3 | 173.81 (11) | C17—C18—N3A—C19A | −3.8 (15) |
| C4—C3—C8—O4 | −7.07 (15) | C17—C16—C20—C19 | −2.0 (7) |
| C4—C5—C6—C1 | 1.56 (16) | C20—C16—C17—C18 | 1.5 (7) |
| C4—C5—C7—O1 | 165.59 (11) | C17A—C18—N3—C19 | −0.5 (14) |
| C4—C5—C7—O2 | −14.70 (15) | C17A—C18—C17—C16 | −179 (3) |
| C6—C1—C2—C3 | 0.43 (16) | C17A—C18—N3A—C19A | −4.3 (18) |
| C6—C5—C7—O1 | −14.15 (16) | C9A—N2A—C13A—C12A | 3.1 (13) |
| C6—C5—C7—O2 | 165.55 (10) | C9A—C10A—C11A—C12A | 2.7 (4) |
| C7—C5—C6—C1 | −178.69 (10) | C9A—C10A—C11A—C14A | −174.9 (3) |
| C8—C3—C4—C5 | 176.09 (10) | N2A—C9A—C10A—C11A | 1.6 (8) |
| C18—N3—C19—C20 | 0.6 (15) | N3A—C18—N3—C19 | −159 (11) |
| C18—C17A—C16A—C20A | 0.9 (9) | N3A—C18—C17—C16 | 2.5 (10) |
| C18—C17A—C16A—C15A | −177.3 (5) | N3A—C18—C17A—C16A | 2.8 (15) |
| C18—N3A—C19A—C20A | 2 (2) | C19A—C20A—C16A—C17A | −2.1 (11) |
| N2—C9—C10—C11 | −2.3 (6) | C19A—C20A—C16A—C15A | 176.0 (8) |
| N3—C18—C17—C16 | 0.0 (8) | C10A—C9A—N2A—C13A | −4.4 (12) |
| N3—C18—C17A—C16A | −0.3 (12) | C10A—C11A—C12A—C13A | −3.8 (5) |
| N3—C18—N3A—C19A | 19 (9) | C10A—C11A—C14A—C15A | 168.9 (3) |
| N3—C19—C20—C16 | 1.0 (12) | C11A—C12A—C13A—N2A | 0.9 (9) |
| C9—N2—C13—C12 | 1.9 (9) | C11A—C14A—C15A—C16A | −177.1 (3) |
| C9—C10—C11—C12 | 4.0 (3) | C12A—C11A—C14A—C15A | −8.5 (5) |
| C9—C10—C11—C14 | −173.2 (2) | C14A—C11A—C12A—C13A | 173.6 (4) |
| C10—C11—C12—C13 | −2.9 (3) | C14A—C15A—C16A—C17A | −172.8 (5) |
| C10—C11—C14—C15 | 3.5 (3) | C14A—C15A—C16A—C20A | 9.1 (6) |
| C11—C12—C13—N2 | −0.1 (6) | C16A—C20A—C19A—N3A | 0.5 (19) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···O1i | 0.899 (17) | 2.062 (17) | 2.9540 (13) | 171.0 (15) |
| N1—H1B···O3ii | 0.894 (17) | 2.157 (17) | 3.0500 (13) | 178.6 (13) |
| O2—H2···N3iii | 0.989 (19) | 1.70 (2) | 2.688 (8) | 173.4 (18) |
| O2—H2···N3Aiii | 0.989 (19) | 1.63 (2) | 2.619 (12) | 177 (2) |
| O4—H4···N2iv | 0.98 (2) | 1.72 (2) | 2.702 (7) | 173.2 (19) |
| O4—H4···N2Aiv | 0.98 (2) | 1.59 (2) | 2.566 (11) | 175 (2) |
Symmetry codes: (i) −x+1/2, y−1/2, −z+1/2; (ii) −x+1/2, y+1/2, −z+1/2; (iii) −x+1, −y+2, −z+1; (iv) x−1/2, −y+1/2, z+1/2.
References
- Brown, J. W., Henderson, B. L., Kiesz, M. D., Whalley, A. C., Morris, W., Grunder, S., Deng, H., Furukawa, H., Zink, J. I., Stoddart, J. F. & Yaghi, O. M. (2013). Chem. Sci. 4, 2858–2864.
- Bruker (2014). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Castellanos, S., Goulet-Hanssens, A., Zhao, F., Dikhtiarenko, A., Pustovarenko, A., Hecht, S., Gascon, J., Kapteijn, F. & Bléger, D. (2016). Chem. Eur. J. 22, 746–752. [DOI] [PubMed]
- Cox, J. M., Walton, I. M., Benson, C. A., Chen, Y.-S. & Benedict, J. B. (2015). J. Appl. Cryst. 48, 578–581.
- Desiraju, G. R. (1995). Angew. Chem. Int. Ed. Engl. 34, 2311–2327.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Eddaoudi, M., Kim, J., Rosi, N., Vodak, D., Wachter, J., O’Keeffe, M. & Yaghi, O. M. (2002). Science, 295, 469–472. [DOI] [PubMed]
- Kongshaug, K. O. & Fjellvåg, H. (2003). J. Solid State Chem. 175, 182–187.
- MacGillivray, L. R., Papaefstathiou, G. S., Friščić, T., Hamilton, T. D., Bučar, D.-K., Chu, Q., Varshney, D. B. & Georgiev, I. G. (2008). Acc. Chem. Res. 41, 280–291. [DOI] [PubMed]
- Patel, D. G. (D.), Walton, I. M., Cox, J. M., Gleason, C. J., Butzer, D. R. & Benedict, J. B. (2014). Chem. Commun. 50, 2653–2656. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Walton, I. M., Cox, J. M., Coppin, J. A., Linderman, C. M., Patel, D. G. (D.) & Benedict, J. B. (2013). Chem. Commun. 49, 8012–8014. [DOI] [PubMed]
- Wang, H.-N., Meng, X., Yang, G.-S., Wang, X.-L., Shao, K.-Z., Su, Z.-M. & Wang, C.-G. (2011). Chem. Commun. 47, 7128–7130. [DOI] [PubMed]
- Zeng, M.-H., Hu, S., Chen, Q., Xie, G., Shuai, Q., Gao, S.-L. & Tang, L.-Y. (2009). Inorg. Chem. 48, 7070–7079. [DOI] [PubMed]
- Zhang, X., Zhu, B. & Guo, F. (2009). Asian J. Chem. 21, 7072–7076.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989016005259/hb7561sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016005259/hb7561Isup2.hkl
CCDC reference: 1471029
Additional supporting information: crystallographic information; 3D view; checkCIF report






