Abstract
Many neurological and psychiatric diseases are associated with clinically detectable, altered brain dynamics. The aberrant brain activity, in principle, can be restored through electrical stimulation. In epilepsies, abnormal patterns emerge intermittently, and therefore, a closed-loop feedback brain control that leaves other aspects of brain functions unaffected is desirable. Here, we demonstrate that seizure-triggered, feedback transcranial electrical stimulation (TES) can dramatically reduce spike-and-wave episodes in a rodent model of generalized epilepsy. Closed-loop TES can be an effective clinical tool to reduce pathological brain patterns in drug-resistant patients.
A successful, although not well-understood, therapy in drug-resistant cases of Parkinson’s disease and depression is deep brain stimulation (1–3), in which high-frequency stimulation is applied continuously. In many diseases, such as epilepsies, events recur unpredictably and often are separated by long interictal intervals (4–6). In such instances, a closed-loop, transient feedback control could abort seizure episodes without inducing detrimental side effects of continuous stimulation (7–13). We attempted to achieve seizure control by means of closed-loop transcranial electrical stimulation (TES) in a rodent model of generalized (“petit mal”) epilepsy (14, 15) because previous experiments have shown that even very weak TES can reliably entrain neurons in widespread cortical areas (16–20).
We first demonstrated the effect of TES on cortical excitability. Local field potentials (LFPs) and multiple-unit activity (MUA) were recorded by chronically implanted tripolar electrodes (Fig. 1A) and placed in the deep and superficial layers of the frontal and parietal cortical areas (21). TES was applied either between the left and right temporal electrodes, placed directly on the skull, or between these bitemporal electrodes, against a frontal midline electrode (Fig. 1B and fig. S1) (18). TES-induced artifacts were reduced by using tripolar electrodes and deriving current source density (CSD) of the recorded traces online (Fig. 1, C and D). TES sinusoid trains at 1 Hz induced significant rate modulation of multiple unit firing patterns both in the absence and presence of spike-and-wave (SW) episodes (P < 0.01; 61 of 103 cortical sites; n = 3 rats; Rayleigh test) (Fig. 2). In addition to affecting the firing rates of neurons, TES at 1 Hz also strongly modulated the spike amplitude of SW patterns (Fig. 2, C and D, and fig. S2) but had no effect on the duration of SW episodes [time (t) = 8.62 s, 21,902 s of TES epochs; t = 9.14 s, 21,775 s of control epochs; n = 9 rats; P > 0.05; t test for dependent samples]. Additional control experiments demonstrated that TES, at the intensities used, neither induced arousal effects when applied during sleep nor affected overt behavior during waking, as demonstrated by the lack of TES-induced head movements (figs. S3 and S4).
Fig. 1.
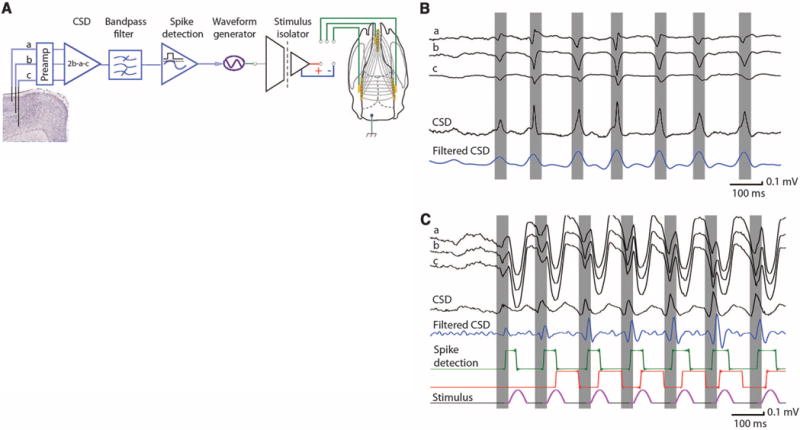
Experimental setup. (A) Artifact reduction by means of tripolar recordings of LFP and unit activity in superficial, mid-, and deep cortical layers. CSD is derived from the three signals (LFPs from channels a and c are subtracted from the 2× amplitude of channel b activity). The derived CSD signal is filtered (10 to 130 Hz), and signals exceeding the predetermined amplitude threshold are detected (spike detection). The thresholded signals are used to trigger TES, applied to the skull either in a bipolar configuration (left versus right hemispheres) or frontal midline versus parietal areas (as shown). (B) Example of LFP signal during a spontaneously occurring SW episode, CSD, and its filtered version in the absence of TES stimulation. (C) Similar SW episode in the presence of TES stimulation. The gating (red) “dead time” pulse (80 ms) was used to prevent prolonged spurious triggering of the stimulator during the wave components of SW episode.
Fig. 2.
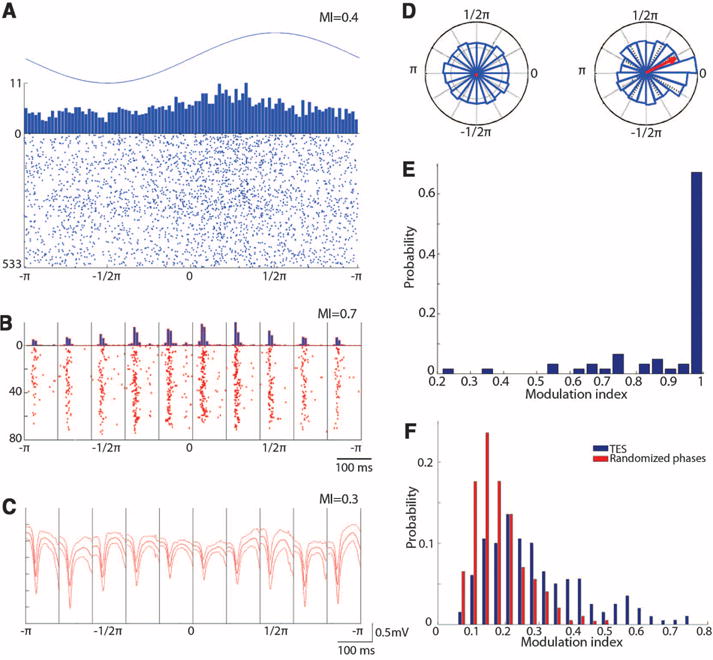
TES modulates cortical neuronal firing and spike component of SW. (A) Raster plot and histogram of multiple-unit firing during interictal 1-Hz TES (sinus). (B) Because both TES and SW strongly modulated unit firing, SW-related discharge was calculated in 10 bins (100-ms windows), using the spike component as reference in each bin. TES-induced cyclic modulation of multiple-unit firing during SW is shown. (C) TES modulation of the LFP spike of SW [different rat from (A) and (B)] and mean and SD of SW in each bin. (D) Polar plots of the magnitude of the LFP spike component in the absence (left, random phase) and presence of TES (right). (A) to (D) are single-session examples. (E) Group data showing the effectiveness of TES stimulation on unit-firing modulation during SW patterns. Modulation index (MI) of 1 corresponds to sessions in which TES completely silenced MUA in at least one bin. (F) Distribution of MI of the LFP spike amplitude compared with randomly shuffling the relationship between LFP spike amplitude and TES phase in all sessions and rats (n = 9 rats).
We next sought to examine how brain activity–triggered stimulation affects SW patterns. Because SW patterns are strongly periodic events, involving reverberatory activity of the thalamocortical loop (14), we applied Gaussian waveforms of 50-ms TES after the detected spike components. SW-triggered TES shortened the duration of SW episodes in an intensity-dependent manner (Fig. 3). Group analysis of closed-loop TES stimulation showed that the mean duration of the SW episodes was significantly shorter in nine of the nine rats (P < 0.01; two sample t test). In addition, the percent time spent in SW episodes in a given session was significantly reduced in seven of the nine rats (P < 0.01). Overall, feedback TES stimulation lead to a >60% decrease of both the duration of SW episodes and the fraction of session time spent in SW across animals (Fig. 4). The percent decrease of time spent in SW episodes in a given session was largely a consequence of the decreased duration of SW episodes because there was a significant correlation between the mean duration and the fraction of time in SW episodes [correlation coefficient (r) =0.60; P < 0.001; Pearson’s correlation test] (fig. S5). This relationship thus shows that TES did not simply fragment or delay SW episodes. The number of SW episodes per unit time was not significantly different between control and TES sessions (P > 0.05; two sample t test), indicating that TES-induced reduction of SW episodes did not lead to a rebound or long-term compensatory increase of their probability of occurrence.
Fig. 3.
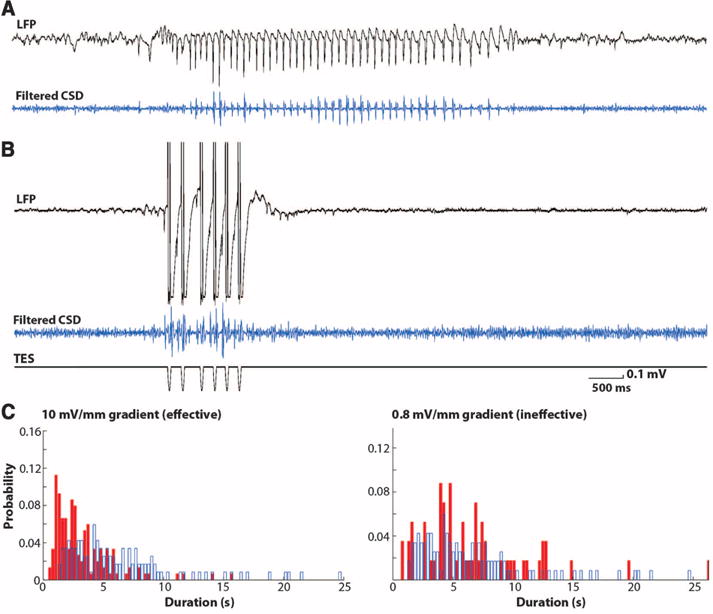
Closed-loop feedback TES stimulation aborts SW episodes. (A and B) SW episodes (LFP and filtered CSD traces) without (top) and with (bottom) feedback TES. (C) Distribution of the duration of SW episodes in the absence (control, blue) and presence of feedback TES. Shown are data from two example sessions with effective (10 mV/mm voltage gradient intensity) and ineffective, subthreshold (0.8 mV/mm) TES stimulation.
Fig. 4.
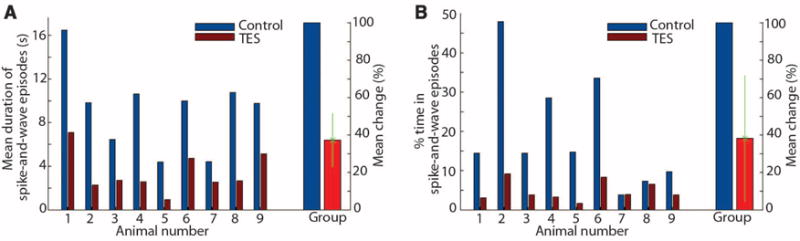
Closed-loop feedback TES stimulation aborts SW episodes. (A) Mean duration of SW episodes in each of the nine rats tested and the group mean (and SD). (B) Percent time spent in SW episodes in a given recording session. Shown are results from individual rats and group. Mean percent change refers to group data.
These findings show that brain pattern–triggered feedback TES of cortical neurons can interfere with thalamocortical reverberation during SW episodes and effectively reduce their duration. SW patterns—the hallmark of generalized petit mal epilepsy—arise from complex interactions between thalamic and neocortical neurons (14, 22–24). During the wave component of the SW cycle, both neocortical and thalamic neurons are largely silent (22–25). We hypothesize that cortical excitatory feedback during this silent period, brought about with TES, quenched the ongoing rhythm by recruiting subsets of thalamic cells, which in turn became refractory during the duty phase of the native SW cycle, as shown through tandem optogenetic activation of the thalamus and neocortex in mice (fig. S6). Brain activity–timed feedback of TES appears critical because 1-Hz sinusoid trains did not affect the duration of SW episodes.
Successful clinical application of closed-loop TES has two fundamental requirements (9, 11, 26, 27). The first is the recording and identifying of causal pathophysiological network patterns. In generalized SW and focal cortical epilepsies, subdural or epidural electrodes, or even electrodes inserted into the skull, may be sufficient. In complex partial seizures, which make up the largest fraction of drug-resistent epilepsies, deep-electrode recordings are required for accurate detection of abnormal patterns (11, 13). The second requirement is closed-loop feedback stimulation of the target circuits, whose activation can interfere with the emerging pathological pattern. Intra-skull plate electrodes may ideally diffuse the applied currents to affect sufficiently large groups of neurons (28). Alternatively, multiple and appropriately placed plates may be used to achieve more focal concentration of current. In contrast to transcranial magnetic stimulation, which requires large and heavy coils, TES electrodes implanted in the skull and powered by ultralight electrical circuits are a cosmetically acceptable solution for long-term clinical applications. Noninvasive, closed-loop TES stimulation may also prove useful for improving mental and mood states.
Supplementary Material
Acknowledgments
This work was supported by NIH grants NS34994, MH54671, and NS074015; the Human Frontier Science Program; and the J. D. McDonnell Foundation. A.B. was supported by a Marie Curie FP7-PEOPLE-2009-IOF grant within the 7th European Community Framework Programme (no. 254780) and by the Rosztoczy Foundation.
Footnotes
Supplementary Materials
www.sciencemag.org/cgi/content/full/337/6095/735/DC1
Materials and Methods
References (29–36)
References and Notes
- 1.Hammond C, Bergman H, Brown P. Trends Neurosci. 2007;30:357. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Mayberg HS, et al. Neuron. 2005;45:651. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Perlmutter JS, Mink JW. Annu Rev Neurosci. 2006;29:229. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makeyev O, Liu X, Koka K, Kay SM, Besio WG. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:7560. doi: 10.1109/IEMBS.2011.6091864. [DOI] [PubMed] [Google Scholar]
- 5.Motamedi GK, et al. Epilepsia. 2002;43:836. doi: 10.1046/j.1528-1157.2002.24901.x. [DOI] [PubMed] [Google Scholar]
- 6.Schiff SJ, et al. Nature. 1994;370:615. doi: 10.1038/370615a0. [DOI] [PubMed] [Google Scholar]
- 7.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Nat Neurosci. 2009;12:1222. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 8.Najib U, Bashir S, Edwards D, Rotenberg A, Pascual-Leone A. Neurosurg Clin N Am. 2011;22:233, ix. doi: 10.1016/j.nec.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosin B, et al. Neuron. 2011;72:370. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Varga ET, et al. Epilepsy Res. 2011;97:142. doi: 10.1016/j.eplepsyres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Morrell MJ, RNS System in Epilepsy Study Group Neurology. 2011;77:1295. [Google Scholar]
- 12.Fisher R, et al. SANTE Study Group Epilepsia. 2010;51:899. [Google Scholar]
- 13.Fisher RS. Ann Neurol. 2012;71:157. doi: 10.1002/ana.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes JR. Epilepsy Behav. 2009;15:404. doi: 10.1016/j.yebeh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Marescaux C, Vergnes M, Depaulis A. J Neural Transm Suppl. 1992;35:37. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]
- 16.Fröhlich F, McCormick DA. Neuron. 2010;67:129. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nitsche MA, Paulus W. Neurotherapeutics. 2009;6:244. doi: 10.1016/j.nurt.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozen S, et al. J Neurosci. 2010;30:11476. doi: 10.1523/JNEUROSCI.5252-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joundi RA, Jenkinson N, Brittain JS, Aziz TZ, Brown P. Curr Biol. 2012;22:403. doi: 10.1016/j.cub.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall L, Helgadóttir H, Mölle M, Born J. Nature. 2006;444:610. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 21.Materials and methods are available as supplementary materials on Science Online.
- 22.Kandel A, Buzsáki G. J Neurosci. 1997;17:6783. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kostopoulos G, Gloor P, Pellegrini A, Gotman J. Exp Neurol. 1981;73:55. doi: 10.1016/0014-4886(81)90045-5. [DOI] [PubMed] [Google Scholar]
- 24.Steriade M. Trends Neurosci. 2005;28:317. doi: 10.1016/j.tins.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Engel J, Jr, et al. Epilepsia. 2003;44:741. doi: 10.1046/j.1528-1157.2003.48202.x. [DOI] [PubMed] [Google Scholar]
- 26.Jackson A, Fetz EE. IEEE Trans Neural Syst Rehabil Eng. 2011;19:534. doi: 10.1109/TNSRE.2011.2158586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatsopoulos NG, Donoghue JP. Annu Rev Neurosci. 2009;32:249. doi: 10.1146/annurev.neuro.051508.135241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotenberg A, Pascual-Leone A. Clin Neurophysiol. 2009;120:1417. doi: 10.1016/j.clinph.2009.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


