Abstract
Cerebrovascular reactivity (CVR) studies have elucidated the physiology and pathophysiology of cerebral blood flow regulation. A non-invasive, high spatial resolution approach uses carbon dioxide (CO2) as the vasoactive stimulus and magnetic resonance techniques to estimate the cerebral blood flow response. CVR is assessed as the ratio response change to stimulus change. Precise control of the stimulus is sought to minimize CVR variability between tests, and show functional differences. Computerized methods targeting end-tidal CO2 partial pressures are precise, but expensive. Simpler, improvised methods that fix the inspired CO2 concentrations have been recommended as less expensive, and so more widely accessible. However, these methods have drawbacks that have not been previously presented by those that advocate their use, or those that employ them in their studies. As one of the developers of a computerized method, I provide my perspective on the trade-offs between these two methods. The main concern is that declaring the precision of fixed inspired concentration of CO2 is misleading: it does not, as implied, translate to precise control of the actual vasoactive stimulus – the arterial partial pressure of CO2. The inherent test-to-test, and therefore subject-to-subject variability, precludes clinical application of findings. Moreover, improvised methods imply widespread duplication of development, assembly time and costs, yet lack uniformity and quality control. A tabular comparison between approaches is provided.
Keywords: Cerebrovascular reactivity, carbon dioxide, cerebral blood flow, carbogen, end-tidal forcing, end-tidal targeting
Introduction
The use of cerebral vascular reactivity (CVR), the change in blood flow normalized for a change in stimulus, has long been used to elucidate underlying brain vascular physiology and pathophysiology in the presence of neurovascular disease.1 Changing the arterial carbon dioxide partial pressure (PaCO2), the actual vasoactive stimulus in a controllable fashion, and magnetic resonance imaging (MRI), to provide a high spatial and time resolved measure of cerebral blood flow, is progressively more popular in non-invasive CVR studies. However, translation from physiological to clinical investigations will require precise PaCO2 changes in order to minimize test–retest variability.
Methods to implement hypercapnic (increased PaCO2) stimuli have long depended heavily on techniques adopted from the field of respiratory physiology. In recent decades, these have included the development of complex and expensive computerized systems targeting end-tidal (end-exhalation) PCO2 (PetCO2) such as dynamic end-tidal forcing,2,3 and prospective targeting.4,5 CVR investigators have recently attempted to circumvent the complexity and expense of these systems by reverting to simpler respiratory circuits that administer fixed concentrations of inspired gases. Although it is readily acknowledged that the performances of these ‘simpler’ circuits do not match those of the computer-controlled systems, a comprehensive presentation of their limitations has not been presented. It has been argued that their simplicity, low cost, MRI compatibility, and ready availability of components are sufficient to commend them as alternative means to generate stimuli for the measure of CVR.
In this opinion essay, I provide (a) a comprehensive list of the limitations of circuits using fixed inspired concentrations of gases to target arterial gas concentrations; (b) general descriptions of the computer-controlled systems of dynamic end-tidal forcing and prospective targeting, and (c) comparisons of the performance, convenience, and cost of a computerized system (prospective targeting, which is still available for research) to those providing fixed inspired concentrations of gases. I conclude that a requirement of an accurately targetable and repeatable stimulus cannot be met using fixed inspired gas concentration circuits.
Fixed inspired gas concentration circuits
Two recent examples of respiratory circuits advocated for the control inspired gases for CVR using MRI are shown in Figure 1. Both methods, adopted from early respiratory experiments,6 use non-rebreathing valves, a fixed flow of a gas with fixed CO2 and O2 concentrations, and an inspired gas reservoir – one a flexible bag7 and the other, a rigid tube8 –to provide a constant inspired fractional concentrations of CO2 (FiCO2) and O2 (FiO2).
Figure 1.
Two examples of circuits that apply a fixed inspired CO2 concentration. Circuit (a) uses a large reservoir of 5% CO2, 21% O2, balance N2, attached to a three-way valve. The position of the valve determines whether inspired gas is room air or the 5% CO2 mix. Circuit (b) applies a fixed CO2 concentration (0–5% CO2 in air or O2) at constant flow as gas input. During exhalation, the input gas fills an open-ended tube. This tube acts as a gas reservoir to make input gas available for inhalation when inspiratory flow exceeds gas input flow. This figure illustrates circuits similar to those used in Lu et al.7+ and Tancredi et al.8
With these circuits it might be assumed that a fixed FiCO2 provides a standardized, predictable PetCO2. However, that is not the case as is evident from a consideration of the alveolar gas equation
VCO2 is minute CO2 production, characteristic of a subject’s metabolism; Va is alveolar ventilation, the volume of gas per minute entering and exiting the alveoli for gas exchange with the blood.
Note that FetCO2 is PetCO2/barometric pressure; similarly FiCO2 = inspired PCO2/barometric pressure.
In a subject at rest, VCO2 is relatively constant, but Va varies from person to person in an unknown manner because the respiratory chemoreflex ventilatory response to CO2 varies from person to person.9 As a result, a constant FiCO2 does not result in a constant,10 or predictable,11 PetCO2. This can be appreciated intuitively by considering that we all breathe a constant FiCO2 (0.039 CO2) in breathing ambient air, yet the PetCO2 varies throughout the population. The same holds true for any fixed FiCO2, as Figure 2 illustrates. More to the point, a fixed FiCO2 does not produce a consistent PaCO2 (see Figure 2). The gradient between PetCO2 and PaCO2 varies between people and varies at different PetCO2.12 Therefore, FiCO2 cannot be applied to target any particular PetCO2 or PaCO2.
Figure 2.

The range of PetCO2 and PaCO2 in subjects breathing carbogen (5% CO2 in O2) from a circuit functionally identical to that shown in 1(A). Note the range of PetCO2 and PaCO2 with this constant inspired PCO2. Also note that when asked to hyperventilate (HV), the PetCO2 decreases to almost that of breathing ambient air, despite continuing to inspire 5% CO2. Data in blue from Prisman et al.13 in healthy subjects. Data in red from Baddeley et al.14 in patients administered carbogen intended as an adjuvant for effectiveness of radiotherapy for cancer.
There are additional confounding aspects to using fixed FiCO2 as a repeatable stimulus. First, step changes in PetCO2 or PaCO2 (i.e., to a plateau value) cannot be achieved because the volume of the bag-lung system buffers the rate of change in lung gas concentrations, producing instead an exponential rise. This is illustrated in Figure 4 in Tancredi et al.,8 using the circuit shown in Figure 1(B) and 5% CO2 as the inspired gas mixture. Second, despite maintenance of a constant FiCO2, a steady stimulus is not achieved, because PetCO2 varies from breath to breath due to normal changes in ventilation and breathing pattern affects PETCO2. Figure 2 in Lu et al.,7 using the circuit shown in Figure 1(A) and Figure 4 in Tancredi et al.,8 using the circuit shown in Figure 1(B) show the breath-by-breath variability of PetCO2, which is apparent even after averaging is applied. Indeed, using an open-ended tube as a reservoir (Figure 1(B)) may not even maintain a constant FiCO2 as Figure 4 in Tancredi et al.8 shows. This variability is caused by the diffusion and mixing of the gas in the reservoir tube with room air during ventilatory excursions, which themselves vary breath to breath. Finally, it should also be apparent that changes in PetCO2 in turn alter minute ventilation, and thereby also PetO2. Isoxia therefore cannot be maintained with fixed inspired gases (e.g. see Bulte et al.16).
Figure 4.
The prospective targeting system (RespirAct™). The system consists of a gas blender and a breathing circuit. Gas A is a blend of O2 and N2. Gas B is a blend of O2, and CO2 with balance N2. Gas C is O2. All gases contain O2 as a safety measure. A computer pre-calculates the breath-by-breath inspired gas concentrations and flow to attain end-tidal gas concentration targets and controls the gas blender delivery to a breathing circuit. The breathing circuit provides sequential gas delivery as follows. The subject exhales to the expiratory reservoir. During exhalation, the inspiratory reservoir fills with blended gas. On inspiration, the inspiratory reservoir is emptied and any additional gas is inhaled from the expiratory reservoir. Gas inhaled from the expiratory reservoir has already equilibrated with the blood and does not affect gas exchange, so that the gas inspired from the blender constitutes entirely of Va (see text). Thus, considering the alveolar gas equation (FetCO2 = FiCO2 + VCO2/Va), VCO2 is a user input function, and Va is imposed by the gas blender; control of these variables enables the targeting of FetCO2 and FetO2 independent of minute ventilation and breathing pattern. For further details see literatures.4,5,15
Implications of unknown, variable and inconsistent stimuli:
Effect of accuracy of vasoactive stimulus on CVR. In considering the accuracy and reproducibility of CVR, we need to consider both the accuracy of the stimulus and that of the blood flow measurement. The inaccuracy of each component is additive in the calculation of CVR. Making one component more precise markedly increases the precision of CVR even if the other is unchanged.
Cost of research. The greater the variability of the stimulus, the greater the number of subjects required in a clinical investigation.
Clinical implications. Few conclusions can be drawn from a single test in a single subject if there is high variability in the test. This precludes any clinical application.
Computer-based gas control systems
Both computer-based gas control systems, dynamic end-tidal forcing and prospective targeting, are capable of providing precise and repeatable sequences of PetCO2 and PetO2, with low breath-to-breath variability. The prospective targeting method employs sequential rebreathing, which has been shown to equalize PetCO2 and PaCO2.17–19 These systems can produce a variety of stimulus patterns, including rapid changes in end-tidal gases, as well as targeting PetCO2 and PetO2 independently. The latter ability allows maintenance of isocapnia with changes of PetO2 and isoxia with changes of PetCO2. Figures 3 and 4 show the dynamic end-tidal forcing, and prospective targeting systems, respectively. These systems are generally capable of providing repeatable, complex, stimulus patterns as Figures 5 and 6 demonstrate for the prospective targeting system. However, some operator expertise and experience with the particular computerized system, and some minimal patient cooperation (i.e., continuing to breathe at their resting rate or greater) are required to obtain consistent, optimal control of gas targeting.
Figure 3.
The dynamic end-tidal forcing system adapted from Wise et al.3 O2, CO2, and N2 are blended breath-by-breath to provide the respective inspired gas concentrations. Inspired gas concentrations are calculated from the respective exhaled gas concentrations of the preceding breath and the target gas concentrations. High inspiratory flows are required to meet peak inspiratory flows. Gases are dry and require efficient humidification.
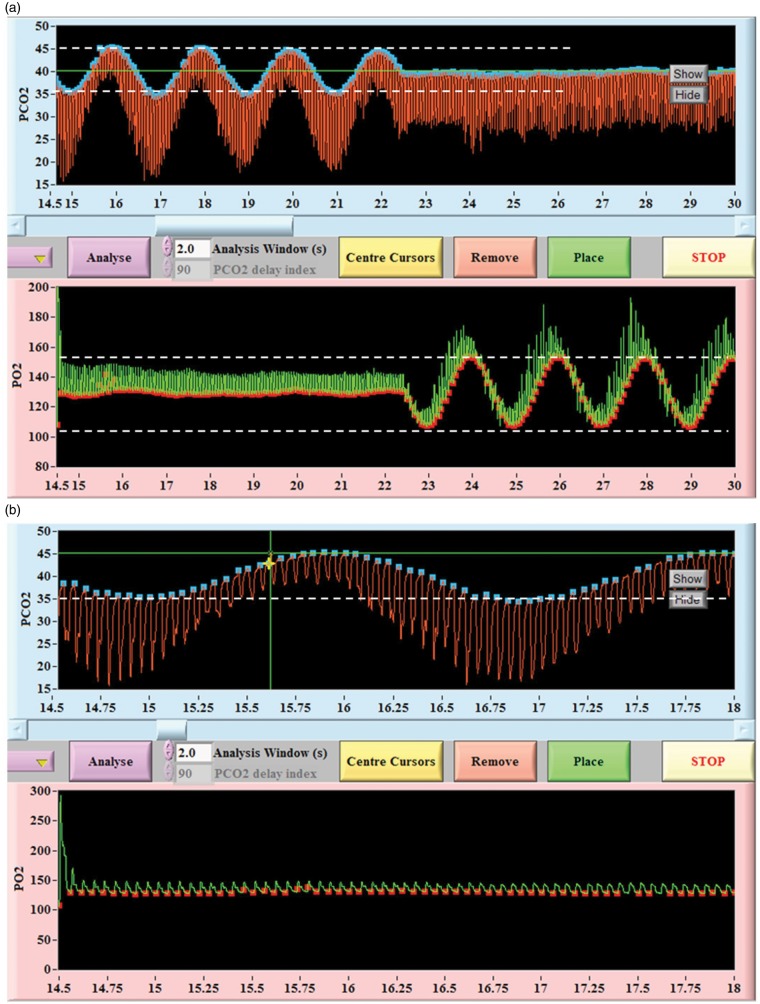
Figure 5.
Screen capture of sinusoidal changes in PetCO2 and PetO2 produced using the RespirAct™. (a) compressed time course; (b) expanded time course. Note the precise breath-to-breath changes and lack of breath-to-breath variability during the steady segments. Note also that changes in PetCO2 and PetO2 are independent of each other. Red tracing is tidal PCO2; blue dots are end-tidal values. Green tracing is tidal PO2; red dots are end-tidal values.
Figure 6.
Screen capture of end-tidal targeting using RespirAct™. Simultaneous tracing from a step algorithm targeting sharp step changes in PCO2 and PO2. Typically (but not always) PCO2 transitions occur within 1–3 breaths. Red tracing is tidal PCO2; blue dots are end-tidal values. Green tracing is tidal PO2; red dots are end-tidal values.
Concluding comments
There are four factors which affect PetCO2: (1) the metabolic CO2 production rate (VCO2); (2) the FiCO2; (3) the time constant of gas exchange in the lungs; and (4) the alveolar ventilation (Va). While (1) and (2) are, or can be made, constant, (3) prevents the possibility of an abrupt change PetCO2 or PetO2 resulting from an abrupt change in inspired gas concentrations. As the extent or pattern of ventilation (and therefore Va) cannot be controlled in spontaneously breathing subjects, so PetCO2, PaCO2, cannot be targeted with any chosen FiCO2 and FiO2. Finally, the gradient between PetCO2 (which is measured) and PaCO2 (which determines the hemodynamic response) is not known. Thus, standardized and repeatable stimuli unfortunately require more complex and expensive computer-controlled systems. A detailed comparison of issues such as complexity/simplicity, effectiveness and cost between the fixed inspired concentration methods and computer-controlled prospective targeting is provided in Table 1.
Table 1.
Comparing fixed inspired gas control with computer controlled targeting.
| Attribute | Fixed inspired CO2 methods | Computerized: e.g. prospective targeting |
|---|---|---|
| Attain a target PaCO2? | NO • Only control inspired PCO2. PaCO2 depends also subject ventilatory response and highly variable end-tidal to arterial difference (both unpredictable) | YES • Can target within 2 mmHg, often ± 1 mmHg (17) • Can target a series of PetCO2 |
| Designate a target PetO2 | NO • Cannot even maintain isoxia | YES. |
| Breath-to-breath PetCO2 variability in the steady state | ∼4 mmHg | <1 mmHg |
| Time to achieve a steady state | Many minutes10,20 | 1–3 breaths (see Figure 6) |
| Maintain steady increase in PCO2 (ramp protocol) | NO • PCO2 rise is a dual exponential form with the first exponential time constant ∼40 s,20 and the second ∼hours | YES • rate of rise can be set |
| Maintain steady change in PO2 | NO • PO2 is ventilatory rate dependent. If inspired PO2 changes abruptly, 95% of new steady state asymptote takes >2 min. Steady state is never reached | YES • A target PO2 is reached within 2–3 breaths and remains steady ±1% of range |
| Automated gas targeting and analysis | NO • Manual switching by attendant in MRI scanner with patient7 • Requires custom computer programming for analysis or manual analysis | YES Targets and durations are a sequence set in the control room computer. No attendant is required in the scanner |
| Breathing circuit | • Requires custom assembly and cleaning | • Comes tested and packaged |
| Quality assurance of device, components, of test, of analysis | NO | YES • ISO 13485 process |
| Cost | Circuit of Figure 1(A): 14 components: • plastics <$150 depending on quality • Three-way respiratory stopcocks and Douglas bags • One custom gas/regulator • MRI shielded pulse oximeter and CO2 analyzer; getting electronic data from CO2 analyzer (or CO2 samples) to control room. Computer and data analysis software Estimated cost: • Capital $9,000 • +per case $100 (including person in scanner, not counting time for gas data analysis) Circuit of Figure 1(B): • three gases: 5% CO2, air, O2 • three automated flow controllers for the three gases • a circuit with mask taped to face • a mixing chamber • a computer-controlled system (i.e. a computer controlled gas blender) • 10 m of tubing • Flow rates of up to 50 L/min and baseline rate of 20 L/min • custom MatLab code for end-tidal picking Estimated cost: Capital and up front cost: $25,000 Cost per case: $100 (including in room attendant, not counting time for gas data analysis) | Prospective targeting • gas blender + computer, • two custom blend gases; pressure regulators, tubes, hosing • Training • Custom mask and breathing circuit Cost • Capital: $45,000 • Per case: $100 (includes circuit, gas data analysis, support and servicing) |
Declaration of conflicting interests
The author declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JAF is Chief Scientist at Thornhill Research Inc. (TRI), a for profit spin-off company from the University Health Network that developed the RespirAct™. RespirAct™ has been a non-commercial research tool assembled, and made available by TRI to research institutions to enable CVR studies.
Funding
The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Reinhard M, Schwarzer G, Briel M, et al. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 2014; 83: 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins PA, Swanson GD, Micco AJ, et al. A fast gas-mixing system for breath-to-breath respiratory control studies. J Appl Physiol 1982; 52: 1358–1362. [DOI] [PubMed] [Google Scholar]
- 3.Wise RG, Pattinson KT, Bulte DP, et al. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J Cereb Blood Flow Metab 2007; 27: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 4.Slessarev M, Han J, Mardimae A, et al. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J Physiol 2007; 581: 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fierstra J, Winter J, Machina M, et al. Non-invasive accurate measurement of arterial PCO2 in a pediatric animal model. J Clin Monit Comp 2013; 27: 147–155. [DOI] [PubMed] [Google Scholar]
- 6.Douglas CG, Haldane JS. The regulation of normal breathing. J Physiol 1909; 38: 420–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H, Liu P, Yezhuvath U, et al. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J Vis Exp 2014; 94. DOI: 10.3791/52306. [DOI] [PMC free article] [PubMed]
- 8.Tancredi FB, Lajoie I, Hoge RD. A simple breathing circuit allowing precise control of inspiratory gases for experimental respiratory manipulations. BMC Res Notes 2014; 7: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffin J. Measuring the respiratory chemoreflexes in humans. Resp Physiol Neurbiol 2011; 177: 71–79. [DOI] [PubMed] [Google Scholar]
- 10.Mark CI, Slessarev M, Ito S, et al. Precise control of end-tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular reactivity measures. Magn Reson Med 2010; 64: 749–756. [DOI] [PubMed] [Google Scholar]
- 11.Farhi LE, Rahn H. Dynamics of changes in carbon dioxide stores. Anesthesiology 1960; 21: 604–614. [DOI] [PubMed] [Google Scholar]
- 12.Jones NL, Robertson DG, Kane JW, et al. Effect of PCO2 level on alveolar-arterial PC02 difference during rebreathing. J Appl Physiol 1972; 32: 782–787. [DOI] [PubMed] [Google Scholar]
- 13.Prisman E, Slessarev M, Azami T, et al. Modified oxygen mask to induce target levels of hyperoxia and hypercarbia during radiotherapy: a more effective alternative to carbogen. Int J Radiat Biol 2007; 83: 457–462. [DOI] [PubMed] [Google Scholar]
- 14.Baddeley H, Brodrick PM, Taylor NJ, et al. Gas exchange parameters in radiotherapy patients during breathing of 2%, 3.5% and 5% carbogen gas mixtures. Br J Radiol 2000; 73: 1100–1104. [DOI] [PubMed] [Google Scholar]
- 15.Fisher JA, Iscoe S and Duffin J. Sequential gas delivery provides precise control of alveolar gas exchange. Respir Physiol Neurobiol. Epub ahead of print 1 February 2016. DOI: 10.1016/j.resp.2016.01.004. [DOI] [PubMed]
- 16.Bulte DP, Chiarelli PA, Wise RG, et al. Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab 2007; 27: 69–75. [DOI] [PubMed] [Google Scholar]
- 17.Ito S, Mardimae A, Han J, et al. Non-invasive prospective targeting of arterial PCO2 in subjects at rest. J Physiol 2008; 586: 3675–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brogan TV, Robertson HT, Lamm WJ, et al. Carbon dioxide added late in inspiration reduces ventilation-perfusion heterogeneity without causing respiratory acidosis. J Appl Physiol 2004; 96: 1894–1898. [DOI] [PubMed] [Google Scholar]
- 19.Fierstra J, Machina M, Battisti-Charbonney A, et al. End-inspiratory rebreathing reduces the end-tidal to arterial PCO(2) gradient in mechanically ventilated pigs. Intensive Care Med 2011; 37: 1543–1550. [DOI] [PubMed] [Google Scholar]
- 20.Berry CB, Myles PS. Preoxygenation in healthy volunteers: a graph of oxygen “washin” using end-tidal oxygraphy. Br J Anaesth 1994; 72: 116–118. [DOI] [PubMed] [Google Scholar]