Erratum to Acta Cryst. (2009), E65, o1325–o1326.
Abstract
In the paper by Rohlicek et al. [Acta Cryst. (2009), E65, o1325–o1326], one H atom was placed incorrectly.
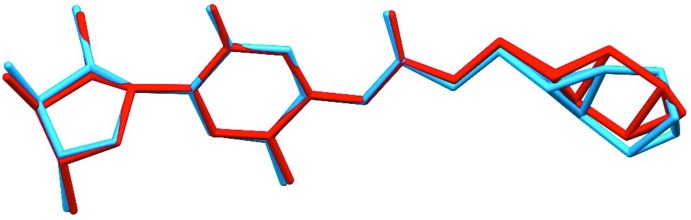
Following our powder-diffraction study of capecitabine (Rohlicek et al., 2009 ▸), Malińska et al. (2014 ▸) published the crystal structure of the same molecule based on single-crystal data. Although they modelled the wrong enantiomer [as was pointed out by Kratochvil et al. (2016 ▸)], the structures are very similar after inverting the single-crystal structure, including the disordered part of the molecule (Fig. 1 ▸). Since single-crystal diffraction is more sensitive to H atoms than powder diffraction, Malinska et al. (2014 ▸) were able to locate the H atoms directly. This indicated a different tautomeric form of capecitabine to that assumed in our study, and as they pointed out, we had therefore placed one H atom wrongly.
Figure 1.
Overlay of the capecitabine molecular structures arising from powder diffraction (blue) and from single-crystal diffraction data (red). Only non-H atoms are shown for clarity.
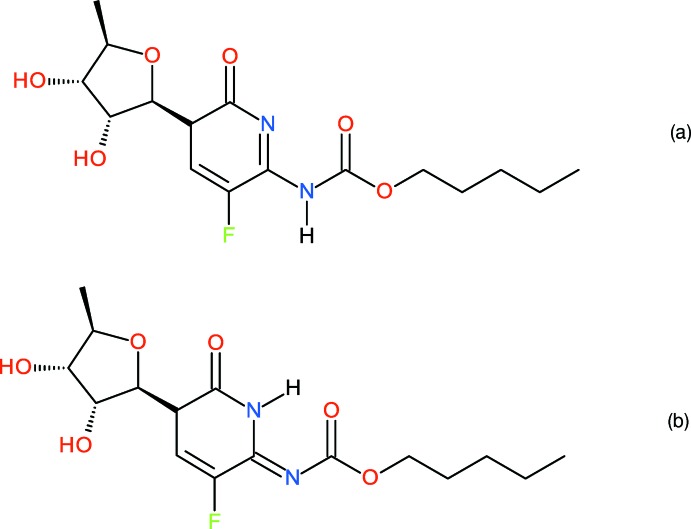
In our defence, in the powder study, we placed the H atoms geometrically according to a reasonable chemical structure for capecitabine, which shows the tautomeric H atom attached to the N atom of the carbamate group and the plausible formation of an intermolecular N—H⋯O hydrogen bond. As shown by Malińska et al. (2014 ▸), the H atom is actually located on the N atom of the pyrimidine ring (Fig. 2 ▸), thereby forming an intramolecular N—H⋯O link.
Figure 2.
Schemes for the tautomeric forms of capecitabine (a) assumed in the powder-diffraction study and (b) established in the single-crystal study of Malinska et al. (2014 ▸).
With respect to the fact that structure solution from powder diffraction data is based on the proposed molecular structure, readers should beware of the incorrectly placed H atom in Rohlicek et al. (2009 ▸) and they should be also beware of the wrong enantiomer in a single-crystal study of Malińska et al. (2014 ▸).
References
- Kratochvil, B., Husak, M., Korotkova, E. I. & Jegorov, A. (2016). Chem. Listy, 110, 40–47.
- Malińska, M., Krzecyński, P., Czerniec-Michalik, E., Trzcińska, K., Cmoch, P., Kutner, A. & Woźniak, K. (2014). J. Pharm. Sci. 103, 587–593. [DOI] [PubMed]
- Rohlicek, J., Husak, M., Gavenda, A., Jegorov, A., Kratochvil, B. & Fitch, A. (2009). Acta Cryst. E65, o1325–o1326. [DOI] [PMC free article] [PubMed]