Abstract
Animal models provide evidence of spleen mediated post-stroke activation of the peripheral immune system. Translation of these findings to stroke patients requires estimation of pre-stroke spleen volume along with quantification of its day-to-day variation. We enrolled a cohort of 158 healthy volunteers and measured their spleen volume over the course of five consecutive days. We also enrolled a concurrent cohort of 158 stroke patients, measured initial spleen volume within 24 h of stroke symptom onset followed by daily assessments. Blood samples for cytokine analysis were collected from a subset of patients. Using data from healthy volunteers, we fit longitudinal quantile regression models to construct gender and body surface area based normograms of spleen volume. We quantified day-to-day variation and defined splenic contraction. Based on our criteria, approximately 40% of stroke patients experienced substantial post-stroke reduction in splenic volume. African Americans, older patients, and patients with past history of stroke have significantly higher odds of post-stroke splenic contraction. All measured cytokine levels were elevated in patients with splenic contraction, with significant differences for interferon gamma, interleukin 6, 10, 12, and 13. Our work provides reference standards for further work, validation of pre-clinical findings, and characterization of patients with post-stroke splenic contraction.
Keywords: Acute stroke, brain ischemia, cerebrovascular disease, immunology, intracerebral hemorrhage
Introduction
There is a growing understanding of post-stroke immunological responses.1 Alongside the inflammatory responses localized to the brain, there is mounting evidence of post-stroke systemic immunological alterations in peripheral organs and tissues such as bone marrow, blood, and the spleen.2,3 Spleen is a lymphoid organ that plays an important role in both innate and adaptive immune responses. Recent pre-clinical studies have focused on characterizing post-stroke splenic responses, and have highlighted the spleen’s role in mediating peripheral immune system related secondary brain injury.4 Multiple mechanisms of post-stroke splenic activation have been described in the literature which include, activation of sympathetic nervous system,5,6 production of chemotactic cytokines,7 and antigen presentation by the damaged brain.8,9 Once activated, the spleen contracts in size,10,11 undergoes cellular changes,12 and releases inflammatory cytokines.7,13 It has also been shown that splenocytes specifically home to the site of primary cerebral injury.14 It has further been demonstrated in experimental stroke models that pre or immediate post-stroke surgical removal or irradiation of the spleen attenuates these responses, and results in reduced stroke lesion size and enhanced recovery.5,14–19 The pre-clinical body of evidence raises an exciting possibility that novel therapies can be designed to target the spleen as an approach to modulate post-stroke inflammation and dampen secondary brain injury.20–24 However, the road to translation of these findings in the clinical setting is beset with challenges.25 Despite mounting pre-clinical evidence, post-stroke splenic responses in the clinical setting remain unknown. Our preliminary work suggested that the initial splenic response observed in patients with acute ischemic stroke (AIS) may substantiate some of the pre-clinical studies, in the absence of known normative data.26 However, determinant factors and reference standards for spleen size and quantification of its day-to-day variability in healthy human populations have not been defined. These standards are a pre-requisite to any further assessment of spleen size in patients with AIS and intracerebral hemorrhage (ICH). We therefore enrolled a cohort of healthy, but at risk population for cerebrovascular disease and followed this cohort serially, assessing spleen volumes daily, over the course of five consecutive days. Furthermore, we expanded the enrollment of stroke patients into our existing protocol, enrolling patients with both AIS and ICH.
We aimed to study the factors that are associated with spleen size in healthy populations, provide estimates of spleen size for a normalized population, explore the day-to-day variation in spleen volume, quantify splenic contraction (SC) in stroke patients, and characterize AIS and ICH patients that undergo SC.
Materials and methods
Selection of healthy volunteers
The age and gender distribution of stroke patients presenting to our hospital from 2007 to 2011 was determined from the University of Texas Houston Stroke Registry (UTHSR).27 A matched cohort of healthy volunteers (HVs) was enrolled for serial measurement of spleen volumes. The HVs were not excluded on the basis of any comorbidities or risk factors for cerebrovascular disease, however they were not included if they had any acute illness, diagnosed cancer, or other known conditions likely to alter spleen size. After obtaining written consent, the HVs were followed for five consecutive days at approximately the same time each day. They were asked not to alter any dietary or daily routine patterns, and the spleen size was measured using abdominal ultrasound (US) in the supine position without breath holding, as per methods previously described.26 Two physicians underwent training and inter-rater reliability assessment with a certified sonographer. All three raters participated in spleen volume assessments; however any given HV was followed by the same rater through the course of five days. Demographic, anthropometric, risk factors, and concomitant medication data were also collected.
Recruitment of patients with AIS and ICH
Patients with suspected AIS and ICH who presented to the Memorial Herman Hospital Emergency Department from April 2010 to December 2013 were screened, and eligible patients were consented for serial measurement of spleen volume for seven days or until hospital discharge. The first spleen scan was obtained within 24 h of symptoms onset followed by one at each 24 ± 6 h for seven days or hospital discharge. As the patients were enrolled in the acute setting, the diagnosis of ischemic stroke was confirmed later by conventional clinical and radiological measures. All AIS and ICH patients received standard of care treatment including intravenous tissue plasminogen activator (IV tPA) for eligible AIS patients, based on American Heart Association (AHA) guidelines. Other than expanding the time window for the first scan from 6 to 24 h, the inclusion and exclusion criteria were followed as per our existing protocol.26 Along with daily spleen volumetric measurements all enrolled patients also underwent daily assessments for the National Institutes of Health Stroke Scale (NIHSS).
Spleen measurements
Details of measurement of spleen size using abdominal ultrasonography have been described.26 Briefly, these measurements were performed using Philips CX 50 US machine and a phased sector array (5–1 MHz) transducer (Philips Medical Systems, Bothell, WA). Multiple measurements in the sagittal (longitudinal) plane for the length (L) and thickness (T), and in the transverse plane for width (W) at the splenic hilum were obtained for all HVs and patients at each time point. Three sets of these measurements having least degree of variation among them were selected, and the final spleen volume was calculated from the average using the standard prolate ellipsoid formula. The formula incorporates product of one-dimensional diameters into the equation , where V is the ellipsoid volume.
Cytokine analysis
Blood samples were obtained from a subset of stroke patients at two time points. The first sample was obtained within 24 h of stroke symptom onset, whereas the second was obtained at 24–48 h. Whole blood was collected into EDTA tubes (Vacutainer, BD). Within 30 min of collection, blood was centrifuged at 1000g for 10 min. The separated plasma was aliquoted into polypropylene tubes and stored at −80℃. Prior to analysis, plasma samples were completely thawed followed by vortex and centrifugation. Cytokines were detected by magnetic bead based assay (HCYTOMAG, Millipore, USA) on a MAGPIX platform (Millipore) according to manufacturer’s protocol. Data were generated and analyzed by xPONENT software.
Institutional Review Board approval
This study was approved by the Institutional Review Board of the University of Texas Health Science center as well as by the research ethics committee of the Memorial Hermann Hospital at Texas Medical Center in Houston, under the International Conference on Harmonization (ICH) Good Clinical Practice (GCP) guidelines.
Statistical analysis
Measures for categorical variables were summarized as frequencies and percentages. Based on the distribution of continuous variables, their summary measures were calculated as either means and standard deviations (SDs) or medians and interquartile range (IQR). Standard published methods were used for derivations of body surface area (BSA).28 Univariate analysis was done to determine demographic, anthropometric, and clinical variables that were most strongly associated with the spleen volume. These factors were then assessed for multicollinearity, confounding, and interactions. Gender and BSA were used to construct percentile based normative predictions of spleen volumes using longitudinal quantile regression.29 Inter-rater reliability for US based spleen measurements was assessed by estimating concordance coefficients.30 Stroke patients were considered to have SC if the observed spleen volume was at least 20 cm3 smaller from the expected volume for the 50th percentile of a given gender and BSA category. Logistic regression models were fit to determine the association of demographic and clinical factors with SC. Serial NIHSS measurements were analyzed for differences between SC and non-SC patients using simultaneous quantile regression. Mean cytokine levels for patients with and without SC were compared using two sample t test under the assumption of unequal variances. Alpha was set at 0.05 for all statistical testing. Statistical analysis and graphical presentation were done using STATA version 13 (StataCorp, College Station, TX), R (R Development Core Team, 2011, Vienna, Austria), and Microsoft Excel (Microsoft, 2013, Redmond, WA).
Results
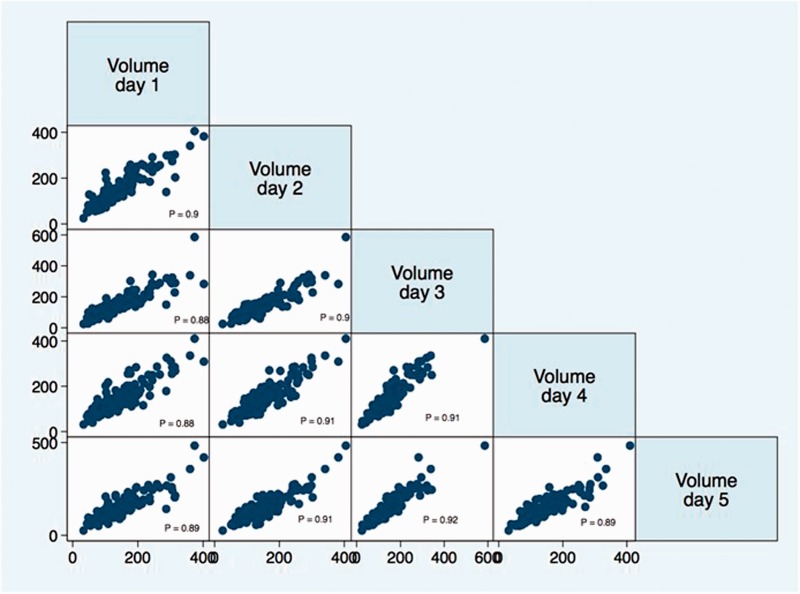
Over a six-month enrollment period, 158 HVs were enrolled and a total of 775 spleen volume assessments via abdominal US were completed. The data were complete (all 5 days of spleen volumes obtained) for 145 (91.7%) HVs. For the remaining 13, two individuals had two days of missing data, whereas 11 had only one missing time point. There was no association of missing data with demographic or other measured variables. The concordance coefficients for two raters with the certified sonographer were 0.95 and 0.99, respectively. The age and gender distribution for HVs was not statistically different from the UTHSR data. The serial spleen measurements for HV over the period of five days showed a high degree of statistically significant correlation (Figure 1). The overall five-day median and IQR for spleen volume was 129.6 (94.8–177.6) cm3. Over the course of five-day period the median (IQR) spleen volume ranged between 122.8 (93.7–176.6) and 133.4 (96.3–177.3) cm3 (Figure 2).
Figure 1.
Day-to-day correlational pattern observed for spleen volumes of individual healthy volunteers. The axes represent spleen volume calculated as volume = length × width × thickness × π/6. P: Pearson correlation coefficient (p value for all comparison < 0.001).
Figure 2.
Median spleen volume for healthy volunteers plotted for each day of observation. Bars indicate interquartile range.
Gender and BSA-based normograms for HVs
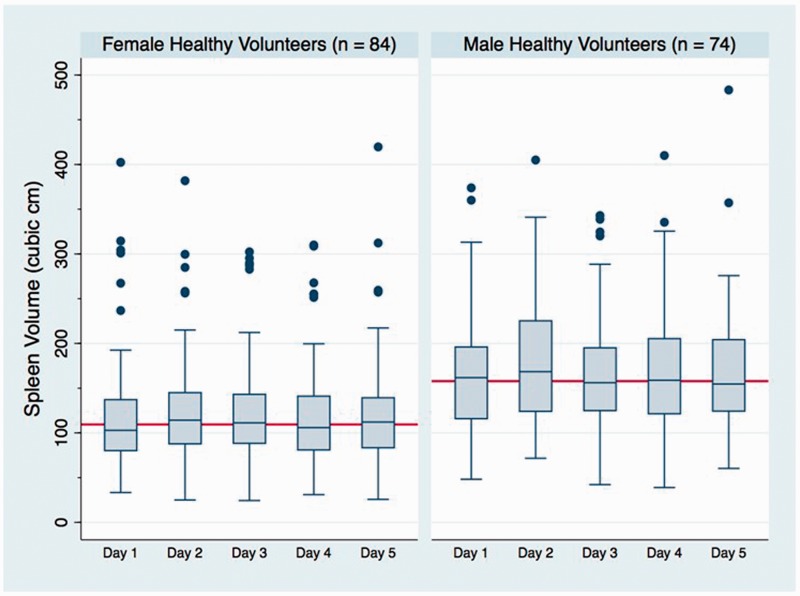
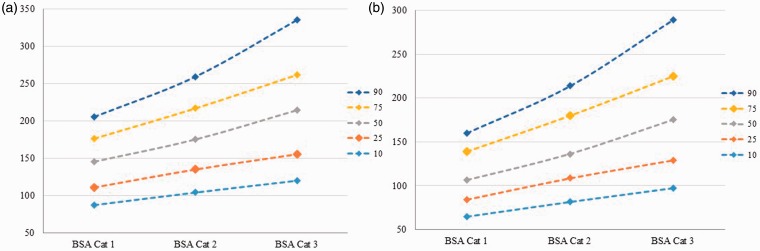
Demographic, anthropometric, and risk factor variables were tested for their association with the spleen volume in HV across five different percentiles (0.1, 0.25, 0.5, 0.75, and 0.9). Gender, height, weight, and BSA were found to be consistently significant across all tested percentiles. The overall median (IQR) volume for females was 109.4 (83.4–143.1) cm3 whereas it was 157.9 (121.8–202.2) cm3 for males. The box plots for the spleen volumes of males and females at each measurement day are presented in Figure 3. Based on statistical association and clinical relevance gender and BSA were selected for estimation of predictive spleen volumes. For the construction of predictive normograms the BSA was categorized into three groups (representing lower half, and upper two quartiles, respectively). Gender-based normograms with estimated spleen volume at 10th, 25th, 50th, 75th, and 90th percentiles for each category of BSA are presented as Figure 4(a) for males, and Figure 4(b) for females.
Figure 3.
Box and Whisker plot for spleen volumes of female and male healthy volunteers at five sequential days of observation. Each box represents the median with 25th and 75th percentile. The bars at the end of whiskers represent upper and lower adjacent lines. Adjacent line distance has been calculated as h-spread ± 2 steps, where h-spread is the difference between 25th and 75th percentile and step is 1.5 × h-spread. One outlier observation greater than 500 cm3 has not been shown to maintain proportional graphical scale, though all values were used for calculation. The solid red line in each sub-graph represents the overall median volume for females and males, respectively.
Figure 4.
Body surface area (BSA)-based normograms for males (a—left panel) and females (b—right panel) showing estimated spleen volume for 10th, 25th, 50th, 75th, and 90th percentiles. BSA Cat: BSA Category.
The maximum difference in median HV spleen volume between any two consecutive days was noted to be 10.5 cm3, whereas the average change in median over the course of five days was 1.5 cm3. Based on these findings, AIS and ICH patients with observed spleen volumes at least 20 cm3 smaller as compared to their expected gender and BSA category volume for the 50th percentile, were considered to have SC.
SC in stroke patients and biochemical markers
Data from 158 stroke patients are included in these analyses. Based on the defined criteria, 64 (40.5%) patients have SC. Table 1 represents univariate comparison of demographic and clinical variables for patients with and without SC. The multivariable logistic regression model showed that African Americans (OR: 5.37, 95% CI: 2.47–11.68), patients older than 75 years (OR: 2.52, 95% CI: 1.13–5.60), and patients with past history of stroke (OR: 2.61, 95% CI: 1.09–6.23) have significantly greater odds of having post-stroke SC. Patients with SC also had significantly higher presentation and serial NIHSS score, as compared to those who did not have SC (difference in median presentation NIHSS: 4 points, 95% CI of difference: 0.26–7.75 and difference in median serial NIHSS: 3 points, 95% CI of difference: 1.16–4.83). However, admission NIHSS was not statistically different between the two groups in the multivariable logistic regression model.
Table 1.
Univariate analysis of demographic, clinical, and outcomes variables for stroke patients with and without splenic contraction.
| Patients without SC (n = 94) | Patients with SC (n = 64) | p value | OR (95% CI) | |
|---|---|---|---|---|
| Gender (Female), n (%) | 48 (51.1) | 29 (45.3) | 0.48 | 1.26 (0.66–2.38) |
| Race, n (%) | ||||
| • White | 70 (76.9) | 27 (44.3) | Referent category | |
| • African American | 21 (23.1) | 34 (55.7) | < 0.01 | 4.19 (2.08–8.47) |
| Ethnicity (Hispanic), n (%) | 19 (20.2) | 13 (20.3) | 0.99 | 1.01 (045–2.22) |
| Age (>75 years), n (%) | 26 (27.7) | 28 (43.8) | 0.04 | 2.03 (1.04–3.97) |
| Stroke type | ||||
| • Ischemic stroke | 69 (73.4) | 49 (76.6) | Referent category | |
| • ICH | 25 (26.6) | 15 (23.4) | 0.66 | 0.84 (0.40–1.77) |
| Stroke onset to first spleen scan (hours), median (IQR) | 5.3 (2.9–13.9) | 4.7 (3.3–12.2) | 0.49 | 0.99 (0.95–1.03) |
| Stroke onset to first spleen scan < 6 hours, n (%) | 49 (55.7) | 38 (60.3) | 0.57 | 1.21 (0.63–2.33) |
| NIHSS, median (IQR) | 7 (4–16) | 11 (7–18) | 0.03 | 4.00 (0.26–7.75)a |
| Serial NIHSS, median (IQR) | 7 (2–13.5) | 10 (4–19) | 0.01 | 3.00 (1.16–4.83)a |
| Hypertension, n (%) | 78 (82.9) | 54 (84.4) | 0.82 | 1.11 (0.46–2.63) |
| Chronic pulmonary disease, n (%) | 3 (3.2) | 5 (7.8) | 0.21 | 2.54 (0.58–11.04) |
| Diabetes mellitus, n (%) | 25 (26.6) | 17 (26.6) | 0.99 | 0.99 (0.48–2.05) |
| Malignancy, n (%) | 7 (7.5) | 3 (4.7) | 0.48 | 0.60 (0.15–2.43) |
| Coronary artery disease, n (%) | 9 (9.7) | 7 (10.9) | 0.79 | 1.15 (0.40–3.25) |
| Hypercholesterolemia, n (%) | 26 (27.9) | 14 (21.9) | 0.39 | 0.72 (0.34–1.52) |
| Smoking, n (%) | 21 (22.3) | 14 (21.9) | 0.95 | 0.97 (0.45–2.09) |
| Atrial fibrillation, n (%) | 14 (15.1) | 12 (18.8) | 0.54 | 1.30 (0.55–3.03) |
| Previous stroke, n (%) | 14 (15.1) | 21 (32.8) | 0.01 | 2.75 (1.27–5.96) |
| Chronic renal disease, n (%) | 4 (4.3) | 2 (3.1) | 0.71 | 0.72 (0.13–4.04) |
| Discharge mRS (median–IQR) | 3.5 (3–4) | 4 (3–5) | 0.17 | 0.5 (−0.41–2.42) |
| UTI or pneumonia, n (%) | 3 (3.2) | 5 (7.8) | 0.21 | 2.57 (0.59–11.16) |
| Mortalityb, n (%) | 6 (6.4) | 7 (10.9) | 0.31 | 1.80 (0.58–5.63) |
SC: splenic contraction. Defined as observed volume < expected 50th percentile for given gender and body surface area category by at least 20 cm3.
Difference in median and the 95% confidence interval of the difference. Null value for 95% confidence interval = 0.
In-patient mortality.
Blood samples for cytokine analysis were collected from a total of 37 stroke patients at two time points. This sub-group of stroke patients did not differ from those on whom cytokine analysis was not performed in terms of demographic factors, risk factor profile, and stroke severity. We compared the patients with (n = 15, 40.5%) and without (n = 22, 59.5%) SC for the difference in mean of 13 different cytokines, these are represented in Figure 5. All 13 cytokines had higher mean values in patients who had SC as compared to those who did not. The differences were statistically significant for interferon gamma (INF γ), interleukin (IL) 6, IL-12, IL-13, and IL-10.
Figure 5.
Comparison of mean cytokine levels for patients with (n = 15) and without (n = 22) splenic contraction. Cytokines were measured at two time points, the mean of two readings is compared. Lines indicate standard error. Different scale of measurement used for IL 4 and IL 10. GM CSF: granulocyte monocyte colony stimulating factor, INF: interferon, TNF: tumor necrosis factor, IL: interleukin. *Indicates statistical significance.
Discussion
The experimental evidence around post-stroke activation of the peripheral immune system is overwhelming.7,31 The intricate pathways of immune system activation lead to a depressive state of peripheral immunity, the so-called post-stroke immunosuppression or post-stroke immunodeficiency.3,32,33 A strong correlation between post-stroke activation of the immune system and changes in spleen have been described in animal models.4 These include a reduction in number and apoptotic cell death of splenocytes, changes in cellularity and phenotype of splenocytes, and splenic atrophy.6,10–14,16,25 The translation of these findings in stroke patients remain challenging, and require a careful estimation of pre-stroke spleen volume as well as other contributory and confounding clinical factors.
We present population-based normograms of estimated spleen volume based on data from age and gender matched HV with our stroke patient population. There are prior studies describing spleen measurements in neonates and children,34,35 or in certain specific adult populations.36,37 However, the assessment of spleen volumes in a stroke-prone aged population has not been carried out. We found significant differences in spleen volumes of males and females even after controlling for height and weight measurements. These findings substantiate prior work in different population groups.36 We further provide an estimate of day-to-day variation in spleen volume by following our cohort of HV serially over a period of five consecutive days. To our knowledge, this approach has not been adapted and is necessary to distinguish noise from the actual signal in terms of post-stroke SC. We feel that these estimates will provide a much needed reference for future inquiries into post-stroke splenic responses in terms of volumetric changes.
Our data also provide evidence of substantial SC in a certain subset of stroke patients. We observed that African American patients, patients greater than 75 years of age, and patients with a prior history of stroke had significantly higher odds of post-stroke SC. Prior studies have reported higher probability of a heightened systemic inflammatory response in African American patients as well as in patients with a previous history of stroke.38 Our findings of SC in these groups potentially substantiate the role of spleen in post-stroke systematic immune response. It is however possible that prior stroke accounts for reduced spleen volume. To our knowledge there are no long-term data for stroke patients on spleen size changes. Worse stroke outcomes for African Americans and older patients have also been reported in the literature.39 Though a number of factors have been described that contribute to disparate cerebrovascular disease burden in terms of incidence, prevalence, and outcomes in African Americans,40,41 differential post-stroke peripheral immune response mediated via the spleen may also represent one of the contributory mechanisms for worse outcomes.
We also observed that patients with SC had higher NIHSS on hospital presentation as compared to those who did not have SC. Furthermore, the overall median NIHSS during the course of hospitalization for patients with SC was significantly higher than those who did not have SC. Though the difference in presentation NIHSS did not retain its statistical significance in multivariate modeling (p = 0.09), the difference of four points in median NIHSS at presentation and a difference of three points in overall median NIHSS through the course of hospitalization substantiate evidence from animal models which show association between SC and disease severity. It is possible that statistical significance for presentation NIHSS was not observed owing to the criteria used for quantifying SC. We have also previously reported correlational change in NIHSS with splenic volume for individual patients over the course of hospitalization.26
Our data demonstrate higher mean values of 13 different cytokines that were analyzed in blood samples of a subset of our stroke patients’ cohort. INF γ, IL-6, IL-10, IL-12, and IL-13 showed significantly raised profiles in patients who had SC. It is noteworthy that INF γ is one of the principal mediators released from the spleen in post-stroke neurodegeneration in experimental stroke models.19 Evidence from our data also corroborates experimental models showing significantly enhanced levels of cytokine secretion (including INF γ & IL-6) from splenocytes following ischemic stroke.7
The findings from our study are to be viewed in the context of certain potential limitations. Though the HVs were matched in age and gender with our stroke patient population, there were differences in risk factor profile with regard to proportion of HV with known hypertension, diabetes, and atrial fibrillation. There are no data to suggest the influence of risk factors on spleen size. Also the risk factor prevalence in HV may be an underestimate as we captured individual stated risk factors in HV, whereas risk factors are captured on the basis of clinical / laboratory parameters for stroke patients. We therefore believe that our HV sample is fairly representative of an at risk stroke population. There could be potential variability between the raters when assessing spleen size using abdominal US. Our raters underwent training and inter-rater reliability assessments before and during the project and consistently showed high (>90%) agreement on all spleen measurements. We also ensured that a single HV undergoes serial daily measurements by the same rater. In general, splenic measurements with abdominal US are shown to be valid as compared to computed tomography42 and autopsy-based assessments.43 We defined SC if the observed spleen volume was at least 20 cm3 smaller as compared to the expected 50th percentile volume for given gender and BSA. Quantification of SC has not been previously reported, and would need to be based on pre-stroke estimates of spleen volume in future inquiries on stroke patients. The basis for this quantification is observing up to 10 cm3 day-to-day variation in the spleen size of HV. It is possible that our definition underestimates the proportion of patients undergoing post-stroke SC. We performed cytokine analyses in a subset of stroke patients, this subset was not different in demographics, risk factors, and stroke severity from those on whom cytokine analysis was not performed. Though we did not find significant association of SC with African American race (p = 0.075), age >75 (p = 0.16), and previous stroke (p = 0.15) in this sub-group, we feel that that this non-significance may be a function of small sample size. The p values and 95% confidence interval limits are generally more indicative of association with these three factors, as compared to all the other variables tested (data not shown). Furthermore, the proportion of patients with SC in this sub-group (40.5%) is similar to the overall proportion of SC in the cohort of stroke patients. We therefore presume that the sub-group of patients on whom cytokine analysis was performed is fairly representative of the larger stroke patients’ cohort. Finally, certain other stressors may contribute to SC in stroke patients. The specificity of SC as a potential bio-marker for stroke remains questionable until splenic responses are concurrently described in a control hospitalized population without cerebral pathology. This remains the focus of our ongoing work. Further work is also underway to characterize cellular responses using flow cytometric analysis, and explore association of SC with evidence of sympathetic nervous system activation.
Acknowledgments
We would like to acknowledge the staff of UT ultrasonology lab and our community partner YMCA of greater Houston for their support and participation.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: in part by Bentsen Stroke Research Center at the University of Texas Health Science Center.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
FSV: Conception and design, drafting the manuscript, analysis and interpretation of data. KNP: Acquisition of data and revising the manuscript. MHR: Analysis and interpretation of data and revising the manuscript for intellectual content. MJL: Analysis and interpretation of data and revising the manuscript for intellectual content. TTB: Acquisition of data and revising the manuscript. CN: Revising the manuscript for intellectual content. ADB: Revising the manuscript for intellectual content. ABB: Acquisition of data and revising the manuscript. PS: Conception and design, acquisition of data and revising the manuscript for intellectual content. BY: Acquisition of data and drafting the manuscript. JAA: Conception and design and revising the manuscript for intellectual content. SIS: Conception and design, drafting the manuscript, interpretation of data, and revising the manuscript for intellectual content.
References
- 1.Kamel H, Iadecola C. Brain-immune interactions and ischemic stroke: clinical implications. Arch Neurol 2012; 69: 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willing AE, Pennypacker KR. Alternate approach to understanding the molecular mechanisms of stroke-induced injury. Histol Histopathol 2007; 22: 697–701. [DOI] [PubMed] [Google Scholar]
- 3.Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience 2009; 158: 1098–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennypacker KR, Offner H. The role of the spleen in ischemic stroke. J Cereb Blood Flow Metab 2015; 35: 186–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajmo CT, Collier LA, Leonardo CC, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol 2009; 218: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasouli J, Lekhraj R, Ozbalik M, et al. Brain-spleen inflammatory coupling: a literature review. Einstein J Biol Med 2011; 27: 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Offner H, Subramanian S, Parker SM, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab 2006; 26: 654–665. [DOI] [PubMed] [Google Scholar]
- 8.Planas AM, Gomez-Choco M, Urra X, et al. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J Immunol 2012; 188: 2156–2163. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchida T, Parker KC, Turner RV, et al. Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc Natl Acad Sci U S A 1994; 91: 10859–10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vendrame M, Gemma C, Pennypacker KR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol 2006; 199: 191–200. [DOI] [PubMed] [Google Scholar]
- 11.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol 2006; 176: 6523–6531. [DOI] [PubMed] [Google Scholar]
- 12.Bao Y, Kim E, Bhosle S, et al. A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflamm 2010; 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seifert HA, Leonardo CC, Hall AA, et al. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis 2012; 27: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seifert HA, Hall AA, Chapman CB, et al. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol 2012; 7: 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrowski RP, Schulte RW, Nie Y, et al. Acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Transl Stroke Res 2012; 3: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ajmo CT, Vernon DOL, Collier L, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res 2008; 86: 2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B-J, Men X-J, Lu Z-Q, et al. Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin Med J (Engl) 2013; 126: 2354–2360. [PubMed] [Google Scholar]
- 18.Fathali N, Ostrowski RP, Hasegawa Y, et al. Splenic immune cells in experimental neonatal hypoxia-ischemia. Transl Stroke Res 2013; 4: 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seifert HA, Collier LA, Chapman CB, et al. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol 2014; 9: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 2009; 7: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarting S, Litwak S, Hao W, et al. Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke 2008; 39: 2867–2875. [DOI] [PubMed] [Google Scholar]
- 22.Keimpema E, Fokkens MR, Nagy Z, et al. Early transient presence of implanted bone marrow stem cells reduces lesion size after cerebral ischaemia in adult rats. Neuropathol Appl Neurobiol 2009; 35: 89–102. [DOI] [PubMed] [Google Scholar]
- 23.Golden JE, Shahaduzzaman M, Wabnitz A, et al. Human umbilical cord blood cells alter blood and spleen cell populations after stroke. Transl Stroke Res 2012; 3: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vendrame M, Gemma C, Pennypacker KR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Experimental neurology 2006; 199: 191–200. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZJ, Chen C, Li FW, et al. Splenic responses in ischemic stroke: New insights into stroke pathology. CNS Neurosci Ther 2015; 21: 320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahota P, Vahidy F, Nguyen C, et al. Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int J Stroke 2013; 8: 60–67. [DOI] [PubMed] [Google Scholar]
- 27.Rahbar MH, Gonzales NR, Ardjomand-Hessabi M, et al. The University of Texas Houston Stroke Registry (UTHSR): implementation of enhanced data quality assurance procedures improves data quality. BMC Neurol 2013; 13: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987; 317: 1098. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Pere A, Koenker R, et al. Quantile regression methods for reference growth charts. Stat Med 2006; 25: 1369–1382. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 31.Vogelgesang A, Becker KJ, Dressel A. Immunological consequences of ischemic stroke. Acta Neurol Scand 2014; 129: 1–12. [DOI] [PubMed] [Google Scholar]
- 32.Famakin BM. The immune response to acute focal cerebral ischemia and associated post-stroke immunodepression: a focused review. Aging Dis 2014; 5: 307–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esmaeili A, Dadkhahfar S, Fadakar K, et al. Post-stroke immunodeficiency: effects of sensitization and tolerization to brain antigens. Int Rev Immunol 2012; 31: 396–409. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg HK, Markowitz RI, Kolberg H, et al. Normal splenic size in infants and children: sonographic measurements. AJR Am J Roentgenol 1991; 157: 119–121. [DOI] [PubMed] [Google Scholar]
- 35.Megremis SD, Vlachonikolis IG, Tsilimigaki AM. Spleen length in childhood with US: normal values based on age, sex, and somatometric parameters. Radiology 2004; 231: 129–134. [DOI] [PubMed] [Google Scholar]
- 36.Loftus WK, Metreweli C. Normal splenic size in a Chinese population. J Ultrasound Med 1997; 16: 345–347. [PubMed] [Google Scholar]
- 37.Hosey RG, Mattacola CG, Kriss V, et al. Ultrasound assessment of spleen size in collegiate athletes. Br J Sports Med 2006; 40: 251–254. discussion 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boehme AK, Kapoor N, Albright KC, et al. Predictors of systemic inflammatory response syndrome in ischemic stroke undergoing systemic thrombolysis with intravenous tissue plasminogen activator. J Stroke Cerebrovasc Dis 2014; 23: e271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radholm K, Arima H, Lindley RI, et al. Older age is a strong predictor for poor outcome in intracerebral haemorrhage: the INTERACT2 study. Age Ageing 2015; 44(3): 422–427. [DOI] [PubMed] [Google Scholar]
- 40.Xian Y, Holloway RG, Smith EE, et al. Racial/Ethnic differences in process of care and outcomes among patients hospitalized with intracerebral hemorrhage. Stroke 2014; 45: 3243–3250. [DOI] [PubMed] [Google Scholar]
- 41.Roth DL, Dhamoon MS. Racial disparities in long-term stroke outcomes: evidence from nationwide epidemiologic studies. Neurology 2014; 83: 384–385. [DOI] [PubMed] [Google Scholar]
- 42.Yetter EM, Acosta KB, Olson MC, et al. Estimating splenic volume: sonographic measurements correlated with helical CT determination. AJR Am J Roentgenol 2003; 181: 1615–1620. [DOI] [PubMed] [Google Scholar]
- 43.Kluhs L, Teichgraber UK, Schneider U, et al. [Accuracy of the sonographic determination of the splenic weight in comparison with the weight at autopsy]. Rofo 2003; 175: 532–535. [DOI] [PubMed] [Google Scholar]