Abstract
Clostridium difficile, a major cause of healthcare-associated diarrhea due to perturbation of the normal gastrointestinal microbiome, is responsible for significant morbidity, mortality, and healthcare expenditures. The incidence and severity of C. difficile infection (CDI) is increasing and recurrent disease is common. Recurrent infection can be difficult to manage with conventional antibiotic therapy. Fecal microbiota transplantation (FMT), which involves instillation of stool from a healthy donor into the gastrointestinal tract of the patient, restores the gut microbiome to a healthy state. FMT has emerged as a promising new treatment for CDI. There are limited data on FMT for treatment of primary CDI, but FMT appears safe and effective for recurrent CDI. The safety and efficacy of FMT in patients with severe primary or recurrent CDI has not been established. Patients with inflammatory bowel disease (IBD) who undergo FMT for CDI may be at increased risk of IBD flare and caution should be exercised with use of FMT in that population. The long-term safety of FMT is unknown; thus, rigorously conducted prospective studies are needed.
Introduction
Epidemiology and pathogenesis
Symptomatic Clostridium difficile infection (CDI) results when C. difficile, a Gram-positive bacillus that is an obligate-anaerobe, produces cytotoxins TcdA and TcdB, causing epithelial and mucosal injury in the gastrointestinal tract.1 Though it was first identified in 1978 as the causative agent of pseudomembranous colitis and several effective treatments have subsequently been discovered,2 nearly three decades later C. difficile remains a major nosocomial pathogen. C. difficile is the most frequent infectious cause of healthcare-associated diarrhea and causes toxin mediated infection. The incidence of CDI in the United States has increased dramatically, especially in hospitals and nursing homes where there are now nearly 500,000 new cases and 30,000 deaths per year.3–6 This increased burden of disease is due both to the emergence of several strains that have led to a worldwide epidemic7 and to a predilection for CDI in older adults, who constitute a growing proportion of hospitalized patients.8 Ninety-two percent of CDI-related deaths occur in adults >65 years,9 and the risk of recurrent CDI is 2-fold higher with each decade of life.10 It is estimated that CDI is responsible for $1.5 billion in excess healthcare costs each year in the US,11 and that much of the additional cost and morbidity of CDI is due to recurrence, with around 83,000 cases per year.6
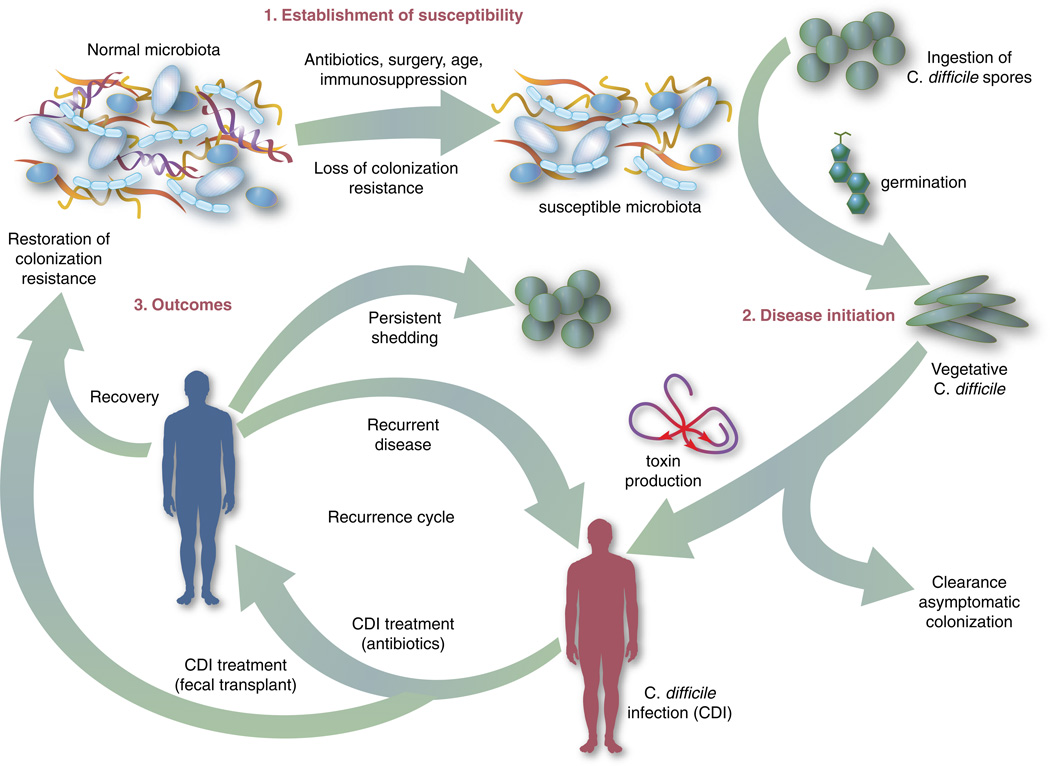
The human gut microbiota which is a diverse ecosystem consisting of thousands of bacterial species,12 protects against invasive pathogens such as C. difficile.13, 14 The pathogenesis of CDI requires disruption of the gut microbiota before onset of symptomatic disease,15 and exposure to antibiotics is the most common precipitant (Figure 1).16 Following exposure, the manifestations can vary from asymptomatic colonization, to a self-limited diarrheal illness, to a fulminant, life-threatening colitis.1 Even among those that recover, recurrent disease is common.10 A first recurrence will occur in 15–20% of successfully treated patients, a second recurrence will occur in 45% of those patients, and up to 5% of all patients enter a prolonged cycle of CDI with multiple recurrences.17–19
Figure 1. Pathophysiology of CDI.
This figure illustrates that an altered intestinal microbiota is a prerequisite to symptomatic infection. Following establishment of susceptibility (1) and exposure to spores, germination occurs, vegetative C. difficile cells produce toxin (2), and this causes injury to the intestinal epithelium and mucosa resulting in symptoms. After recovery following conventional therapy, disruption of the intestinal microbiota may continue and patients remain at risk for CDI (3).
Abbreviations: CDI, Clostridium difficile infection.
The need for better treatment modalities: rationale
Conventional treatments (Table 1) utilize antibiotics with activity against C. difficile20, 21 but these antibiotics have activity against other gut bacteria, limiting the ability of the microbiota to fully recover following CDI and predisposing patients to recurrence.22 Traditional treatments for CDI result in a high incidence of recurrence (35%), with up to 65% of these patients that are again treated with conventional approaches developing a chronic pattern of recurrent CDI.23 Though other factors may also explain why patients have recurrence (such as low serum antibody response to C. difficile toxins,24 use of medications such as proton pump inhibitors,10 and the specific strain of C. difficile causing infection10, 21), restoration of the gut microbiome through fecal microbiota transplantation (FMT) is the treatment strategy that has garnered the most attention and has gained acceptance among practitioners in the treatment of recurrent CDI when conventional treatments have failed.25 A review of the practices and evidence for use of FMT in the treatment of CDI in hospitalized patients is presented here, with recommendations shown in Table 2.
Table 1.
Conventional treatment strategies for primary and recurrent CDI.
| Type of CDI | Associated Signs / Symptoms |
Usual Treatment(s)20 |
|---|---|---|
| Primary CDI, non-severe | Diarrhea without signs of systemic infection, WBC <15,000 cells/mL, and serum creatinine <1.5 times the premorbid level |
metronidazole 500 mg by mouth three times daily for 10–14 days OR vancomycin 125 mg by mouth three times daily for 10–14 days OR fidaxomicin 200 mg by mouth twice daily for 10 daysa |
| Primary CDI, severe | Signs of systemic infection and/or WBC ≥15,000 cells/mL, or serum creatinine ≥1.5 times the premorbid level |
vancomycin 125 mg by mouth three times daily for 10–14 days OR fidaxomicin 200 mg by mouth twice daily for 10 daysa |
| Primary CDI, complicated | signs of systemic infection including hypotension, ileus, or megacolon |
vancomycin 500 mg by mouth four times daily AND vancomycin 500 mg by rectum four times daily AND intravenous metronidazole 500 mg three times daily |
| Recurrent CDI | Return of symptoms with positive C. difficile testing within 8 weeks of onset, but after initial symptoms resolved with treatment |
First recurrence same as initial treatment, based on severity. Second recurrence Start treatment based on severity, followed by a vancomycin pulsed and/or tapered regimen over six or more weeks |
Abbreviations: CDI, Clostridium difficile infection; WBC, white blood cell count.
Footnotes:
afidaxomicin is considerably more expensive than vancomycin and not currently included in US guidelines, but is approved by the FDA for the treatment of CDI.21
Table 2.
Recommendation for the use of FMT in the treatment of primary, severe, and recurrent CDI.
| Type of CDI | Recommendation on Use of FMT |
|---|---|
| Primary CDI, non-severe | Insufficient data on safety / efficacy to make a recommendation; effective conventional treatments exist |
| Primary CDI, severe | Not recommended due to insufficient data on safety / efficacy with documented adverse events |
| Primary CDI, complicated | Not recommended due to insufficient data on safety / efficacy with documented adverse events |
| Recurrent CDI (usually 2nd recurrence) | Recommended based on data from case reports, systematic reviews, and two randomized, controlled clinical trials demonstrating safety and efficacy |
Abbreviations: CDI, Clostridium difficile infection; FMT, fecal microbiota transplantation.
Overview of FMT
FMT is not new to modern times as there are reports of its use in ancient China for various purposes.26 It was first described as a treatment for pseudomembranous colitis in the 1950s27 and in the past several years the use of FMT for CDI has increasingly gained acceptance as a safe and effective treatment. The optimal protocol for FMT is unknown: there are numerous published methods of stool preparation, infusion, and recipient and donor preparation. Diluents include tap water, normal saline, or even yogurt.23, 28, 29 Sites of instillation of the stool include the stomach, small intestine and large intestine.23, 29, 30 Methods of recipient preparation for the infusion include cessation of antibiotic therapy for 24–48 hours prior to FMT, a bowel preparation or lavage, and use of antimotility agents, such as loperamide, to aid in retention of transplanted stool.28 Donors may include friends or family members of the patients or one or more universal donors for an entire center. In both cases, screening for blood-borne and fecal pathogens is performed before one can donate stool, though the tests performed vary between centers. FMT has been performed in both inpatient and outpatient settings, and a published study that instructed patients on self-administration of fecal enema at home also demonstrated success.30
Although there are numerous variables to consider in designing a protocol, as discussed further below it is encouraging that FMT appears to be highly effective regardless of the specific details of the protocol.28 If the first procedure fails, evidence suggests a second or third treatment can be quite effective.28 In a recent advance, successful FMT via administration of frozen stool oral capsules has been demonstrated,31 which potentially removes many system- and patient-level barriers to receipt of this treatment.
Clinical Evidence for Efficacy of FMT in Treatment of CDI
Recurrent CDI
The clinical evidence for FMT is most robust for recurrent CDI, consisting of case reports or case series, recently aggregated by two large systematic reviews, as well as several clinical trials.23, 29 Gough et al. published the larger of the two reviews with data from 317 patients treated via FMT for recurrent CDI,23 including FMT via retention enema (35%), colonoscopic infusion (42%), and gastric infusion (23%). Though the authors noted differences in resolution proportions among routes of infusion, types of donors, and types of infusates, it is not possible to draw definite conclusions form these data given their anecdotal nature. Regardless of the specific protocol’s details, 92% of patients in the review had resolution of recurrent CDI overall after one or more treatments, with 89% improving after only one treatment. Another systematic review of FMT, both for CDI and non-CDI indications, reinforced its efficacy in CDI and overall benign safety profile.32 Other individual case series and reports of FMT for CDI not included in these reviews have been published; they too demonstrate an excellent resolution rate.33–38 As with any case reports / series, generalizing from these data to arrive at conclusions about the safety and efficacy of FMT for CDI is limited by potential confounding and publication bias; thus, there emerged a need for high-quality prospective trials.
The first randomized, controlled clinical trial (RCT) of FMT for recurrent CDI was reported in 2013.39 Three treatment groups were compared: vancomycin for 5 days followed by FMT (n=16), vancomycin alone for 14 days (n=13), or vancomycin for 14 days with bowel lavage (n=13). Despite a strict definition of cure (absence of diarrhea or persistent diarrhea from another cause with three consecutive negative stool tests for C. difficile toxin), the study was stopped early after an interim analysis due to resolution of CDI in 94% of patients in the FMT arm (81% after just one infusion) versus 23–31% in the others. Off-protocol FMT was offered to the patients in the other two groups and 83% of them were also cured.
Youngster et al. conducted a pilot RCT with 10 patients in each group where patients were randomized to receive FMT via either colonoscopy or nasogastric tube from a frozen fecal suspension and no difference in efficacy was seen between administration routes, with an overall cure rate of 90%.40 Subsequently, Youngster et al. conducted an open-label non-comparative study with frozen fecal capsules for FMT in 20 patients with recurrent CDI.31 Resolution occurred in 14 (70%) patients after a single treatment and four of the six non-responders had resolution upon retreatment for an overall efficacy of 90%.
Finally, Cammarota et al. conducted an open-label RCT on FMT for recurrent CDI,41 comparing FMT to a standard course of vancomycin for ten days followed by pulsed dosing every 2–3 days for three weeks. The study was stopped after a 1-year interim analysis as 18 of 20 patients (90%) treated by FMT exhibited resolution of CDI-associated diarrhea compared to only five of 19 patients (26%) in the vancomycin-treated group (P <.001).
Primary and severe CDI
There are few data on the use of FMT for primary, non-recurrent CDI aside from a few case reports, which are included in the data presented above. A mathematical model of CDI in an ICU assessed the role of FMT on primary CDI,42 and predicted a decreased median incidence of recurrent CDI in patients treated with FMT for primary CDI. In addition to the general limitations inherent in any mathematical model, the study had specific assumptions for model parameters that limited generalizability, such as lack of incorporation of known risk factors for CDI and assumed immediate, persistent disruption of the microbiota after any antimicrobial exposure until FMT occurred.43
Lagier et al.44 conducted a non-randomized, open-label, before and after prospective study comparing mortality between two intervention periods: conventional antibiotic treatment for CDI vs. early FMT via nasogastric infusion. This shift happened due to clinical need, as their hospital in Marseille developed a ribotype 027 outbreak with a dramatic global mortality rate (50.8%). Mortality in the FMT group was significantly less (64.4% vs. 18.8%, P <.01). This was an older cohort (mean age 84), suggesting that in an epidemic setting with a high mortality rate, early FMT may be beneficial, but one cannot extrapolate these data to support a position of early FMT for primary CDI in a non-epidemic setting.
Similarly, the evidence for use of FMT in severe CDI (defined in Table 1) consists of published case reports, which suggest efficacy.45–48 Similarly, the study by Lagier et al.44 does not provide data on severity classification, but had a high mortality rate and found a benefit of FMT vs. conventional therapy, suggesting that at least some patients presented with severe CDI and benefited. However, one documented death (discussed further below) following FMT for severe CDI highlights the need for caution before this treatment is used in that setting.49
Patient and Provider perceptions regarding acceptability of FMT as a treatment option for CDI
A commonly cited reason for a limited role of FMT is the aesthetics of the treatment. However, few studies exist on the perceptions of patients and providers regarding FMT. Zipursky et. al surveyed 192 outpatients on their attitudes towards FMT using hypothetical case scenarios.50 Only one patient had a history of CDI. The results were largely positive, with 81% of respondents agreeing to FMT for CDI. However, the need to handle stool and the nasogastric route of administration were identified as the most unappealing aspects of FMT. More respondents (90%, P = .002) agreed to FMT when offered as a pill.
The same group of investigators undertook an electronic survey to examine physician attitudes toward FMT51 and found that 83 of 135 physicians (65%) in their sample had not offered or referred a patient for FMT. Frequent reasons for this included institutional barriers, concern that patients would find it too unappealing, and uncertainty regarding indications for FMT. Only 8% of physicians believed that patients would choose FMT if given the option. As the role of FMT in CDI continues to grow, it is likely that patient and provider perceptions and attitudes regarding this treatment will evolve to better align.
Safety of FMT
Short-term complications
Serious adverse effects directly attributable to FMT in patients with normal immune function are uncommon. Symptoms of an irritable bowel (constipation, diarrhea, cramping, bloating) shortly after FMT are observed and usually last less than 48 hours.23 A recent case series of immunocompromised patients (excluding those with IBD) treated for CDI with FMT did not find many adverse events in this group.35 However, patients with IBD may have a different risk profile; the same case series noted adverse events occurred in 14% of IBD patients, who experienced disease flare requiring hospitalization in some cases.35 No cases of septicemia or other infections were observed in this series. An increased risk of IBD flare, fever, and elevation in inflammatory markers following FMT has also been observed in other studies.52–54 However, the interaction between IBD and the microbiome is complex, and a recent RCT for patients with ulcerative colitis (without CDI) treated via FMT did not show any significant adverse events.55 FMT side effects may vary by the administration method and may be related to complications of the method itself rather than FMT (for example misplacement of a nasogastric tube, perforation risk with colonoscopy).
Deaths following FMT are rare and often are not directly attributed to FMT. One reported death occurred as a result of aspiration pneumonia during sedation for colonoscopy for FMT.35 In another case, a patient with severe CDI was treated with FMT, did not achieve cure, and developed toxic megacolon and shock, dying shortly after. The authors speculate that withdrawal of antibiotics with activity against CDI following FMT contributed to the outcome, rather than FMT itself.49 FMT is largely untested in patients with severe CDI45–48 and this fatal case of toxic megacolon warrants caution.
Long-term complications
The long-term safety of FMT is unknown. There is an incomplete understanding of the interaction between the gut microbiome and the host, but this is a complex system and associations with disease processes have been demonstrated. The gut microbiome may be associated with colon cancer, diabetes, obesity, and atopic disorders.56 The role of FMT in contributing to these conditions is unknown. It is also not known whether targeted screening / selection of stool for infusion can mitigate these potential risks.
In the only study to capture long-term outcomes after FMT, 77 patients were followed for 3–68 months (mean 17 months).57 New conditions such as ovarian cancer, myocardial infarction, autoimmune disease, and stroke were observed. Although it is not possible to establish causality from this study or infer an increased risk of these conditions from FMT, the results underscore the need for long-term follow-up after FMT.
Regulatory status
The increased use of FMT for CDI and interest in non-CDI indications led the FDA in 2013 to publish an initial guidance statement regulating stool as a biologic agent.58 However, subsequently the United States Department of Health and Human Services’ Food and Drug Administration (FDA) issued guidance stating that it would exercise enforcement discretion for physicians administering FMT to treat patients with C. difficile infections; thus an investigational new drug (IND) approval is not required but appropriate informed consent from the patient indicating that FMT is an investigational therapy is needed. Revision to this guidance is in progress.59
Future Directions
Expansion of the indications for FMT and use of synthetic and/or frozen stool are directions currently under active exploration. There are a number of clinical trials studying FMT for CDI underway that are not yet completed,60–65 and these may shed light on the safety and efficacy of FMT for primary CDI, severe CDI and FMT as a pre-emptive therapy for high risk patients on antibiotics. Frozen stool preparations, often from a known set of pre-screened donors and recently in capsule form, have been used for FMT and are gaining popularity.31, 33 A synthetic intestinal microbiota suspension for use in FMT is currently being tested.62 There also exists a non-profit organization, OpenBiome (www.OpenBiome.org), which performs all donor selection, screening, and stool preparation tasks. OpenBiome will ship prepared stool that can be used immediately for FMT or stored at −20°C for up to six months. However, the FDA published a proposed guidance statement on FMT, which requires that the donor be known to the treating physician or recipient; this statement is currently under review and will likely shed light on whether donors anonymous to both providers and patients are acceptable for FMT.59
Conclusions
Based on several prospective trials and observational data, FMT appears to be a safe and effective treatment for recurrent CDI that is superior to conventional approaches. Despite recent pivotal advances in the field of FMT, there remain many unanswered questions and further research is needed to examine the optimal parameters, indications, and outcomes with FMT.
Acknowledgments
Funding
KR is supported by grants from the Claude D. Pepper Older Americans Independence Center [grant number AG-024824] and the Michigan Institute for Clinical and Health Research [grant number 2UL1TR000433]. NS is supported by a VA MERIT award. The contents of this paper do not necessarily represent the views of the Department of Veterans Affairs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest
The authors have nothing to disclose.
References
- 1.Kuijper EJ, Coignard B, Tüll P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 2006;12:2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 3.Robert J, Campbell P, Lynn Giljahn MPH, Kim Machesky MPH, et al. Clostridium difficile Infection in Ohio Hospitals and Nursing Homes During 2006. Infect. Control Hosp. Epidemiol. 2009;30(6):526–533. doi: 10.1086/597507. [DOI] [PubMed] [Google Scholar]
- 4.Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. Attributable Burden of Hospital-Onset Clostridium difficile Infection: A Propensity Score Matching Study. Infect. Control Hosp. Epidemiol. 2013;34(6):588–596. doi: 10.1086/670621. [DOI] [PubMed] [Google Scholar]
- 5.Making Health Care Safer: Centers for Disease Control and Prevention Vital Signs. [Accessed January 15, 2015]; http://www.cdc.gov/VitalSigns/Hai/StoppingCdifficile/.
- 6.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015 Feb 26;372(9):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He M, Miyajima F, Roberts P, et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat. Genet. 2013;45(1):109–113. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louie TJ, Miller MA, Crook DW, et al. Effect of Age on Treatment Outcomes in Clostridium difficile Infection. J. Am. Geriatr. Soc. 2013;61(2):222–230. doi: 10.1111/jgs.12090. [DOI] [PubMed] [Google Scholar]
- 9.Lessa FC, Gould CV, McDonald LC. Current Status of Clostridium difficile Infection Epidemiology. Clin. Infect. Dis. 2012 Aug 1;55(suppl 2):S65–S70. doi: 10.1093/cid/cis319. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk Factors for Recurrence, Complications and Mortality in Clostridium difficile Infection: A Systematic Review. PLoS ONE. 2014;9(6):e98400. doi: 10.1371/journal.pone.0098400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013 Dec 9–23;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 12.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Waaij D, Berghuis-de Vries JM, Lekkerkerk-van der Wees JEC. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. Epidemiol. Infect. 1971;69(03):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vollaard E, Clasener H. Colonization resistance. Antimicrob. Agents Chemother. 1994;38(3):409. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterol. 2014 May;146(6):1547–1553. doi: 10.1053/j.gastro.2014.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theriot CM, Koenigsknecht MJ, Carlson PE, Jr, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15(6):285–289. doi: 10.1016/j.anaerobe.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Huebner ES, Surawicz CM. Treatment of recurrent Clostridium difficile diarrhea. Gastroenterol. Hepatol. 2006;2(3):203–208. [PMC free article] [PubMed] [Google Scholar]
- 19.Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy Using Fecal Flora: Toying With Human Motions. J. Clin. Gastroenterol. 2004;38(6):475–483. doi: 10.1097/01.mcg.0000128988.13808.dc. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect. Control Hosp. Epidemiol. 2010 May;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 21.Crook DW, Walker AS, Kean Y, et al. Fidaxomicin Versus Vancomycin for Clostridium difficile Infection: Meta-analysis of Pivotal Randomized Controlled Trials. Clin. Infect. Dis. 2012 Aug 1;55(suppl 2):S93–S103. doi: 10.1093/cid/cis499. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased Diversity of the Fecal Microbiome in Recurrent Clostridium difficile—Associated Diarrhea. J. Infect. Dis. 2008 Feb 1;197(3):435–438. doi: 10.1086/525047. 2008. [DOI] [PubMed] [Google Scholar]
- 23.Gough E, Shaikh H, Manges AR. Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2011 Nov 15;53(10):994–1002. doi: 10.1093/cid/cir632. 2011. [DOI] [PubMed] [Google Scholar]
- 24.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 25.Bakken JS, Polgreen PM, Beekmann SE, Riedo FX, Streit JA. Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe. 2013;24(0):20–24. doi: 10.1016/j.anaerobe.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should We Standardize the 1,700-Year-Old Fecal Microbiota Transplantation? Am. J. Gastroenterol. 2012;107(11):1755–1755. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 27.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958 Nov;44(5):854–859. [PubMed] [Google Scholar]
- 28.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011 Dec;9(12):1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal Microbiota Transplantation for Clostridium difficile Infection: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2013;108(4):500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 30.Silverman MS, Davis I, Pillai DR. Success of Self-Administered Home Fecal Transplantation for Chronic Clostridium difficile Infection. Clinical Gastroenterology and Hepatology. 2010;8(5):471–473. doi: 10.1016/j.cgh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. JAMA. 2014 Oct 11; doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 32.Sha S, Liang J, Chen M, et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment. Pharmacol. Ther. 2014;39(10):1003–1032. doi: 10.1111/apt.12699. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized Frozen Preparation for Transplantation of Fecal Microbiota for Recurrent Clostridium difficile Infection. Am. J. Gastroenterol. 2012;107(5):761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 34.Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch. Intern. Med. 2012 Jan 23;172(2):191–193. doi: 10.1001/archinte.172.2.191. [DOI] [PubMed] [Google Scholar]
- 35.Kelly CR, Ihunnah C, Fischer M, et al. Fecal Microbiota Transplant for Treatment of Clostridium difficile Infection in Immunocompromised Patients. Am. J. Gastroenterol. 2014 Jul;109(7):1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutta SK, Girotra M, Garg S, et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol. 2014 Sep;12(9):1572–1576. doi: 10.1016/j.cgh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Friedman-Moraco RJ, Mehta AK, Lyon GM, Kraft CS. Fecal Microbiota Transplantation for Refractory Clostridium difficile Colitis in Solid Organ Transplant Recipients. American Journal of Transplantation. 2014;14(2):477–480. doi: 10.1111/ajt.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emanuelsson F, Claesson BEB, Ljungström L, Tvede M, Ung K-A. Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: A retrospective evaluation of 31 patients. Scand. J. Infect. Dis. 2014;46(2):89–97. doi: 10.3109/00365548.2013.858181. [DOI] [PubMed] [Google Scholar]
- 39.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N. Engl. J. Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 40.Youngster I, Sauk J, Pindar C, et al. Fecal Microbiota Transplant for Relapsing Clostridium difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin. Infect. Dis. 2014 Jun 1;58(11):1515–1522. doi: 10.1093/cid/ciu135. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2015 Mar 1; doi: 10.1111/apt.13144. [DOI] [PubMed] [Google Scholar]
- 42.Lofgren ET, Moehring RW, Anderson DJ, Weber DJ, Fefferman NH. A Mathematical Model to Evaluate the Routine Use of Fecal Microbiota Transplantation to Prevent Incident and Recurrent Clostridium difficile Infection. Infect. Control Hosp. Epidemiol. 2013;35(1):18–27. doi: 10.1086/674394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao K, Young VB, Aronoff DM. Commentary: Fecal Microbiota Therapy: Ready for Prime Time? Infect. Control Hosp. Epidemiol. 2014;35(1):28–30. doi: 10.1086/674395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagier JC, Delord M, Million M, et al. Dramatic reduction in Clostridium difficile ribotype 027-associated mortality with early fecal transplantation by the nasogastric route: a preliminary report. Eur. J. Clin. Microbiol. Infect. Dis. 2015 May 7; doi: 10.1007/s10096-015-2394-x. [DOI] [PubMed] [Google Scholar]
- 45.Neemann K, Eichele DD, Smith PW, Bociek R, Akhtari M, Freifeld A. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transplant Infect Dis. 2012;14(6):E161–E165. doi: 10.1111/tid.12017. [DOI] [PubMed] [Google Scholar]
- 46.Trubiano JA, Gardiner B, Kwong JC, Ward P, Testro AG, Charles PGP. Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. Eur. J. Gastroenterol. Hepatol. 2013;25(2):255–257. doi: 10.1097/MEG.0b013e32835b2da9. [DOI] [PubMed] [Google Scholar]
- 47.Gallegos-Orozco J, Paskvan-Gawryletz C, Gurudu S, Orenstein R. Successful colonoscopic fecal transplant for severe acute Clostridium difficile pseudomembranous colitis. Rev. Gastroenterol. Mex. 2011;77(1):40–42. [PubMed] [Google Scholar]
- 48.You DM, Franzos MA, Holman RP. Successful Treatment of Fulminant Clostridium difficile Infection with Fecal Bacteriotherapy. Ann. Intern. Med. 2008;148(8):632–633. doi: 10.7326/0003-4819-148-8-200804150-00024. [DOI] [PubMed] [Google Scholar]
- 49.Solari PR, Fairchild PG, Noa LJ, Wallace MR. Tempered Enthusiasm for Fecal Transplant. Clin. Infect. Dis. 2014 Apr 23;59(2):319. doi: 10.1093/cid/ciu278. 2014. [DOI] [PubMed] [Google Scholar]
- 50.Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Patient Attitudes Toward the Use of Fecal Microbiota Transplantation in the Treatment of Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2012 Dec 15;55(12):1652–1658. doi: 10.1093/cid/cis809. 2012. [DOI] [PubMed] [Google Scholar]
- 51.Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Physician attitudes toward the use of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection. Can J Gastroenterol Hepatol. 2014 Jun;28(6):319–324. doi: 10.1155/2014/403828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Leon LM, Watson JB, Kelly CR. Transient Flare of Ulcerative Colitis After Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. Clin. Gastroenterol. Hepatol. 2013;11(8):1036–1038. doi: 10.1016/j.cgh.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 53.Angelberger S, Reinisch W, Makristathis A, et al. Temporal Bacterial Community Dynamics Vary Among Ulcerative Colitis Patients After Fecal Microbiota Transplantation. Am. J. Gastroenterol. 2013;108(10):1620–1630. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 54.Kump PK, Gröchenig H-P, Lackner S, et al. Alteration of Intestinal Dysbiosis by Fecal Microbiota Transplantation Does not Induce Remission in Patients with Chronic Active Ulcerative Colitis. Inflamm. Bowel Dis. 2013;19(10):2155–2165. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 55.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a Randomized Controlled Trial of Fecal Transplantation for Patients with Ulcerative Colitis. Gastroenterol. 2015 doi: 10.1053/j.gastro.2015.03.045. Available online: http://dx.doi.org/10.1053/j.gastro.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 56.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut Microbiota in Health and Disease. Physiol. Rev. 2010 Jul 1;90(3):859–904. doi: 10.1152/physrev.00045.2009. 2010. [DOI] [PubMed] [Google Scholar]
- 57.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-Term Follow-Up of Colonoscopic Fecal Microbiota Transplant for Recurrent Clostridium difficile Infection. Am. J. Gastroenterol. 2012;107(7):1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 58.Guidance for Industry: Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. [Accessed 2014 July 1]; http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm361379.htm.
- 59.Draft Guidance for Industry: Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. [Accessed 2014 July 1]; http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm387023.htm.
- 60.University Health Network Toronto. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2014 July 1]. Oral Vancomycin Followed by Fecal Transplant Versus Tapering Oral Vancomycin. Available from: http://clinicaltrials.gov/ct2/show/NCT01226992. NLM Identifier: NCT01226992. [Google Scholar]
- 61.Tel-Aviv Sourasky Medical Center. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2014 July 1]. Transplantation of Fecal Microbiota for Clostridium difficile Infection. Available from: http://clinicaltrials.gov/ct2/show/NCT01958463. NLM Identifier: NCT01958463. [Google Scholar]
- 62.Rebiotix Inc. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2014 July 1]. Microbiota Restoration Therapy for Recurrent Clostridium difficile-associated Diarrhea (PUNCH CD) Available from: http://clinicaltrials.gov/ct2/show/NCT01925417. NLM Identifier: NCT01925417. [Google Scholar]
- 63.Hadassah Medical Organization. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2014 July 1]. Efficacy and Safety of Fecal Microbiota Transplantation for Severe Clostridium difficile Associated Colitis. Available from: http://clinicaltrials.gov/ct2/show/NCT01959048. NLM Identifier: NCT01959048. [Google Scholar]
- 64.University Hospital Tuebingen. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2014 July 1]. Fecal Microbiota Transplantation in Recurrent or Refractory Clostridium difficile Colitis (TOCSIN) Available from: http://clinicaltrials.gov/ct2/show/NCT01942447. NLM Identifier: NCT01942447. [Google Scholar]
- 65.Duke University. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2014 July 1]. Stool Transplants to Treat Refractory Clostridium difficile Colitis. Available from: http://clinicaltrials.gov/ct2/show/NCT02127398. NLM Identifier: NCT02127398. [Google Scholar]