Abstract
Isoniazid (INH), a front-line antituberculosis agent, is activated by mycobacterial catalase-peroxidase KatG, converting INH into bactericidal reactive species. Here we investigated the requirements and the pathway of nitric oxide (NO ˙) generation during oxidative activation of INH by Mycobacterium tuberculosis KatG in vitro. We also provide in vivo evidence that INH-derived NO ˙ can inhibit key mycobacterial respiratory enzymes, which may contribute to the overall antimycobacterial action of INH.
Mycobacterium tuberculosis infections are of serious concern; they cause 2 million deaths every year and latently persist in over a billion individuals worldwide (41). Isoniazid (isonicotinic acid hydrazide [INH]) remains a front-line antituberculosis agent some 50 years after its development, and millions of doses are prescribed worldwide. M. tuberculosis is exceptionally sensitive to INH (12, 44), a prodrug, which is peroxidatively activated intracellularly by the M. tuberculosis catalase-peroxidase KatG to produce a range of reactive radicals that act as damaging species within the bacteria. Although the mechanism(s) of action and cellular targets of KatG-activated INH continue to be uncovered (22, 27), its full range of effects on mycobacterial cells still remains to be resolved (20, 33).
The critical role of INH activation via KatG is clearly apparent based on the findings that the katG gene represents the main site for mutations causing INH resistance in M. tuberculosis (24, 45). Several INH-derived intermediates generated from isoniazid activation, such as isonicotinic acyl NADH (27), and mycobacterial targets including enzymes from the mycobacterial type II fatty acid synthase system (1, 22) have been identified. Other work on INH activation has centered upon INH-derived free radicals as important antimycobacterial intermediates (18, 31, 38). Despite these advances, the exact mechanism(s) of INH action that results in its exceptional and specific potency against M. tuberculosis are not yet fully delineated, as multiple targets and pathways have been considered (12, 20, 23, 32).
A range of reactive nitrogen species, such as nitric oxide (NO ˙) and peroxynitrite (ONOO−) are known to have various levels of activity against M. tuberculosis (8, 21, 43). In addition to the action of exogenously added NO ˙ immune-derived NO ˙ from the action of inducible nitric oxide synthase is considered to contribute to defenses against mycobacterial infection (29). Previous reports of tyrosine nitration during oxidation of INH (34) and NO ˙ formation from peroxidative activation of hydroxyurea (16, 17) led us to hypothesize that NO ˙ might be generated as a result of INH activation by KatG. We further hypothesized that the exceptional sensitivity of M. tuberculosis to NO ˙ could potentially account for at least some of the potency of INH (8, 21, 43). Here we used spin trapping techniques to document KatG generation of nitric oxide during activation of INH and to probe potential pathways for its production from INH. We also present in vivo analysis indicating that NO ˙ production during INH activation acts against important respiratory enzymes and that this may potentially contribute to the antimycobacterial action of INH.
MATERIALS AND METHODS
In vitro spin trapping of INH-derived NO ˙.
An NO ˙-specific electron paramagnetic resonance (EPR) spin trapping technique was used (19). Ten millimolar Fe(II) (N-methyl-d-glucamine dithiocarbamate)2 complex was prepared by anoxic mixing of FeSO4 and N-methyl-d-glucamine dithiocarbamate and was incubated with 0.471 mg of purified M. tuberculosis H37Rv KatG ml−1 (37) with 10 mM INH and 10 mM H2O2 in 10 mM phosphate buffer, pH 7, at 37°C for 5 min. These reaction conditions were similar to those previously described (38), with one modification: the overwhelming catalase activity of KatG necessitated higher levels of H2O2 than the previously used catalase-insensitive tert-butyl hydroperoxide (38). N-Methyl-d-glucamine dithiocarbamate was synthesized by the method of Shinobu et al. (30), EPR spectrometry of incubated samples was then performed using a Bruker Elexsys series spectrometer operating at X-band frequencies at 25°C with samples held in 20-μl capillaries. Authentic NO was from a commercial source (Sigma-Aldrich). To produce an authentic NO spin adduct, NO gas was bubbled through an aqueous solution (10 mM) of the spin trap Fe(II) (N-methyl-d-glucamine dithiocarbamate)2 complex.
In vitro spin trapping of oxygen and carbon-centered INH-derived free radicals.
EPR spectra were recorded after incubation of 0.471 mg of KatG ml−1 with 10 mM INH, 5,5-dimethyl-1-pyrolline-N-oxide (DMPO; Sigma, St. Louis, Mo.) and 0.4 mM tert-butylhydroperoxide. DMPO was purified by charcoal treatment (6) and was used at a final concentration of 0.058 M. Anaerobic samples were prepared by deoxygenation of reagents with O2-free argon and sample loading under argon. Adduct assignments were made upon the hyperfine couplings (7), with peroxyl radical-derived adducts being dependent upon the presence of O2.
Aconitase and cytochrome c oxidase activities upon exposure to INH.
Exponentially growing cultures of Mycobacterium bovis BCG were treated overnight with various concentrations of INH. After treatment, cell extracts were obtained by beadbeating with 0.1-mm zirconia beads (two 30-s cycles) in a minibeadbeater (Biospec Products Inc., Bartsville, Okla.). Cell extracts were assayed for protein with a Pierce bicinchoninic acid (BCA) kit (Pierce, Rockford, Ill.). For aconitase activity, exponentially growing cultures of M. bovis BCG (as above) were treated overnight with 73 μM INH at 37°C, and aconitase was assayed in cell extracts with a Oxis Bioxytech Aconitase-340 kit (Oxis International Inc., Portland, Oreg.). For cytochrome c oxidase activity, exponentially growing cultures of M. bovis BCG were treated overnight with 5.5 μM INH at 37°C. Cytochrome c oxidase (in cell extracts, as previously described) was assayed using a Sigma cytochrome c oxidase kit (Sigma).
Isocitrate dehydrogenase assay.
Exponentially growing cultures of M. bovis BCG (as above) were treated overnight with 73 μM INH at 37°C, and isocitrate dehydrogenase was assayed in cell extracts by using components of the Oxis Bioxytech Aconitase-340 kit (Oxis International Inc.), except for the substitution of citrate with 230 μM isocitrate (Sigma).
RESULTS AND DISCUSSION
Generation of NO ˙ from INH depends on KatG and H2O2.
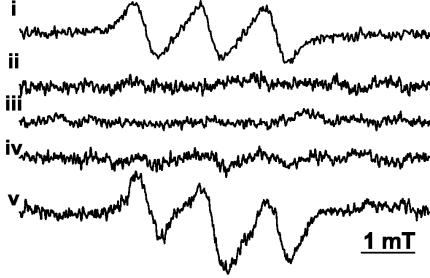
To examine the requirements for NO ˙ production during INH activation, we used an in vitro system consisting of purified components. NO ˙ production was detected during KatG-mediated oxidation of INH in a reaction mixture comprised of the following: (i) purified and previously characterized M. tuberculosis H37Rv KatG (38), (ii) INH, and (iii) H2O2 (39) (Fig. 1, spectrum v). This mixture, consisting of purified components, was identical to the previously published INH activation system (38), with the single modification of substituting the previously used catalase-insensitive tert-butyl hydroperoxide (38) with H2O2, at concentrations bypassing the intrinsic catalase activity of KatG. Identification of NO ˙ was based on the 14N-hyperfine coupling (1.25 mT), the g value (2.04), and by the identity of the spectra with an authentic NO ˙ standard (Fig. 1, spectrum i). The generation of NO ˙ was reproducible and had absolute requirements (Fig. 1, spectra ii to v) for enzyme (KatG), substrate (INH), and oxidant (H2O2).
FIG. 1.
Nitric oxide generated during INH activation requires KatG and hydrogen peroxide. EPR spectra of NO ˙ derived from KatG activation of INH are shown. NO ˙ was spin trapped with 10 mM Fe(II) (N-methyl-d-glucamine dithiocarbamate)2 complex after incubation of 0.471 mg of KatG ml−1 with 10 mM INH and 10 mM H2O2 in 10 mM phosphate buffer, pH 7, at 37°C for 5 min. Spectra: i, authentic NO ˙ adduct; ii, +KatG, +H2O2, −INH (with + or − indicating presence or absence); iii, −KatG, +H2O2, +INH; iv, +KatG, −H2O2, +INH; v, +KatG, +H2O2, +INH. EPR spectrometer settings were as follows: microwave power, 10 mW; modulation, 0.4 mT at 100 kHz; x-axis resolution, 1,024 points; conversion time, 82 ms; time constant, 164 ms; sweep, 8 mT (average of 20 scans).
Initial investigations of the potential pathway of NO ˙ production from INH.
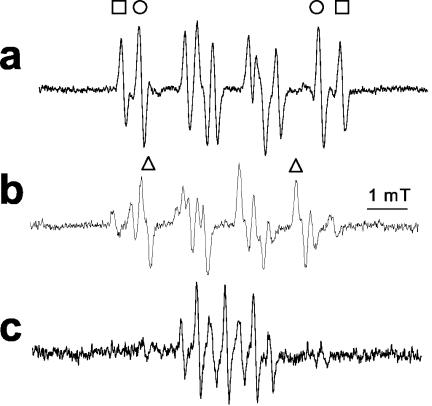
We next addressed the pathway of NȮ production from INH. From the analogy with hydroxyurea, the NO ˙ must derive from oxidation occurring at the nitrogen atoms of the hydrazide group. Prior work also indicated the importance of oxidation at the inner nitrogen atom of the hydrazide group, since alkyl substitution here destroys activity in vivo, whereas that at the terminal nitrogen does not (14). Furthermore, since INH is only effective against aerobic mycobacteria (36), we hypothesized that addition of molecular oxygen, O2, to one of the initial radicals might be important. To follow up on this lead, we examined the formation of other free radical species formed by KatG oxidation of INH, by using EPR spin trapping with DMPO, a spin trap able to form stable adducts with a wide range of radical species. DMPO is superior to the α-phenylbutylnitrone previously used in such studies (38) as it possesses a much wider dynamic range of hyperfine coupling constants that can allow much better identification of the radical adducts (7). To allow for anoxic incubations, we used tert-butylhydroperoxide as the oxidant, as previously described (38), to avoid the O2 formation that occurs from the catalase domain acting upon H2O2. In the absence of oxygen, we could observe species assigned (on the basis of their hyperfine couplings) as carbon-centered (Ṙ) (AN = 1.6 mT, AH = 2.3 mT) and alkoxyl (RȮ) (AN = 1.5 mT, AH = 1.6 mT) radical adducts (Fig. 2a and reactions 1 and 2).
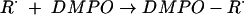 |
(1) |
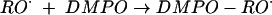 |
(2) |
As predicted, in the presence of oxygen, an additional species assigned as deriving from peroxyl radical (ROȮ) (AN = 1.46 mT, AH = 1.1 mT) was formed (Fig. 2b and reaction 3). It can be seen that the ratio of intensity of the carbon-centered to alkoxyl radical adducts is not greatly changed by addition of O2. This indicates that either (i) both alkoxyl and carbon-centered species react with O2 to form peroxyl radicals at similar rates (which is unlikely, as oxygen addition to alkoxyl radicals is not at all favorable) or (ii) one of the species is a precursor to the other, so that lowered levels of one from its scavenging by O2 equally lowers levels of both. The latter is much more probable.
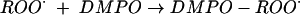 |
(3) |
Thus, we confirmed that O2 reacted with an initial INH-derived radical, formed by KatG oxidation of INH, to form a peroxyl radical. The requirement for O2 for optimal activity of INH (42) would suggest that this peroxyl species may be an important intermediate in INH activation. In the absence of INH, the only product observed was simply the oxidation product of DMPO, DMPOX (13, 26) (Fig. 2c), demonstrating that the adducts detected in Fig. 2a and b are INH derived. No EPR signals at all were observed in the absence of KatG.
FIG. 2.
Analysis of the pathway of nitric oxide production from INH. (a) EPR spectra derived from anaerobic KatG activation of INH using DMPO (oxygen concentration, 0 mM). Spectra recorded incubation of 0.471 mg of KatG ml−1 with 10 mM INH, 58 mM DMPO, and 0.4 mM tert-butylhydroperoxide (final addition). EPR spectrometer settings were as follows: microwave power, 10 mW; modulation, 0.1 mT at 100 kHz; x-axis resolution, 1,024 points; conversion time, 164 ms; time constant, 164 ms; sweep, 10 mT (average of 10 scans). Adduct assignments are shown as carbon centered (R·; indicated by squares) (with the following hyperfine coupling constants [HFCs]: AN = 1.6 mT, AH = 2.3 mT) and alkoxyl (RȮ; indicated by circles) (HFCs: AN = 1.5 mT, AH = 1.6 mT). Not all lines are assigned for clarity. (b) EPR spectra derived from aerobic KatG activation of INH using DMPO. All conditions are as in panel a, except that the reaction mixture was equilibrated with air (oxygen concentration, 0.21 mM). Adduct assignments as in panel a, with addition of peroxyl-derived adducts (ROȮ; indicated by triangles) (HFCs: AN = 1.46 mT, AH = 1.1 mT). (c) EPR spectra derived from aerobic KatG and tert-butylhydroperoxide in the absence of INH (all other conditions are as in panel a) assigned as DMPO oxidation product DMPOX (HFCs: AN = 0.71 mT, AH(2) = 0.42 mT).
Biological activity of NO ˙ generated during INH-derived NO ˙ activation.
NO ˙ exerts its antimicrobial activities primarily through two different mechanisms. Firstly, NO ˙ reacts with superoxide (O2̇−) at diffusion-controlled rates to form peroxynitrite (ONOO−; reaction 4), a reactive species capable of oxidizing and nitrating biomolecules (28). Secondly, NO ˙ acts directly upon metalloproteins such as aconitases (15), cytochrome c oxidases (10), and a range of other targets (11), thereby inhibiting respiratory activity and iron homeostasis.
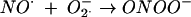 |
(4) |
However, there are many reports that M. tuberculosis has potent detoxifying systems against peroxynitrite, including the peroxynitritase activity of KatG (4, 5, 21, 37, 43). Furthermore, the importance of inhibition of M. tuberculosis respiration by NO ˙ was recently demonstrated, even under conditions when the inhibition of respiration was modest (35). We therefore focused our attention upon NO ˙-sensitive respiratory metalloproteins.
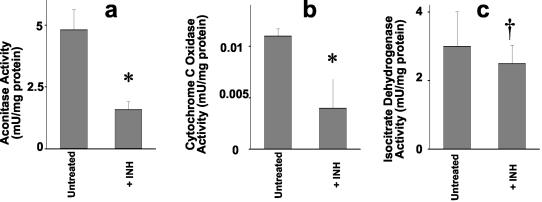
Aconitase is an important respiratory enzyme and also has additional roles in iron homeostasis (40). Aconitase contains an 4S-4Fe iron-sulfur center and is known to be sensitive to NO ˙-induced damage (15). A significant decrease in the aconitase activity was detected in M. bovis BCG treated overnight with 73 μM INH (Fig. 3a). This finding is consistent with the predicted effects of INH-derived NO ˙ upon metalloproteins, although this concentration of INH is approximately 10 times the MIC.
FIG. 3.
Inhibition of respiratory enzymes by INH-derived nitric oxide. (a) Aconitase activity upon exposure to INH. Exponentially growing cultures of M. bovis BCG were treated overnight with 73 μM INH at 37°C. Cell extracts were assayed for protein with a Pierce BCA kit, and aconitase was assayed with an Oxis Bioxytech Aconitase-340 kit. Data represent means of six replicate cultures. *, P = 0.008. (b) Cytochrome c oxidase activity upon exposure to INH. Exponentially growing cultures of M. bovis BCG (as in panel a) were treated overnight with 5.5 μM INH at 37°C. Cell extracts were assayed for protein with a Pierce BCA kit, and cytochrome c oxidase was assayed using a Sigma cytochrome c oxidase kit. Data represent means of three replicate cultures. *, P = 0.037. (c) Isocitrate dehydrogenase activity in extracts of M. bovis BCG treated with INH. Isocitrate dehydrogenase was assayed as described in Materials and Methods. †, P > 0.05.
Next, we tested another prototypical NO ˙ target, the respiratory enzyme cytochrome c oxidase, which is exquisitely sensitive to NO ˙ (10). Although whole-cell oxygen consumption measurements can prove useful (35), there are many potential sources of oxygen consumption other than cytochrome c oxidase, not least of which is oxidative detoxification of NO ˙ by the truncated hemoglobins trHbN and trHbO (25). Thus, we used a specific assay for cytochrome c oxidase, based upon cytochrome c oxidation, to study its inhibition by INH. Overnight treatment with 5.5 μM INH, a value close to the MIC for M. tuberculosis (3.7 to 7.3 μM in our hands) caused a 64% inhibition of cytochrome c oxidase activity (Fig. 3b). The much greater sensitivity of cytochrome c oxidase compared to that of aconitase is in accord with its known greater sensitivity to NO ˙ over FeS proteins (2, 3). As a control, isocitrate dehydrogenase was assayed in the same extracts, and no differences upon INH treatment were observed (Fig. 3c).
Conclusions.
In this work we have demonstrated that NO ˙ is formed by oxidative activation of INH by KatG, in a reaction that requires KatG and H2O2. We have also provided initial evidence for a pathway of KatG oxidation of INH. Our enzymological assays suggest that the NO ˙ generated in vivo during oxidation of INH can have appreciable activity against respiratory enzymes. It is evident from the experimental data, however, that INH-derived NO ˙ did not fully inhibit either aconitase or cytochrome c oxidase. This is consistent with the importance of other known antimycobacterial products of INH, such as isonicotinic acyl NADH (27). Nevertheless, the generation of NO ˙ from INH and its detectable effects on the bacteria suggest the prospects of enhancing this property of INH as a potential strategy for generating new antituberculosis drugs. The addition of NO ˙-releasing groups to another antibiotic, ciprofloxacin, greatly increases its activity against M. tuberculosis (9), supporting the hypothesis that NO ˙ release can synergize with other antimycobacterial activities. Since even modest inhibition (∼50%) of respiration by NO ˙ has profound effects on the physiology of M. tuberculosis (35), the levels of inhibition observed in this study may prove similarly important.
Acknowledgments
We thank E. J. H. Bechara, K. J. Liu, and T. Wilson for discussions. EPR facilities were provided by the UNMHSC Center of Biomedical Research Excellence, NCRR P20-R15636.
This work was supported by NIH grant AI42999.
REFERENCES
- 1.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 2.Brown, G. C. 1997. Nitric oxide inhibition of cytochrome oxidase and mitochondrial respiration: implications for inflammatory, neurodegenerative and ischaemic pathologies. Mol. Cell. Biochem. 174:189-192. [PubMed] [Google Scholar]
- 3.Brown, G. C. 2001. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys. Acta 1504:46-57. [DOI] [PubMed] [Google Scholar]
- 4.Bryk, R., P. Griffin, and C. Nathan. 2000. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407:211-215. [DOI] [PubMed] [Google Scholar]
- 5.Bryk, R., C. D. Lima, H. Erdjument-Bromage, P. Tempst, and C. Nathan. 2002. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 295:1073-1077. [DOI] [PubMed] [Google Scholar]
- 6.Buettner, G., and L. Oberley. 1978. Considerations in spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem. Biophys. Res. Commun. 83:69-74. [DOI] [PubMed] [Google Scholar]
- 7.Buettner, G. R. 1987. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 3:259-303. [DOI] [PubMed] [Google Scholar]
- 8.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccone, R., F. Mariani, A. Cavone, T. Persichini, G. Venturini, E. Ongini, V. Colizzi, and M. Colasanti. 2003. Inhibitory effect of NO-releasing ciprofloxacin (NCX 976) on Mycobacterium tuberculosis survival. Antimicrob. Agents Chemother. 47:2299-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clementi, E., G. C. Brown, N. Foxwell, and S. Moncada. 1999. On the mechanism by which vascular endothelial cells regulate their oxygen consumption. Proc. Natl. Acad. Sci. USA 96:1559-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Autréaux, B., D. Touati, B. Bersch, J. M. Latour, and I. Michaud-Soret. 2002. Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron. Proc. Natl. Acad. Sci. USA 99:16619-16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deretic, V., E. Pagan-Ramos, Y. Zhang, S. Dhandayuthapani, and L. E. Via. 1996. The extreme sensitivity of Mycobacterium tuberculosis to the front-line antituberculosis drug isoniazid. Nat. Biotechnol. 14:1557-1561. [DOI] [PubMed] [Google Scholar]
- 13.Floyd, R., and L. Soong. 1977. Spin trapping in biological systems—oxidation of spin trap 5,5-dimethyl-1-pyrroline-1-oxide by a hydroperoxide-hematin-system. Biochem. Biophys. Res. Commun. 74:79-84. [DOI] [PubMed] [Google Scholar]
- 14.Fox, H. H., and J. Y. Gibas. 1955. Synthetic tuberculostats. IX. Dialkyl derivatives of isonicotinylhydrazine. J. Org. Chem. 20:60-69. [Google Scholar]
- 15.Gardner, P. R., G. Costantino, C. Szabo, and A. L. Salzman. 1997. Nitric oxide sensitivity of the aconitases. J. Biol. Chem. 272:25071-25076. [DOI] [PubMed] [Google Scholar]
- 16.Huang, J., E. M. Sommers, D. B. Kim-Shapiro, and S. B. King. 2002. Horseradish peroxidase catalyzed nitric oxide formation from hydroxyurea. J. Am. Chem. Soc. 124:3473-3480. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, J., S. J. Jordan, D. P. Barr, M. R. Gunther, H. Maeda, and R. P. Mason. 1997. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol. Pharmacol. 52:1081-1086. [DOI] [PubMed] [Google Scholar]
- 18.Johnsson, K., and P. G. Schultz. 1994. Mechanistic studies of the oxidation of isoniazid by the catalase peroxidase from Mycobacterium tuberculosis. J. Am. Chem. Soc. 116:7425-7426. [Google Scholar]
- 19.Komarov, A. M., and C. S. Lai. 1995. Detection of nitric oxide production in mice by spin-trapping electron paramagnetic resonance spectroscopy. Biochim. Biophys. Acta 1272:29-36. [DOI] [PubMed] [Google Scholar]
- 20.Larsen, M. H., C. Vilcheze, L. Kremer, G. S. Besra, L. Parsons, M. Salfinger, L. Heifets, M. H. Hazbon, D. Alland, J. C. Sacchettini, and W. R. Jacobs. 2002. Overexpression of inhA, but not kasA, confers resistance to isoniazid and ethionamide in Mycobacterium smegmatis, M. bovis BCG and M. tuberculosis. Mol. Microbiol. 46:453-466. [DOI] [PubMed] [Google Scholar]
- 21.Master, S. S., B. Springer, P. Sander, E. C. Boettger, V. Deretic, and G. S. Timmins. 2002. Oxidative stress response genes in Mycobacterium tuberculosis: role of ahpC in resistance to peroxynitrite and stage-specific survival in macrophages. Microbiology 148:3139-3144. [DOI] [PubMed] [Google Scholar]
- 22.Mdluli, K., R. A. Slayden, Y. Zhu, S. Ramaswamy, X. Pan, D. Mead, D. D. Crane, J. M. Musser, and C. E. Barry III. 1998. Inhibition of a Mycobacterium tuberculosis beta-ketoacyl ACP synthase by isoniazid. Science 280:1607-1610. [DOI] [PubMed] [Google Scholar]
- 23.Miesel, L., D. A. Rozwarski, J. C. Sacchettini, and W. R. Jacobs, Jr. 1998. Mechanisms for isoniazid action and resistance. Novartis Found. Symp. 217:209-220. [DOI] [PubMed] [Google Scholar]
- 24.Musser, J. M. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8:496-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouellet, H., Y. Ouellet, C. Richard, M. Labarre, B. Wittenberg, J. Wittenberg, and M. Guertin. 2002. Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc. Natl. Acad. Sci. USA 99:5902-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen, G., and E. Rauckman. 1980. Spin trapping of the primary radical involved in the activation of the carcinogen N-hydroxy-2-acetylaminofluorene by cumene hydroperoxide-hematin. Mol. Pharmacol. 17:233-238. [PubMed] [Google Scholar]
- 27.Rozwarski, D. A., G. A. Grant, D. H. Barton, W. R. Jacobs, Jr., and J. C. Sacchettini. 1998. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279:98-102. [DOI] [PubMed] [Google Scholar]
- 28.Rubbo, H., R. Radi, M. Trujillo, R. Telleri, B. Kalyanaraman, S. Barnes, M. Kirk, and B. A. Freeman. 1994. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 269:26066-26075. [PubMed] [Google Scholar]
- 29.Scanga, C., V. Mohan, K. Tanaka, D. Alland, J. Flynn, and J. Chan. 2001. The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice. Infect. Immun. 69:7711-7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinobu, L., S. Jones, and M. Jones. 1984. Sodium N-methyl-D-glucamine dithiocarbamate and cadmium intoxication. Acta Pharmacol. Toxicol. 54:189-194. [DOI] [PubMed] [Google Scholar]
- 31.Shoeb, H. A., B. U. Bowman, A. C. Ottolenghi, and A. J. Merola. 1985. Enzymatic and nonenzymatic superoxide-generating reactions of isoniazid. Antimicrob. Agents Chemother. 27:408-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slayden, R. A., and C. E. Barry III. 2000. The genetics and biochemistry of isoniazid resistance in Mycobacterium tuberculosis. Microbes Infect. 2:659-669. [DOI] [PubMed] [Google Scholar]
- 33.Slayden, R. A., R. E. Lee, and C. E. Barry III. 2000. Isoniazid affects multiple components of the type II fatty acid synthase system of Mycobacterium tuberculosis. Mol. Microbiol. 38:514-525. [DOI] [PubMed] [Google Scholar]
- 34.Vanzyl, J. M., and B. J. Vanderwalt. 1994. Apparent hydroxyl radical generation without transition metal catalysis and tyrosine nitration during oxidation of the anti-tubercular drug, isonicotinic acid hydrazide. Biochem. Pharmacol. 48:2033-2042. [DOI] [PubMed] [Google Scholar]
- 35.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wayne, L. G., and H. A. Sramek. 1994. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 38:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wengenack, N. L., M. P. Jensen, F. Rusnak, and M. K. Stern. 1999. Mycobacterium tuberculosis KatG is a peroxynitritase. Biochem. Biophys. Res. Commun. 256:485-487. [DOI] [PubMed] [Google Scholar]
- 38.Wengenack, N. L., and F. Rusnak. 2001. Evidence for isoniazid-dependent free radical generation catalyzed by Mycobacterium tuberculosis KatG and the isoniazid-resistant mutant KatG(S315T). Biochemistry 40:8990-8996. [DOI] [PubMed] [Google Scholar]
- 39.Wengenack, N. L., J. R. Uhl, A. L. St. Amand, A. J. Tomlinson, L. M. Benson, S. Naylor, B. C. Kline, F. R. Cockerill III, and F. Rusnak. 1997. Recombinant Mycobacterium tuberculosis KatG(S315T) is a competent catalase-peroxidase with reduced activity toward isoniazid. J. Infect. Dis. 176:722-727. [DOI] [PubMed] [Google Scholar]
- 40.Wong, D. K., B. Y. Lee, M. A. Horwitz, and B. W. Gibson. 1999. Identification of Fur, aconitase, and other proteins expressed by Mycobacterium tuberculosis under conditions of low and high concentrations of iron by combined two-dimensional gel electrophoresis and mass spectrometry. Infect. Immun. 67:327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization. 2002. Report on infectious diseases. World Health Organization, Geneva, Switzerland.
- 42.Youatt, J. 1969. A review of the action of isoniazid. Am. Rev. Respir. Dis. 99:729-749. [DOI] [PubMed] [Google Scholar]
- 43.Yu, K., C. Mitchell, Y. Xing, R. S. Magliozzo, B. R. Bloom, and J. Chan. 1999. Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber. Lung Dis. 79:191-198. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, Y., S. Dhandayuthapani, and V. Deretic. 1996. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc. Natl. Acad. Sci. USA 93:13212-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]