Abstract
Prostaglandin E2 EP3 receptor is the only prostaglandin E2 receptor that couples to multiple G-proteins, but its role in thrombin-induced brain injury is unclear. In the present study, we exposed mouse hippocampal slice cultures to thrombin in vitro and injected mice with intrastriatal thrombin in vivo to investigate the role of EP3 receptor in thrombin-induced brain injury and explore its underlying cellular and molecular mechanisms. In vitro, EP3 receptor inhibition reduced thrombin-induced hippocampal CA1 cell death. In vivo, EP3 receptor was expressed in astrocytes and microglia in the perilesional region. EP3 receptor inhibition reduced lesion volume, neurologic deficit, cell death, matrix metalloproteinase-9 activity, neutrophil infiltration, and the number of CD68+ microglia, but increased the number of Ym-1+ M2 microglia. RhoA-Rho kinase levels were increased after thrombin injection and were decreased by EP3 receptor inhibition. In mice that received an intrastriatal injection of autologous arterial blood, inhibition of thrombin activity with hirudin decreased RhoA expression compared with that in vehicle-treated mice. However, EP3 receptor activation reversed this effect of hirudin. These findings show that prostaglandin E2 EP3 receptor contributes to thrombin-induced brain damage via Rho-Rho kinase–mediated cytotoxicity and proinflammatory responses.
Keywords: Microglia, matrix metalloproteinase-9, prostaglandin receptor, Rho-Rho kinase, slice culture, thrombin
Introduction
Prostaglandin E2 (PGE2) has been implicated in inflammation and secondary injury after brain insults, including intracerebral hemorrhage (ICH),1 which accounts for 15% of all strokes. After an injurious event, PGE2 is released largely in the perilesional region of the brain because of the increased expression of cyclooxygenase-2 and PGE2 synthases.2 PGE2 acts through its four receptor subtypes known as EP1R–EP4R.2,3 For many years, investigators have been researching ways to either reduce PGE2 production or regulate the four receptors to minimize progressive brain damage.4–6
PGE2 receptor EP3 (EP3R) is expressed mainly by neurons and activated glial cells in brain7,8 and is associated with neuronal death.8,9 Of the four G-protein–coupled PGE2 receptors, EP3R is unique in its ability to bind to multiple G-proteins based on different spliced carboxy-terminal tail isoforms.10 Although it signals primarily via Gi/Gs, EP3R can also be linked to the G12/13 protein, resulting in activation of the small G-protein Rho.11 The Rho-Rho kinase (ROCK) pathway is thought to contribute to cell migration, distribution, and inflammation.12–14 Preclinical data regarding the effect of EP3R deletion on ischemic stroke outcomes are limited and conflicting and lack mechanistic insight.8,15,16 To our knowledge, no one has explored the molecular target of EP3R in inflammatory responses after ICH, which has a different pathogenesis from that of ischemic stroke.
Thrombin is a serine protease that converts fibrinogen into fibrin in blood coagulation. Much evidence has confirmed that thrombin acts as a major contributor to acute ICH injury by promoting blood–brain barrier disruption, brain edema, and neuroinflammation.17 Thrombin has been shown to stimulate macrophages and vascular smooth muscle cells to induce cyclooxygenase-2 expression and PGE2 production.18,19 Moreover, thrombin-induced neuronal death may involve PGE2.20 Thus, in this study, we investigated the role of EP3R in thrombin toxicity, a major contributor to ICH injury, and the possibility that the Rho-ROCK pathway is involved.
Materials and methods
Animals
C57BL/6 mice (male, 3-month-old, 23–25 g) were obtained from Charles River Laboratories (Wilmington, MA). Transgenic Cx3cr1GFP/+ mice on C57BL/6 background (male and female, 3-month-old, 23–25 g) were obtained from Dr Jonathan Bromberg (University of Maryland, Baltimore, MD). Cx3cr1GFP/+ mice express green fluorescent protein (GFP) in monocytes, dendritic cells, NK cells, and microglia under control of the endogenous chemokine receptor Cx3cr1 locus.21 In this study, we used heterozygous Cx3cr1GFP/+ mice. All experimental procedures were conducted in accordance with guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine. The animals were allowed free access to water and food before and after surgery. Animal experiments were reported in accordance with the ARRIVE guidelines.
Intrastriatal injections of thrombin and autologous arterial blood
C57BL/6 mice were injected in the left striatum with 10 U of thrombin (T6634, Sigma, St. Louis, MO) dissolved in sterile saline (10 U/2 µL per mouse) or 30 µL of autologous arterial blood without any anticoagulant, as previously reported.22,23 These animal models are commonly used to study thrombin and blood toxicity.24,25
Experimental design
Three main experiments were conducted. All mice were randomly allocated into separate study groups by using the randomizer form at http://www.randomizer.org. Investigators blinded to the treatment groups evaluated outcomes in all mice and performed calculations and analysis. EP3R agonist ONO-AE3-248 (AE248, Ki values: 7.5 nM for the EP3 receptor and more than 3 µM for the other receptors; EC50, 5 nM) and antagonist ONO-AE3-240 (AE240, Ki values: 590, 0.23, and 58 nM for the EP1, EP3, and EP4 receptors, and >10 mM for the EP2 receptor; mouse EP3/EP1 selectivity ratio = 2500; IC50, 5 nM) were kind gifts from ONO Pharmaceuticals (Osaka, Japan). The selectivity of AE240 and AE248 for EP3R has been established.4,8,26 Thrombin inhibitor hirudin (H7016, Sigma) and ROCK2 inhibitor HA1077 (H-2330, LC Laboratories, Woburn, MA) were used as indicated in the schematic outline of the different experimental groups (Supplemental Figure 1).
Experiment 1
EP3R antagonist AE240 was administered by intraperitoneal injection at 20 min and 6 h after striatal thrombin injection and then twice daily for up to 72 h. EP3R agonist AE248 was administered by tail vein injection at 20 min after thrombin injection and then once daily for up to 72 h. We chose dosing regimens for AE240 and AE248 based on previous work in an ischemic stroke model8,11 and our pilot studies. Both AE240 (3 mg/kg) and AE248 (0.3 mg/kg) were dissolved in DMSO, stored at a concentration of 0.2 M, and diluted with sterilized saline immediately before use; the final DMSO concentration was less than 0.5% for in vivo injections. Endpoint assessment included histology, Western blotting, zymography, behavior tests, and brain edema measurement.
Experiment 2
At 20 min after thrombin injection, ROCK2 inhibitor HA1077 (10 mg/kg, dissolved in sterile saline)27 was injected intraperitoneally and AE248 (0.3 mg/kg) was administered by tail vein injection. Tissue was collected for Western blotting at 4 h.
Experiment 3
Thrombin was injected into the left striatum. Thrombin inhibitor hirudin (5 U) was co-injected with 30 µL of autologous arterial blood into the left striatum.28–30 AE248 (0.3 mg/kg) was administered by tail vein injection at 20 min after blood injection. Tissue was collected for Western blotting at 4 h.
Tissue preparation and histology
The mice were anesthetized and perfused with 4% paraformaldehyde. Brains were dissected, post-fixed overnight at 4℃, and then transferred to 30% sucrose. Brains were cut into 14-µm coronal cryosections for staining with Luxol Fast Blue/Cresyl violet, propidium iodide (PI, nonviable cells), Fluoro-Jade B (FJB, degenerating neurons; Millipore, Billerica, MA), or TUNEL (DNA fragmentation; Roche, Indianapolis, IN) or for use in immunohistochemistry, as previously described.23 The primary antibodies used were anti-NeuN (1:200, Millipore), anti-glial fibrillary acidic protein (GFAP, astrocyte marker, 1:500, Sigma), anti-Iba1 (1:500, Wako Chemicals, Richmond, VA), anti-myeloperoxidase (MPO, neutrophil marker, 1:200, DAKO, Carpinteria, CA), anti-CD8a (1:100, AbD Serotec, Raleigh, NC), anti-CD11b (1:500, AbD Serotec), anti-Ym-1 (1:500, Stem Cell Technologies, Vancouver, Canada), anti-CD68 (1:200, AbD Serotec), anti-EP3R (1:200, Cayman Chemical, Ann Arbor, MI), and anti-RhoA (1:500, Cell Signaling Technology, Danvers, MA). All fluorescent-conjugated secondary antibodies (Life Technologies, Grand Island, NY) were used at a dilution of 1:1000. Nuclei were labeled with DAPI (1:1000, Life Technologies). Control sections were processed without primary antibodies. The specificity of the anti-EP3R antibody was confirmed by incubation of the antibody with EP3R blocking peptide (Cayman Chemical). Images were collected with a fluorescence microscope (Eclipse 90i, Nikon, Japan) at constant parameters (section position, image fields, gain, offset, exposure time) for fair comparisons among groups. Image J software (NIH, Image J 1.47t) was used for analyzing images from the region of interest. The average fluorescence intensity was expressed as percent intensity increase in the peri-injury area with respect to the contralateral side of the same section. Any positive signal not accompanied with DAPI staining was rejected as a false signal during cell counting.
Quantification of lesion volume
For quantification of thrombin-induced lesion volume, we stained a group of cryosections with Luxol Fast Blue and Cresyl violet. Areas in individual sections were measured by Image J software. The total lesion volume was calculated as the sum of the total lesion area multiplied by the distance between the sections (140 µm).31
Neurologic function
We used a 24-point scoring test, rota-rod test, corner turn test, and wire-hanging test to assess neurologic deficits, as previously reported.22,32,33
Brain water content
The brain water content was measured as previously reported.23
Organotypic hippocampal slice culture
Organotypic hippocampal slices were cultured as described previously.11,34,35 Brains were rapidly removed from 5- to 7-day-old C57BL/6 mice or Cx3cr1GFP/+ mice and cut coronally into 350-µm thick slices with a McIlwain tissue chopper (Ted Pella, Redding, CA). Hippocampal slices were placed on a 30-mm Millicell-CM insert membrane (Millipore) in six-well culture plates and cultured with 1 mL of culture medium consisting of 50% Minimal Essential Medium, 25% horse serum, and 25% Hanks’ Balanced Salt Solution, supplemented with 6.5 mg/mL D-glucose, 2 mM L-glutamine, 100 U/mL penicillin G potassium, and 100 µg/mL streptomycin sulfate (all from Life Technologies). Slices were cultured at 37℃ in a humidified incubator with 5% CO2 atmosphere.
At 12–14 days in vitro, cultured slices were incubated for 48 h in serum-free medium before being exposed to 300 U/mL thrombin (T4648, Sigma) with AE240 or AE248 for 24, 48, or 72 h. AE240 and AE248, stored in DMSO, were dissolved in culture medium immediately before use and had a final DMSO concentration of <0.1%. PI (5 µg/mL) was added for cell death assessment. The PI fluorescence intensity before thrombin treatment was recorded as P0. At 72 h after thrombin exposure, slices were exposed to 100 μM N-methyl-D-aspartate (NMDA) to induce maximal cell death, which was recorded as Pmax. PI fluorescence was observed with an inverted fluorescence microscope (TE2000-E, Nikon, Japan). Fluorescence intensity was analyzed by Image J (NIH, 1.47t) and normalized to the maximal cell injury as percentage. Cell death was calculated by the formula: (PX – P0) / (Pmax – P0) × 100%. The morphology of the Cx3cr1-GFP+ microglia in Cx3cr1GFP/+ mice was analyzed by using NeuroLucida software (MBF Bioscience, USA).
Western blotting
Proteins from the ipsilateral striatum (2 mm sagittal distance from the bregma [1.2 mm to –0.8 mm]) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes by electroblotting. Membranes were incubated with rabbit anti-cleaved caspase-3, anti-caspase-3, anti-RhoA, anti-ROCK2, anti-phosphorylated-MYPT1 (p-MYPT1), and anti-total-MYPT1 (t-MYPT1) antibodies (1:1000; all from Cell Signaling Technology) or anti-RhoB (1:1000, Sigma), anti-ZO-1, and mouse anti-claudin-5 (1:1000, Life Technologies). β-actin (1:3000, Santa Cruz Biotechnology, Dallas, TX) was used as a loading control. Bound antibodies were visualized by using a chemiluminescence detection system (LAS-4000, GE Healthcare, Piscataway, NJ). Images were analyzed by Image J.
Gelatin gel zymography
At 24 h after thrombin-induced ICH, protein samples obtained as described above were purified with gelatin-sepharose 4B (GE Healthcare) and separated on a 10% Tris-glycine gel with 0.1% gelatin. After 36-h incubation with developing buffer, the gel was stained with 0.5% Coomassie blue and then photographed (LAS-4000, GE Healthcare). Gelatinase standard was mixed with mouse pro-matrix metalloproteinase (MMP)-9 and pro-MMP-2 (R&D systems, Minneapolis, MN). MMP-9/2 activity was measured by optical density and quantified as fold increase over that of sham controls.36
Statistical analysis
All data are presented as means ± SD. Student’s t test was used to compare differences between two groups, and one-way or two-way analysis of variance (ANOVA) with Bonferroni post hoc test was used to compare differences between multiple groups. A probability value of p < 0.05 was considered statistically significant.
Results
EP3R is expressed in neurons in intact brain and induced in astrocytes and microglia after thrombin injection into the striatum
To clarify the cell type that expresses EP3R after thrombin injection, we performed double-immunolabeling with cell-type–specific antibodies. In intact brain, EP3R was present exclusively in NeuN+ cells. After thrombin injection, EP3R expression in neurons appeared scattered in the ipsilateral striatum (Supplemental Figure 2a). Moreover, glia-like, not vascular-like, EP3R+ cells were observed around the lesion at 72 h after thrombin injection. Double-labeling revealed that they were GFAP+ astrocytes (Supplemental Figure 2b) and CD11b+ microglia (n = 6; Supplemental Figure 2c).
EP3R inhibition mitigates thrombin-induced brain injury, brain edema, and neurobehavioral deficits
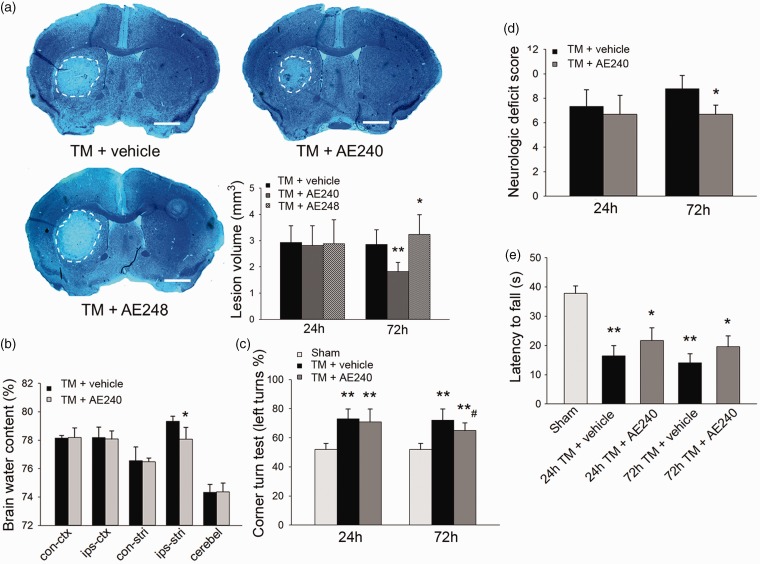
Treatment with 3 mg/kg EP3R antagonist AE240 reduced the lesion volume by 36% compared to that of vehicle-treated mice (1.82 ± 0.36 vs. 2.8 ± 0.56 mm3, n = 8/group, p = 0.002; Figure 1a) at 72 h after thrombin injection. A lower dose of 1 mg/kg was not protective (2.73 ± 0.71 vs. 2.8 ± 0.56 mm3, n = 8, p = 0.81; Supplemental Figure 3a and b), and 5 mg/kg AE240 did not expand the protection (1.68 ± 0.48 vs. 1.82 ± 0.36 mm3, n = 8, p = 0.32; Supplemental Figure 3a and b). At the same time point, EP3R agonist AE248 increased the injury volume by 14% (n = 8/group, p = 0.04; Figure 1a). At 72 h after thrombin injection, AE240 also decreased brain edema in the ipsilateral striatum compared to that in the vehicle group (n = 5, p = 0.03, t-test; Figure 1b).
Figure 1.
EP3 receptor (EP3R) inhibition reduces lesion volume and improves neurobehavioral outcomes after thrombin injection (TM). (a) Luxol Fast Blue and Cresyl violet-stained brain sections after thrombin injection. Lesions are circled in white; scale bar: 1 mm. EP3R antagonist AE240 reduced and agonist AE248 increased lesion volume at 72 h after thrombin injection (n = 8, *p < 0.05, **p < 0.01 vs. thrombin + vehicle; one-way ANOVA with Bonferroni post hoc test at each time point). (b) AE240 reduced striatal edema at 72 h after thrombin injection (n = 5, *p < 0.05 vs. thrombin + vehicle; t-test). Con-ctx: contralateral cortex; ips-ctx: ipsilateral cortex; con-stri: contralateral striatum; ips-stri: ipsilateral striatum; cerebel: cerebellum. (c) AE240 improved corner turn test performance of mice at 72 h after thrombin injection (n = 10, **p < 0.01 vs. sham; #p < 0.05 vs. thrombin + vehicle; one-way ANOVA with Bonferroni post hoc test at each time point). (d) AE240 decreased neurologic deficit score at 72 h after thrombin injection (n = 13–24, *p < 0.05, t-test at each time point). (e) Mice subjected to thrombin injection exhibited reduced latency to fall in the wire-hanging test at 24 and 72 h after thrombin injection; AE240-treated mice tended to have a longer falling latency than mice in the vehicle-treated group, but the difference was not statistically significant (n = 11–16, *p < 0.05, **p < 0.01 vs. sham; two-way ANOVA with Bonferroni test). Values are presented as mean ± SD.
Next we assessed neurologic function with the 24-point neurologic deficit score, corner turn test, wire-hanging test, and rota-rod test. At 24 and 72 h after thrombin injection, vehicle- treated mice had severe deficits in the corner turn test compared with sham animals (n = 10/group, p = 0.008 at 24 h, p = 0.007 at 72 h). Compared with vehicle-treated mice, AE240 treatment improved performance in the corner turn test at 72 h after thrombin injection (65.0 ± 3.27% vs. 72.0 ± 6.89%, n = 10, p = 0.01; Figure 1c). Neurologic deficit scores were also lower in the AE240-treated group than in the vehicle-treated group at 72 h after thrombin injection (6.7 ± 0.8 vs. 8.8 ± 1.1, n = 13–24, p = 0.01; Figure 1d). Moreover, 5 mg/kg AE240 treatment did not provide any additional improvement on this test over the 3-mg/kg dose (6.4 ± 0.5 vs. 6.7 ± 0.8, n = 8, p = 0.32; Supplemental Figure 3b). In the wire-hanging test, the latency to fall was shorter in all thrombin-injected groups than in the sham-operated group (n = 11-16, p < 0.05 vs. sham, two-way ANOVA; Figure 1e); AE240-treated mice tended to have a longer falling latency than mice in the vehicle-treated group, but the difference was not statistically significant (n = 11–16, p = 0.34 at 24 h, p = 0.14 at 72 h; Figure 1e). Because the reduction in lesion volume and neurologic deficits at 5 mg/kg did not differ from that at 3 mg/kg, we used 3 mg/kg of AE240 in subsequent studies.
Considering the potential role of EP3R in controlling core temperature,37 we recorded the rectal temperature before and after thrombin injection and 3 mg/kg AE240 injections. We detected no significant difference between the vehicle- and AE240-treated groups at any time point (n = 6, p = 0.96; Supplemental Figure 4).
EP3R inhibition improves neuronal survival in organotypic hippocampal slices
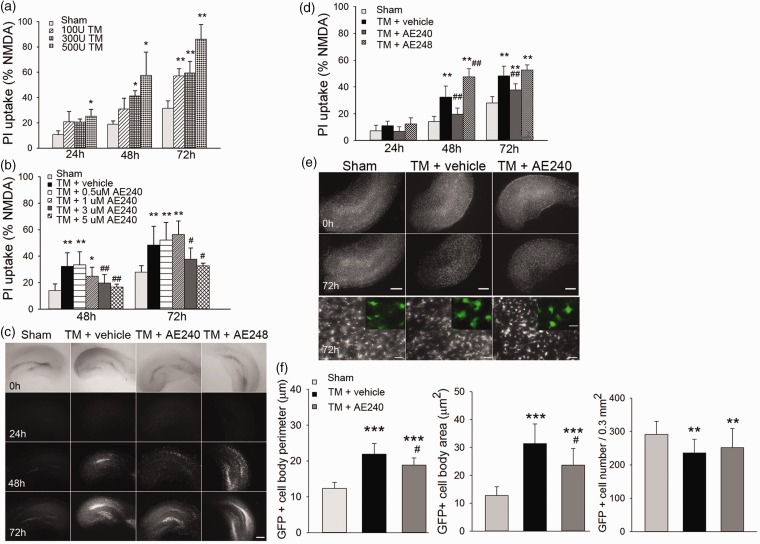
To assess whether EP3R inhibition provides neuroprotection against thrombin-induced injury, we first established an organotypic hippocampal slice culture assay to measure neuronal survival via PI labeling. A dose-response experiment revealed that 300 U/mL of thrombin was optimal to induce neuronal injury in the CA1 area. The injury became apparent at 48 h and continued to worsen until at least 72 h (n = 8–13, p = 0.04 at 48 h, p = 0.01 at 72 h vs. sham; Figure 2a). To assess the effect of sex on thrombin-induced toxicity, we examined PI uptake after thrombin exposure in hippocampal slices from age-matched male and female pups (5- to 7-day-old C57BL/6 mice). PI uptake did not differ between the slices from male and female pups (n = 8–10, p = 0.14 at 48 h, p = 0.10 at 72 h; Supplemental Figure 5a).
Figure 2.
EP3 receptor (EP3R) inhibition improves neuronal survival in organotypic hippocampal slice cultures. (a) Hippocampal injury was dose-dependent after thrombin (TM) exposure for 24, 48, and 72 h (n = 8–13, *p < 0.05, **p < 0.01 vs. sham group; one-way ANOVA with Bonferroni post hoc test). (b) EP3R antagonist (AE240) provided dose-dependent protection against hippocampal injury induced by 300 U/mL thrombin at 24, 48, and 72 h (n = 8–23; *p < 0.05, **p < 0.01 vs. sham group; #p < 0.05, ##p < 0.01 vs. thrombin + vehicle group; one-way ANOVA with Bonferroni post hoc test). PI: propidium iodide. (c) Representative images of PI fluorescence in hippocampal slices exposed to saline (sham), 300 U/mL thrombin with vehicle, 300 U/mL thrombin with EP3R antagonist (AE240, 3 µM), or 300 U/mL thrombin with EP3R agonist (AE248, 0.1 µM) for 24, 48, and 72 h. Scale bar: 500 µm. (d) Hippocampal injury was quantified by intensity of PI fluorescence (n = 8–23, **p < 0.01 vs. sham group; ##p < 0.01 vs. thrombin + vehicle group; one-way ANOVA with Bonferroni post hoc test). (e) Representative images of hippocampus from Cx3cr1GFP/+ mice exposed to saline (sham), 300 U/mL thrombin with vehicle, or 300 U/mL thrombin with 3 µM AE240. Upper panels, scale bar: 500 µm. Bottom panel shows that Cx3cr1-GFP–positive cells were activated at 72 h after thrombin exposure compared with those in the sham group. Scale bar: 50 µm. Inset: representative higher magnification images of Cx3cr1-GFP+ cells. Scale bar: 10 µm. (f) Quantification analysis shows that the cell body perimeter and cell body area of microglia in slices were increased at 72 h after thrombin exposure. Treatment with AE240 partially blocked this increase (n = 17–30, **p < 0.01, ***p < 0.001 vs. sham group; #p < 0.05 vs. thrombin + vehicle group; one-way ANOVA with Bonferroni post hoc test). All data are presented as means ± SD.
Simultaneous exposure of slices to thrombin and 3 μM or 5 μM AE240 reduced thrombin-induced CA1 cell death at 48 and 72 h (n = 8–23, p < 0.05 vs. thrombin + vehicle; one-way ANOVA; Figure 2b). Compared to PI uptake in the thrombin-exposed, vehicle-treated group, PI uptake in thrombin-exposed slices treated with 3 μM AE240 was decreased at 48 h (19.7 ± 3.1% vs. 32.4 ± 5.2%, n = 8–23, p = 0.004) and 72 h (37.8 ± 4.1% vs. 48.4 ± 5.2%, n = 8-23, p = 0.006; Figure 2c and d). In contrast, 0.1 μM EP3R agonist AE248 increased PI labeling in the CA1 region by 15.1 ± 2.1% (n = 8–23, p = 0.002; Figure 2c and d) at 48 h compared to that in the vehicle group. These results indicate that EP3R contributes to thrombin-induced hippocampal neuronal damage ex vivo.
To further clarify thrombin-induced cell death in hippocampal slice cultures, we used DR2313 (5 μM) to inhibit poly ADP-ribose polymerase (PARP), a protein associated with cell death.38 However, it did not provide any neuronal protection as assessed by PI labeling (n = 17, p = 0.173, Supplemental Figure 5b). Thus, it seems unlikely that PARP contributes to cell death in this thrombin-induced ex vivo model.
Next we assessed activated microglia in hippocampal slice cultures from Cx3cr1GFP/+ mice, which have GFP-labeled microglia. After 72 h of incubation with 300 U/mL thrombin, the cell soma of microglia in hippocampal slices was substantially expanded (Figure 2e). Microglial cell body perimeter and cell area were increased at 72 h compared to those in sham controls (21.9 ± 3.0 µm vs. 12.4 ± 1.7 µm and 31.34 ± 7.1 µm2 vs. 11.71 ± 3.17 µm2, respectively, n = 30, p < 0.001, Figure 2f). Treatment with AE240 reduced the expansion of the cell soma of microglia compared to that in the vehicle-treated group (n = 30, p = 0.02 and p = 0.015, respectively, at 72 h; Figure 2f). The density of Cx3cr1-GFP+microglia was decreased (19.1 ± 3.4%) in slices after 72 h of thrombin exposure (n = 17, p = 0.002, Figure 2f); however, the AE240 treatment did not significantly reverse this change (n = 17, p = 1.0, Figure 2f).
Of note, 300 U/mL thrombin caused a delayed shrinkage of the slices. Hippocampal area was decreased after 72 h of thrombin exposure (62.0 ± 15.5% vs. 96.2 ± 13.5%, n = 8, p = 0.008). The hippocampal shrinkage was likely caused by cell shrinkage and/or death, as no shrinkage was apparent in the sham group. Contrary to our expectations, EP3R inhibition did not significantly reverse this effect (n = 8, p = 0.78; Supplemental Figure 6).
EP3R inhibition reduces thrombin-induced cell death in vivo
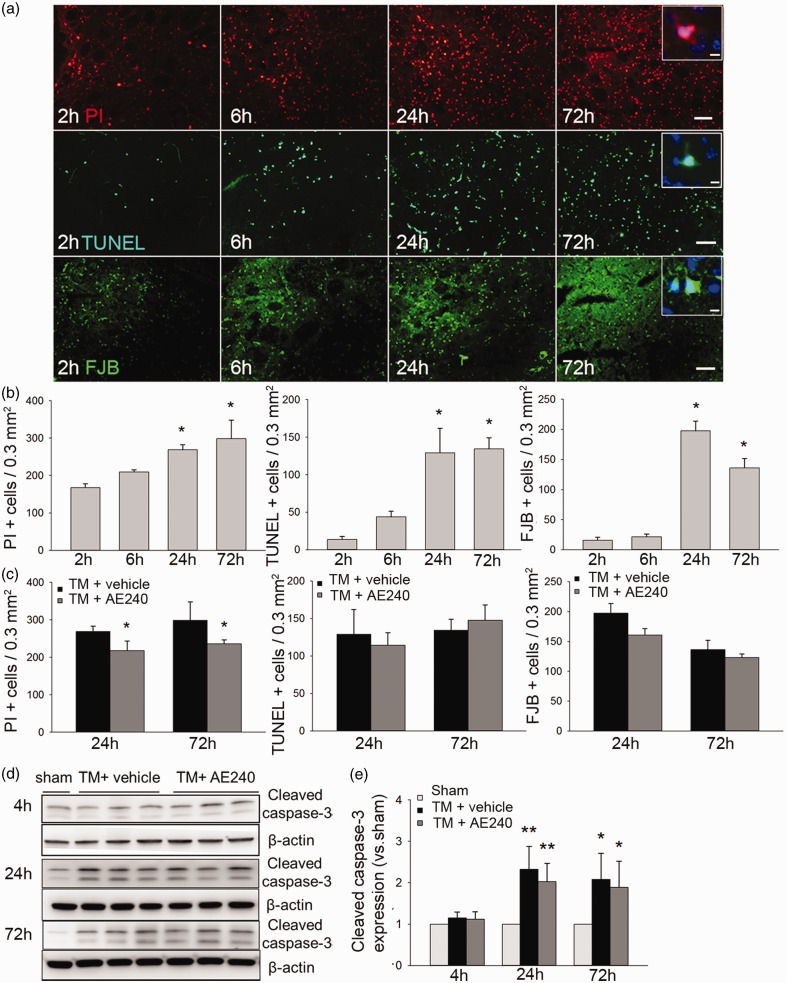
Based on the slice culture results in vitro, we next studied whether EP3R inhibition preserves cell viability after thrombin injection in vivo. In the perilesional region, PI+ cells were apparent at 2 h (167.5 ± 10.7/0.3 mm2) compared with only a few TUNEL+ and FJB+ cells (13.7 ± 3.9/0.3 mm2 and 16.0 ± 4.7/0.3 mm2, respectively). PI+, TUNEL+, and FJB+ cells were all elevated at 24 h after thrombin injection (n = 8, p = 0.01; one-way ANOVA; Figure 3a and b). EP3R inhibition significantly reduced PI+ cells at 24 and 72 h after thrombin injection (n = 8, p = 0.03) but had no effect on TUNEL+ (n = 8, p = 0.46) or FJB+ cell number (n = 8, p = 0.33; Figure 3c; related images are shown in Supplemental Figure 7). These data support our PI results in slice culture. Next, we used Western blotting to assess the possible involvement of cleaved caspase-3. Cleaved caspase-3 expression was elevated at 24 and 72 h after thrombin injection (n = 6, p = 0.001 at 24 h, p = 0.02 at 72 h vs. sham), but we observed no difference between vehicle- and AE240-treated groups (n = 6, p = 0.42 at 24 h, p = 0.70 at 72 h; Figure 3d and e).
Figure 3.
EP3 receptor (EP3R) inhibition reduces cell death after thrombin injection in vivo. (a) Images show propidium iodide (PI)-, TUNEL-, and Fluoro-Jade B (FJB)-stained brain sections at different time points after thrombin injection. Scale bars: 50 µm, inset: 10 µm. (b) Quantification of PI+, TUNEL+, and FJB+ cells (n = 8, *p < 0.05 vs. 2-h group; one-way ANOVA with Bonferroni post hoc test). (c) AE240-treated mice had fewer PI+ cells than did vehicle-treated mice at 24 and 72 h after thrombin injection (n = 8, *p < 0.05; t-test). Numbers of TUNEL+and FJB+ cells did not differ between the two groups. (d) Representative immunoblots of cleaved caspase-3 protein from sham, vehicle-treated, and AE240-treated groups at 4, 24, and 72 h after thrombin injection (n = 6). (e) Quantification analysis showed that cleaved caspase-3 protein levels were significantly elevated in vehicle-treated and AE240-treated groups at 24 and 72 h after thrombin injection, but not at 4 h. There was no difference between the vehicle-treated and AE240-treated groups (n = 6, *p < 0.05, **p < 0.01 vs. sham group; one-way ANOVA with Bonferroni post hoc test).
EP3R inhibition reduces microglial activation and neutrophil infiltration and increases M2 microglia number
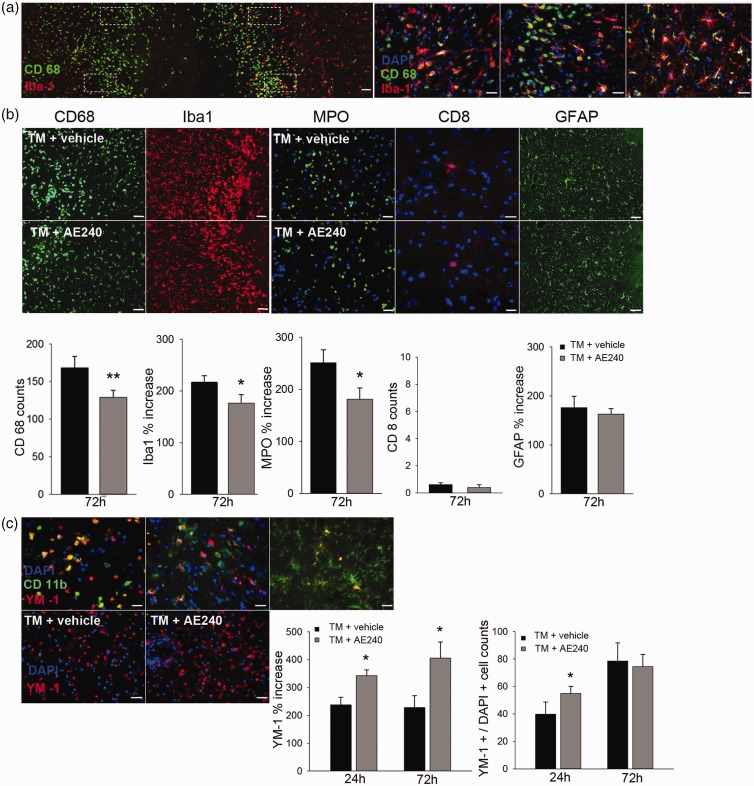
To evaluate the impact of EP3R antagonism on the inflammatory reaction after thrombin injection, we first quantified activated microglia by immunostaining the perilesional region for CD68 (activated microglia) and Iba1 (quiescent and activated microglia). We observed an accumulation of CD68+ microglia in the perilesional region at 72 h after thrombin injection. Whereas the resting microglia had long, ramified processes, the CD68+/Iba1+ cells around the injury center exhibited transformation from ramified toward amoeboid morphology (n = 8; Figure 4a). AE240 caused reductions in the number of CD68+ microglia (n = 8, p = 0.002) and Iba1 expression in the perilesional area at 72 h after thrombin injection (n = 8, p = 0.04, t-test; Figure 4b).
Figure 4.
EP3 receptor (EP3R) inhibition attenuates the inflammatory response and increases M2 microglial activation after thrombin injection. (a) Transverse section of striatum at the lesion site. Immunoreactivity of CD68 and Iba1 was assessed in four regions (outlined with white dotted lines) close to the lesion. The right three panels from left to right indicate activated CD68+/Iba1+ microglia located at the lesion border to 0.3 mm and 1 mm from the border. Green, CD68; red, Iba1; blue, DAPI. Scale bars: left panel, 100 µm; right three panels, 25 µm. (b) AE240 reduced the number of CD68+ microglia and the intensity of Iba1 immunoreactivity at 72 h after thrombin injection (TM). Green, CD68; red, Iba1 (n = 8). AE240 also reduced the infiltrating neutrophils (MPO, green, n = 8) but did not affect the number of infiltrating cytotoxic T-lymphocytes (CD8, red, n = 8) or the GFAP immunoreactivity (green, n = 8). Blue, DAPI. Scale bars: MPO, 25 µm; CD8, 10 µm; CD68, Iba1, GFAP, 50 µm. (c) Top panel: images from left to right show the different morphologies of CD11b+/Ym-1+ cells at three preselected regions in the striatum (lesion border, 0.2 mm and 1 mm from the border). Bottom panel: images show Ym-1+-cells in the perilesional region at 24 h after thrombin injection. At right, the bar graphs indicate that AE240 increased the intensity of Ym-1 at 24 and 72 h and the number of Ym-1+-cells at 24 h after thrombin injection. Green, CD11b; red, Ym-1; blue, DAPI (n = 8). Scale bars: top panel, 10 µm; bottom panel, 25 µm. The data in B and C are plotted as the relative ratio of the immunoreactivity near the injury site to the signal in the contralateral striatum in the same section for Iba1, MPO, GFAP, and Ym-1, or as the number of CD68+ or Ym-1+ cells. *p < 0.05, **p < 0.01; t-test. Data are presented as means ± SD.
As part of the systemic immune response, neutrophils and lymphocytes infiltrate the hematoma.1 At 72 h after thrombin injection, the intensity of signal for the neutrophil marker MPO was markedly increased, but AE240 diminished this increase (n = 8, p = 0.03; Figure 4b). Of note, few CD8+ cells were detected in either group (n = 8; Figure 4b). Activated astrocytes exhibited a higher GFAP intensity at 72 h after thrombin injection (175.72 ± 23.6% vs. contralateral striatum) that was not attenuated by AE240 treatment (n = 8, p = 0.29; Figure 4b). These data indicate that AE240 treatment reduces microglial activation and neutrophil infiltration in the perilesional region.
Recent studies have indicated that microglia can undergo polarized activation to M1 (inflammatory) or M2 (phagocytotic) phenotype, which each releases different cytokines.39 Therefore, we next evaluated cells positive for Ym-1, an M2 cell marker. Ym-1+/CD11b+ cells were detected from 24 to 72 h after thrombin injection and comprised ramified and globular microglia around the lesion (Figure 4c). Because Ym-1 is a secretory protein that binds heparin, we quantified Ym-1 by both counting Ym-1+ cells and measuring Ym-1+ intensity. The Ym-1+ cells continued to increase from 24 to 72 h after thrombin injection. AE240 treatment increased Ym-1+ intensity at 24 h and 72 h and Ym-1+ cells at 24 h compared with vehicle treatment (n = 8, p = 0.02; Figure 4c).
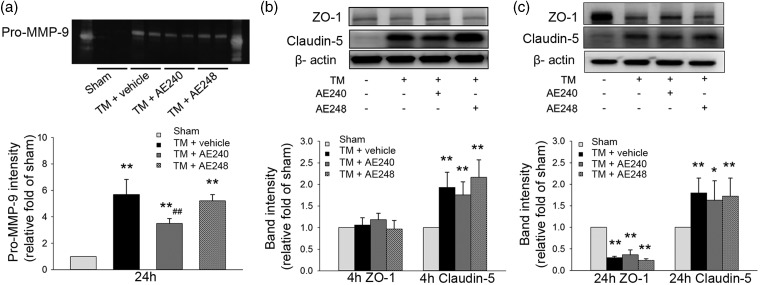
EP3R inhibition reduces MMP-9 activity
The inflammatory response after ICH is related to an increase in MMP-9 activity, which contributes to blood–brain barrier disruption and brain edema.36 We found that pro-MMP-9 activity was reduced at 24 h after AE240 treatment (n = 6, p = 0.006; Figure 5a). Next we evaluated the expression of tight junction proteins ZO-1 and claudin-5. Compared with expression in the sham group, claudin-5 expression was upregulated as early as 4 h after thrombin injection (n = 6, p = 0.003; Figure 5b). In contrast, ZO-1 expression was unchanged at 4 h (n = 6, p = 0.26; Figure 5b) and decreased at 24 h (n = 6, p = 0.002 vs. sham; Figure 5c). AE240 treatment tended to decrease claudin-5 expression at 4 and 24 h and increase ZO-1 expression at 24 h after thrombin injection, but it did not significantly reverse the changes in claudin-5 (n = 6, p = 0.25) or ZO-1 expression (n = 6, p = 0.39) at either time point (Figure 5b and c).
Figure 5.
EP3 receptor (EP3R) inhibition reduces matrix metalloproteinase (MMP)-9 activity after thrombin injection (TM) but does not change the expression of tight junction proteins of the blood–brain barrier. (a) EP3R inhibition with AE240 reduced the gelatinase activity of pro-MMP-9 at 24 h after thrombin injection. pro-MMP-2 was not detectable in any group (n = 6, **p < 0.01 vs. sham; ##p < 0.01 vs. thrombin + vehicle; one-way ANOVA with Bonferroni post hoc test). (b) Degraded claudin-5, but not ZO-1, was increased at 4 h after thrombin injection. AE240 treatment did not change the level of claudin-5 expression (n = 6, **p < 0.01 vs. sham; one-way ANOVA with Bonferroni post hoc test). (c) At 24 h after thrombin injection, expression of ZO-1, but not claudin-5, was reduced. Neither EP3R inhibition nor activation altered ZO-1 or claudin-5 expression (n = 6, *p < 0.05, **p < 0.01 vs. sham; one-way ANOVA with Bonferroni post hoc test).
The protective effect of EP3R inhibition is achieved through the Rho-ROCK signal pathway
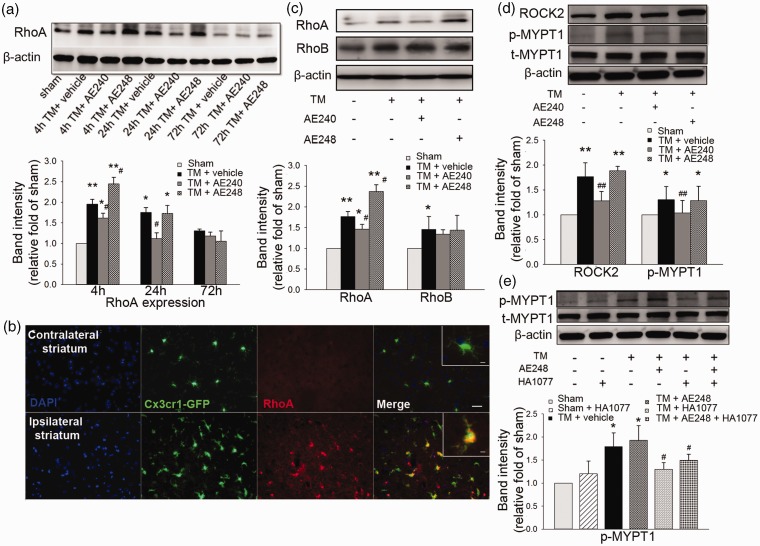
The Rho-ROCK pathway is known to be associated with neurotoxicity.40 Accordingly, we evaluated the total RhoA expression in brain after thrombin injection. RhoA was elevated at 4 h after thrombin injection (n = 12, p = 0.003) but had returned to baseline at 72 h (n = 12, p = 0.32; Figure 6a). EP3R inhibition with AE240 decreased RhoA expression at 4 and 24 h compared to that in the vehicle-treated group (n = 12, p = 0.04 at 4 h, p = 0.02 at 24 h; Figure 6a); EP3R agonist AE248 increased RhoA level at 4 h (n = 12, p = 0.03; Figure 6a). Immunohistochemistry from Cx3cr1GFP/+ mice confirmed that RhoA expression was increased in activated Cx3cr1-GFP+ cells in the perilesional region (n = 8; Figure 6b). We then tested whether RhoB, another member of the Rho family, was changed as early as RhoA after thrombin injection. RhoB expression was increased after thrombin injection (n = 12, p = 0.03), but neither AE240 nor AE248 changed its expression (n = 12, p = 0.84 and p = 0.91, respectively; Figure 6c).
Figure 6.
RhoA/ROCK2/p-MYPT1 pathway after thrombin toxicity. (a) RhoA expression was increased at 4 and 24 h and returned to the basal level at 72 h after thrombin injection (TM). EP3 receptor (EP3R) inhibition with AE240 decreased RhoA expression at 4 and 24 h, whereas its activation with AE248 increased RhoA expression at 4 h after thrombin injection (n = 12). (b) Colocalization of RhoA+ and Cx3cr1-GFP+ microglia in the perilesional region at 72 h after thrombin injection in Cx3cr1GFP/+ mice. Red, RhoA; green, Cx3cr1-GFP; blue, DAPI. Scale bar: 25 µm. Inset: 5 µm. (c) Expression levels of RhoA and RhoB were increased at 4 h after thrombin injection. Inhibition of EP3R with AE240 reduced RhoA expression at 4 h after thrombin injection, but activation with AE248 increased RhoA expression; neither AE240 nor AE248 altered RhoB expression (n = 12). (d) EP3R inhibition with AE240 lessened the thrombin-induced increase in expression of ROCK2 and phosphorylated MYPT1 (p-MYPT1) at 4 h, but EP3R activation with AE248 did not further increase ROCK2 and p-MYPT1 expression after thrombin injection (n = 12). t-MYPT1, total MYPT1. (e) ROCK2 antagonist HA1077 decreased p-MYPT1 expression at 4 h after thrombin injection (n = 8), but the addition of AE248 did not reverse this trend (n = 8, p = 0.49 vs. thrombin + HA1077). Data are presented as means ± SD. *p < 0.05, **p < 0.01 vs. sham; #p < 0.05, ##p < 0.01 vs. thrombin + vehicle; one-way ANOVA with Bonferroni post hoc test.
We next assessed ROCK2 expression and MYPT1 phosphorylation because these proteins lie downstream of RhoA signaling. Both ROCK2 and p-MYPT1 were increased at 4 h after thrombin injection. EP3R inhibition with AE240 reversed these increases (n = 12, p = 0.002 for ROCK2, p = 0.006 for p-MYPT1; Figure 6d). Stimulation with AE248 had no significant effect, although we observed a trend toward increased ROCK2 expression (n = 12, p = 0.36 for ROCK2, p = 0.81 for p-MYPT1; Figure 6d). Finally, we co-treated mice with ROCK inhibitor HA1077 and EP3R agonist AE248. HA1077 reduced p-MYPT1 level at 4 h after thrombin injection (n = 8, p = 0.04), but the addition of AE248 did not reverse this change (n = 8, p = 0.49; Figure 6e). Together, these data suggest that EP3R-mediated toxicity involves the RhoA–ROCK2–p-MYPT1 pathway.
EP3R activation reverses the thrombin-induced reduction in RhoA expression after blood injection
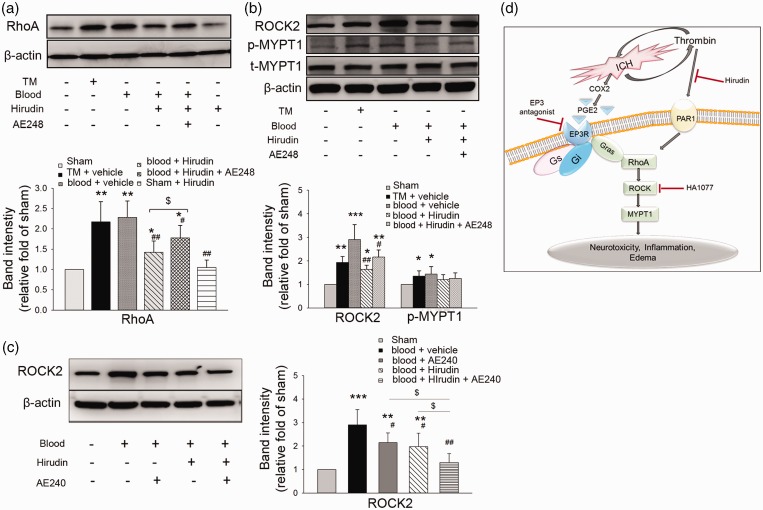
Thrombin signaling is mediated by the cleavage of protease-activated receptors, which couple to G-proteins to trigger multiple intracellular signaling pathways.41 To eliminate the effect of thrombin on RhoA expression induced by protease-activated receptor-G-proteins, we used the thrombin inhibitor hirudin to block thrombin activity after blood injection. At 4 h after blood injection, hirudin-treated mice exhibited a decrease in RhoA compared to that in vehicle-treated mice (n = 6, p = 0.003 vs. blood + vehicle). The addition of AE248 reversed the hirudin-induced decrease in RhoA expression (n = 6, p = 0.04 vs. blood + hirudin; Figure 7a). Hirudin also decreased ROCK2 level (n = 6, p = 0.002 vs. blood + vehicle) but had no effect on p-MYPT1. The addition of AE248 tended to increase ROCK2 expression above that with hirudin alone, but the difference did not reach statistical significance (n = 6, p = 0.27 vs. blood + hirudin; Figure 7b). Thus, thrombin inhibition did not completely block EP3R activation-induced increases in RhoA expression. Moreover, when administered separately, hirudin and EP3R antagonist AE240 each decreased ROCK2 expression at 4 h after blood injection; combined treatment with hirudin and AE240 further decreased ROCK2 expression (n = 6, p = 0.04 vs. blood + hirudin or AE240 alone; Figure 7c). Together, these data suggest that the activation of the RhoA-ROCK pathway is not entirely thrombin-dependent in the blood model (Figure 7d).
Figure 7.
EP3 receptor (EP3R)/RhoA/ROCK2 signaling in mice after intrastriatal blood injection. (a) RhoA expression was increased at 4 h after thrombin injection (TM) or blood injection. Hirudin treatment after blood injection decreased RhoA expression compared to that with vehicle treatment (n = 6). EP3R agonist AE248 increased RhoA expression after blood injection in the presence of thrombin inhibitor hirudin (n = 6, *p < 0.05, **p < 0.01 vs. sham; #p < 0.05, ##p < 0.01 vs. blood + vehicle; $p < 0.05 vs. blood + hirudin; one-way ANOVA with Bonferroni post hoc test). (b) Expression levels of ROCK2 and phosphorylated MYPT1 (p-MYPT1) were increased after thrombin or blood injection (n = 6, *p < 0.05, **p < 0.01, ***p < 0.001 vs. sham; one-way ANOVA with Bonferroni post hoc test). Hirudin treatment decreased ROCK2 expression at 4 h after blood injection (n = 6, #p < 0.05, ##p < 0.01 vs. blood + vehicle; one-way ANOVA with Bonferroni post hoc test), but it had no effect on p-MYPT1. EP3R agonist AE248 tended to increase ROCK2 expression after blood injection compared with expression after hirudin alone, but the difference was not statistically significant (n = 6, p = 0.27 vs. blood + hirudin). Hirudin with or without AE248 did not change the expression of p-MYPT1 compared to that with vehicle treatment. t-MYPT1: total MYPT1. Values in (a) and (b) are presented as means ± SD. (c) ROCK2 expression was decreased at 4 h after blood injection in mice treated with hirudin or EP3R antagonist AE240. Co-treatment with hirudin and AE240 further decreased ROCK2 expression (n = 6, **p < 0.01, ***p < 0.001 vs. sham; #p < 0.05, ##p < 0.01 vs. blood + vehicle; $p < 0.05 vs. blood + hirudin or blood + AE240; one-way ANOVA with Bonferroni post hoc test). (d) Schematic outline of the potential EP3R–RhoA–ROCK–MYPT1 pathway after ICH/thrombin-induced brain injury.
Discussion
In this study, we utilized both in vivo and in vitro models to address the mechanisms of EP3R involved in thrombin-induced brain injury. We found that after striatal injection of thrombin, EP3R is induced in glial cells, mostly in astrocytes and microglia. Moreover, EP3R inhibition (1) mitigated thrombin-induced brain injury volume, brain edema, and neurologic deficits; (2) reduced thrombin-induced cell death in ex vivo (organotypic slice culture) and in vivo models; (3) reduced cellular inflammatory responses, including microglial activation and neutrophil infiltration; (4) increased the number of M2 microglia; and (5) reduced MMP-9 activity. Finally, we provide novel evidence that EP3R-mediated toxicity involves the RhoA–ROCK2–p-MYPT1 pathway but is not completely thrombin-dependent in the blood model. Together, these findings indicate that EP3R inhibition is protective, that the RhoA–ROCK2–p-MYPT1 pathway might contribute to EP3R-mediated thrombin-induced brain injury, and that promoting the neuroprotective M2 microglia phenotype and inhibiting the RhoA–ROCK2–p-MYPT1 signaling pathway could be potential therapeutic options for treating ICH.
EP3R is believed to be expressed exclusively in neurons in healthy brain. It has been shown that EP3R protein expression is increased in microglia after ischemic stroke in vivo.8 After thrombin injection, we found that astrocytes and microglia in the perilesional region of the striatum expressed EP3R, consistent with the appearance of brain injury-induced local astrogliosis and microgliosis. However, EP3R inhibition did not change GFAP immunoreactivity at 72 h after thrombin injection, though the number of activated microglia (CD68+ cells) was decreased. Our results suggest that EP3R inhibition may affect microglia and astrocytes differently in the early period of thrombin-induced brain injury. Moreover, a prior study42 showed that 60% of microglia expresses EP3R in striatum at 5 days after quinolinic acid injection and that this percentage increases to 80% at 10 days. In contrast, our results showed EP3R+/CD11b+ cells in the perilesional area at 72 h after thrombin injection. Additional studies are needed to determine whether expression of EP3R in astrocytes and microglia changes over time and whether EP3R activation by thrombin affects astrocyte, microglial, and neuronal function.
Thrombin is released from hematomas and is a major contributor to acute ICH injury.43 In our study, thrombin (300 U/mL) induced CA1 neuronal damage in hippocampal slice cultures at 48 h. The damage continued to increase for at least 72 h after thrombin exposure, indicating delayed neuronal death. In contrast, cell death in mouse brain appeared as early as 2 h after thrombin injection. This result indicates that thrombin exposure may cause acute microenvironmental damage to the surrounding brain tissue that initiates and amplifies the toxic signals in vivo. PI labels the cellular DNA in necrotic cells after the cell membrane has been compromised. Although AE240 exposure caused a decrease in PI+ cells after thrombin insult in vitro and in vivo, we observed no evidence of a decrease in TUNEL+ cells, FJB+ cells, or caspase-3 level in the perilesional region. Additionally, the PARP inhibitor DR2313 provided no protection in vitro. These data indicate that EP3R may play a critical role in mitigating cell membrane damage and necrotic-like cell death rather than apoptotic-like cell death in thrombin-induced ICH.
Increasing evidence indicates that inflammatory responses contribute to secondary injury after ICH.1,44 Therefore, we addressed whether EP3R inhibition reduces inflammation after thrombin-induced injury. In hippocampal slice cultures, morphologic evidence suggested that microglia were activated after thrombin exposure but that cell soma expansion of microglia was decreased by EP3R inhibition. We further assessed whether systemic administration of EP3R inhibitor AE240 affects the inflammatory response after striatal injection of thrombin. AE240 reduced the number of activated CD68+/Iba1+ microglia but increased the number of YM-1+/Iba1+ microglia. It is known that microglia can differentiate into an M1 (classically activated) or M2 (alternatively activated) phenotype. M2 microglia are considered to be beneficial because they release neuroprotective factors.39 The increase in M2 microglia, as reflected by an increase in the YM-1+ marker, suggests that EP3R inhibition with AE240 may promote conversion of microglia to an anti-inflammatory and neuroprotective phenotype that inhibits the proinflammatory reaction. Infiltrating neutrophils release reactive oxygen species and cytokines that augment secondary injury.1 We found that AE240 treatment also suppressed the invasion of MPO+ neutrophils. Although we have reported previously that pro-MMP-2 and pro-MMP-9 are present in the brain after collagenase-induced ICH,36 we did not detect pro-MMP-2 activity in brain tissue after thrombin-induced brain injury. These differing results could be related to the different animal models used. Furthermore, EP3R inhibition reduced pro-MMP-9 activity but, surprisingly, did not mitigate the thrombin-induced damage to tight junction proteins. Thus, considering that EP3R was not observed in vascular-like structures, EP3R inhibition may play a bigger role in reducing inflammation than in preserving the microvasculature after thrombin injection. The increased M2 microglial polarization and reduced MMP-9 activity likely contribute to the protection conferred by EP3R inhibition at 72 h after thrombin injection, as we assessed both at 24 h, when the lesion volume did not differ between the vehicle- and AE240-treated groups.
EP3R can couple with small G-proteins, such as Rho, Rac, and CDC42.11 We focused on the RhoA-ROCK pathway because it affects cell apoptosis, migration, and inflammation.12 ROCK has two known isoforms; ROCK1 is expressed mostly in non-neuronal tissue, whereas ROCK2 is expressed abundantly in brain.45 Our data indicate that the RhoA-ROCK pathway is involved in EP3R-mediated thrombin- and blood-induced brain injury. Expression levels of RhoA, as well as its downstream effector proteins ROCK2 and p-MYPT1, were increased as early as 4 h after thrombin injection. These increases were prevented by EP3R inhibition. Although Jiang et al.46 found that RhoA was expressed in neurons after ischemic stroke, we found that RhoA was expressed in activated microglia in the perilesional region after thrombin injection. Considering that EP3R is also expressed in microglia, EP3R-RhoA-ROCK signaling may be associated primarily with inflammatory responses after thrombin-induced brain injury. Whether RhoA-ROCK signaling regulates the microglial phenotype (M1 and M2) after thrombin injection warrants investigation. Previous ex vivo studies have shown consistently that thrombin causes striatal shrinkage that is thought to be mediated by protease pathways, such as PKC and ERK.34 We confirmed the thrombin-induced shrinkage of hippocampus in our cultured brain slices. Unexpectedly, EP3R inhibition did not reduce hippocampal shrinkage, possibly because it mitigates necrotic-like but not apoptotic-like cell death.
Thrombin could be a double-edged sword in ICH pathogenesis. On the one hand, thrombin promotes the coagulation cascade; on the other hand, thrombin generated during hematoma formation can directly damage the blood–brain barrier and cause cytotoxicity and tissue damage, partly by acting through protease-activated receptors.30 Indeed, thrombin inhibition has been protective in ICH models.30 To address the concern that thrombin may directly induce the Rho-ROCK2 pathway by activating its receptors, we used the blood model with hirudin treatment. Hirudin alone reduced RhoA protein level after blood injection, but EP3R agonist administered with hirudin partially blocked this reduction. Thus, thrombin may elicit an increase in RhoA expression through its receptors, but EP3R may also be involved. It is noteworthy that in addition to thrombin, the hematoma releases other blood components such as hemoglobin and its metabolites iron and bilirubin.17 In vitro and in vivo studies have shown that hemoglobin is able to stimulate PGE2 production47,48 and activate the Rho-ROCK2 pathway.49 Therefore, hemoglobin might contribute to EP3R activation of the Rho-ROCK2 pathway in the absence of thrombin activity. The fact that hirudin and AE240 have an additive effect on ROCK2 expression further supports the existence of a thrombin-independent Rho-ROCK2 pathway in ICH-induced brain injury. It is also important to note that PGE2 acts through several receptors (EP1R–EP4R), and we recently reported that EP1R activation also plays a toxic role after ICH through mechanisms that involve the Src kinases and the MMP-9 signaling pathway.5 Therefore, the synergistic or antagonistic effects of EP3R with other EP receptors need to be determined.
Together, our results provide proof of concept that PGE2 receptor EP3 contributes to thrombin/blood-induced brain injury by augmenting necrotic-like cell death and inflammatory responses. These effects may be mediated, at least in part, by the Rho-ROCK2 signaling pathway. Additionally, we found that EP3R inhibition promotes the neuroprotective M2 microglial phenotype and downregulates the Rho–ROCK2–p-MYPT1 signaling pathway. Given the increasing preclinical and clinical evidence that inflammation is a key contributor to ICH-induced secondary brain injury,50 these findings suggest a potential novel strategy for therapeutic intervention against ICH.
Supplementary Material
Acknowledgements
We thank Suzy Cho and Weizhu Tang for blind data analysis. We thank Dr Raymond Koehler for helpful suggestions and Claire Levine, MS, ELS, for assistance with manuscript preparation.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by AHA 13GRNT15730001, NIH K01AG031926, R01NS078026, and R01AT007317 (JW) and an AHA postdoctoral fellowship award (14POST20140003, X. Han).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
JW conceived, designed, and coordinated the research; XH participated in the research design; XH, XL, QL, YG, WZ, and TC performed the research; XH and JW analyzed data and wrote the paper; TM contributed the drugs and reviewed the paper; JW obtained funding. All authors read and approved the final draft.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data.
References
- 1.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 2007; 27: 894–908. [DOI] [PubMed] [Google Scholar]
- 2.Wu T, Wu H, Wang J, et al. Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain. J Neuroinflammation 2011; 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat 2010; 91: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RL, Giembycz MA, Woodward DF. Prostanoid receptor antagonists: development strategies and therapeutic applications. Br J Pharmacol 2009; 158: 104–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, Wu T, Chang CF, et al. Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain Behav Immun 2015; 46: 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H, Wu T, Hua W, et al. PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice. Neurobiol Aging 2015; 36: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takemiya T, Yamagata K. Intercellular signaling pathway among endothelia, astrocytes and neurons in excitatory neuronal damage. Int J Mol Sci 2013; 14: 8345–8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda-Matsuo Y, Tanji H, Narumiya S, et al. Inhibition of prostaglandin E2 EP3 receptors improves stroke injury via anti-inflammatory and anti-apoptotic mechanisms. J Neuroimmunol 2011; 238: 34–43. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Wang Q, Johansson JU, et al. Inflammatory prostaglandin E2 signaling in a mouse model of Alzheimer disease. Ann Neurol 2012; 72: 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin receptors: advances in the study of EP3 receptor signaling. J Biochem 2002; 131: 781–784. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda-Matsuo Y, Tanji H, Ota A, et al. Microsomal prostaglandin E synthase-1 contributes to ischaemic excitotoxicity through prostaglandin E2 EP3 receptors. Br J Pharmacol 2010; 160: 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azzarelli R, Pacary E, Garg R, et al. An antagonistic interaction between PlexinB2 and Rnd3 controls RhoA activity and cortical neuron migration. Nat Commun 2014; 5: 3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villar-Cheda B, Dominguez-Meijide A, Joglar B, et al. Involvement of microglial RhoA/Rho-kinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors. Neurobiol Dis 2012; 47: 268–279. [DOI] [PubMed] [Google Scholar]
- 14.Dusaban SS, Purcell NH, Rockenstein E, et al. Phospholipase C epsilon links G protein-coupled receptor activation to inflammatory astrocytic responses. Proc Natl Acad Sci U S A 2013; 110: 3609–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Liang X, Wang Q, et al. Misoprostol, an anti-ulcer agent and PGE2 receptor agonist, protects against cerebral ischemia. Neurosci Lett 2008; 438: 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saleem S, Kim YT, Maruyama T, et al. Reduced acute brain injury in PGE2 EP3 receptor-deficient mice after cerebral ischemia. J Neuroimmunol 2009; 208: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 2012; 11: 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo HM, Chen CL, Tsai YJ, et al. Thrombin induces cyclooxygenase-2 expression and prostaglandin E2 release via PAR1 activation and ERK1/2- and p38 MAPK-dependent pathway in murine macrophages. J Cell Biochem 2009; 108: 1143–1152. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh HL, Sun CC, Wang TS, et al. PKC-delta/c-Src-mediated EGF receptor transactivation regulates thrombin-induced COX-2 expression and PGE(2) production in rat vascular smooth muscle cells. Biochim Biophys Acta 2008; 1783: 1563–1575. [DOI] [PubMed] [Google Scholar]
- 20.Thirumangalakudi L, Rao HV, Grammas P. Involvement of PGE2 and PGDH but not COX-2 in thrombin-induced cortical neuron apoptosis. Neurosci Lett 2009; 452: 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung S, Aliberti J, Graemmel P, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000; 20: 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W, Gao Y, Chang CF, et al. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLoS One 2014; 9: e97423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CF, Cho S, Wang J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol 2014; 1: 258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue M, Hollenberg MD, Demchuk A, et al. Relative importance of proteinase-activated receptor-1 versus matrix metalloproteinases in intracerebral hemorrhage-mediated neurotoxicity in mice. Stroke 2009; 40: 2199–2204. [DOI] [PubMed] [Google Scholar]
- 25.Liu DZ, Ander BP, Xu H, et al. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Ann Neurol 2010; 67: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueta M, Matsuoka T, Narumiya S, et al. Prostaglandin E receptor subtype EP3 in conjunctival epithelium regulates late-phase reaction of experimental allergic conjunctivitis. J Allergy Clin Immunol 2009; 123: 466–471. [DOI] [PubMed] [Google Scholar]
- 27.Hara M, Takayasu M, Watanabe K, et al. Protein kinase inhibition by fasudil hydrochloride promotes neurological recovery after spinal cord injury in rats. J Neurosurg 2000; 93: 94–101. [DOI] [PubMed] [Google Scholar]
- 28.Ma Q, Huang B, Khatibi N, et al. PDGFR-alpha inhibition preserves blood-brain barrier after intracerebral hemorrhage. Ann Neurol 2011; 70: 920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Song S, Hua Y, et al. Effects of thrombin on neurogenesis after intracerebral hemorrhage. Stroke 2008; 39: 2079–2084. [DOI] [PubMed] [Google Scholar]
- 30.Xue M, Hollenberg MD, Yong VW. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J Neurosci 2006; 26: 10281–10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H, Wu T, Li M, et al. Efficacy of the lipid-soluble iron chelator 2,2′-dipyridyl against hemorrhagic brain injury. Neurobiol Dis 2012; 45: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Zhuang H, Dore S. Heme oxygenase 2 is neuroprotective against intracerebral hemorrhage. Neurobiol Dis 2006; 22: 473–476. [DOI] [PubMed] [Google Scholar]
- 33.Abe T, Kunz A, Shimamura M, et al. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab 2009; 29: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujimoto S, Katsuki H, Kume T, et al. Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol Dis 2006; 22: 130–142. [DOI] [PubMed] [Google Scholar]
- 35.Katayama T, Kobayashi H, Okamura T, et al. Accumulating microglia phagocytose injured neurons in hippocampal slice cultures: involvement of p38 MAP kinase. PLoS One 2012; 7: e40813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain 2005; 128: 1622–1633. [DOI] [PubMed] [Google Scholar]
- 37.Hanada R, Leibbrandt A, Hanada T, et al. Central control of fever and female body temperature by RANKL/RANK. Nature 2009; 462: 505–509. [DOI] [PubMed] [Google Scholar]
- 38.Baek SH, Bae ON, Kim EK, et al. Induction of mitochondrial dysfunction by poly(ADP-ribose) polymer: implication for neuronal cell death. Mol Cells 2013; 36: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012; 43: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 40.Fujimura M, Usuki F, Kawamura M, et al. Inhibition of the Rho/ROCK pathway prevents neuronal degeneration in vitro and in vivo following methylmercury exposure. Toxicol Appl Pharmacol 2011; 250: 1–9. [DOI] [PubMed] [Google Scholar]
- 41.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000; 407: 258–264. [DOI] [PubMed] [Google Scholar]
- 42.Slawik H, Volk B, Fiebich B, et al. Microglial expression of prostaglandin EP3 receptor in excitotoxic lesions in the rat striatum. Neurochem Int 2004; 45: 653–660. [DOI] [PubMed] [Google Scholar]
- 43.Sharp F, Liu DZ, Zhan X, et al. Intracerebral hemorrhage injury mechanisms: glutamate neurotoxicity, thrombin, and Src. Acta Neurochir Suppl 2008; 105: 43–46. [DOI] [PubMed] [Google Scholar]
- 44.Wu H, Zhang Z, Hu X, et al. Dynamic changes of inflammatory markers in brain after hemorrhagic stroke in humans: a postmortem study. Brain Res 2010; 1342: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffy P, Schmandke A, Schmandke A, et al. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci 2009; 29: 15266–15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang W, Xia F, Han J, et al. Patterns of Nogo-A, NgR, and RhoA expression in the brain tissues of rats with focal cerebral infarction. Transl Res 2009; 154: 40–48. [DOI] [PubMed] [Google Scholar]
- 47.Ciuffi M, Tarlini L, Mugnai S, et al. Hemoglobin affects lipid peroxidation and prostaglandin E2 formation in rat corticocerebral tissues in vitro. Biochem Pharmacol 1996; 52: 97–103. [DOI] [PubMed] [Google Scholar]
- 48.Frosini M, Sesti C, Valoti M, et al. Rectal temperature and prostaglandin E2 increase in cerebrospinal fluid of conscious rabbits after intracerebroventricular injection of hemoglobin. Exp Brain Res 1999; 126: 252–258. [DOI] [PubMed] [Google Scholar]
- 49.Fu Z, Chen Y, Qin F, et al. Increased activity of Rho kinase contributes to hemoglobin-induced early disruption of the blood-brain barrier in vivo after the occurrence of intracerebral hemorrhage. Int J Clin Exp Pathol 2014; 7: 7844–7853. [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Wang Y, Wang J, et al. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol 2014; 115: 25–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.