The crystal structure of anhydrous trisodium citrate has been solved and refined using synchrotron X-ray powder diffraction data, and optimized using density functional techniques. The five-, six-, and five-coordinate Na polyhedra share edges and corners to form a three-dimensional framework.
Keywords: crystal structure, powder diffraction, density functional theory, sodium citrate
Abstract
The crystal structure of anhydrous trisodium citrate, Na3(C6H5O7), has been solved and refined using synchrotron X-ray powder diffraction data, and optimized using density functional theory (DFT). There are two independent five-coordinate Na+ and one six-coordinate Na+ cations in the asymmetric unit. The [NaO5] and [NaO6] polyhedra share edges and corners to form a three-dimensional framework. There are channels parallel to the a and b axes in which the remainder of the citrate anions reside. The only hydrogen bonds are an intramolecular one between the hydroxy group and one of the terminal carboxylate O atoms and an intermolecular one between a methylene group and the hydroxyl O atom.
Chemical context
In the course of a systematic study of the crystal structures of Group 1 (alkali metal) citrate salts to understand the anion’s conformational flexibility, ionization, coordination tendencies, and hydrogen bonding, we have determined several new crystal structures. Most of the new structures were solved using powder diffraction data (laboratory and/or synchrotron), but single crystals were used where available. The general trends and conclusions about the 16 new compounds and 12 previously characterized structures are being reported separately (Rammohan & Kaduk, 2016a
▸). Two of the new structures containing multiple Group 1 cations) – NaKHC6H5O7 and NaK2C6H5O7 – have been published recently (Rammohan & Kaduk, 2016b
▸,c
▸).
Structural commentary
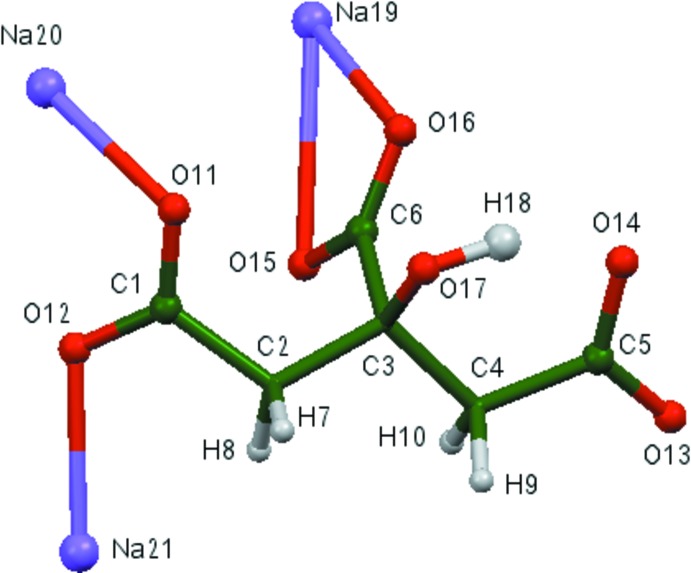
The asymmetric unit of the title compound is shown in Fig. 1 ▸. The root-mean-square deviation of the non-hydrogen atoms in the Rietveld refined and the optimized structure using density functional theory (DFT) is only 0.057 Å. The maximum deviation is 0.103 Å, at Na19. The excellent agreement between the two structures (Fig. 2 ▸) is strong evidence that the experimental structure is correct (van de Streek & Neumann, 2014 ▸). This discussion uses the DFT-optimized structure. All of the bond lengths, bond angles, and torsion angles fall within the normal ranges indicated by a Mercury Mogul geometry check (Macrae et al., 2008 ▸). The hydroxyl group bridges atoms Na20 and Na21. The citrate anion occurs in the trans,trans-conformation (about C2—C3 and C3—C4), which is one of the two low-energy conformations of an isolated citrate. The central carboxylate group and the hydroxyl group occur in the normal planar arrangement. The central carboxylate group C6–O15–O16 chelates to Na19, and the terminal carboxylate C5–O13–O14 chelates to Na21. The citrate chelates to Na20 through the hydroxyl group O17 and the terminal carboxylate C1–O11–O12, and to a second Na19 through the terminal carboxylate oxygen atom O14 and the central carboxylate oxygen atom O16. Na19 is five-coordinate (irregular) with a bond-valence sum of 1.08. Na20 is six-coordinate (distorted octahedral) with a bond-valence sum of 1.14. Na21 is five-coordinate (trigonal–bipyramidal) with a bond-valence sum of 1.01. The metal–oxygen bonding is ionic, based on the cation charges and Mulliken overlap populations.
Figure 1.
The asymmetric unit, showing the atom numbering. The atoms are represented by 50% probability spheroids.
Figure 2.
Comparison of the refined and optimized structures of trisodium citrate. The refined structure is in red, and the DFT-optimized structure is in blue.
Supramolecular features
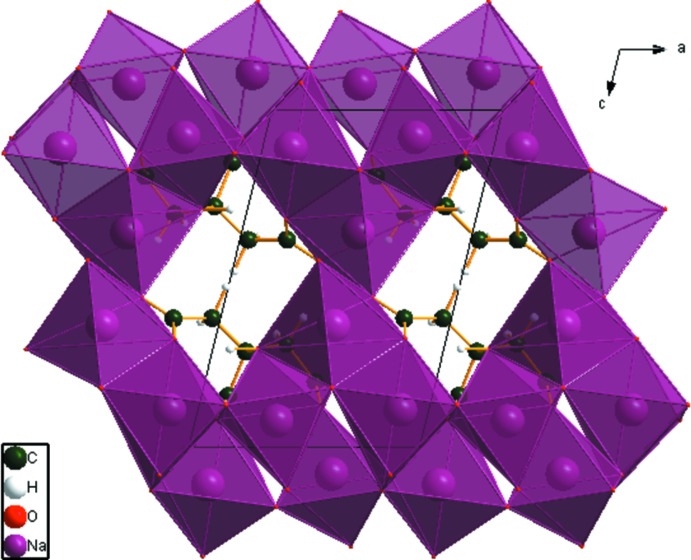
There are two independent five-coordinate and one six-coordinate Na+ cations in the asymmetric unit. The [NaO5] and [NaO6] polyhedra share edges and corners to form a three-dimensional framework (Fig. 3 ▸). There are channels parallel to the a and b axes in which the remainder of the citrate anions reside. The only hydrogen bond is an intramolecular O17–H18⋯O14 one between the hydroxy group and one of the terminal carboxylate O atoms (Table 1 ▸). One intermolecular C—H⋯O hydrogen bond also apparently contributes to the crystal packing.
Figure 3.
Crystal structure of Na3(C6H5O7), viewed down the b axis.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O17—H18⋯O14 | 0.987 | 1.805 | 2.671 | 144.0 |
| C2—H8⋯O17i | 1.086 | 2.356 | 3.355 | 152.2 |
Symmetry code: (i)  .
.
Database survey
Details of the comprehensive literature search for citrate structures are presented in Rammohan & Kaduk (2016a ▸). A reduced cell in the Cambridge Structural Database (Groom et al., 2016 ▸) search (increasing the default tolerance from 1.5 to 2.0%) yielded 19 hits, but limiting the chemistry to C, H, Na, and O only resulted in no hits. The powder pattern matched no entry in the Powder Diffraction File (ICDD, 2015 ▸).
Synthesis and crystallization
The sample was purchased from Sigma–Aldrich (lot #119K0107V) as anhydrous Na3(C6H5O7). A laboratory powder pattern confirmed its phase purity. In the one year between this measurement and the measurement of the synchrotron pattern, the sample had partially hydrated to contain Na3(C6H5O7)(H2O)2 (UMOGAE; Fischer & Palladino, 2003 ▸).
Refinement details
Both laboratory and synchrotron patterns could be indexed (DICVOL06; Louër & Boultif, 2007 ▸) on a primitive monoclinic cell having a = 7.34705 (5), b = 5.43481 (4), c = 11.03449 (7) Å, β = 103.8 (6)°, and V = 427.740 (5) Å3. The systematic absences were consistent with space group P21 (No. 4). All attempts to solve the structure using direct methods, charge flipping, and Monte Carlo simulated annealing (using a citrate and 3 Na) failed using this unit cell. Using the synchrotron pattern was complicated by the presence of 12.8 (1) wt% Na3(C6H5O7)(H2O)2 (UMOGAE; Fischer & Palladino, 2003 ▸). Since the cell of the anhydrous compound is approximately ½a, ½b, c that of the C2/c cell of UMOGAE, unsuccessful attempts to solve the structure were also made in 2× and 4× supercells of the observed cell. The powder pattern (Fig. 4 ▸) was indexed using Jade 9.5 (MDI, 2012 ▸). Pseudo-Voigt profile coefficients were as parameterized in Thompson et al. (1987 ▸), and the asymmetry correction of Finger et al. (1994 ▸) was applied and microstrain broadening by Stephens (1999 ▸).
Figure 4.
Rietveld plot for the refinement of Na3(C6H5O7). The red crosses represent the observed data points, and the green line is the calculated pattern. The magenta curve is the difference pattern, plotted at the same scale as the other patterns. The vertical scale has been multiplied by a factor of 5 for 2θ > 12.8°. The lower row of black tick marks indicates the reflection positions for the major phase and the upper row of red tick marks is for the dihydrate impurity.
The structure was ultimately solved with FOX (Favre-Nicolin & Černý, 2002 ▸) using laboratory data from a single-phase dehydrated sample. A single Na3(C6H5O7) fragment was derived from UMOGAE, with Na bound to the hydroxyl group, the central carboxyl group, and one of the terminal carboxyl groups. Attempts were made using both bump-check and bond-valence restraints, but the ultimate solution came without applying these restraints. This model refined reasonably well, but the bond-valence sums of the Na atom were unreasonable. A Hartree–Fock geometry optimization was carried out using CRYSTAL09 (Dovesi et al., 2005 ▸), and the resulting model (which had Na bond-valence sums 2) led to a successful refinement. All C—C and C—O bond lengths were restrained, as were all bond angles. The hydrogen atoms were included at fixed positions, which were re-calculated using Materials Studio (Dassault Systemes, 2014 ▸) during the course of the refinement. The U iso of C2, C3, and C4 were constrained to be equal, and those of H7, H8, H9, and H10 were constrained to be 1.3 × that of these carbon atoms. The U iso of C1, C5, C6 and the oxygen atoms were constrained to be equal, and that of H18 was constrained to be 1.3 × this value. Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The structure of the UMOGAE impurity was not refined.
Table 2. Experimental details.
| Phase_1 | Phase_2 | |
|---|---|---|
| Crystal data | ||
| Chemical formula | Na3(C6H5O7) | C6H5O7·2H2O |
| M r | 258.07 | 98.03 |
| Crystal system, space group | Monoclinic, P21 | Monoclinic, C2/c |
| Temperature (K) | 293 | 293 |
| a, b, c (Å) | 7.34705 (5), 5.43482 (4), 11.03447 (7) | 15.7057 (5), 12.5045 (5), 11.2945 (8) |
| β (°) | 103.8797 (6) | 103.611 (4) |
| V (Å3) | 427.74 (1) | 2155.84 (12) |
| Z | 2 | 2 |
| Radiation type | Synchrotron, λ = 0.41307 Å | Synchrotron, λ = 0.41307 Å |
| μ (mm−1) | 0.02 | 0.02 |
| Specimen shape, size (mm) | Cylinder, 1.5 × 1.5 | Cylinder, 1.5 × 1.5 |
| Data collection | ||
| Diffractometer | 11-BM APS | 11-BM APS |
| Specimen mounting | Kapton capillary | Kapton capillary |
| Data collection mode | Transmission | Transmission |
| Scan method | Step | Step |
| 2θ values (°) | 2θmin = 0.5 2θmax = 50.0 2θstep = 0.001 | 2θmin = 0.5 2θmax = 50.0 2θstep = 0.001 |
| Refinement | ||
| R factors and goodness of fit | R p = 0.059, R wp = 0.073, R exp = 0.062, R(F 2) = 0.06382, χ2 = 1.416 | R p = 0.059, R wp = 0.073, R exp = 0.062, R(F 2) = 0.06382, χ2 = 1.416 |
| No. of parameters | 73 | 73 |
| No. of restraints | 29 | 29 |
The Bravais–Friedel–Donnay–Harker (Bravais, 1866 ▸; Friedel, 1907 ▸; Donnay & Harker, 1937 ▸) morphology suggests that we might expect platy morphology for trisodium citrate, with {001} as the principal faces. No texture model was necessary in the refinement, showing that preferred orientation was not significant for the rotated capillary specimen.
DFT calculations
After the Rietveld refinement, a density functional geometry optimization (fixed experimental unit cell) was carried out using CRYSTAL09 (Dovesi et al., 2005 ▸). The basis sets for the C, H, and O atoms were those of Gatti et al. (1994 ▸), and the basis set for Na was that of Dovesi et al. (1991 ▸). The calculation used 8 k-points and the B3LYP functional, and took about 42 h on a 2.8 GHz PC. The U iso from the Rietveld refinement were assigned to the optimized fractional coordinates.
Supplementary Material
Crystal structure: contains datablock(s) NA3CITRATE_publ, Na3Citrate_DFT, NA3CITRATE_overall, Na3Citrate_phase_1, Na3Citrate_phase_2, NA3CITRATE_p_01. DOI: 10.1107/S2056989016007453/vn2111sup1.cif
Supporting information file. DOI: 10.1107/S2056989016007453/vn2111Na3Citrate_phase_1sup2.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| Na3C6H5O7 | β = 103.8797° |
| Mr = 258.07 | V = 427.72 Å3 |
| Monoclinic, P21 | Z = 2 |
| a = 7.3471 Å | None radiation, λ = 1.5418 Å |
| b = 5.4348 Å | T = 300 K |
| c = 11.0345 Å |
Data collection
| Density functional calculation |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.52139 | 0.36379 | 0.19408 | 0.01570* | |
| C2 | 0.68978 | 0.38591 | 0.30743 | 0.00670* | |
| C3 | 0.85441 | 0.53721 | 0.28438 | 0.00670* | |
| C4 | 1.03620 | 0.47678 | 0.38367 | 0.00670* | |
| C5 | 1.20386 | 0.64379 | 0.38297 | 0.01570* | |
| C6 | 0.88406 | 0.47193 | 0.15320 | 0.01570* | |
| H7 | 0.64280 | 0.46663 | 0.38548 | 0.00870* | |
| H8 | 0.74029 | 0.20195 | 0.33589 | 0.00870* | |
| H9 | 1.00831 | 0.48799 | 0.47670 | 0.00870* | |
| H10 | 1.08026 | 0.28777 | 0.37225 | 0.00870* | |
| O11 | 0.47255 | 0.54929 | 0.12466 | 0.01570* | |
| O12 | 0.44290 | 0.15314 | 0.17676 | 0.01570* | |
| O13 | 1.36310 | 0.57603 | 0.44517 | 0.01570* | |
| O14 | 1.17738 | 0.84623 | 0.32240 | 0.01570* | |
| O15 | 0.88375 | 0.24614 | 0.12627 | 0.01570* | |
| O16 | 0.90355 | 0.64680 | 0.08168 | 0.01570* | |
| O17 | 0.80788 | 0.79240 | 0.29212 | 0.01570* | |
| H18 | 0.92601 | 0.87924 | 0.29256 | 0.02050* | |
| Na19 | −0.12326 | 0.46644 | −0.11139 | 0.02321* | |
| Na20 | −0.35267 | −0.09861 | 0.07904 | 0.02321* | |
| Na21 | 0.50371 | −0.08736 | 0.36215 | 0.02321* |
Bond lengths (Å)
| C1—C2 | 1.539 | C4—C5 | 1.532 |
| C1—O11 | 1.265 | C4—H9 | 1.096 |
| C1—O12 | 1.276 | C4—H10 | 1.093 |
| C2—C3 | 1.533 | C5—O13 | 1.261 |
| C2—H7 | 1.094 | C5—O14 | 1.278 |
| C2—H8 | 1.086 | C6—O15 | 1.262 |
| C3—C4 | 1.546 | C6—O16 | 1.265 |
| C3—C6 | 1.556 | O17—H18 | 0.987 |
| C3—O17 | 1.436 |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O17—H18···O14 | 0.987 | 1.805 | 2.671 | 144.0 |
| C2—H8···O17i | 1.086 | 2.356 | 3.355 | 152.2 |
Symmetry code: (i) x, y−1, z.
References
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Bravais, A. (1866). In Études Cristallographiques. Paris: Gauthier Villars.
- Bruker (2009). DIFFRAC. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dassault Systemes (2014). Materials Studio. BIOVIA, San Diego, CA, USA.
- Donnay, J. D. H. & Harker, D. (1937). Am. Mineral. 22, 446–467.
- Dovesi, R., Orlando, R., Civalleri, B., Roetti, C., Saunders, V. R. & Zicovich-Wilson, C. M. (2005). Z. Kristallogr. 220, 571–573.
- Dovesi, R., Roetti, C., Freyria-Fava, C., Prencipe, M. & Saunders, V. R. (1991). Chem. Phys. 156, 11–19.
- Favre-Nicolin, V. & Černý, R. (2002). J. Appl. Cryst. 35, 734–743.
- Finger, L. W., Cox, D. E. & Jephcoat, A. P. (1994). J. Appl. Cryst. 27, 892–900.
- Fischer, A. & Palladino, G. (2003). Acta Cryst. E59, m1080–m1082.
- Friedel, G. (1907). Bull. Soc. Fr. Mineral. 30, 326–455.
- Gatti, C., Saunders, V. R. & Roetti, C. (1994). J. Chem. Phys. 101, 10686–10696.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- ICDD (2015). PDF-4+ 2015 and PDF-4 Organics 2016 (Databases), edited by Dr Soorya Kabekkodu. International Centre for Diffraction Data, Newtown Square PA, USA.
- Kourkoumelis, N. (2013). Powder Diffr. 28, 137–48.
- Larson, A. C. & Von Dreele, R. B. (2004). General Structure Analysis System, (GSAS). Report LAUR, 86–784 Los Alamos National Laboratory, New Mexico, USA.
- Louër, D. & Boultif, A. (2007). Z. Kristallogr. Suppl. 2007, 191–196.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- MDI (2012). JADE. Materials Data Inc., Livermore, CA, USA.
- Rammohan, A. & Kaduk, J. A. (2016a). Acta Cryst. B. Submitted.
- Rammohan, A. & Kaduk, J. A. (2016b). Acta Cryst. E72, 170–173. [DOI] [PMC free article] [PubMed]
- Rammohan, A. & Kaduk, J. A. (2016c). Acta Cryst. E72, 403–406. [DOI] [PMC free article] [PubMed]
- Stephens, P. W. (1999). J. Appl. Cryst. 32, 281–289.
- Streek, J. van de & Neumann, M. A. (2014). Acta Cryst. B70, 1020–1032. [DOI] [PMC free article] [PubMed]
- Thompson, P., Cox, D. E. & Hastings, J. B. (1987). J. Appl. Cryst. 20, 79–83.
- Toby, B. H. (2001). J. Appl. Cryst. 34, 210–213.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) NA3CITRATE_publ, Na3Citrate_DFT, NA3CITRATE_overall, Na3Citrate_phase_1, Na3Citrate_phase_2, NA3CITRATE_p_01. DOI: 10.1107/S2056989016007453/vn2111sup1.cif
Supporting information file. DOI: 10.1107/S2056989016007453/vn2111Na3Citrate_phase_1sup2.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report