Abstract
Purpose
Insomnia is a burdensome, commonly comorbid condition. How patients value various aspects of the safety and efficacy of available drugs has not been studied. The aim of the present study was to quantify patient-rated utility by studying willingness to pay (WTP) for attributes of symptom relief via a discrete choice experiment (DCE).
Methodology
Adult primary care patients (West Virginia University Hospital) with comorbid insomnia were enrolled. The attributes and levels examined were sleep onset latency (SOL; 10, 20, 30 minutes), awakenings (1, 2, 3), wake time after sleep onset (WASO; 15, 45, 60 minutes), total sleep time (TST; 6, 7, 8 hours), hangover (none, mild, moderate), FDA-approved duration of use (short term, not restricted to short term, no restrictions), and out-of-pocket cost per month ($20, $35, $50). Willingness to pay (WTP) data were analyzed using a random effects binary logistic regression model.
Results
A total of 82 patients completed the DCE (74 analyzed). SOL, WASO, TST, and cost were all found to predict treatment choice. Higher values of SOL, WASO, and cost resulted in decreased preference for a particular treatment, while higher TST predicted increased preference. Modeling revealed an estimated marginal WTP of $66.69 for an example product that improved SOL by 10 minutes, reduced WASO by 15 minutes, and improved TST by 1 hour.
Conclusion
Patient WTP for symptomatic relief in insomnia can help clinicians fine-tune interventions based on patient preferences, provide evidence for drug formulary and reimbursement decisions, and potentially guide the development of novel drugs.
BACKGROUND
Insomnia is an underreported (Benca 2005), underrecognized (Winkelman 2005, Rosenberg 2006), under-diagnosed, and undertreated condition, likely due to the fact that about three-fourths of cases are comorbid with other “primary” illnesses (Roth 2003, Thase 2005). Despite the individual, social, and economic burden it imposes, with annual costs estimated in the tens of billions of dollars in the United States alone (Walsh 1999, Stoller 1994), and a prevalence among adults of 6% to 30% (Roth 2007), insomnia remains inconsistently understood among health care professionals, primarily owing to the disorder’s myriad presentations and etiologies. Although pharmacological treatment options are widely available (Winkelman 2005, Rosenberg 2006), insomnia’s multifarious nature has made defining the condition difficult, and in turn has complicated evaluation of drug effectiveness. Prescription sleep aids offer varying levels of symptom relief along a number of dimensions, including improvements in sleep onset latency, sleep maintenance, and number of awakenings (Dündar 2004). Furthermore, disparities in safety profiles factor into both physicians’ and patients’ treatment decision making.

As presentation varies from patient to patient, so too does the relative benefit and value patients derive from treatment. Consequently, out-of-pocket willingness to pay (WTP) may represent the best method for evaluating therapeutic utility for both patients and payers (see glossary on page 43). Although WTP for symptom relief or cure has been examined in a number of conditions, including urinary incontinence (O’Conor 1998), psoriasis or atopic eczema (Lundberg 1999), and asthma (Blumenschein 1998), WTP studies of pharmacological interventions of insomnia remain scant in the literature.
To remedy this gap in understanding, a discrete choice experiment (DCE) was conducted to estimate patients’ WTP for various attributes of pharmacologically mediated symptom relief in insomnia. Determining the price that patients place on improvement of various symptoms and on complete symptom relief would enable an understanding of how patients value the impacts of insomnia drugs in improving disease symptoms. Attributes for which patients are likely to pay greater amounts of money would be the ones that patients value more. These highly valued attributes could provide critical evidence for drug formulary and reimbursement decisions, as well as potentially guiding novel drug development. As such, an assessment of differential WTP for various insomnia treatment attributes and complete symptom relief would constitute a valuable addition to the literature.
METHODS
Inclusion criteria/recruitment
Patients visiting the Clark K. Sleeth Family Medicine Center (West Virginia University, Morgantown) during the 6 months prior to the study were recruited via the clinic’s database. Adults (≥18 years of age) with an ICD-9 diagnosis code pertaining to any of five predefined classes of chronic conditions (cardiovascular, diabetes, gastrointestinal, musculoskeletal, and obstructive airways disease) were eligible to participate. Patients diagnosed with psychiatric conditions (eg, depression) or with other sleep disorders (eg, obstructive sleep apnea) were excluded.
A survey packet, comprising a demographic and general health questionnaire and a screening questionnaire for insomnia (the Insomnia Severity Index [ISI]—a validated 7-item instrument [Bastien 2001]), was initially mailed to all selected participants. A cut-off score of >15 on the ISI was used to identify patients suffering from insomnia (Bastien 2001). Results of this survey have been described elsewhere (Roy 2014). Respondents identified as having insomnia via the ISI were subsequently mailed the DCE survey.
Rationale behind study design
WTP can be estimated using a DCE, an attribute-based stated preference valuation technique used to quantify preferences (ie, utilities) for commodities. By including a “price” attribute in a DCE, a monetary measure of benefit (ie, WTP) may be estimated (Kleinman 2002). In a DCE, subjects choose their preferred alternative from a number of hypothetical choices. Each choice is described by a unique combination of attribute levels. For example, in a hypothetical DCE examining three levels of both dosing (once, twice, or thrice daily) and method of administration (oral, intravenous, or topical), a potential choice-set presented to subjects might be an oral medication administered once daily versus a topical medication administered thrice daily. Using a DCE, it is possible to identify the relative importance of the individual attributes, and quantify how individuals weight the tradeoffs between them. Including cost as an attribute can yield marginal WTP estimates for the various attributes as well as total WTP for combinations of attribute levels (Ryan 2001). Although WTP may be directly estimated by other means, DCEs may provide richer information than such methods by accounting for how constituent attributes affect utility (Louviere 2000).
KEY POINTS.
Sleep onset latency (time it takes to fall asleep), wake time after sleep onset (cumulative amount of time spent awake during a sleep period), total sleep time, and cost are useful in predicting sleep aid preferences.
People are willing to pay $2.22 per month for every minute of reduction in sleep onset latency.
People are willing to pay $1.44 per month for each minute less they are awake during the sleep period.
An increase in total sleep time from 6 to 7 hours is highly valued, but people are indifferent to adding sleep time over 7 hours.
Willingness to pay changes with financial circumstances, so these findings might not apply to people in different circumstances.
The study did not include cognitive debriefing of the respondents that would help identify issues related to respondent understanding of the discrete choice experiment (DCE) scenarios.
People in this study were recruited at an outpatient clinic of a hospital, and their insomnia was associated with other health conditions. These findings may not generalize to other populations.
Design of the discrete choice experiment
The attributes and levels examined in the DCE were determined via a review of the literature and expert input (interviews with three sleep specialists and a focus group discussion with eight general practitioners). Specifically, experts were queried regarding hypnotics in the context of patients seeking treatment for insomnia comorbid with other somatic conditions.
Based on the results of these investigations, the following attributes and levels were chosen as representative of the important determinants in choosing a prescription sleep aid: sleep onset latency (SOL; 10, 20, 30 minutes), awakenings (1, 2, 3), wake time after sleep onset (WASO; 15, 45, 60 minutes), total sleep time (TST; 6, 7, 8 hours), hangover (none, mild, moderate), FDA-approved duration of use (short term, not restricted to short term, no restrictions), and out-of-pocket cost per month ($20, $35, $50). Out-of-pocket costs were estimated based on input from a pharmacy manager regarding typical monthly payments made by patients for prescription sleep aids.
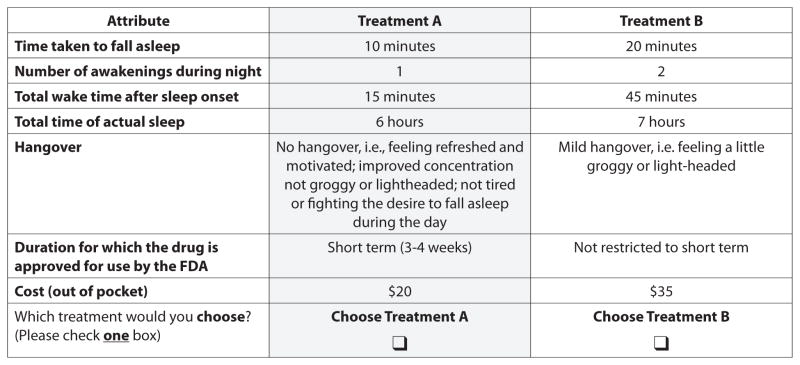
Because it wasn’t feasible to present all unique choice-sets to respondents, a set of 18 unique, two-choice scenarios (Table 1, page 46) was chosen based on an experimental design using the Addelman-Kempthorne orthogonal array, the simplest specification with the correct number of attributes and levels (Addelman 1961). Choice-sets were generated based on input from clinicians and an expert in DCE methodology. Scenarios were designed to exhaustively examine attributes of potential interest to patients and to be easy to understand. Figure 1 on page 45 is an example of one of the choice scenarios presented to the study subjects.
TABLE 1.
Orthogonal array of attribute level choice-sets
| CHOICE A | CHOICE B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Question | Sleep onset latency (minutes) | No. of awakenings | Wake time after sleep on-set (minutes) | Total sleep time (hours) | Hangover | Duration of use approved | Cost | Sleep onset latency (minutes) | No. of awakenings | Wake time after sleep on-set (minutes) | Total sleep time (hours) | Hangover | Duration of use approved | Cost |
| 1 | 10 | 1 | 15 | 6 | None | 3–4 weeks | $20 | 20 | 2 | 45 | 7 | Mild | 6 months | $35 |
| 2 | 20 | 2 | 45 | 7 | Mild | 6 months | $20 | 30 | 3 | 60 | 8 | Moderate | Indefinite | $35 |
| 3 | 30 | 3 | 60 | 8 | Moderate | Indefinite | $20 | 10 | 1 | 15 | 6 | None | 3–4 weeks | $35 |
| 4 | 10 | 1 | 45 | 8 | Mild | Indefinite | $20 | 20 | 2 | 60 | 6 | Moderate | 3–4 weeks | $35 |
| 5 | 20 | 2 | 60 | 6 | Moderate | 3–4 weeks | $20 | 30 | 3 | 15 | 7 | None | 6 months | $35 |
| 6 | 30 | 3 | 15 | 7 | None | 6 months | $20 | 10 | 1 | 45 | 8 | Mild | Indefinite | $35 |
| 7 | 10 | 2 | 15 | 8 | Moderate | 6 months | $35 | 20 | 3 | 45 | 6 | None | Indefinite | $50 |
| 8 | 20 | 3 | 45 | 6 | None | Indefinite | $35 | 30 | 1 | 60 | 7 | Mild | 3–4 weeks | $50 |
| 9 | 30 | 1 | 60 | 7 | Mild | 3–4 weeks | $35 | 10 | 2 | 15 | 8 | Moderate | 6 months | $50 |
| 10 | 10 | 3 | 60 | 6 | Mild | 6 months | $35 | 20 | 1 | 15 | 7 | Moderate | Indefinite | $50 |
| 11 | 20 | 1 | 15 | 7 | Moderate | Indefinite | $35 | 30 | 2 | 45 | 8 | None | 3–4 weeks | $50 |
| 12 | 30 | 2 | 45 | 8 | None | 3–4 weeks | $35 | 10 | 3 | 60 | 6 | Mild | 6 months | $50 |
| 13 | 10 | 2 | 60 | 7 | None | Indefinite | $50 | 20 | 3 | 15 | 8 | Mild | 3–4 weeks | $20 |
| 14 | 20 | 3 | 15 | 8 | Mild | 3–4 weeks | $50 | 30 | 1 | 45 | 6 | Moderate | 6 months | $20 |
| 15 | 30 | 1 | 45 | 6 | Moderate | 6 months | $50 | 10 | 2 | 60 | 7 | None | Indefinite | $20 |
| 16 | 10 | 3 | 45 | 7 | Moderate | 3–4 weeks | $50 | 20 | 1 | 60 | 8 | None | 6 months | $20 |
| 17 | 20 | 1 | 60 | 8 | None | 6 months | $50 | 30 | 2 | 15 | 6 | Mild | Indefinite | $20 |
| 18 | 30 | 2 | 15 | 6 | Mild | Indefinite | $50 | 10 | 3 | 45 | 7 | Moderate | 3–4 weeks | $20 |
FIGURE 1.
An example of one of the 18 choice scenarios presented to study subjects
Administration of the discrete choice experiment
Participants were mailed paper-and-pencil format DCE questionnaires and an instruction booklet. Patients were instructed to imagine a situation in which they wanted to get a prescription for a sleep aid either for the first time or because their current sleep aid was not helping, and to select one treatment they preferred from each set of treatment choices offered. A scenario superior on all attributes was posed as a consistency check. Data from respondents failing the consistency check (ie, those not choosing the clearly superior scenario) were discarded. Respondents were offered a gift card of $15 as a gesture of appreciation for completing the questionnaires.
Ethics and blinding
All research activities were approved by the Institutional Review Board (IRB) of West Virginia University prior to study commencement. Privacy safeguards in compliance with the IRB and the Health Insurance Portability and Accountability Act were instituted for the study, and all patient-level data (related to diagnoses, age, and insurance status) were obtained from patient records extracted by participating clinic staff.
In accordance with IRB regulations, the target sample was assigned alphanumeric codes by the study clinic. Codes were based on current diagnoses of the participants and were used to track responses. Identifiers corresponding to these codes were available only to clinic staff, and only anonymized data were used for analysis.
Statistical analyses
WTP data were analyzed by a random effects binary logistic regression model, which was implemented using SPSS (version 14.0). First, odds and the logarithm of the odds of choosing each particular scenario within a profile were calculated. Subsequently, the marginal means (or utilities) of each particular attribute were calculated. Finally, a logit model was employed to estimate binary response probabilities among scenario pairs.
RESULTS
Altogether, 82 patients completed the DCE. Eight respondents failed the consistency check and were discarded, for a total of 74 submitted to analysis. Over 60% of the respondents were women; the average age was 52 years. Demographic and socioeconomic data are presented in Table 2.
TABLE 2.
Demographic and socioeconomic characteristics
| Demographic characteristic | Value |
|---|---|
| Age, mean (SD), years | 51.7 (±25.1) |
| Sex, female | 62.2% |
| Race, white | 94.6% |
| Married currently | 55.4% |
| Educational status | |
| High school or less | 47.3% |
| Some college | 18.9% |
| College graduate | 32.4% |
| Employment status | |
| Employed full time | 41.9% |
| Employed part time | 8.1% |
| Not employed | 48.6% |
| Annual household income | |
| <$25,000 | 40.5% |
| $25,001–$50,000 | 29.7% |
| $50,001–$100,000 | 13.5% |
| >$100,000 | 12.2% |
The marginal means (ie, utilities) of the log odds for the various attributes were calculated based on the odds of a respondent choosing a particular attribute profile and are summarized in Table 3. The WTP model based on binary logistic regression analyses of these data is presented in Table 4. Estimates of WTP for changes in each of the attributes are provided in Table 5.
TABLE 3.
Marginal means
| Attributes and levels | Marginal means (utilities) of levels* | ||
|---|---|---|---|
| Sleep onset latency (10, 20, 30 minutes) | −0.1 | 0.3 | −0.2 |
| Awakenings (1, 2, 3) | −0.2 | 0.3 | 0.0 |
| Wake time after sleep onset (15, 45, 60 minutes) | 0.3 | 0.2 | −0.3 |
| Total sleep time (6, 7, 8 hours) | 0.0 | 0.1 | 0.1 |
| Hangover (none, mild, moderate) | 1.6 | −1.4 | 1.6 |
| FDA-approved duration of use (short term, not restricted to short term, no restrictions) | −0.2 | 0.0 | 0.2 |
| Costs, out-of-pocket per month ($20, $35, $50) | 0.2 | 0.3 | 1.0 |
Marginal means represent the utilities attached to the different levels of the attributes.
TABLE 4.
Variables and their parameters estimates in the equation (results from the basic logistic regression analyses)
| Attributes | B | SE | Wald | df | P value | Exp(B) |
|---|---|---|---|---|---|---|
| Sleep onset latency | −0.02 | 0.01 | 10.80 | 1 | <.001 | 0.98 |
| Awakenings | −0.05 | 0.06 | 0.57 | 1 | .45 | 0.96 |
| Wake time after sleep onset | −0.01 | 0.00 | 24.25 | 1 | .01 | 0.99 |
| Total sleep time | 0.21 | 0.06 | 11.53 | 1 | .001 | 1.23 |
| Hangover, none | 668.51 | 2 | <.001 | |||
| Hangover, mild | 3.52 | 0.14 | 667.48 | 1 | <.001 | 33.77 |
| Hangover, moderate | 1.97 | 0.13 | 232.81 | 1 | <.001 | 7.14 |
| FDA, short term | 31.57 | 2 | <.001 | |||
| FDA, not restricted to short term | −0.54 | 0.12 | 19.44 | 1 | <.001 | 0.58 |
| FDA, no restrictions | 0.13 | 0.12 | 1.16 | 1 | 0.82 | 1.14 |
| Costs, out-of-pocket per month | −0.01 | 0.00 | 5.30 | 1 | .02 | 0.99 |
| Constant | −1.95 | 0.53 | 13.57 | 1 | <.001 | 0.14 |
B=logistic coefficient, df=degrees of freedom, Exp(B)=change in odds ratio, SE=standard error, Wald=Wald statistic
TABLE 5.
WTP results from the basic logistic regression model
| Attributes | (SE) | Marginal WTP/month ($) |
|---|---|---|
| Sleep onset latency (per minute) | 0.01 | $2.22 |
| Wake time after sleep onset (per minute) | 0.00 | $1.44 |
| Total sleep time (per hour) | 0.06 | $22.89 |
| Hangover (none=reference category) | ||
| Mild | 0.14 | $391.00 |
| Moderate | 0.13 | $218.44 |
| FDA-approved duration of use (short term=reference category) | ||
| Not short term | 0.12 | $60.33 |
| Costs (out of pocket per month) | 0.00 | NA |
Relative utility varied among the attribute levels examined (Table 3). A positive trend with increasing level was observed in FDA-approved duration and cost, initially indicating a progressive increase in estimated utility among respondents for increasing values of those attributes (log odds ratios are linearly related to the unknown utilities for each attribute level [Louviere 2000]). The opposite trend, however, was observed in WASO, which proceeded from a positive marginal mean of 0.3 for the 15-minute level to −0.3 at 45 minutes. Similarly, SOL, awakenings, and TST all showed initial increases followed by a decreasing or flattening trend, while hangover exhibited the opposite (ie, an initial decrease followed by an increase across attribute levels).
Statistically significant Wald statistics (P≤0.05) for the parameter estimates for SOL, WASO, TST, and cost indicated that all these attributes were useful in predicting choice of hypnotic in the model. Also, values of Exp(B), a measure of the likelihood of choosing a product with increasing levels of a particular attribute, revealed that, consistent with expectations, higher values of SOL, WASO, and cost resulted in a decreased preference (Exp[B] <1.0), while higher values of TST resulted in an increased preference (Exp[B] >1.0) for a particular profile.
With respect to WTP, values of marginal WTP ranged from $1.44 per month for each minute decrease of WASO to $22.89 per month for each additional hour of total sleep time. For FDA-approved duration of use and hangover, the simplest and most appropriate way to interpret the data is that the participants placed no value on the avoidance of hangover or restrictions on use in this sample of patients in which the DCE was conducted. Based on these estimates, the marginal WTP for an example product that improved SOL by 10 minutes, reduced WASO by 15 minutes, and improved TST by 1 hour would be: ($2.22 × 10) + ($1.44 × 15) + ($22.89 × 1) = $66.69 per month.
DISCUSSION
The objective of the study was to determine patients’ preferences for various types of treatment-induced symptom relief by assessing WTP, which has not been studied previously. Based on the results, the attributes of SOL, WASO, and TST were useful in predicting choice of hypnotic, while awakenings were not. Specifically, higher levels of SOL or WASO (ie, the amount of time trying to fall asleep or the time awake following a sleep interruption) were associated with a lower preference, while a greater TST (ie, the overall amount of sleep) was associated with an increased preference.
All attributes, with the exception of hangover and one level of FDA-approved duration of use, exhibited the expected sign (positive or negative), thereby validating the theoretical constructs underlying the model. Attributes demonstrating statistical significance may be considered to be especially useful in explaining the DCE model.
Individually, findings reveal some nuance among patient preference (or lack thereof) for particular attributes. The awakenings attribute, for example, did not reach statistical significance; however, WASO, which did, incorporates the concept of both number of awakenings and the duration of such awakenings, and therefore the concept of awakenings was validated and remained accounted for in the model. With respect to SOL, the negative sign on this attribute indicated that respondents preferred a reduction in SOL. Similarly, the negative coefficients on WASO suggest that respondents preferred a reduction in the attribute and would be willing to pay for it. Also, as anticipated, marginal utilities decreased with increasing WASO (ie, patients preferred a decrease in WASO) and increased with lengthening TST. Although the increase in sleep time by 1 hour was valued very highly, as shown by the increase in utilities moving from 6 to 7 hours, patients seemed to be relatively indifferent to an increase in total sleep time over 7 hours.
FDA-approved duration of use performed inconsistently, and while the coefficient for one level of this attribute was positive, indicating that longer approved duration of use may be of value to patients, the attribute did not reach statistical significance. Hangover similarly performed inconsistently, as sign varied counter to expectations. These results were possibly the consequence of the study participants misinterpreting the attributes and levels as they were responding to questions in a mailed survey. Therefore, we chose to interpret this data in the simplest and most appropriate way and treat it as though the participants did not place any value on the two attributes.
Despite the minor deviations noted, overall the model appears consistent with a priori expectations that, in choosing an insomnia treatment, patients have different preferences for the various aspects of symptom relief examined.
Limitations
Discrepancies have been observed between what patients claim they will pay and what they actually pay (Blumenschein 2001). Although the results of the present study indicate a WTP for symptom relief in insomnia, true WTP may differ in real-world scenarios, especially in the face of other financial commitments. This may be particularly true among the population sample described in the present study, of which 48.6% were unemployed and a further 8.1% employed only part-time—figures likely related to the relatively high proportion of subjects (47.3%) completing only high school or less. Generally, WTP is most influenced by ability to pay and the severity of the disease condition. As such, similar studies employing samples more representative of the nation as a whole, or with varying proportions of more well off or severely afflicted individuals, may differ considerably in their findings.
As previously noted, respondents were a small convenience sample of patients from the outpatient clinics of a university hospital, which, in addition to the considerations noted above, may limit its generalizability and statistical power. In particular, owing to the predominately Caucasian makeup of the sample (94.6%), it is unclear whether WTP might differ across a more racially diverse population.
It is also worth noting that, in the absence of an understanding of what constitutes an ideal response burden for the survey population, the number of questions asked may not have been optimal and thus had an unpredictable effect on response rates. Nevertheless, these findings are instructive with respect to the relative importance patients ascribe to the various attributes of symptom relief.
Importance
Knowledge of WTP for symptomatic relief in insomnia can help clinicians understand the preferences of patients and fine-tune an intervention based on characteristics of the prescribed treatment. Data with respect to patient WTP may help assess whether important benefits from treatment are in line with patients’ preferences and how much patients value those benefits.
Such knowledge can also help guide decision makers in the cost-effective provisioning of health care. As estimates of direct and indirect costs of untreated insomnia have pegged 6-month expenditures as $1,253 greater for patients with insomnia (Ozminkowski 2007), the incentives to effectively treat the condition are strong. Payers and hospital policy makers can use WTP information as a relevant outcome measure to create a monetary rank-order based on stated user value, and thereby make decisions concerning formulary inclusions and pharmacy benefits as new products are introduced into clinical practice. Manufacturers can also use information on patient preferences and WTP for various product-related attributes to guide product development and marketing. Such informed decisions are especially critical in an age of increasing accountability for health care dollars spent and patient-centered health care.
Acknowledgments
Ramon Iovin, PhD, provided writing assistance in the preparation of this manuscript.
Funding source: Sepracor (now Sunovion Pharmaceuticals) provided funding in the form of a grant awarded to Roy to support research conducted as part of her doctoral dissertation at West Virginia University. Roy’s current employer, Novartis, did not fund or influence the manuscript in any way.
Glossary
- Approved Duration of Use
The period of time over which a drug may be safely used, as specified by the FDA. The FDA approves some sleep aids for short-term use (3–4 weeks), and some are approved with no short-term restrictions, so they may be used for a few months or years. Other sleep aids are approved with no restrictions on duration of use whatsoever, meaning they can be used indefinitely. Sleep aids approved with lesser restrictions presumably would be considered safer and thus favored by patients
- Awakenings
The number of disruptions in a sleep period. Sleep aids that reduce the number of awakenings in a given sleep period presumably would be favored by patients
- Discrete Choice Experiment (DCE)
A means of elucidating preferences across a given set of attributes. In a DCE, subjects select their preferred alternative from a number of hypothetical alternatives, where each option is described by a unique combination of attribute levels
- Hangover
Residual sedation attributed to a pharmacological sleep aid. Sleep aids with less of a hangover presumably would be favored by patients
- Sleep Onset Latency (SOL)
The delay between the attempt to sleep and actual sleep onset. Sleep aids that reduce SOL presumably would be favored by patients
- Total Sleep Time (TST)
The cumulative amount of time spent asleep in a sleep period. Sleep aids that increase the total amount of time spent asleep presumably would be favored by patients
- Wake Time After Sleep Onset (WASO)
The cumulative amount of time spent awake in a sleep period. Sleep aids that reduce the amount of time awake following an awakening presumably would be favored by patients
- Willingness to Pay (WTP)
A measure of the utility or value, in monetary terms, assigned to a particular therapeutic attribute or set of attributes
Footnotes
Conflict disclosure: None
References
- Addelman S, Kempthorne O. Some main-effect plans and orthogonal arrays of strength two. Ann Math Statist. 1961;32:1167–1176. [Google Scholar]
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Benca RM. Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv. 2005;56:332–343. doi: 10.1176/appi.ps.56.3.332. [DOI] [PubMed] [Google Scholar]
- Blumenschein K, Johannesson M, Yokoyama KK, Freeman PR. Hypothetical versus real willingness to pay in the health care sector: results from a field experiment. J Health Econ. 2001;20:441–457. doi: 10.1016/s0167-6296(01)00075-3. [DOI] [PubMed] [Google Scholar]
- Blumenschein K, Johannesson M. Relationship between quality of life instruments, health state utilities, and willingness to pay in patients with asthma. Ann Allergy Asthma Immunol. 1998;80:189–194. doi: 10.1016/S1081-1206(10)62954-7. [DOI] [PubMed] [Google Scholar]
- Dündar Y, Boland A, Strobl J, et al. Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation. Health Technol Assess. 2004;8(24):iii–x. 1–125. doi: 10.3310/hta8240. [DOI] [PubMed] [Google Scholar]
- Kleinman L, McIntosh E, Ryan M, et al. Willingness to pay for complete symptom relief of gastroesophageal reflux disease. Arch Intern Med. 2002;162:1361–1366. doi: 10.1001/archinte.162.12.1361. [DOI] [PubMed] [Google Scholar]
- Louviere JJ, Hensher DA, Swait DJ. Experimental design. In: Louviere JJ, Hensher DA, Swait DJ, editors. Stated Choice Methods. Analysis and Applications. 1. Cambridge, UK: Cambridge University Press; 2000. pp. 83–110. [Google Scholar]
- Lundberg L, Johannesson M, Silverdahl M, et al. Quality of life, health-state utilities and willingness to pay in patients with psoriasis and atopic eczema. Br J Dermatol. 1999;141:1067–1075. doi: 10.1046/j.1365-2133.1999.03207.x. [DOI] [PubMed] [Google Scholar]
- O’Conor RM, Johannesson M, Hass SL, Kobelt-Nguyen G. Urge incontinence. Quality of life and patients’ valuation of symptom reduction. Pharmacoeconomics. 1998;14:531–539. doi: 10.2165/00019053-199814050-00005. [DOI] [PubMed] [Google Scholar]
- Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in the United States. Sleep. 2007;30:263–273. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18:49–56. doi: 10.1080/10401230500464711. [DOI] [PubMed] [Google Scholar]
- Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone. 2003;5:5–15. doi: 10.1016/s1098-3597(03)90031-7. [DOI] [PubMed] [Google Scholar]
- Roth T. Insomnia: Definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 suppl):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- Roy AN, Madhavan S. Patient reported health-related quality of life in co-morbid insomnia: results from a survey of primary care patients in the United States. Primary Health Care. 2014;4(2):160. doi: 10.4172/2167-1079.1000160. [DOI] [Google Scholar]
- Ryan M, Bate A, Eastmond CJ, Ludbrook A. Use of discrete choice experiments to elicit preferences. Qual Health Care. 2001;10(suppl 1):i55–i60. doi: 10.1136/qhc.0100055... [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller MK. Economic effects of insomnia. Clin Ther. 1994;16:873–897. [PubMed] [Google Scholar]
- Thase ME. Correlates and consequences of chronic insomnia. Gen Hosp Psychiatry. 2005;27:100–112. doi: 10.1016/j.genhosppsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Engelhardt CL. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999;22(suppl 2):S386–S393. [PubMed] [Google Scholar]
- Winkelman J, Pies R. Current patterns and future directions in the treatment of insomnia. Ann Clin Psychiatry. 2005;17(1):31–40. doi: 10.1080/10401230590905344. [DOI] [PubMed] [Google Scholar]