Abstract
Activin, a member of the transforming growth factor-β family, exerts multiple functions in the nervous system. Originally identified as a neurotrophic and -protective agent, increasing evidence implicates activin also in the regulation of glutamatergic and GABAergic neurotransmission in brain regions associated with cognitive and affective functions. To explore how activin impacts on ethanol potentiation of GABA synapses and related behavioral paradigms, we used an established transgenic model of disrupted activin receptor signaling, in which mice express a dominant-negative activin receptor IB mutant (dnActRIB) under the control of the CaMKIIα promoter. Comparison of GABAA receptor currents in hippocampal neurons from dnActRIB mice and wild-type mice showed that all concentrations of ethanol tested (30–150 mM) produced much stronger potentiation of phasic inhibition in the mutant preparation. In dentate granule cells of dnActRIB mice, tonic GABA inhibition was more pronounced than in wild-type neurons, but remained insensitive to low ethanol (30 mM) in both preparations. The heightened ethanol sensitivity of phasic inhibition in mutant hippocampi resulted from both pre- and postsynaptic mechanisms, the latter probably involving PKCɛ. At the behavioral level, ethanol produced significantly stronger sedation in dnActRIB mice than in wild-type mice, but did not affect consumption of ethanol or escalation after withdrawal. We link the abnormal narcotic response of dnActRIB mice to ethanol to the excessive potentiation of inhibitory neurotransmission. Our study suggests that activin counteracts oversedation from ethanol by curtailing its augmenting effect at GABA synapses.
INTRODUCTION
Activins are members of the TGF-β family and serve as multifunctional regulatory proteins in many tissues and organs (Werner and Alzheimer, 2006). In the brain, activin A acts as a neurotrophic factor during development (Andreasson and Worley, 1995). Upon acute injury of the adult brain, expression of activin A is strongly upregulated, which was shown to afford potent neuroprotection in various lesion models (Tretter et al, 2000; Mukerji et al, 2007). Activin A has also been implicated in the daily operations of adult brain circuits under normal conditions. On the basis of a combination of findings from electrophysiological and behavioral studies in normal and transgenic mice, activin A emerged as a multi-faceted modulator that tunes glutamatergic and GABAergic neurotransmission in ways that serve to improve cognitive functions as well as to balance affective responses (Krieglstein et al, 2011).
Structurally, activins are homo- or heterodimers of βA and/or βB subunits, with activin A (βA/βA) being the most abundant and best-characterized variant in the brain (Chen et al, 2006). In their canonical pathway, activins signal through heteromeric complexes of type II (ActRIIA, ActRIIB) and type I receptors (ActRIB, ActRIC). The latter phosphorylate the intracellular signaling proteins SMAD2/3, which then co-assemble with SMAD4 and translocate to the nucleus, where they bind to specific target genes to modulate their expression (Ten Dijke and Hill, 2004; Link et al, 2015). In addition, other signaling pathways are also activated by activin receptors, including mitogen-activated kinase signaling (Moustakas and Heldin, 2005).
To interrogate the role of activin in adult brain, we had previously generated transgenic mice, which express a dominant-negative activin receptor IB mutant (dnActRIB) under the control of the CaMKIIα-promoter (Muller et al, 2006). Using this strategy, we found that disruption of activin receptor signaling gives rise to a low-anxiety phenotype, alters GABAergic neurotransmission and reduces allosteric modulation of GABAA receptors (GABAARs) by diazepam (Zheng et al, 2009). As the effects of ethanol at GABAergic synapses and their behavioral correlates strongly resemble those of diazepam (Luddens and Korpi, 1995), we wondered whether activin would also have an impact on the responsiveness of GABAARs to ethanol. We report here the unexpected finding that disruption of activin receptor signaling renders fast GABA transmission more sensitive to ethanol. At the behavioral level, we link the enhanced potentiation of GABAergic synapses to the prolonged sedative effect of ethanol in dnActRIB mice.
MATERIALS AND METHODS
For additional details, please see Supplementary Materials and Methods.
Animals and Slice Electrophysiology
Transverse hippocampal slices were prepared from 2–6 months old wild-type (wt) mice and transgenic mice expressing dnActRIB under the control of the CaMKII-α promoter as described before (Muller et al, 2006). Whole-cell recordings were performed from visually identified neurons in a submerged recording chamber perfused with artificial cerebrospinal fluid at room temperature or at 35 °C (see Zheng et al, 2009). Patch pipettes were filled with (in mM) CsCl 130, MgCl2 3, EGTA 5, Hepes 5, Na2-ATP 2, Na3-GTP 0.3 and QX-314 5 (pH 7.3). In some experiments, PKCɛ translocation inhibiting protein (PKCɛ TIP, 200 μM) or its negative control (200 μM, obtained from Calbiochem, Merck Millipore, Germany) was included in the recording pipette. Inhibitory postsynaptic currents (IPSCs) were recorded with kynurenic acid (KA, 2 mM) in the bath. Tonic GABA inhibition was determined as the difference in steady-state current and current variance before and during application of the GABAAR antagonists bicuculline (50 μM) or picrotoxin (100 μM). Ethanol stock solution (5 M) was prepared freshly before experiments and kept on ice. Ethanol perfusion lasted 8–12 min before washout. Only a single concentration of ethanol (30, 80, or 150 mM) was tested per slice.
Loss of Righting Reflex (LORR)
All behavioral procedures were carried out according to the guidelines and with the approval of the local government. Ethanol naive animals were used for this test. Animals were administered with an ethanol injection of 3.5 g/kg (i.p.) to induce LORR, and immediately placed in an empty cage. LORR was observed when the animal became ataxic and stopped moving for at least 30 s. The animal was then placed on its back. Recovery from ethanol administration was defined as the animal being able to right itself three times within a minute.
Ethanol Drinking and Ethanol Deprivation Effect
Ethanol drinking was tested in naive dnActRIB and wt mice using a two-bottle free-choice drinking paradigm. Animals received ethanol at increasing concentrations of 2, 4, 8, 12 and 16 vol.% for 4 days each and then maintained at 16 vol.% for 2 weeks. In order to measure the ethanol deprivation effect (Spanagel and Hölter, 2000), baseline consumption of 16 vol.% ethanol was measured. Ethanol was removed for 3 weeks and reintroduced for 4 days. Ethanol-experienced animals were used to examine taste preference. Sucrose (0.5 and 5%) and quinine (2 and 20 mg/dl) preference was measured in a two-bottle free-choice test vs water. Each dose was offered for 3 days.
Statistical Analysis
Data are expressed as means±SEM. Statistical comparisons of data were performed using ANOVA or Student's t-test. Significance was assumed for P<0.05.
RESULTS
Activin Attenuates Ethanol Potentiation of Evoked IPSCs in CA1 Pyramidal Cells
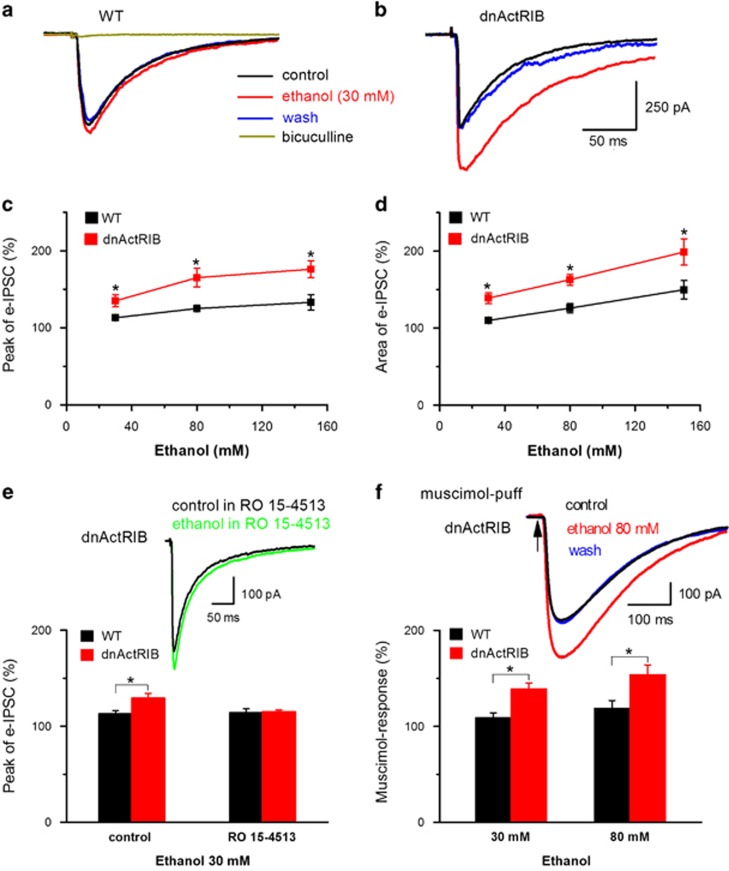
We first recorded pharmacologically isolated bicuculline-sensitive IPSCs from CA1 pyramidal cells, which were evoked by electrical stimulation in stratum radiatum (Figure 1a). Increasing concentrations of ethanol (30–150 mM) produced a dose-dependent enhancement of IPSCs in wt hippocampi (Figure 1a and c–d). When the same concentrations of ethanol were examined in hippocampi from dnActRIB mice, we obtained a significant leftward shift of the dose–response relationships for peak amplitude and area of IPSCs (Figure 1b–d). Ro 15-4513, which is an antagonist of low-dose ethanol effects at GABAARs (Wallner et al, 2006; Linden et al, 2011), abrogated the anomalous potentiation of IPSCs by 30 mM ethanol in mutant neurons (Figure 1e). In control experiments, Ro 15-4513 (0.3 μM) alone had no significant effect on the peak amplitude of eIPSCs in either group (wt 112±14% of control peak amplitude, n=8; dnActRIB 90±6% of control peak amplitude, n=5, P=0.19).
Figure 1.
Ethanol potentiation of IPSCs is stronger in dnActRIB CA1 pyramidal cells than in their wt counterparts. Representative current traces illustrate the differential enhancement by low ethanol (30 mM) of IPSCs in neurons from wt (a) and transgenic mice (b). Evoked IPSCs were sensitive to bicuculline indicating that they were mediated by GABAARs (a). Dose–response curves summarize the significantly stronger effects of ethanol on IPSC peak amplitude (c) and area (d) in mutant neurons compared with wt neurons at all concentrations (n=7–14 for each data point). (e) Ro 15-4513 (300 nM) abrogated the pronounced enhancement of IPSCs by low ethanol in mutant neurons (30 mM, n=6–7). (f) Just like IPSCs, current responses to local brief application of the GABAAR agonist, muscimol (20 μM), were more sensitive to ethanol in dnActRIB neurons than in wt neurons. Inset above histogram depicts current responses evoked by brief muscimol pulses delivered to the dendritic region of the recorded neuron through a second pipette, which was attached to a pressure application system (n=5–7). *P<0.05. dnActRIB, dominant-negative activin receptor IB mutant; IPSC, inhibitory postsynaptic current; wt, wild type.
To examine the enhanced sensitivity of GABAARs to ethanol in dnActRIB neurons in the absence of possibly confounding presynaptic effects, we superfused slices with the Na+ channel blocker TTX (1 μM) and applied brief pulses of the GABAAR agonist muscimol (20 μM) from a second pipette, which was positioned close to the recorded neuron (see Supplementary Materials and Methods). As illustrated in Figure 1f, current responses to brief puffs of muscimol received much stronger ethanol potentiation in dnActRIB neurons than in wt neurons. This finding points to a prominent postsynaptic mechanism through which activin constrains the augmenting effects of ethanol at GABAARs.
Activin Targets PKCɛ to Regulate Effect of Ethanol on IPSCs
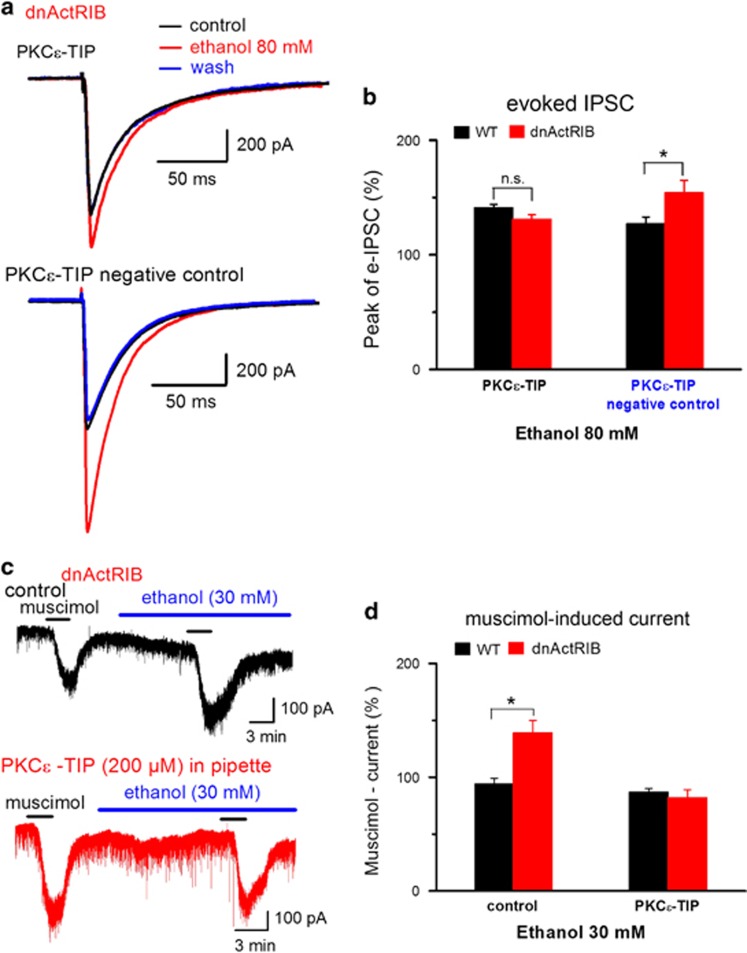
Previous work had implicated protein kinase C epsilon (PKCɛ) in the regulation of the sensitivity of GABAARs to ethanol (Hodge et al, 1999; Proctor et al, 2003; Qi et al, 2007). It is of note that activin might also recruit PKC-dependent pathways, in addition to its canonical signaling pathway through SMAD2/3 proteins (Moustakas and Heldin, 2005). In particular, activin has been shown to sensitize TRPV1 channels of dorsal root ganglion neurons through PKCɛ signaling (Zhu et al, 2007). We therefore wondered whether activin would also act through PKCɛ to control the response of GABAARs to ethanol. To specifically suppress the effects of PKCɛ, we used the PKCɛ-translocation inhibiting protein (PKCɛ-TIP) (Jiang and Ye, 2003; Dai et al, 2004; Lawrence et al, 2005). In wt pyramidal cells, addition of 200 μM PKCɛ TIP or its negative control to the pipette solution did not significantly alter the peak amplitude of evoked IPSCs, when compared with normal pipette solution within 20–40 min of whole-cell recording at a stimulus intensity of around 60 μA (696±180 pA with PKCɛ TIP, n=9, 819±120 pA with PKCɛ TIP-negative control, n=7, and 580±74 pA for wt-control, n=13, P>0.05).
In wt neurons, PKCɛ-TIP enhanced the ethanol effect on IPSCs from a relative potentiation of 124±4% (n=10, Figure 1c) to 141±3% (n=8, P=0.01, Figure 2b, black column on the left), consistent with previous work (Proctor et al, 2003), whereas scrambled PKCɛ-TIP did not affect ethanol potentiation of IPSCs (127±6%, n=6, Figure 2b, black column on the right). In dnActRIB neurons, PKCɛ-TIP abrogated the excessive ethanol potentiation of IPSCs. Figure 2a (upper panel) depicts the strong attenuation that PKCɛ-TIP exerted on the effect of ethanol (80 mM) on IPSCs in a mutant CA1 neuron. Compared with control recordings with scrambled PKCɛ-TIP as negative control (Figure 2a, lower panel), the translocation inhibitor reduced relative ethanol potentiation of IPSC peak amplitude from 154±11% of control (n=4, Figure 2b red column on the right, not different from ethanol potentiation of IPSCs in mutant neurons without scrambled protein, 151±7%, n=14, Figure 1c, P=0.80) to 131±4% (n=6, P=0.03, Figure 2b, red column on the left). Thus, inhibition of PKCɛ translocation equalized the difference in ethanol potentiation of IPSCs between normal and dnActRIB neurons (Figure 2b, black vs red column on the left).
Figure 2.
Enhanced sensitivity of GABAARs of mutant pyramidal cells to ethanol is abrogated by PKCɛ translocation inhibiting peptide (PKCɛ-TIP). (a) Superimposition of evoked IPSCs, recorded from a mutant neuron with PKCɛ-TIP (200 μM) in the pipette solution before, during and after ethanol (80 mM) superfusion (upper traces). Lower traces show lacking effect of scrambled PKCɛ-TIP on enhanced ethanol responsiveness in another mutant neuron. (b) Histogram summarizes effect of ethanol on IPSC amplitude in wt and mutant neurons recorded with pipette solutions containing either PKCɛ-TIP or its inactive variant (PKCɛ-TIP: wt n=8, dnActRIB n=6; scrambled PKCɛ-TIP: wt n=6, dnActRIB n=4). (c) Representative current traces from two whole-cell recordings showing that the strong augmenting effect of low ethanol (30 mM) on muscimol (0.5 μM)-induced inward current in dnActRIB neuron (upper trace) was abrogated with PKCɛ-TIP in the pipette solution (lower trace). (d) Histogram summarizes effect of intracellular PKCɛ-TIP on ethanol-mediated increase of muscimol response (n=6–8). *P<0.05. dnActRIB, dominant-negative activin receptor IB mutant; IPSC, inhibitory postsynaptic current; PKCɛ-TIP, PKCɛ-translocation inhibiting protein.
The notion that inhibition of PKCɛ restored normal sensitivity of GABAARs to ethanol in dnActRIB neurons was substantiated in a second experiment, in which we measured Cl- inward currents evoked by bath-applied muscimol (0.2–0.5 μM) in the absence and presence of low ethanol. Muscimol alone induced inward Cl− currents of comparable amplitude in wt neurons (n=8, 96±19 pA) and in dnActRIB neurons (n=7, 99±20 pA). In the absence of ethanol, a second application of muscimol produced virtually identical current responses (wt, n=5, 98±2% of 1st current amplitude), making this a suitable paradigm to determine the modulatory effect of ethanol. Low ethanol (30 mM) did not appreciably alter the current response to muscimol in wt neurons, whereas a significant increase was observed in dnActRIB neurons (Figure 2c and d), consistent with our previous findings using eIPSCs or muscimol puffs (Figure 1c). When we repeated the experiment with PKCɛ-TIP (200 μM) in the recording pipette, the overly augmenting effect of ethanol on the muscimol response in dnActRIB neurons was completely abrogated (Figure 2c and d).
Activin Regulates Presynaptic Effects of Ethanol at CA1 GABAergic Synapses
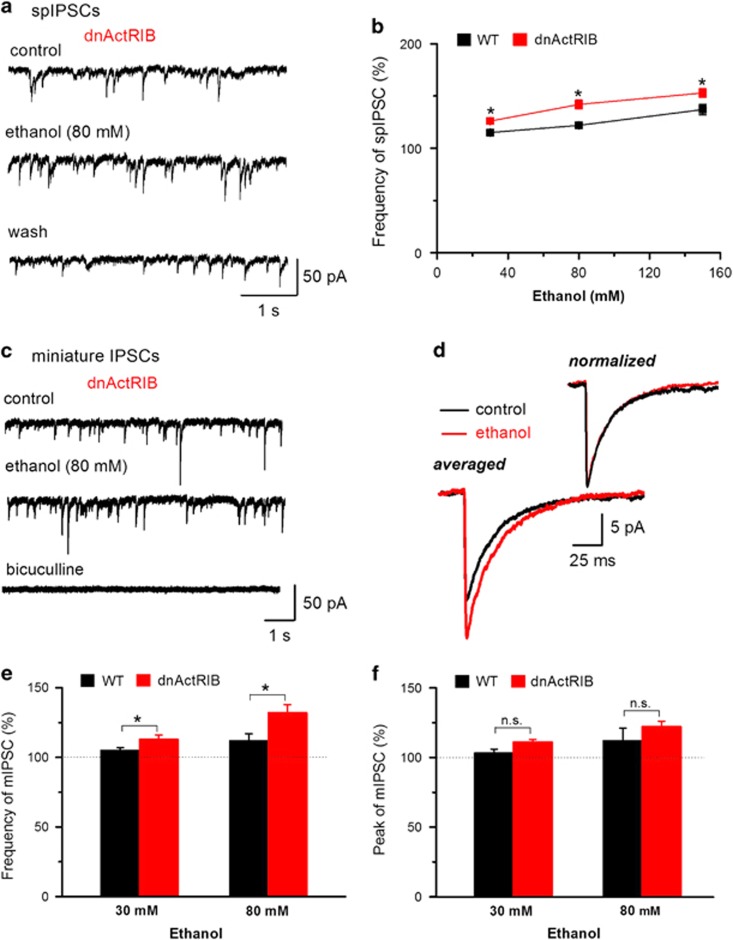
Increasing concentrations of ethanol (30–150 mM) produced a moderate, dose-dependent enhancement of the frequency of spIPSCs in CA1 pyramidal cells. Compared with wt neurons, dnActRIB neurons exhibited a higher frequency of spIPSCs (wt 2.71±0.36 Hz, n=16; dnActRIB 3.80±0.39 Hz, n=23; P=0.048) under control conditions (see Zheng et al, 2009) and exhibited a significantly stronger increase in spIPSC frequency in response to each concentration of ethanol (Figure 3a and b). We then added TTX (1 μM) to the bathing solution to isolate action potential-independent, miniature IPSCs (mIPSCs; Figure 3c and d). In wt neurons, 30 mM ethanol produced no marked change in the frequency and peak amplitude of mIPSCs. 80 mM ethanol enhanced frequency, but not peak amplitude of mIPSCs in wt neurons. In contrast, 30 and 80 mM ethanol significantly enhanced frequency and peak amplitude of mIPSCs in dnActRIB neurons (Figure 3c, e and f): 30 mM ethanol (n=10) increased the frequency of mIPSCs from 2.9±0.5 Hz to 3.2±0.5 Hz (P=0.002) and the mean peak amplitude of mIPSCs from 23.0±2.0 pA to 25.5±2.5 pA (P=0.008), and 80 mM ethanol (n=6) enhanced the frequency of mIPSCs from 2.7±0.4 Hz to 3.4±0.5 Hz (P=0.001) and the mean peak amplitude of mIPSCs from 20.3±1.1 pA to 24.6±1.1 pA (P=0.003). Overlay of mIPSC traces, which were normalized to peak amplitude, showed that the ethanol-induced increase in mIPSC amplitude in dnActRIB neurons was not accompanied by a change in kinetic properties (Figure 3d). This notion was further substantiated when we quantified the half-width of mIPSCs during application of 80 mM ethanol, which was not altered in either group (wt 16.4±0.6 ms, ethanol 15.9±1.0 ms, n=7, P=0.64; dnActRIB 16.5±1.5 ms, ethanol 17.1±1.1 ms, n=6, P=0.22).
Figure 3.
Presynaptic effects of ethanol are enhanced in dnActRIB hippocampi. (a, b) More pronounced effect of ethanol on spontaneous IPSC (spIPSC) frequency in dnActRIB neurons than in wt neurons. Raw traces of (a) depict spIPSCs recorded from a dnActRIB neuron before, during and after ethanol superfusion. Graph in (b) plots spIPSC frequency as function of ethanol concentration, demonstrating the stronger effect of ethanol in dnActRIB neurons (n=5–14 for each data point). (c) Representative current traces from a dnActRIB neuron, displaying mIPSCs before and during ethanol application (top and middle traces), and their suppression in the presence of bicuculline (50 μM, bottom trace). (d) Superimposition of averaged mIPSCs obtained in the absence and presence of ethanol (80 mM) before and after normalization to peak amplitude. Histograms summarize effects of 30 and 80 mM ethanol on mIPSC frequency (e) and peak amplitude (f) (n=6–10); (e) ANOVA F[1, 26]=12.3, P=0.002; Tukey post-hoc test: P=0.004 wt vs dnActRIB 30 mM ethanol, P=0.007 wt vs dnActRIB 80 mM ethanol, (f) ANOVA F[1, 25]=3.5, P=0.07. *P<0.05. ANOVA, analysis of variance; dnActRIB, dominant-negative activin receptor IB mutant; IPSC, inhibitory postsynaptic current; mIPSC, miniature IPSCs; wt, wild type.
Activin Controls Phasic, But Not Tonic GABA Inhibition in Granule Cells of Dentate Gyrus
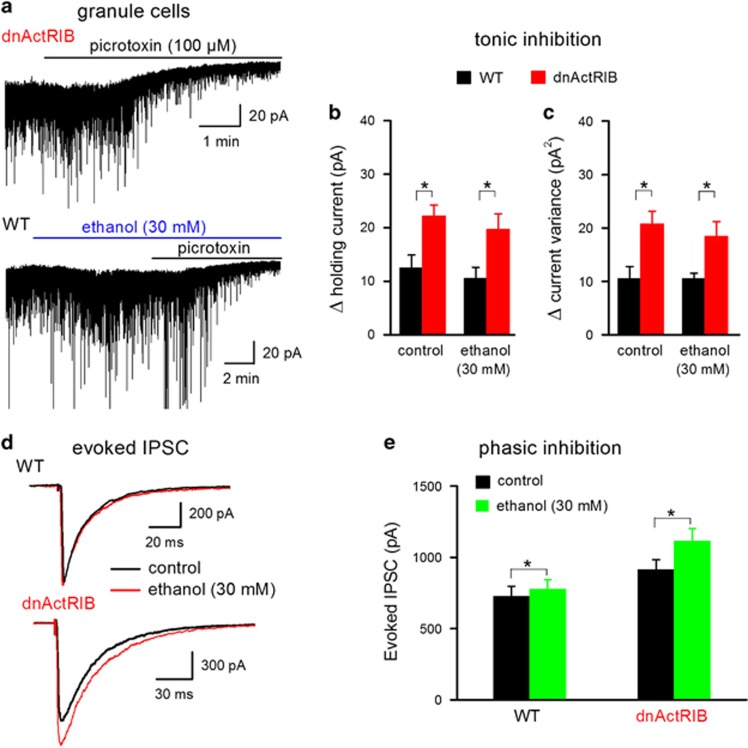
It remains disputed whether the behavioral effects of low ethanol are attributable to the enhancement of extrasynaptic δ subunit-containing GABAARs currents of DG granule cells (Wallner et al, 2003; Wei et al, 2004; Borghese et al, 2006). In contrast to synaptic GABAARs, which mediate phasic inhibition, ie IPSCs, activation of extrasynaptic GABAARs by ambient GABA produces tonic inhibition in DG granule cells (Farrant and Nusser, 2005). Disruption of activin receptor signaling led to a significant increase in tonic GABA inhibition of DG granule cells. Under our recording conditions, the steady-state inward current, which is sensitive to the GABAAR antagonists bicuculline (50 μM) or picrotoxin (100 μM), is the equivalent of tonic GABA inhibition. This current shift was significantly larger in dnActRIB granule cells than in wt granule cells (Figure 4a and b; wt 12.5±2.4 pA, n=11; dnActRIB 22.2±2.0 pA, n=15; P=0.01). Complementary analysis of tonic GABA current using current variance revealed a similar stronger GABAergic inhibition in dnActRIB cells (Figure 4c; wt 10.5±2.4 pA2, n=11; dnActRIB 20.7±2.4 pA2, n=15; P=0.01).
Figure 4.
Differential effect of ethanol on phasic vs tonic inhibition in dentate granule cells. (a) Representative traces illustrate tonic GABA current of a dnActRIB granule cell in the absence of ethanol (upper trace) and of a wt granule cell in the presence of ethanol (30 mM; bottom trace), respectively. (b, c) Histograms summarize enhanced GABAergic tone (expressed as holding current shift and current variance change) in dnActRIB granule cells (wt n=11, dnActRIB n=15) and the lack of effect of low ethanol on tonic inhibition (wt ethanol n=12, dnActRIB ethanol n=9). (d) Superimposed IPSC traces recorded at 35 °C before and during low ethanol (30 mM) from a wt and a dnActRIB granule cell. (e) Histogram summarizes the significantly stronger effects of ethanol on IPSC amplitude in dnActRIB granule cells (wt n=4, dnActRIB n=8). *P<0.05. dnActRIB, dominant-negative activin receptor IB mutant; IPSC, inhibitory postsynaptic currents; wt, wild type.
Low-dose ethanol (30 mM) produced similar inward shifts of holding current in wt (Figure 4a, bottom trace) and mutant neurons (wt 6.4±1.0 pA, n=12; dnActRIB 7.2±1.0 pA, n=9; P=0.55). However, these ethanol-induced current shifts were most likely engendered by membrane conductances other than extrasynaptic GABAARs, since bicuculline- or picrotoxin-induced changes in holding current and current variance were not affected by ethanol in either preparation (Figure 4b and c). Presumably owing to more efficient GABA re-uptake, tonic inhibition was reduced in both preparations when we raised recording temperature to 35 oC, but the significant bias toward dnActRIB granule cells was preserved (wt 5.2±1.7 pA, n=5; dnActRIB 11.9±1.7 pA, n=9; P=0.02). At this quasi-physiological temperature, low ethanol (30 mM) failed again to enhance tonic GABA currents in either group (wt 6.6±0.7 pA, n=6; dnActRIB 9.3±2.9 pA, n=3). This finding demonstrates that, although activin receptor signaling dampens tonic GABA current, it does not appear to regulate the effect of ethanol on extrasynaptic GABAARs. At the same quasi-physiological temperature, phasic inhibition in mutant granule cells was found to be overly potentiated by low ethanol (30 mM), as it was in mutant CA1 neurons (dnActRIB granule cells n=8, 122.3±3.1% of control peak amplitude; wt granule cells n=4, 107.2±1.9% of control peak amplitude; P=0.01, Figure 4d and e). These data strengthen our hypothesis that phasic, but not tonic inhibition serves as a prime target of activin to control ethanol effects in the brain.
Activin Diminishes the Sedating, but not Rewarding Effects of Ethanol
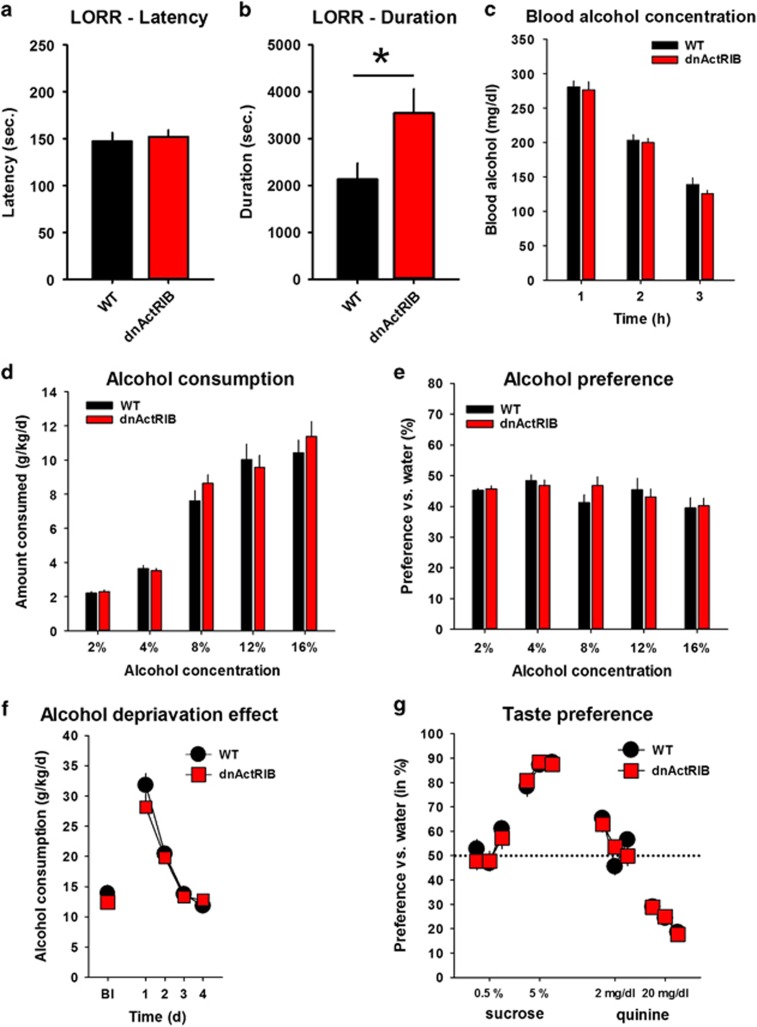
The LORR test was used to estimate the role of activin in the sedating effects of ethanol. We found no difference in the time to LORR between dnActRIB and wt mice (wt n=12, 147±9 s; dnActRIB n=9, 152±7 s; P=0.71, Figure 5a). However, the duration of sedation was significantly enhanced in the dnActRIB mice (wt n=12, 2132±344 s; dnActRIB n=9, 3542±516 s; t=−2.36, d.f.=19, P=0.03; Figure 5b). Blood alcohol levels were not different between genotypes (F[1,14]=0.813, P=0.38; Figure 5c). These findings suggest that activin limits the sedating effects of ethanol without influencing its bioavailability.
Figure 5.
The sedating effects of ethanol are enhanced while ethanol consumption, preference over water and escalation after prolonged access are preserved in dnActRIB mice. (a–b) Loss of righting reflex (LORR) latency to sedation (a) and LORR duration (b) after an acute ethanol (3.5 g/kg, i.p.) treatment (wt n=12, dnActRIB n=9, *P<0.05). (c) Histogram summarizes blood alcohol concentration in wt (n=8) and dnActRIB mice (n=8) after alcohol injection (3.5 g/kg i.p.). Values were determined 1, 2 and 3 h after injection. (d) Ethanol consumption shown as mean consumption over 4 days of drinking for each dose of ethanol (wt n=14, dnActRIB n=15). (e) Ethanol preference vs water shown as mean preference over 4 days of drinking for each dose of ethanol. (f) Mean consumption of a 16 vol.% ethanol solution per day during ethanol deprivation effect on consumption after a 3-week withdrawal period (Bl—baseline). Ethanol consumption increased for 2 days in dnActRIB and wt mice compared with Bl. (g) Mean preference of sucrose and quinine solution over water measured over three consecutive days, respectively. dnActRIB, dominant-negative activin receptor IB mutant; i.p., intraperitoneally.
Ethanol consumption is an indicator for the rewarding effects of ethanol and was measured in a two-bottle free-choice test with continuous access to water and ethanol (wt n=14; dnActRIB n=15). We did not find differences in ethanol consumption (P=0.79) or preference (P=0.94) between dnActRIB and wt mice (Figure 5d and e). The alcohol deprivation effect (ADE) is an indicator for escalating consumption after withdrawal from prolonged access to ethanol. Both dnActRIB and wt mice showed a profound ADE after withdrawal (day1 and 2 vs baseline, dnActRIB P<0.0001; wt P<0.0001; Figure 5f), but no genotype difference (P=0.76). The preference of sweet taste as well as avoidance of bitter taste, were also comparable between dnActRIB and wt mice (0.5%: P=0.68; 5%: P=0.30; 2 mg/dl: P=0.61; 20 mg/dl: P=0.92; Figure 5g). These findings suggest that activin is not required for the reinforcing effects of ethanol, the escalation of consumption after withdrawal, or for taste perception.
DISCUSSION
We report here that disruption of activin receptor signaling enhances the sensitivity of synaptic GABAARs to ethanol in a behaviorally relevant manner. In the hippocampus from dnActRIB mice, both presynaptic and postsynaptic effects of ethanol at GABAergic synapses were altered in favor of more efficient transmission. In dnActRIB CA1 pyramidal neurons, electrically evoked IPSCs as well as GABAAR current responses to brief muscimol puffs showed a significantly stronger response to increasing concentrations of ethanol when compared with their wt counterparts. As a consequence of this pronounced leftward shift of the dose–response relationship of IPSCs to ethanol in dnActRIB CA1 pyramidal cells, their GABAARs, which normally do not mediate the effects of low (⩽30 mM) ethanol, are now also recruited at concentrations of ethanol typically achieved during social drinking. Since we observed the same undue ethanol potentiation of IPSCs in dentate granule cells of dnActRIB mice, control over how ethanol augments phasic inhibition might emerge as a widespread feature in the functional repertoire of activin in the brain.
The abnormally heightened sensitivity of synaptic GABAA receptors to ethanol was abrogated by Ro 15-4513. This imidazobenzodiazepine compound has been reported to reverse ethanol actions through two distinct mechanisms: First, Ro 15-4513 was found to reverse the effects of low ethanol on recombinant GABAARs expressed in Xenopus oocytes containing the δ subunit, most likely by competing for the same binding site (Wallner et al, 2006). Because δ subunit-containing GABAARs are located extrasynaptically (Farrant and Nusser, 2005), this effect of Ro 15-4513 would primarily suppress the enhancement of tonic GABAergic inhibition by low ethanol. Second, Ro 15-4513 was reported to antagonize the sedative effects of relatively low doses of ethanol on αβγ2-type GABAARs, which are located synaptically (Linden et al, 2011). In our mutant preparation, ethanol effect on tonic inhibition was not different from wt neurons, and, from previous work, we do not have evidence for aberrant expression of δ subunit-containing GABAARs (Zheng et al, 2009). We therefore conclude that Ro 15-4513 targeted αβγ2-type GABAARs to counteract the excessive augmentation of phasic inhibition by ethanol in dnActRIB hippocampi.
Notably, genetic disruption of activin receptor signaling did not alter the increase of IPSCs by pentobarbital, which belongs to another class of positive modulators at GABAARs (Supplementary Figure 1). Altogether with our previous work demonstrating an attenuation of diazepam sensitivity of GABAARs in dnActRIB hippocampi (Zheng et al, 2009), these findings indicate that activin receptor signaling can regulate the efficacy of different allosteric modulators of GABAARs in an apparently site-specific manner, with positive modulation being augmented, as is the case for diazepam, diminished, as is the case for ethanol, or left unchanged, as is the case for pentobarbital.
How does activin prevent synaptic GABAARs from being augmented by low ethanol in wt neurons? Previous immunohistochemistry did not provide evidence for an altered expression pattern of GABAAR subunits in any of the hippocampal subfields of dnActRIB mice (Zheng et al, 2009). It seems therefore likely that differences in subunit assembly, receptor trafficking and/or altered receptor phosphorylation might come into play. Supporting a role for the latter, we found that the enhanced sensitivity of mutant neurons to ethanol involved PKCɛ, suggesting that activin makes use of a non-canonical, ie SMAD2/3-independent signaling pathway. Phosphorylation of the GABAAR γ2 subunit by PKCɛ regulates the allosteric modulation of GABAARs by ethanol, with PKCɛ inhibition rendering these receptors more sensitive to ethanol, as reported previously by Hodge et al (1999) and confirmed here in wt neurons intracellularly perfused with PKCɛ-TIP. As predicted from these findings, PKCɛ-deficient mice displayed overly enhanced ethanol potentiation of their GABAARs and proved more susceptible to the acute behavioral effects of ethanol including increased duration of the LORR (Proctor et al, 2003; Qi et al, 2007). To account for the heightened ethanol sensitivity of dnActRIB mice, a scheme would thus come to mind in which activin receptor signaling normally promotes PKCɛ translocation/activation to preclude the supersensitivity of GABAARs to ethanol. However, when we suppressed the translocation of PKCɛ in dnActRIB neurons, we found that the exceeding response of GABAARs to ethanol was restored to the lower level seen in wt neurons. This seemingly paradoxical finding suggests that the effect of PKCɛ on ethanol potentiation was reversed in mutant neurons, possibly reflecting (mal)adaptive processes caused by the lack of activin signaling.
In addition to allosteric modulation of GABAARs, ethanol has also been reported to enhance GABA release (Roberto et al, 2006; Weiner and Valenzuela, 2006; Kelm et al, 2011). We wondered therefore, whether activin would also have an impact on the operation of GABAergic terminals. Previous work from our laboratory has shown that activin interferes with several presynaptic features of GABAergic synapses including spontaneous release, paired-pulse depression, and GABABR-mediated feedback inhibition (Zheng et al, 2009). With its influence on several essential properties of GABA release, it seemed plausible to assume that activin is also in a position to regulate the presynaptic action of ethanol. In fact, the frequency of spontaneous and miniature (TTX independent) events at GABA synapses were much stronger enhanced by ethanol in dnActRIB hippocampi than in their wt counterparts. Again, this effect attained significance already at a low ethanol concentration (30 mM), a concentration that was virtually ineffective in normal hippocampus. The possible presynaptic loci of ethanol action are still not fully resolved. Likely candidates are presynaptic voltage-dependent Ca2+ channels and large conductance, Ca2+-activated K+ channels (BK channels) as well as mechanisms upstream or downstream of G-protein-coupled receptors (Kelm et al, 2011; Li et al, 2014). Gaining insight into the signaling pathways involved in the interplay between activin and ethanol at GABAergic terminals will require substantial future work, given that the presynaptic effects of each substance alone are far from being understood at the mechanistic level.
A key finding of this study at the behavioral level is that activin is involved in the sedating, but not reinforcing effects of ethanol. In view of recent evidence implicating activin receptor signaling in cocaine addiction (Gancarz et al, 2015), it may be argued that the expression of dnActRIB under the control of the CaMKIIα promoter predominantly occurred in forebrain areas and, therefore, did not affect the dopaminergic projection from the ventral tegmental area (VTA) to the nucleus accumbens, which is essential to drug reinforcement. However, we have recently shown that CaMKIIα function has a direct impact on ethanol-induced activation of GABAergic neurons of the VTA (Easton et al, 2013) and postsynaptic neurons in projection areas (Schöpf et al, 2015). Despite the expression of endogenous CAMKIIα in this region, we cannot entirely exclude that the activity of the promoter used for driving transgene expression may not be sufficiently high to allow expression of high levels of the dominant-negative receptor, which are essential for efficient blockade of activin receptor signaling.
Compared with wt mice, dnActRIB mice exhibited an enhanced duration of ethanol-induced sedation. On the basis of our electrophysiological findings, we relate the prolonged sedative response to ethanol to the overly potentiated GABAergic neurotransmission in the mutant mice. Although it is not known which brain areas are actually responsible for drug-induced sedation, GABAARs have a major role as exemplified in the PKCɛ-deficient mice mentioned above, in which the ethanol supersensitivity of their GABAARs was linked to the enhanced behavioral sedation in the same behavioral paradigm we used (Hodge et al, 1999). Interestingly, serum activin A levels were enhanced in patients with alcoholic cirrhosis, but not in patients with cirrhosis of other etiologies (Voumvouraki et al, 2012). This may suggest an activin increase as a marker for excessive ethanol consumption and as a possible mediator for tolerance development for the sedative effects of ethanol.
FUNDING AND DISCLOSURE
This work was supported by the Deutsche Forschungsgemeinschaft (DFG AL 294/10-1 to CA), the Johannes und Frieda Marohn-Stiftung (to FZ, CPM and CA), the Neurotrition Project of the FAU Emerging Field Initiative (to CA), the Dr Ernst und Anita Bauer Stiftung (to ASL), and the Jürgen Manchot Stiftung (to ASL). The authors declare no conflict of interest.
Acknowledgments
We thank Maria Schulte for technical assistance. The present work was performed in (partial) fulfillment of the requirements for obtaining the degree ‘Dr. med.' for AP.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Andreasson K, Worley PF (1995). Induction of beta-A activin expression by synaptic activity and during neocortical development. Neuroscience 69: 781–796. [DOI] [PubMed] [Google Scholar]
- Banks MI, Li TB, Pearce RA (1998). The synaptic basis of GABAA,slow. J Neurosci 18: 1305–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ et al (2006). The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther 316: 1360–1368. [DOI] [PubMed] [Google Scholar]
- Chen YG, Wang Q, Lin SL, Chang CD, Chuang J, Ying SY (2006). Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp Biol Med (Maywood) 231: 534–544. [DOI] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H et al (2004). Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci 24: 4293–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton AC, Lucchesi W, Lourdusamy A, Lenz B, Solati J, Golub Y et al (2013). alphaCaMKII autophosphorylation controls the establishment of alcohol drinking behavior. Neuropsychopharmacology 38: 1636–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z (2005). Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6: 215–229. [DOI] [PubMed] [Google Scholar]
- Gancarz AM, Wang ZJ, Schroeder GL, Damez-Werno D, Braunscheidel KM, Mueller LE et al (2015). Activin receptor signaling regulates cocaine-primed behavioral and morphological plasticity. Nat Neurosci 18: 959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF et al (1999). Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci 2: 997–1002. [DOI] [PubMed] [Google Scholar]
- Jiang ZL, Ye JH (2003). Protein kinase C epsilon is involved in ethanol potentiation of glycine-gated Cl(-) current in rat neurons of ventral tegmental area. Neuropharmacology 44: 493–502. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR (2011). Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev 65: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Zheng F, Unsicker K, Alzheimer C (2011). More than being protective: functional roles for TGF-beta/activin signaling pathways at central synapses. Trends Neurosci 34: 421–429. [DOI] [PubMed] [Google Scholar]
- Lawrence KM, Kabir AM, Bellahcene M, Davidson S, Cao XB, McCormick J et al (2005). Cardioprotection mediated by urocortin is dependent on PKCepsilon activation. FASEB J 19: 831–833. [DOI] [PubMed] [Google Scholar]
- Li Q, Madison R, Moore SD (2014). Presynaptic BK channels modulate ethanol-induced enhancement of GABAergic transmission in the rat central amygdala nucleus. J Neurosci 34: 13714–13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden AM, Schmitt U, Leppa E, Wulff P, Wisden W, Luddens H et al (2011). Ro 15-4513 Antagonizes Alcohol-Induced Sedation in Mice Through alphabetagamma2-type GABAA Receptors. Front Neurosci 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link AS, Kurinna S, Havlicek S, Lehnert S, Reichel M, Kornhuber J et al (2015). Kdm6b and Pmepa1 as targets of bioelectrically and behaviorally induced activin A signaling. Mol Neurobiol (e-pub ahead of print 28 July 2015; doi:10.1007/s12035-015-9363-3). [DOI] [PubMed]
- Luddens H, Korpi ER (1995). Biological function of GABAA/benzodiazepine receptor heterogeneity. J Psychiatr Res 29: 77–94. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH (2005). Non-Smad TGF-beta signals. J Cell Sci 118: 3573–3584. [DOI] [PubMed] [Google Scholar]
- Mukerji SS, Katsman EA, Wilber C, Haner NA, Selman WR, Hall AK (2007). Activin is a neuronal survival factor that is rapidly increased after transient cerebral ischemia and hypoxia in mice. J Cereb Blood Flow Metab 27: 1161–1172. [DOI] [PubMed] [Google Scholar]
- Muller MR, Zheng F, Werner S, Alzheimer C (2006). Transgenic mice expressing dominant-negative activin receptor IB in forebrain neurons reveal novel functions of activin at glutamatergic synapses. J Biol Chem 281: 29076–29084. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Poelchen W, Bowers BJ, Wehner JM, Messing RO, Dunwiddie TV (2003). Ethanol differentially enhances hippocampal GABAA receptor-mediated responses in protein kinase C gamma (PKC gamma) and PKC epsilon null mice. J Pharmacol Exp Ther 305: 264–270. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T et al (2007). Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J Biol Chem 282: 33052–33063. [DOI] [PubMed] [Google Scholar]
- Roberto M, Treistman SN, Pietrzykowski AZ, Weiner J, Galindo R, Mameli M et al (2006). Actions of acute and chronic ethanol on presynaptic terminals. Alcohol Clin Exp Res 30: 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöpf I, Easton AC, Solati J, Golub Y, Kornhuber J, Giese KP et al (2015). alphaCaMKII autophosphorylation mediates neuronal activation in the hippocampal dentate gyrus after alcohol and cocaine in mice. Neurosci Lett 591: 65–68. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Hölter SM (2000). Pharmacological validation of a new animal model of alcoholism. J Neural Transm 107: 669–680. [DOI] [PubMed] [Google Scholar]
- Ten Dijke P, Hill CS (2004). New insights into TGF-beta-Smad signalling. Trends Biochem Sci 29: 265–273. [DOI] [PubMed] [Google Scholar]
- Tretter YP, Hertel M, Munz B, ten Bruggencate G, Werner S, Alzheimer C (2000). Induction of activin A is essential for the neuroprotective action of basic fibroblast growth factor in vivo. Nat Med 6: 812–815. [DOI] [PubMed] [Google Scholar]
- Voumvouraki A, Notas G, Koulentaki M, Georgiadou M, Klironomos S, Kouroumalis E (2012). Increased serum activin-A differentiates alcoholic from cirrhosis of other aetiologies. Eur J Clin Invest 42: 815–822. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW (2003). Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA 100: 15218–15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW (2006). Low-dose alcohol actions on alpha4beta3delta GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci USA 103: 8540–8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I (2004). Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci 24: 8379–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF (2006). Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther 111: 533–554. [DOI] [PubMed] [Google Scholar]
- Werner S, Alzheimer C (2006). Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev 17: 157–171. [DOI] [PubMed] [Google Scholar]
- Zheng F, Adelsberger H, Muller MR, Fritschy JM, Werner S, Alzheimer C (2009). Activin tunes GABAergic neurotransmission and modulates anxiety-like behavior. Mol Psychiatry 14: 332–346. [DOI] [PubMed] [Google Scholar]
- Zhu W, Xu P, Cuascut FX, Hall AK, Oxford GS (2007). Activin acutely sensitizes dorsal root ganglion neurons and induces hyperalgesia via PKC-mediated potentiation of transient receptor potential vanilloid I. J Neurosci 27: 13770–13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.