Crystallographic analysis of a nucleoside analog of the 2′-deoxyguanosine/abasic site cross-link is presented. This structure corroborates an earlier two-dimensional NMR analysis, concluding that the 2-deoxyribose unit attached at the exocyclic N 2-amino group of the guanine residue exists in the cyclic aminoglycoside form.
Keywords: crystal structure, purine-6(9H)-one, 2′-deoxyguanosine, deoxy-d-ribofuranose, glycosidic linkage, nucleobase, hydrogen bonding
Abstract
The title compound, 9-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-2-{[(2R,4S,5R)-4-methoxy-5-(methoxymethyl)tetrahydrofuran-2-yl]amino}-1H-purin-6(9H)-one, C17H25N5O7, crystallizes with two independent molecules (A and B) in the asymmetric unit. In the crystal, the guanosine moieties of molecules A and B are linked by N—H⋯N and O—H⋯N hydrogen-bonding interactions, forming ribbons which are stacked to form columns along [100]. These columns are then linked by O—H⋯O hydrogen bonds between the ribose moieties and numerous C—H⋯O interactions to complete the three-dimensional structure.
Chemical context
Recent work has characterized a structurally novel set of interstrand DNA–DNA cross-links involving reaction of the ubiquitous DNA abasic lesion with a nucleobase on the opposing strand of the double helix (Catalano et al., 2015 ▸; Dutta et al., 2007 ▸; Gamboa Varela & Gates, 2015 ▸; Johnson et al., 2013 ▸; Price et al., 2014 ▸, 2015 ▸; Yang et al., 2015 ▸; Zhang et al., 2015 ▸). Evidence indicates that the covalent attachment is forged between the anomeric carbon of the abasic sugar and the exocyclic amino group of either a guanine, adenine, or N
4-aminocytosine residue (Catalano et al., 2015 ▸; Dutta et al., 2007 ▸; Gamboa Varela & Gates, 2015 ▸; Johnson et al., 2013 ▸; Price et al., 2014 ▸, 2015 ▸; Yang et al., 2015 ▸). This type of glycosidic linkage involving the exocyclic amino group of a nucleobase is reminiscent of that found in the natural products anicemycin, spicamycin, and septacidin (Acton et al., 1977 ▸; Igarashi et al., 2005 ▸; Suzuki et al., 2002 ▸). 
Here we present single crystal X-ray crystallographic analysis of a nucleoside analog, (I), of the 2′-deoxyguanosine/abasic site cross-link. This structure corroborates an earlier two-dimensional NMR analysis (Catalano et al., 2015 ▸) concluding that the 2-deoxyribose unit attached at the exocyclic N 2-amino group of the guanine residue exists in the cyclic aminoglycoside form.
Structural commentary
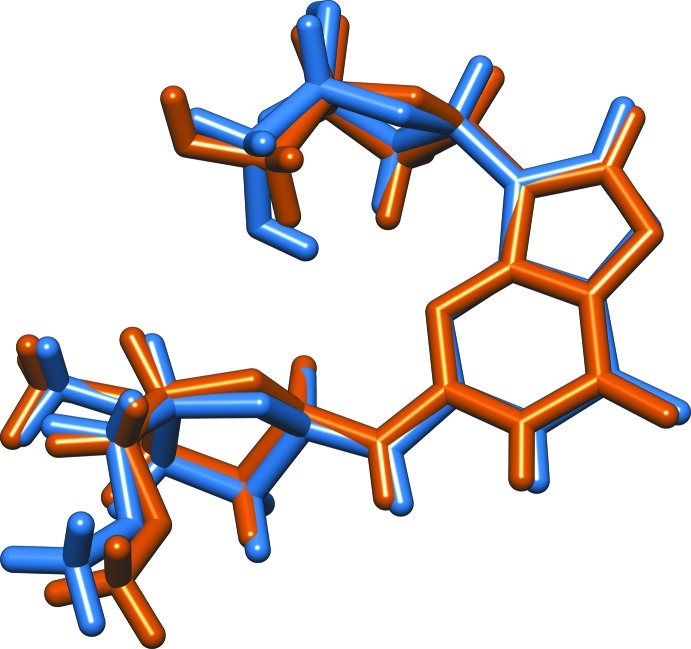
The two independent molecules (A and B) of (I) are shown in Fig. 1 ▸ as they are oriented in the crystal, while Fig. 2 ▸ shows an overlay to illustrate the differences in orientation and conformation of the furanose rings. Ring puckering analysis, after Cremer & Pople as calculated using PLATON (Spek, 2009 ▸) indicates the furanose rings attached to N4 positions in the two molecules to be half-chairs in both molecules, but with the maximum variance from planarity occurring between C7 and C8 in molecule A and C6 and C7 in molecule B [Q(2) = 0.367 (2), Φ(2) = 88.0 (4)° for molecule A and Q(2) = 0.347 (2), Φ(2) = 60.6 (4)° for molecule B]. The disposition of these furanose rings relative to the purine rings can be described by the torsion angle C2—N4—C6—O2, which is 70.9 (3)° in molecule A and 61.7 (3)° in molecule B. The furanose ring attached to the N5 position in molecule A is again a half-chair, with the maximum deviation from planarity between C11A and C12A [Q(2) = 3.41 (2), Φ(2) = 62.2 (3)°], while this furanose ring in molecule B is an envelope with C11B at the flap [Q(2) = 0.422 (2), Φ(2) = 45.4 (3)°]. The disposition of these furanose rings relative to the purine rings can be described by the angle C1—N5—C11—O5, which is −87.4 (2)° in molecule A and −93.7 (2)° in molecule B.
Figure 1.
The molecular structure of (I) showing 50% displacement ellipsoids.
Figure 2.
Overlay plot of the two molecules in (I). A molecule in orange and B molecule in blue.
Supramolecular features
In the crystal, the two molecules form infinite ribbons along the a–c diagonal of the unit cell, with the A molecules on one side of the ribbon and the B molecules on the other. The molecules are staggered such that each A molecule forms hydrogen bonds to two B molecules and each B molecule forms hydrogen bonds (Table 1 ▸) to two A molecules, fully involving the N1, N3, N5 and O1 atoms. These ribbons are then stacked to form slabs propagating in the ac plane and one half the b dimension in thickness. The deoxyribose moieties occupy the outsides of these slabs and are linked via hydrogen bonds to twofold screw-related slabs, resulting in a herringbone pattern in the three-dimensional structure as seen in Fig. 3 ▸.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1A—H1A⋯N3B | 0.88 | 1.92 | 2.789 (2) | 170 |
| O3A—H3A⋯O5A i | 0.84 | 2.07 | 2.897 (2) | 167 |
| O4A—H4A⋯O3B ii | 0.84 | 2.01 | 2.847 (2) | 178 |
| N5A—H5A⋯O1B | 0.88 | 2.23 | 3.058 (2) | 157 |
| C5A—H5A1⋯O1B iii | 0.95 | 2.63 | 3.284 (3) | 126 |
| C7A—H7A1⋯N2A | 0.99 | 2.46 | 3.172 (3) | 128 |
| C8A—H8A⋯O7A i | 1.00 | 2.39 | 3.316 (3) | 153 |
| C12A—H12A⋯O1A iv | 0.99 | 2.61 | 3.432 (3) | 141 |
| C12A—H12B⋯O1B | 0.99 | 2.55 | 3.426 (3) | 147 |
| C16A—H16A⋯O4A v | 0.98 | 2.47 | 3.401 (3) | 158 |
| C16A—H16B⋯O6A v | 0.98 | 2.54 | 3.222 (3) | 127 |
| C16A—H16C⋯O2A vi | 0.98 | 2.50 | 3.356 (3) | 146 |
| C17A—H17A⋯O3A vi | 0.98 | 2.65 | 3.610 (3) | 168 |
| C17A—H17B⋯O2A iv | 0.98 | 2.60 | 3.573 (3) | 175 |
| N1B—H1B⋯N3A vi | 0.88 | 1.94 | 2.808 (2) | 166 |
| O3B—H3B⋯O5B vii | 0.84 | 1.99 | 2.817 (2) | 169 |
| O4B—H4B⋯N2B | 0.84 | 2.38 | 3.180 (3) | 158 |
| N5B—H5B⋯O1A vi | 0.88 | 2.19 | 3.027 (2) | 159 |
| C5B—H5B1⋯O1A | 0.95 | 2.60 | 3.269 (3) | 127 |
| C8B—H8B⋯O7B vii | 1.00 | 2.49 | 3.363 (3) | 146 |
| C11B—H11B⋯O4B | 1.00 | 2.59 | 3.251 (3) | 124 |
| C12B—H12C⋯O1A vi | 0.99 | 2.55 | 3.363 (3) | 140 |
| C12B—H12D⋯O1B v | 0.99 | 2.45 | 3.424 (3) | 167 |
| C14B—H14B⋯O4B | 1.00 | 2.61 | 3.272 (3) | 123 |
| C17B—H17E⋯O2B v | 0.98 | 2.48 | 3.456 (3) | 176 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi)  ; (vii)
; (vii)  .
.
Figure 3.
The packing in (I) along the c axis showing the formation of hydrogen-bonded chains (A molecules green, B molecules blue).
Database survey
A search of the Cambridge Structural Database (CSD, Version 5.36, update February 2015; Groom & Allen, 2014 ▸) for deoxyguanosine analogues with exocyclic amine substitution revealed three crystal structures (Morr et al., 1991 ▸; Fujino et al., 2010 ▸). In all these crystal structures, the five-membered 2-deoxyribofuranose rings have envelope conformations, as in the title compound.
Synthesis and crystallization
2′-Deoxyguanosine (199 mg, 0.75 mmol) and 3,5-bis-O-methyl-2-deoxy-d-ribofuranose (110 mg, 0.74 mmol) were dissolved in 0.8 ml of a 3:1 mixture of DMSO and 25 mM sodium phosphate buffer (pH 7.0) in a round-bottom flask. The flask was heated to 333 K and the mixture stirred for 22 h. The solvent removed in vacuo and the product purified by column chromatography on silica gel eluted with 0–15% methanol in dichloromethane (Rf = 0.30, 15% methanol/dichloromethane) to yield 36 mg (12% yield) of the title compound as a colorless oil. The precursor 3,5-bis-O-methyl-2-deoxy-d-ribofuranose was synthesized according to previously reported procedures (Deriaz et al., 1949 ▸; Olsson et al., 1998 ▸). The title compound was crystallized by vapour diffusion, a 2 ml vial containing the title compound in methanol being placed in a 20 ml vial containing hexanes at room temperature for several days.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. H atoms were placed geometrically (C—H = 0.95 or 0.98 Å) and refined as riding with U iso(H) = 1.2U eq(C).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C17H25N5O7 |
| M r | 411.42 |
| Crystal system, space group | Monoclinic, P21 |
| Temperature (K) | 100 |
| a, b, c (Å) | 8.1817 (1), 26.4033 (5), 8.8800 (2) |
| β (°) | 98.023 (1) |
| V (Å3) | 1899.52 (6) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 0.96 |
| Crystal size (mm) | 0.15 × 0.08 × 0.08 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Sheldrick, 2008 ▸) |
| T min, T max | 0.86, 0.93 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 26696, 6862, 6644 |
| R int | 0.029 |
| (sin θ/λ)max (Å−1) | 0.617 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.027, 0.072, 1.04 |
| No. of reflections | 6862 |
| No. of parameters | 531 |
| No. of restraints | 1 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.23, −0.17 |
| Absolute structure | Flack x determined using 2923 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) |
| Absolute structure parameter | 0.08 (5) |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698901600517X/hb7568sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901600517X/hb7568Isup2.hkl
CCDC reference: 1448235
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| C17H25N5O7 | F(000) = 872 |
| Mr = 411.42 | Dx = 1.439 Mg m−3 |
| Monoclinic, P21 | Cu Kα radiation, λ = 1.54178 Å |
| a = 8.1817 (1) Å | Cell parameters from 9940 reflections |
| b = 26.4033 (5) Å | θ = 5.3–72.2° |
| c = 8.8800 (2) Å | µ = 0.96 mm−1 |
| β = 98.023 (1)° | T = 100 K |
| V = 1899.52 (6) Å3 | Prism, colourless |
| Z = 4 | 0.15 × 0.08 × 0.08 mm |
Data collection
| Bruker APEXII CCD diffractometer | 6644 reflections with I > 2σ(I) |
| Radiation source: Incoatec micro focus Cu tube | Rint = 0.029 |
| ω and phi scans | θmax = 72.2°, θmin = 3.4° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2008) | h = −10→10 |
| Tmin = 0.86, Tmax = 0.93 | k = −31→31 |
| 26696 measured reflections | l = −9→10 |
| 6862 independent reflections |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.027 | w = 1/[σ2(Fo2) + (0.0424P)2 + 0.3476P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.072 | (Δ/σ)max < 0.001 |
| S = 1.04 | Δρmax = 0.23 e Å−3 |
| 6862 reflections | Δρmin = −0.17 e Å−3 |
| 531 parameters | Absolute structure: Flack x determined using 2923 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 1 restraint | Absolute structure parameter: 0.08 (5) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1A | 0.34576 (17) | 0.46915 (6) | −0.12995 (17) | 0.0168 (3) | |
| N1A | 0.3499 (2) | 0.50765 (6) | 0.1022 (2) | 0.0146 (3) | |
| H1A | 0.4493 | 0.4955 | 0.1317 | 0.017* | |
| C1A | 0.2806 (2) | 0.53738 (8) | 0.2030 (2) | 0.0140 (4) | |
| O2A | −0.1409 (2) | 0.64849 (6) | 0.01984 (18) | 0.0223 (3) | |
| N2A | 0.1339 (2) | 0.55894 (7) | 0.1729 (2) | 0.0155 (4) | |
| C2A | 0.0560 (3) | 0.54690 (8) | 0.0330 (2) | 0.0150 (4) | |
| O3A | −0.44313 (19) | 0.65756 (6) | 0.18573 (19) | 0.0221 (3) | |
| H3A | −0.5051 | 0.6460 | 0.2454 | 0.027* | |
| N3A | −0.0017 (2) | 0.51309 (7) | −0.2041 (2) | 0.0170 (4) | |
| C3A | 0.1129 (2) | 0.51624 (8) | −0.0744 (2) | 0.0148 (4) | |
| O4A | 0.0015 (2) | 0.72195 (6) | 0.3635 (2) | 0.0298 (4) | |
| H4A | −0.0253 | 0.7501 | 0.3235 | 0.036* | |
| N4A | −0.0978 (2) | 0.56295 (7) | −0.0324 (2) | 0.0177 (4) | |
| C4A | 0.2740 (2) | 0.49535 (7) | −0.0439 (2) | 0.0138 (4) | |
| O5A | 0.37552 (18) | 0.62783 (6) | 0.42933 (16) | 0.0176 (3) | |
| N5A | 0.3711 (2) | 0.54372 (7) | 0.3418 (2) | 0.0163 (4) | |
| H5A | 0.4647 | 0.5272 | 0.3642 | 0.020* | |
| C5A | −0.1246 (3) | 0.54152 (8) | −0.1750 (2) | 0.0192 (4) | |
| H5A1 | −0.2220 | 0.5468 | −0.2449 | 0.023* | |
| O6A | 0.23353 (18) | 0.61868 (6) | 0.75564 (18) | 0.0227 (3) | |
| C6A | −0.2089 (3) | 0.59897 (8) | 0.0258 (3) | 0.0185 (4) | |
| H6A | −0.3179 | 0.5980 | −0.0410 | 0.022* | |
| O7A | 0.69231 (18) | 0.63615 (6) | 0.60640 (19) | 0.0221 (3) | |
| C7A | −0.2373 (3) | 0.59060 (8) | 0.1890 (3) | 0.0184 (4) | |
| H7A1 | −0.1372 | 0.5770 | 0.2514 | 0.022* | |
| H7A2 | −0.3309 | 0.5673 | 0.1948 | 0.022* | |
| C8A | −0.2764 (3) | 0.64379 (8) | 0.2388 (2) | 0.0182 (4) | |
| H8A | −0.2507 | 0.6476 | 0.3516 | 0.022* | |
| C9A | −0.1612 (3) | 0.67599 (8) | 0.1574 (2) | 0.0189 (4) | |
| H9A | −0.2152 | 0.7093 | 0.1293 | 0.023* | |
| C10A | 0.0085 (3) | 0.68527 (9) | 0.2482 (3) | 0.0245 (5) | |
| H10A | 0.0518 | 0.6531 | 0.2951 | 0.029* | |
| H10B | 0.0853 | 0.6970 | 0.1787 | 0.029* | |
| C11A | 0.3171 (2) | 0.57690 (8) | 0.4527 (2) | 0.0156 (4) | |
| H11A | 0.1938 | 0.5767 | 0.4429 | 0.019* | |
| C12A | 0.3901 (3) | 0.56396 (8) | 0.6145 (2) | 0.0182 (4) | |
| H12A | 0.3228 | 0.5381 | 0.6581 | 0.022* | |
| H12B | 0.5045 | 0.5513 | 0.6190 | 0.022* | |
| C13A | 0.3861 (2) | 0.61397 (8) | 0.6978 (2) | 0.0167 (4) | |
| H13A | 0.4804 | 0.6161 | 0.7823 | 0.020* | |
| C14A | 0.4064 (3) | 0.65359 (8) | 0.5738 (2) | 0.0175 (4) | |
| H14A | 0.3230 | 0.6811 | 0.5769 | 0.021* | |
| C15A | 0.5775 (3) | 0.67638 (9) | 0.5938 (3) | 0.0218 (4) | |
| H15A | 0.5917 | 0.6979 | 0.5053 | 0.026* | |
| H15B | 0.5947 | 0.6976 | 0.6866 | 0.026* | |
| C16A | 0.8590 (3) | 0.65335 (11) | 0.6422 (3) | 0.0301 (5) | |
| H16A | 0.8799 | 0.6802 | 0.5711 | 0.045* | |
| H16B | 0.9346 | 0.6250 | 0.6338 | 0.045* | |
| H16C | 0.8766 | 0.6666 | 0.7463 | 0.045* | |
| C17A | 0.2364 (3) | 0.65893 (11) | 0.8622 (3) | 0.0289 (5) | |
| H17A | 0.3324 | 0.6551 | 0.9407 | 0.043* | |
| H17B | 0.1352 | 0.6581 | 0.9096 | 0.043* | |
| H17C | 0.2435 | 0.6913 | 0.8097 | 0.043* | |
| O1B | 0.72354 (17) | 0.51384 (5) | 0.47267 (17) | 0.0168 (3) | |
| N1B | 0.9716 (2) | 0.47201 (6) | 0.50184 (19) | 0.0139 (3) | |
| H1B | 0.9971 | 0.4855 | 0.5927 | 0.017* | |
| C1B | 1.0833 (2) | 0.43911 (7) | 0.4530 (2) | 0.0136 (4) | |
| O2B | 0.9310 (2) | 0.32766 (6) | 0.07593 (19) | 0.0219 (3) | |
| N2B | 1.0610 (2) | 0.41620 (6) | 0.3193 (2) | 0.0153 (4) | |
| C2B | 0.9168 (3) | 0.42949 (8) | 0.2340 (2) | 0.0150 (4) | |
| O3B | 1.09413 (19) | 0.31769 (6) | −0.23244 (18) | 0.0220 (3) | |
| H3B | 1.1571 | 0.3306 | −0.2890 | 0.026* | |
| N3B | 0.6634 (2) | 0.46447 (7) | 0.1606 (2) | 0.0188 (4) | |
| C3B | 0.7979 (2) | 0.46230 (8) | 0.2729 (2) | 0.0159 (4) | |
| O4B | 1.2568 (3) | 0.31747 (9) | 0.2436 (2) | 0.0452 (5) | |
| H4B | 1.1920 | 0.3377 | 0.2779 | 0.054* | |
| N4B | 0.8528 (2) | 0.41146 (7) | 0.0918 (2) | 0.0186 (4) | |
| C4B | 0.8210 (2) | 0.48559 (8) | 0.4184 (2) | 0.0143 (4) | |
| O5B | 1.32491 (17) | 0.34807 (6) | 0.57774 (18) | 0.0175 (3) | |
| N5B | 1.2221 (2) | 0.43116 (7) | 0.5529 (2) | 0.0159 (3) | |
| H5B | 1.2334 | 0.4468 | 0.6413 | 0.019* | |
| C5B | 0.7002 (3) | 0.43335 (9) | 0.0558 (3) | 0.0215 (5) | |
| H5B1 | 0.6287 | 0.4266 | −0.0358 | 0.026* | |
| O6B | 1.67925 (19) | 0.36348 (6) | 0.43876 (18) | 0.0216 (3) | |
| C6B | 0.9187 (3) | 0.37405 (8) | −0.0034 (2) | 0.0185 (4) | |
| H6B | 0.8402 | 0.3699 | −0.0995 | 0.022* | |
| O7B | 1.5367 (2) | 0.31343 (6) | 0.84948 (19) | 0.0252 (3) | |
| C7B | 1.0893 (3) | 0.38469 (8) | −0.0431 (3) | 0.0198 (4) | |
| H7B1 | 1.1615 | 0.4002 | 0.0435 | 0.024* | |
| H7B2 | 1.0845 | 0.4072 | −0.1328 | 0.024* | |
| C8B | 1.1484 (3) | 0.33176 (8) | −0.0781 (2) | 0.0191 (4) | |
| H8B | 1.2709 | 0.3287 | −0.0525 | 0.023* | |
| C9B | 1.0581 (3) | 0.29760 (9) | 0.0248 (3) | 0.0226 (5) | |
| H9B | 1.0071 | 0.2683 | −0.0357 | 0.027* | |
| C10B | 1.1657 (4) | 0.27835 (11) | 0.1641 (3) | 0.0360 (6) | |
| H10C | 1.0958 | 0.2618 | 0.2322 | 0.043* | |
| H10D | 1.2427 | 0.2526 | 0.1337 | 0.043* | |
| C11B | 1.3507 (3) | 0.39806 (8) | 0.5188 (2) | 0.0157 (4) | |
| H11B | 1.3500 | 0.3963 | 0.4062 | 0.019* | |
| C12B | 1.5222 (3) | 0.41144 (8) | 0.5959 (3) | 0.0191 (4) | |
| H12C | 1.5218 | 0.4204 | 0.7041 | 0.023* | |
| H12D | 1.5700 | 0.4398 | 0.5436 | 0.023* | |
| C13B | 1.6149 (3) | 0.36211 (8) | 0.5792 (2) | 0.0177 (4) | |
| H13B | 1.7050 | 0.3572 | 0.6664 | 0.021* | |
| C14B | 1.4792 (3) | 0.32117 (8) | 0.5795 (3) | 0.0178 (4) | |
| H14B | 1.4738 | 0.3009 | 0.4838 | 0.021* | |
| C15B | 1.5051 (3) | 0.28550 (9) | 0.7128 (3) | 0.0227 (5) | |
| H15C | 1.4055 | 0.2643 | 0.7142 | 0.027* | |
| H15D | 1.5995 | 0.2628 | 0.7033 | 0.027* | |
| C16B | 1.5735 (3) | 0.28046 (11) | 0.9765 (3) | 0.0331 (6) | |
| H16D | 1.4856 | 0.2552 | 0.9752 | 0.050* | |
| H16E | 1.5814 | 0.3002 | 1.0708 | 0.050* | |
| H16F | 1.6788 | 0.2633 | 0.9711 | 0.050* | |
| C17B | 1.7854 (3) | 0.32160 (10) | 0.4226 (3) | 0.0271 (5) | |
| H17D | 1.8756 | 0.3212 | 0.5077 | 0.041* | |
| H17E | 1.8312 | 0.3248 | 0.3267 | 0.041* | |
| H17F | 1.7225 | 0.2900 | 0.4221 | 0.041* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1A | 0.0171 (7) | 0.0182 (7) | 0.0155 (7) | 0.0035 (6) | 0.0033 (5) | −0.0025 (6) |
| N1A | 0.0120 (7) | 0.0159 (8) | 0.0155 (9) | 0.0028 (6) | 0.0006 (6) | −0.0015 (6) |
| C1A | 0.0148 (9) | 0.0128 (9) | 0.0142 (10) | −0.0002 (7) | 0.0018 (7) | −0.0013 (7) |
| O2A | 0.0304 (9) | 0.0195 (8) | 0.0191 (8) | 0.0039 (6) | 0.0108 (6) | 0.0007 (6) |
| N2A | 0.0152 (8) | 0.0175 (9) | 0.0134 (9) | 0.0031 (6) | 0.0008 (7) | −0.0026 (7) |
| C2A | 0.0152 (9) | 0.0136 (10) | 0.0158 (10) | 0.0012 (7) | 0.0005 (7) | −0.0019 (7) |
| O3A | 0.0198 (8) | 0.0247 (8) | 0.0234 (8) | 0.0066 (6) | 0.0078 (6) | 0.0027 (6) |
| N3A | 0.0174 (8) | 0.0183 (9) | 0.0148 (9) | 0.0033 (7) | 0.0001 (7) | −0.0032 (7) |
| C3A | 0.0170 (9) | 0.0137 (9) | 0.0134 (10) | 0.0018 (8) | 0.0014 (8) | −0.0032 (7) |
| O4A | 0.0448 (10) | 0.0194 (8) | 0.0243 (9) | −0.0047 (7) | 0.0011 (7) | 0.0001 (7) |
| N4A | 0.0172 (8) | 0.0195 (9) | 0.0154 (9) | 0.0053 (7) | −0.0012 (7) | −0.0036 (7) |
| C4A | 0.0152 (10) | 0.0127 (10) | 0.0139 (10) | −0.0012 (7) | 0.0032 (8) | −0.0005 (7) |
| O5A | 0.0227 (7) | 0.0174 (7) | 0.0123 (7) | −0.0008 (6) | 0.0009 (5) | −0.0013 (6) |
| N5A | 0.0139 (8) | 0.0201 (9) | 0.0142 (9) | 0.0039 (6) | −0.0003 (6) | −0.0034 (7) |
| C5A | 0.0169 (10) | 0.0228 (11) | 0.0166 (11) | 0.0045 (8) | −0.0027 (8) | −0.0048 (8) |
| O6A | 0.0163 (7) | 0.0333 (9) | 0.0194 (8) | −0.0025 (6) | 0.0060 (6) | −0.0082 (7) |
| C6A | 0.0163 (9) | 0.0200 (11) | 0.0185 (11) | 0.0069 (8) | 0.0005 (8) | −0.0024 (8) |
| O7A | 0.0153 (7) | 0.0240 (8) | 0.0268 (9) | −0.0015 (6) | 0.0027 (6) | 0.0011 (6) |
| C7A | 0.0172 (10) | 0.0172 (11) | 0.0211 (11) | 0.0001 (8) | 0.0037 (8) | 0.0002 (8) |
| C8A | 0.0203 (10) | 0.0201 (11) | 0.0148 (10) | 0.0015 (8) | 0.0047 (8) | −0.0008 (8) |
| C9A | 0.0257 (11) | 0.0157 (10) | 0.0165 (10) | 0.0038 (8) | 0.0073 (8) | 0.0009 (8) |
| C10A | 0.0259 (12) | 0.0182 (11) | 0.0297 (13) | 0.0001 (9) | 0.0052 (10) | 0.0023 (9) |
| C11A | 0.0150 (9) | 0.0167 (10) | 0.0149 (10) | 0.0005 (7) | 0.0015 (7) | −0.0028 (8) |
| C12A | 0.0206 (10) | 0.0201 (10) | 0.0137 (10) | 0.0017 (8) | 0.0024 (8) | −0.0002 (8) |
| C13A | 0.0139 (9) | 0.0227 (11) | 0.0134 (10) | 0.0007 (8) | 0.0012 (7) | −0.0029 (8) |
| C14A | 0.0185 (10) | 0.0172 (10) | 0.0163 (10) | 0.0037 (8) | 0.0004 (8) | −0.0042 (8) |
| C15A | 0.0257 (11) | 0.0193 (11) | 0.0207 (11) | −0.0025 (9) | 0.0043 (9) | −0.0023 (9) |
| C16A | 0.0209 (11) | 0.0458 (15) | 0.0230 (12) | −0.0085 (10) | 0.0010 (9) | 0.0059 (11) |
| C17A | 0.0229 (11) | 0.0410 (14) | 0.0238 (12) | 0.0011 (10) | 0.0065 (9) | −0.0138 (10) |
| O1B | 0.0172 (7) | 0.0172 (7) | 0.0163 (7) | 0.0052 (6) | 0.0031 (6) | −0.0026 (6) |
| N1B | 0.0149 (8) | 0.0147 (8) | 0.0119 (8) | 0.0022 (6) | 0.0009 (6) | −0.0038 (6) |
| C1B | 0.0148 (9) | 0.0122 (9) | 0.0140 (10) | 0.0001 (7) | 0.0033 (7) | 0.0005 (7) |
| O2B | 0.0261 (8) | 0.0176 (8) | 0.0243 (8) | −0.0016 (6) | 0.0113 (6) | −0.0048 (6) |
| N2B | 0.0137 (8) | 0.0171 (9) | 0.0148 (9) | 0.0019 (6) | 0.0010 (6) | −0.0028 (7) |
| C2B | 0.0169 (9) | 0.0146 (10) | 0.0136 (10) | −0.0007 (8) | 0.0022 (7) | −0.0021 (8) |
| O3B | 0.0278 (8) | 0.0215 (8) | 0.0183 (8) | −0.0035 (6) | 0.0094 (6) | −0.0033 (6) |
| N3B | 0.0168 (8) | 0.0222 (9) | 0.0166 (9) | 0.0042 (7) | −0.0003 (7) | −0.0038 (7) |
| C3B | 0.0144 (9) | 0.0162 (10) | 0.0168 (10) | 0.0017 (8) | 0.0014 (8) | −0.0016 (8) |
| O4B | 0.0386 (11) | 0.0665 (15) | 0.0278 (10) | 0.0170 (10) | −0.0056 (8) | −0.0101 (10) |
| N4B | 0.0168 (9) | 0.0227 (9) | 0.0153 (9) | 0.0048 (7) | −0.0013 (7) | −0.0068 (7) |
| C4B | 0.0154 (9) | 0.0128 (9) | 0.0147 (10) | −0.0004 (7) | 0.0024 (7) | 0.0014 (7) |
| O5B | 0.0139 (7) | 0.0165 (7) | 0.0226 (8) | 0.0023 (6) | 0.0037 (6) | 0.0013 (6) |
| N5B | 0.0172 (8) | 0.0162 (8) | 0.0137 (8) | 0.0038 (7) | 0.0004 (6) | −0.0042 (6) |
| C5B | 0.0185 (10) | 0.0270 (12) | 0.0174 (11) | 0.0039 (9) | −0.0028 (8) | −0.0053 (9) |
| O6B | 0.0225 (8) | 0.0239 (8) | 0.0200 (8) | 0.0048 (6) | 0.0084 (6) | 0.0045 (6) |
| C6B | 0.0212 (11) | 0.0186 (11) | 0.0151 (10) | 0.0027 (8) | 0.0002 (8) | −0.0061 (8) |
| O7B | 0.0264 (8) | 0.0281 (9) | 0.0207 (8) | 0.0003 (7) | 0.0018 (6) | 0.0081 (7) |
| C7B | 0.0246 (11) | 0.0175 (10) | 0.0181 (11) | −0.0018 (8) | 0.0055 (8) | −0.0025 (8) |
| C8B | 0.0204 (10) | 0.0202 (11) | 0.0175 (11) | 0.0020 (8) | 0.0054 (8) | −0.0025 (8) |
| C9B | 0.0305 (12) | 0.0183 (11) | 0.0208 (11) | 0.0022 (9) | 0.0098 (9) | −0.0036 (8) |
| C10B | 0.0506 (16) | 0.0338 (14) | 0.0241 (13) | 0.0162 (12) | 0.0067 (11) | 0.0026 (11) |
| C11B | 0.0174 (10) | 0.0146 (10) | 0.0151 (10) | 0.0013 (7) | 0.0018 (8) | 0.0003 (7) |
| C12B | 0.0156 (10) | 0.0195 (11) | 0.0220 (11) | 0.0002 (8) | 0.0023 (8) | −0.0036 (8) |
| C13B | 0.0149 (10) | 0.0209 (11) | 0.0174 (11) | 0.0028 (8) | 0.0028 (8) | 0.0004 (8) |
| C14B | 0.0160 (9) | 0.0167 (10) | 0.0210 (11) | 0.0035 (8) | 0.0040 (8) | −0.0011 (8) |
| C15B | 0.0201 (10) | 0.0200 (11) | 0.0280 (12) | 0.0021 (8) | 0.0034 (9) | 0.0031 (9) |
| C16B | 0.0261 (12) | 0.0417 (15) | 0.0298 (13) | −0.0035 (11) | −0.0019 (10) | 0.0187 (11) |
| C17B | 0.0263 (12) | 0.0291 (13) | 0.0281 (12) | 0.0092 (10) | 0.0118 (9) | 0.0021 (10) |
Geometric parameters (Å, º)
| O1A—C4A | 1.238 (3) | O1B—C4B | 1.237 (3) |
| N1A—C1A | 1.371 (3) | N1B—C1B | 1.374 (3) |
| N1A—C4A | 1.397 (3) | N1B—C4B | 1.393 (3) |
| N1A—H1A | 0.8800 | N1B—H1B | 0.8800 |
| C1A—N2A | 1.321 (3) | C1B—N2B | 1.323 (3) |
| C1A—N5A | 1.357 (3) | C1B—N5B | 1.356 (3) |
| O2A—C6A | 1.425 (3) | O2B—C6B | 1.409 (3) |
| O2A—C9A | 1.450 (3) | O2B—C9B | 1.432 (3) |
| N2A—C2A | 1.353 (3) | N2B—C2B | 1.356 (3) |
| C2A—N4A | 1.377 (3) | C2B—C3B | 1.382 (3) |
| C2A—C3A | 1.380 (3) | C2B—N4B | 1.382 (3) |
| O3A—C8A | 1.427 (3) | O3B—C8B | 1.429 (3) |
| O3A—H3A | 0.8400 | O3B—H3B | 0.8400 |
| N3A—C5A | 1.309 (3) | N3B—C5B | 1.308 (3) |
| N3A—C3A | 1.382 (3) | N3B—C3B | 1.379 (3) |
| C3A—C4A | 1.420 (3) | C3B—C4B | 1.420 (3) |
| O4A—C10A | 1.417 (3) | O4B—C10B | 1.405 (4) |
| O4A—H4A | 0.8400 | O4B—H4B | 0.8400 |
| N4A—C5A | 1.376 (3) | N4B—C5B | 1.373 (3) |
| N4A—C6A | 1.460 (3) | N4B—C6B | 1.452 (3) |
| O5A—C14A | 1.443 (2) | O5B—C11B | 1.446 (3) |
| O5A—C11A | 1.452 (3) | O5B—C14B | 1.447 (2) |
| N5A—C11A | 1.433 (3) | N5B—C11B | 1.433 (3) |
| N5A—H5A | 0.8800 | N5B—H5B | 0.8800 |
| C5A—H5A1 | 0.9500 | C5B—H5B1 | 0.9500 |
| O6A—C13A | 1.420 (3) | O6B—C13B | 1.420 (3) |
| O6A—C17A | 1.421 (3) | O6B—C17B | 1.426 (3) |
| C6A—C7A | 1.515 (3) | C6B—C7B | 1.513 (3) |
| C6A—H6A | 1.0000 | C6B—H6B | 1.0000 |
| O7A—C15A | 1.412 (3) | O7B—C15B | 1.413 (3) |
| O7A—C16A | 1.431 (3) | O7B—C16B | 1.424 (3) |
| C7A—C8A | 1.520 (3) | C7B—C8B | 1.525 (3) |
| C7A—H7A1 | 0.9900 | C7B—H7B1 | 0.9900 |
| C7A—H7A2 | 0.9900 | C7B—H7B2 | 0.9900 |
| C8A—C9A | 1.524 (3) | C8B—C9B | 1.544 (3) |
| C8A—H8A | 1.0000 | C8B—H8B | 1.0000 |
| C9A—C10A | 1.525 (3) | C9B—C10B | 1.503 (4) |
| C9A—H9A | 1.0000 | C9B—H9B | 1.0000 |
| C10A—H10A | 0.9900 | C10B—H10C | 0.9900 |
| C10A—H10B | 0.9900 | C10B—H10D | 0.9900 |
| C11A—C12A | 1.516 (3) | C11B—C12B | 1.514 (3) |
| C11A—H11A | 1.0000 | C11B—H11B | 1.0000 |
| C12A—C13A | 1.516 (3) | C12B—C13B | 1.525 (3) |
| C12A—H12A | 0.9900 | C12B—H12C | 0.9900 |
| C12A—H12B | 0.9900 | C12B—H12D | 0.9900 |
| C13A—C14A | 1.544 (3) | C13B—C14B | 1.550 (3) |
| C13A—H13A | 1.0000 | C13B—H13B | 1.0000 |
| C14A—C15A | 1.512 (3) | C14B—C15B | 1.505 (3) |
| C14A—H14A | 1.0000 | C14B—H14B | 1.0000 |
| C15A—H15A | 0.9900 | C15B—H15C | 0.9900 |
| C15A—H15B | 0.9900 | C15B—H15D | 0.9900 |
| C16A—H16A | 0.9800 | C16B—H16D | 0.9800 |
| C16A—H16B | 0.9800 | C16B—H16E | 0.9800 |
| C16A—H16C | 0.9800 | C16B—H16F | 0.9800 |
| C17A—H17A | 0.9800 | C17B—H17D | 0.9800 |
| C17A—H17B | 0.9800 | C17B—H17E | 0.9800 |
| C17A—H17C | 0.9800 | C17B—H17F | 0.9800 |
| C1A—N1A—C4A | 124.64 (17) | C1B—N1B—C4B | 124.95 (17) |
| C1A—N1A—H1A | 117.7 | C1B—N1B—H1B | 117.5 |
| C4A—N1A—H1A | 117.7 | C4B—N1B—H1B | 117.5 |
| N2A—C1A—N5A | 119.73 (18) | N2B—C1B—N5B | 120.96 (18) |
| N2A—C1A—N1A | 124.14 (18) | N2B—C1B—N1B | 123.93 (18) |
| N5A—C1A—N1A | 116.13 (17) | N5B—C1B—N1B | 115.11 (18) |
| C6A—O2A—C9A | 109.66 (16) | C6B—O2B—C9B | 109.15 (17) |
| C1A—N2A—C2A | 112.51 (17) | C1B—N2B—C2B | 112.52 (17) |
| N2A—C2A—N4A | 126.92 (19) | N2B—C2B—C3B | 127.64 (19) |
| N2A—C2A—C3A | 127.70 (19) | N2B—C2B—N4B | 127.58 (19) |
| N4A—C2A—C3A | 105.38 (18) | C3B—C2B—N4B | 104.73 (18) |
| C8A—O3A—H3A | 109.5 | C8B—O3B—H3B | 109.5 |
| C5A—N3A—C3A | 104.51 (18) | C5B—N3B—C3B | 104.43 (17) |
| C2A—C3A—N3A | 110.89 (18) | N3B—C3B—C2B | 111.34 (19) |
| C2A—C3A—C4A | 119.30 (18) | N3B—C3B—C4B | 129.16 (19) |
| N3A—C3A—C4A | 129.73 (19) | C2B—C3B—C4B | 119.33 (19) |
| C10A—O4A—H4A | 109.5 | C10B—O4B—H4B | 109.5 |
| C5A—N4A—C2A | 106.28 (17) | C5B—N4B—C2B | 106.50 (17) |
| C5A—N4A—C6A | 124.53 (18) | C5B—N4B—C6B | 123.40 (18) |
| C2A—N4A—C6A | 128.96 (18) | C2B—N4B—C6B | 129.95 (18) |
| O1A—C4A—N1A | 121.03 (18) | O1B—C4B—N1B | 121.23 (19) |
| O1A—C4A—C3A | 127.38 (19) | O1B—C4B—C3B | 127.16 (19) |
| N1A—C4A—C3A | 111.59 (17) | N1B—C4B—C3B | 111.59 (17) |
| C14A—O5A—C11A | 109.25 (15) | C11B—O5B—C14B | 106.31 (15) |
| C1A—N5A—C11A | 121.20 (17) | C1B—N5B—C11B | 121.92 (18) |
| C1A—N5A—H5A | 119.4 | C1B—N5B—H5B | 119.0 |
| C11A—N5A—H5A | 119.4 | C11B—N5B—H5B | 119.0 |
| N3A—C5A—N4A | 112.93 (18) | N3B—C5B—N4B | 112.99 (18) |
| N3A—C5A—H5A1 | 123.5 | N3B—C5B—H5B1 | 123.5 |
| N4A—C5A—H5A1 | 123.5 | N4B—C5B—H5B1 | 123.5 |
| C13A—O6A—C17A | 111.90 (16) | C13B—O6B—C17B | 112.00 (17) |
| O2A—C6A—N4A | 108.59 (17) | O2B—C6B—N4B | 107.89 (18) |
| O2A—C6A—C7A | 106.39 (17) | O2B—C6B—C7B | 105.90 (17) |
| N4A—C6A—C7A | 115.46 (18) | N4B—C6B—C7B | 116.03 (18) |
| O2A—C6A—H6A | 108.7 | O2B—C6B—H6B | 108.9 |
| N4A—C6A—H6A | 108.7 | N4B—C6B—H6B | 108.9 |
| C7A—C6A—H6A | 108.7 | C7B—C6B—H6B | 108.9 |
| C15A—O7A—C16A | 112.43 (18) | C15B—O7B—C16B | 110.76 (19) |
| C6A—C7A—C8A | 102.12 (17) | C6B—C7B—C8B | 101.90 (17) |
| C6A—C7A—H7A1 | 111.3 | C6B—C7B—H7B1 | 111.4 |
| C8A—C7A—H7A1 | 111.3 | C8B—C7B—H7B1 | 111.4 |
| C6A—C7A—H7A2 | 111.3 | C6B—C7B—H7B2 | 111.4 |
| C8A—C7A—H7A2 | 111.3 | C8B—C7B—H7B2 | 111.4 |
| H7A1—C7A—H7A2 | 109.2 | H7B1—C7B—H7B2 | 109.3 |
| O3A—C8A—C7A | 111.70 (18) | O3B—C8B—C7B | 111.64 (18) |
| O3A—C8A—C9A | 109.09 (17) | O3B—C8B—C9B | 107.79 (17) |
| C7A—C8A—C9A | 102.03 (17) | C7B—C8B—C9B | 102.92 (17) |
| O3A—C8A—H8A | 111.2 | O3B—C8B—H8B | 111.4 |
| C7A—C8A—H8A | 111.2 | C7B—C8B—H8B | 111.4 |
| C9A—C8A—H8A | 111.2 | C9B—C8B—H8B | 111.4 |
| O2A—C9A—C8A | 105.75 (17) | O2B—C9B—C10B | 107.12 (19) |
| O2A—C9A—C10A | 108.82 (17) | O2B—C9B—C8B | 107.00 (17) |
| C8A—C9A—C10A | 114.59 (18) | C10B—C9B—C8B | 114.3 (2) |
| O2A—C9A—H9A | 109.2 | O2B—C9B—H9B | 109.4 |
| C8A—C9A—H9A | 109.2 | C10B—C9B—H9B | 109.4 |
| C10A—C9A—H9A | 109.2 | C8B—C9B—H9B | 109.4 |
| O4A—C10A—C9A | 111.51 (19) | O4B—C10B—C9B | 111.9 (2) |
| O4A—C10A—H10A | 109.3 | O4B—C10B—H10C | 109.2 |
| C9A—C10A—H10A | 109.3 | C9B—C10B—H10C | 109.2 |
| O4A—C10A—H10B | 109.3 | O4B—C10B—H10D | 109.2 |
| C9A—C10A—H10B | 109.3 | C9B—C10B—H10D | 109.2 |
| H10A—C10A—H10B | 108.0 | H10C—C10B—H10D | 107.9 |
| N5A—C11A—O5A | 109.19 (17) | N5B—C11B—O5B | 109.36 (17) |
| N5A—C11A—C12A | 113.26 (17) | N5B—C11B—C12B | 115.06 (18) |
| O5A—C11A—C12A | 104.50 (16) | O5B—C11B—C12B | 102.84 (16) |
| N5A—C11A—H11A | 109.9 | N5B—C11B—H11B | 109.8 |
| O5A—C11A—H11A | 109.9 | O5B—C11B—H11B | 109.8 |
| C12A—C11A—H11A | 109.9 | C12B—C11B—H11B | 109.8 |
| C13A—C12A—C11A | 103.50 (17) | C11B—C12B—C13B | 101.47 (17) |
| C13A—C12A—H12A | 111.1 | C11B—C12B—H12C | 111.5 |
| C11A—C12A—H12A | 111.1 | C13B—C12B—H12C | 111.5 |
| C13A—C12A—H12B | 111.1 | C11B—C12B—H12D | 111.5 |
| C11A—C12A—H12B | 111.1 | C13B—C12B—H12D | 111.5 |
| H12A—C12A—H12B | 109.0 | H12C—C12B—H12D | 109.3 |
| O6A—C13A—C12A | 109.43 (17) | O6B—C13B—C12B | 108.29 (17) |
| O6A—C13A—C14A | 112.71 (17) | O6B—C13B—C14B | 111.87 (18) |
| C12A—C13A—C14A | 103.35 (16) | C12B—C13B—C14B | 103.26 (16) |
| O6A—C13A—H13A | 110.4 | O6B—C13B—H13B | 111.0 |
| C12A—C13A—H13A | 110.4 | C12B—C13B—H13B | 111.0 |
| C14A—C13A—H13A | 110.4 | C14B—C13B—H13B | 111.0 |
| O5A—C14A—C15A | 109.66 (17) | O5B—C14B—C15B | 110.05 (17) |
| O5A—C14A—C13A | 106.95 (16) | O5B—C14B—C13B | 106.37 (16) |
| C15A—C14A—C13A | 112.09 (17) | C15B—C14B—C13B | 114.55 (18) |
| O5A—C14A—H14A | 109.4 | O5B—C14B—H14B | 108.6 |
| C15A—C14A—H14A | 109.4 | C15B—C14B—H14B | 108.6 |
| C13A—C14A—H14A | 109.4 | C13B—C14B—H14B | 108.6 |
| O7A—C15A—C14A | 107.74 (17) | O7B—C15B—C14B | 109.74 (18) |
| O7A—C15A—H15A | 110.2 | O7B—C15B—H15C | 109.7 |
| C14A—C15A—H15A | 110.2 | C14B—C15B—H15C | 109.7 |
| O7A—C15A—H15B | 110.2 | O7B—C15B—H15D | 109.7 |
| C14A—C15A—H15B | 110.2 | C14B—C15B—H15D | 109.7 |
| H15A—C15A—H15B | 108.5 | H15C—C15B—H15D | 108.2 |
| O7A—C16A—H16A | 109.5 | O7B—C16B—H16D | 109.5 |
| O7A—C16A—H16B | 109.5 | O7B—C16B—H16E | 109.5 |
| H16A—C16A—H16B | 109.5 | H16D—C16B—H16E | 109.5 |
| O7A—C16A—H16C | 109.5 | O7B—C16B—H16F | 109.5 |
| H16A—C16A—H16C | 109.5 | H16D—C16B—H16F | 109.5 |
| H16B—C16A—H16C | 109.5 | H16E—C16B—H16F | 109.5 |
| O6A—C17A—H17A | 109.5 | O6B—C17B—H17D | 109.5 |
| O6A—C17A—H17B | 109.5 | O6B—C17B—H17E | 109.5 |
| H17A—C17A—H17B | 109.5 | H17D—C17B—H17E | 109.5 |
| O6A—C17A—H17C | 109.5 | O6B—C17B—H17F | 109.5 |
| H17A—C17A—H17C | 109.5 | H17D—C17B—H17F | 109.5 |
| H17B—C17A—H17C | 109.5 | H17E—C17B—H17F | 109.5 |
| C4A—N1A—C1A—N2A | −1.4 (3) | C4B—N1B—C1B—N2B | 0.2 (3) |
| C4A—N1A—C1A—N5A | 177.84 (18) | C4B—N1B—C1B—N5B | −179.83 (18) |
| N5A—C1A—N2A—C2A | −176.82 (19) | N5B—C1B—N2B—C2B | −178.87 (19) |
| N1A—C1A—N2A—C2A | 2.4 (3) | N1B—C1B—N2B—C2B | 1.1 (3) |
| C1A—N2A—C2A—N4A | 179.8 (2) | C1B—N2B—C2B—C3B | −0.8 (3) |
| C1A—N2A—C2A—C3A | −0.3 (3) | C1B—N2B—C2B—N4B | −177.6 (2) |
| N2A—C2A—C3A—N3A | −179.8 (2) | C5B—N3B—C3B—C2B | 0.2 (3) |
| N4A—C2A—C3A—N3A | 0.1 (2) | C5B—N3B—C3B—C4B | −175.1 (2) |
| N2A—C2A—C3A—C4A | −2.9 (3) | N2B—C2B—C3B—N3B | −176.7 (2) |
| N4A—C2A—C3A—C4A | 177.03 (18) | N4B—C2B—C3B—N3B | 0.7 (2) |
| C5A—N3A—C3A—C2A | 0.2 (2) | N2B—C2B—C3B—C4B | −0.9 (3) |
| C5A—N3A—C3A—C4A | −176.3 (2) | N4B—C2B—C3B—C4B | 176.47 (19) |
| N2A—C2A—N4A—C5A | 179.5 (2) | N2B—C2B—N4B—C5B | 176.1 (2) |
| C3A—C2A—N4A—C5A | −0.4 (2) | C3B—C2B—N4B—C5B | −1.3 (2) |
| N2A—C2A—N4A—C6A | 4.8 (4) | N2B—C2B—N4B—C6B | 0.6 (4) |
| C3A—C2A—N4A—C6A | −175.1 (2) | C3B—C2B—N4B—C6B | −176.8 (2) |
| C1A—N1A—C4A—O1A | 178.41 (19) | C1B—N1B—C4B—O1B | 176.86 (19) |
| C1A—N1A—C4A—C3A | −1.7 (3) | C1B—N1B—C4B—C3B | −1.8 (3) |
| C2A—C3A—C4A—O1A | −176.6 (2) | N3B—C3B—C4B—O1B | −1.6 (4) |
| N3A—C3A—C4A—O1A | −0.3 (4) | C2B—C3B—C4B—O1B | −176.5 (2) |
| C2A—C3A—C4A—N1A | 3.6 (3) | N3B—C3B—C4B—N1B | 177.0 (2) |
| N3A—C3A—C4A—N1A | 179.8 (2) | C2B—C3B—C4B—N1B | 2.0 (3) |
| N2A—C1A—N5A—C11A | −5.3 (3) | N2B—C1B—N5B—C11B | 0.3 (3) |
| N1A—C1A—N5A—C11A | 175.39 (18) | N1B—C1B—N5B—C11B | −179.74 (18) |
| C3A—N3A—C5A—N4A | −0.5 (3) | C3B—N3B—C5B—N4B | −1.0 (3) |
| C2A—N4A—C5A—N3A | 0.6 (3) | C2B—N4B—C5B—N3B | 1.5 (3) |
| C6A—N4A—C5A—N3A | 175.6 (2) | C6B—N4B—C5B—N3B | 177.4 (2) |
| C9A—O2A—C6A—N4A | −138.45 (17) | C9B—O2B—C6B—N4B | −153.74 (17) |
| C9A—O2A—C6A—C7A | −13.6 (2) | C9B—O2B—C6B—C7B | −28.9 (2) |
| C5A—N4A—C6A—O2A | −103.0 (2) | C5B—N4B—C6B—O2B | −113.2 (2) |
| C2A—N4A—C6A—O2A | 70.9 (3) | C2B—N4B—C6B—O2B | 61.7 (3) |
| C5A—N4A—C6A—C7A | 137.7 (2) | C5B—N4B—C6B—C7B | 128.3 (2) |
| C2A—N4A—C6A—C7A | −48.4 (3) | C2B—N4B—C6B—C7B | −56.9 (3) |
| O2A—C6A—C7A—C8A | 31.8 (2) | O2B—C6B—C7B—C8B | 36.8 (2) |
| N4A—C6A—C7A—C8A | 152.34 (18) | N4B—C6B—C7B—C8B | 156.41 (19) |
| C6A—C7A—C8A—O3A | 79.6 (2) | C6B—C7B—C8B—O3B | 85.4 (2) |
| C6A—C7A—C8A—C9A | −36.8 (2) | C6B—C7B—C8B—C9B | −30.0 (2) |
| C6A—O2A—C9A—C8A | −10.4 (2) | C6B—O2B—C9B—C10B | 131.8 (2) |
| C6A—O2A—C9A—C10A | 113.18 (18) | C6B—O2B—C9B—C8B | 8.8 (2) |
| O3A—C8A—C9A—O2A | −88.60 (19) | O3B—C8B—C9B—O2B | −103.77 (19) |
| C7A—C8A—C9A—O2A | 29.7 (2) | C7B—C8B—C9B—O2B | 14.3 (2) |
| O3A—C8A—C9A—C10A | 151.55 (18) | O3B—C8B—C9B—C10B | 137.8 (2) |
| C7A—C8A—C9A—C10A | −90.2 (2) | C7B—C8B—C9B—C10B | −104.1 (2) |
| O2A—C9A—C10A—O4A | 165.83 (17) | O2B—C9B—C10B—O4B | −68.9 (3) |
| C8A—C9A—C10A—O4A | −76.0 (2) | C8B—C9B—C10B—O4B | 49.4 (3) |
| C1A—N5A—C11A—O5A | −87.4 (2) | C1B—N5B—C11B—O5B | −93.7 (2) |
| C1A—N5A—C11A—C12A | 156.64 (19) | C1B—N5B—C11B—C12B | 151.2 (2) |
| C14A—O5A—C11A—N5A | −148.25 (16) | C14B—O5B—C11B—N5B | −163.87 (16) |
| C14A—O5A—C11A—C12A | −26.8 (2) | C14B—O5B—C11B—C12B | −41.2 (2) |
| N5A—C11A—C12A—C13A | 154.32 (17) | N5B—C11B—C12B—C13B | 162.67 (18) |
| O5A—C11A—C12A—C13A | 35.6 (2) | O5B—C11B—C12B—C13B | 43.9 (2) |
| C17A—O6A—C13A—C12A | 167.04 (19) | C17B—O6B—C13B—C12B | 172.60 (18) |
| C17A—O6A—C13A—C14A | −78.6 (2) | C17B—O6B—C13B—C14B | −74.3 (2) |
| C11A—C12A—C13A—O6A | 89.7 (2) | C11B—C12B—C13B—O6B | 88.8 (2) |
| C11A—C12A—C13A—C14A | −30.6 (2) | C11B—C12B—C13B—C14B | −30.0 (2) |
| C11A—O5A—C14A—C15A | 128.97 (18) | C11B—O5B—C14B—C15B | 146.08 (17) |
| C11A—O5A—C14A—C13A | 7.2 (2) | C11B—O5B—C14B—C13B | 21.5 (2) |
| O6A—C13A—C14A—O5A | −102.90 (18) | O6B—C13B—C14B—O5B | −109.88 (18) |
| C12A—C13A—C14A—O5A | 15.1 (2) | C12B—C13B—C14B—O5B | 6.4 (2) |
| O6A—C13A—C14A—C15A | 136.89 (18) | O6B—C13B—C14B—C15B | 128.32 (19) |
| C12A—C13A—C14A—C15A | −105.08 (19) | C12B—C13B—C14B—C15B | −115.5 (2) |
| C16A—O7A—C15A—C14A | −173.67 (18) | C16B—O7B—C15B—C14B | −175.77 (18) |
| O5A—C14A—C15A—O7A | −66.7 (2) | O5B—C14B—C15B—O7B | −69.6 (2) |
| C13A—C14A—C15A—O7A | 51.9 (2) | C13B—C14B—C15B—O7B | 50.2 (2) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1A—H1A···N3B | 0.88 | 1.92 | 2.789 (2) | 170 |
| O3A—H3A···O5Ai | 0.84 | 2.07 | 2.897 (2) | 167 |
| O4A—H4A···O3Bii | 0.84 | 2.01 | 2.847 (2) | 178 |
| N5A—H5A···O1B | 0.88 | 2.23 | 3.058 (2) | 157 |
| C5A—H5A1···O1Biii | 0.95 | 2.63 | 3.284 (3) | 126 |
| C7A—H7A1···N2A | 0.99 | 2.46 | 3.172 (3) | 128 |
| C8A—H8A···O7Ai | 1.00 | 2.39 | 3.316 (3) | 153 |
| C12A—H12A···O1Aiv | 0.99 | 2.61 | 3.432 (3) | 141 |
| C12A—H12B···O1B | 0.99 | 2.55 | 3.426 (3) | 147 |
| C16A—H16A···O4Av | 0.98 | 2.47 | 3.401 (3) | 158 |
| C16A—H16B···O6Av | 0.98 | 2.54 | 3.222 (3) | 127 |
| C16A—H16C···O2Avi | 0.98 | 2.50 | 3.356 (3) | 146 |
| C17A—H17A···O3Avi | 0.98 | 2.65 | 3.610 (3) | 168 |
| C17A—H17B···O2Aiv | 0.98 | 2.60 | 3.573 (3) | 175 |
| N1B—H1B···N3Avi | 0.88 | 1.94 | 2.808 (2) | 166 |
| O3B—H3B···O5Bvii | 0.84 | 1.99 | 2.817 (2) | 169 |
| O4B—H4B···N2B | 0.84 | 2.38 | 3.180 (3) | 158 |
| N5B—H5B···O1Avi | 0.88 | 2.19 | 3.027 (2) | 159 |
| C5B—H5B1···O1A | 0.95 | 2.60 | 3.269 (3) | 127 |
| C8B—H8B···O7Bvii | 1.00 | 2.49 | 3.363 (3) | 146 |
| C11B—H11B···O4B | 1.00 | 2.59 | 3.251 (3) | 124 |
| C12B—H12C···O1Avi | 0.99 | 2.55 | 3.363 (3) | 140 |
| C12B—H12D···O1Bv | 0.99 | 2.45 | 3.424 (3) | 167 |
| C14B—H14B···O4B | 1.00 | 2.61 | 3.272 (3) | 123 |
| C17B—H17E···O2Bv | 0.98 | 2.48 | 3.456 (3) | 176 |
Symmetry codes: (i) x−1, y, z; (ii) −x+1, y+1/2, −z; (iii) x−1, y, z−1; (iv) x, y, z+1; (v) x+1, y, z; (vi) x+1, y, z+1; (vii) x, y, z−1.
References
- Acton, E. M., Ryan, K. J. & Luetzow, A. E. (1977). J. Med. Chem. 20, 1362–1371. [DOI] [PubMed]
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Bruker (2008). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Catalano, M. J., Liu, S., Andersen, N., Yang, Z., Johnson, K. M., Price, N. A., Wang, Y. & Gates, K. S. (2015). J. Am. Chem. Soc. 137, 3933–3945. [DOI] [PMC free article] [PubMed]
- Deriaz, R. E., Overend, W. G., Stacey, M. & Wiggins, L. F. (1949). J. Chem. Soc. pp. 2836–2841.
- Dutta, S., Chowdhury, G. & Gates, K. S. (2007). J. Am. Chem. Soc. 129, 1852–1853. [DOI] [PMC free article] [PubMed]
- Fujino, T., Tsunaka, N., Guillot-Nieckowski, M., Nakanishi, W., Iwamoto, T., Nakamura, E. & Isobe, H. (2010). Tetrahedron Lett. 51, 2036–2038.
- Gamboa Varela, J. & Gates, K. S. (2015). Angew. Chem. Int. Ed. 54, 7666–7669. [DOI] [PMC free article] [PubMed]
- Groom, C. R. & Allen, F. H. (2014). Angew. Chem. Int. Ed. 53, 662–671. [DOI] [PubMed]
- Igarashi, Y., Ootsu, K., Onaka, H., Fujita, T., Uehara, Y. & Furumai, T. (2005). J. Antibiot. 58, 322–326. [DOI] [PubMed]
- Johnson, K. M., Price, N. E., Wang, J., Fekry, M. I., Dutta, S., Seiner, D. R., Wang, Y. & Gates, K. S. (2013). J. Am. Chem. Soc. 135, 1015–1025. [DOI] [PMC free article] [PubMed]
- Morr, M., Ernst, L. & Schomburg, D. (1991). Liebigs Ann. Chem. 1991, 615–631.
- Olsson, R., Rundström, P. & Frejd, T. (1998). J. Chem. Soc. Perkin Trans. 1, pp. 785–790.
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Price, N. E., Catalano, M. J., Liu, S., Wang, Y. & Gates, K. S. (2015). Nucleic Acids Res. 43, 3434–3441. [DOI] [PMC free article] [PubMed]
- Price, N. E., Johnson, K. M., Wang, J., Fekry, M. I., Wang, Y. & Gates, K. S. (2014). J. Am. Chem. Soc. 136, 3483–3490. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Suzuki, T., Suzuki, S. T., Yamada, I., Koashi, Y., Yamada, K. & Chida, N. (2002). J. Org. Chem. 67, 2874–2880. [DOI] [PubMed]
- Yang, Z., Price, N. E., Johnson, K. M. & Gates, K. S. (2015). Biochemistry, 54, 4259–4266. [DOI] [PMC free article] [PubMed]
- Zhang, X., Price, N. E., Fang, X., Yang, Z., Gu, L. Q. & Gates, K. S. (2015). ACS Nano, 9, 11812–11819. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S205698901600517X/hb7568sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901600517X/hb7568Isup2.hkl
CCDC reference: 1448235
Additional supporting information: crystallographic information; 3D view; checkCIF report