Abstract
Background:
The use of medicinal plants in modern medicine for the prevention and treatment of cancer is an important aspect. For this reason, it is important to identify antitumor promoting agents present in medicinal plants commonly used by the human population.
Materials and Methods:
We used in vivo and in vitro methods using chromosomal aberrations (CAs), sister chromatid exchange (SCE) and replication index (RI) as markers, exposed by methyl methanesulfonate (MMS) as well as alcoholic extract of Alstonia scholaris in five increasing concentrations (200, 250, 300, 350 and 400 mg/kg body weight for in vivo and 150, 200, 250 and 300 μg/ml of culture) and of three different durations of 24, 48 and 72 h in the presence as well absence of S9 mix.
Results:
Extracts of Alstonia reduces the total aberrant cells ranges from 10.0% to 41.84% and frequencies of aberration in the aberrant cells ranges from 220 to 124 against 290 aberrations causes due to MMS in vivo. Similarly in the in vitro, it reduces CAs (39.62%, 32.83%, and 38.48%) and (45.31%, 44.46%, and 38.34%) at 24, 48, and 72 h of exposure respectively; in the absence as well as presence of liver S9 fraction. It also reduces SCE from 7.70 to 4.20 per cell and enhances RI from 1.45 to 1.64.
Conclusion:
Extracts of Alstonia significantly reduces the number of aberrant cells and frequency of aberration per cell at each concentration and duration of exposure in vivo; and CAs and SCE in vitro and enhances RI.
Keywords: Alstonia scholaris, anticarcinogenic, antimutagenic, antitumor, ayurvedic medicine, chromosomal aberration
INTRODUCTION
Cancer is among the most dreaded of human diseases. It is considered as an adversary of modernization and the pattern of socioeconomic life dominated by western medicine. The use of medicinal plants in modern medicine for the prevention or treatment of cancer is an important aspect. For this reason, it is important to identify antitumor promoting agents present in medicinal plants commonly used by the human population, which can inhibit the initiation, progression and promotion of the tumor. Recent development of medical treatment of human disease will be intimately connected to the natural products and greater emphasis has been given towards the researches on complementary and alternative medicine that deals with cancer management.[1] Many herbs have been evaluated in clinical studies and are currently being investigated phytochemically to understand their antitumor actions against various cancers. Some herbs protect the body from cancer by enhancing detoxification functions of the body.[2] Certain biological response modifiers derived from herbs are known to inhibit the growth of cancer by modulating the activity of specific hormones and enzymes. The traditional Indian system of medicines, Ayurveda, uses about 2000 plant species, while the Chinese pharmacopeia lists over 5700 traditional medicines, most of which are of plant origin.[3]
Alstonia scholaris (also known as Devils tree) belonging to the family Apocynaceae, has been used since time immemorial in the folklore and traditional systems of medicine in India. The plant is grown in the lowland and mountain rainforests of India, the Asia-Pacific, Southern China, and Queensland. The plant is used in Ayurvedic, Unani and Siddha types of alternative medicinal systems.[4,5] The methanolic extract of this plant was found to exhibit pronounced antiplasmodial activity. The plant is reported to have antimutagenic effect.[6]
Alstonia scholaris is used in various Ayurvedic preparations like Saptaparnasatvadi vati, Saptachadadi vati, Saptacchadadi kvatha and Saptaparna ghanasara for uses of A. scholaris mainly in whooping cough, malaria, jaundice, gastric complaint, headache, asthma, stomach ache and fever. Ethanolic extract of A. scholaris using various concentrations under in vitro tests were found to have significant (P = 0.01) free radical scavenging and metal ion chelating properties.[6]
The bark extract of A. scholaris has immune-stimulating effects. The aqueous extracts at low dose induced the cellular immune response while at high dose inhibited the delayed type of hypersensitivity reaction.[7] Echitamine chloride, an indole alkaloid, extracted from the bark of A. scholaris has promising anticancer effect against sarcoma.[8] The plant A. scholaris is reported to possess in vitro nitric oxide scavenging activity in preliminary studies.[9] Several studies have demonstrated that plants produce potent antioxidants and represent important sources of natural antioxidants.[10,11]
The ethanolic extract of the leaves of A. scholaris (30, 300, 1000 and 2000 mg/kg body weight [bw]) intraperitoneally did not elicit any changes in the behavior and autonomic responses of mice compared to controls. None of the mice treated with up to 2 g/kg of the extract died during the 48 h observation period following the administration of the extract. The higher dose caused lethargy in the rats.[12] Ethnomedicinal practices suggest it to be of use in treating cancer, and preclinical studies performed with cultured neoplastic cells and tumor-bearing animals having validated these observations. In addition to the cytotoxic effects, A. scholaris has also been observed to possess radiomodulatory, chemomodulatory, and chemopreventive effects and free radical scavenging, antioxidant, anti-inflammatory, antimutagenic, and immunomodulatory activities, all of which are properties efficacious in the treatment and prevention of cancer.[13]
MATERIALS AND METHODS
The whole plant of A. scholaris was shade dried at room temperature. Then the shade dried samples were powdered, 60 g of coarse powder was defatted with petroleum ether and extracted exhaustively with 95% of methanol at a temperature of 60°C. The extract was air dried by vacuum evaporator. Methanol extract of A. scholaris was dissolved in dimethyl sulfoxide (DMSO) to prepare different optimum concentrations for studies [Tables 1A and 1B].
Table 1A.
Table of chemical concentration

Table 1B.
In vivo concentrations of phyto-chemicals

In vivo method
Albino mice 8–10 weeks old (25–35 g in weight) were exposed to different test chemicals by appropriate routes (intraperitoneal injection) and were sacrificed at sequential intervals of 16, 24, and 32 h of stipulated treatment time. Animals were treated with each test substance as shown in the tables. Three replicates of treatment were used. The central sampling interval was 24 h since cell cycle kinetics could be influenced by the test substances. The earlier and late sampling interval was adequately spaced within the range of 6–48 h. The additional dose levels were tested in subsequent experiments, the samples being taken for the scheduled duration.
Preparation of slides
Immediately after sacrifice, the bone marrows have been obtained by standard procedure and exposed to hypotonic solution and cells were fixed in Carnoy's fixative. Chromosome preparations were made from bone marrow cells following the standard procedure as described below. The stained slides were examined and metaphase cells were scored for chromosomal aberrations (CAs). Prior to sacrifice, mice were further treated with colchicines, a spindle inhibitor to arrest the cells in C-metaphase. The slides were stained with 10% aqueous Giemsa solution and about 100 bone marrow metaphase cells from each animal were scored under the code. The types of CAs considered were chromatid and chromosome gaps, breaks, and fragments, exchanges and pulverization (severely damaged cells). The reduction factors due to test chemicals treatment were calculated using the formula:

Control groups
Concurrent positive and negative controls were included in this study.
Positive control
A single dose of methyl methanesulfonate (MMS), a compound known to produce CAs in vivo, was used as positive control showing a significant response.
In vitro lymphocytes culture method
Most of the cytogenetic studies being carried out involve the examination of metaphase chromosomes. The evaluation of chromosomal damage at metaphase stage gives more precise and detailed picture of the clastogenic agent than those at anaphase or telophase stage. Human peripheral blood lymphocytes are extremely sensitive indicators of the in vitro assay system. The chromosomal changes (numerical and structural) were utilized for investigation of the genotoxic as well as the antigenotoxic potentiality of test chemicals. The parameters studied included CAs, sister chromatid exchanges (SCEs) and cell growth kinetics replication index (RI) both in the presence as well as in the absence of exogenous metabolic activation system.
Preparation of S9 liver/microsome fraction
For preparing S9 fraction, the standard procedures as recommended by Maron and Ames [14] were followed. Swiss albino healthy rats (Wister strain, obtained from Animal house, Biotech, Varanasi, India), each weighing about 200 g were given 0.1% (1 mg/ml) of phenobarbital in drinking water for 1-week for the induction of liver enzymatic activities. The removed livers were immediately placed in 0.15 M KCl chilled solution in the culture tube. The whole procedure was carried out at 0–4°C using sterilized solutions and glassware. The livers were washed in chilled KCl several times so as to remove the traces of hemoglobin, which inhibits enzyme activity. The washed livers were transferred to a beaker containing three volumes of 0.15 M KCl (3 ml/g wet liver) and after mincing with sterile scissors, these were homogenized by a tissue homogenizer at 4°C. The homogenate was centrifuged in a refrigeration centrifuge for 10 min at 9000 rpm. The supernatant (S9 fraction) was decanted and saved as 1 ml aliquots in polypropylene storage vials and stored in liquid nitrogen till further use.
The S9 mix from S9 fraction was prepared fresh every time for use in the culture. The S9 fraction was complemented with 8 μm of NADP, 100 μm of Na2HPO4 buffer with 7.4 pH, 0.8 ml of the S9 mix was added every time along with the test chemicals in the cultures.
Chromosomal aberrations
Preparation of culture media
Tissue culture medium RPMI–1640 (flow Laboratories) with L-glutamine and Hepes buffer without NaHCO3 was prepared in advance and stored at 4°C, but the storage period never lasted longer than a week. About 1.574 g of the medium was dissolved in 100 ml of double distilled water by gentle shaking. Antibiotics, penicillin (100 IU/ml) and streptomycin (100 IU/ml) (Hoechst) were also added and pH was adjusted from 6.8 to 7.2 with N/10 NaHCO3 and HCl. The medium was filtered and sterilized using Millipore filtration assembly by 0.45 μm Millipore filters. The filtered medium was then stored in sterilized and tightly capped glass bottles.
Collection of blood samples
Peripheral blood from the healthy donors was taken fresh every time through veinal puncture under aseptic conditions (disposable needle and disposable syringes, Unitech) and Heparin (500 IU/ml; Micro Lab) was used as anticoagulant. The tightly capped glass vials were gently mixed and stored at 4°C for half an hour to separate blood cells from plasma.
Setting of the cultures
Lymphocyte culture was carried out by adding 0.8 ml of plasma containing white blood cells in 4.5 ml of culture medium supplemented with 0.1 ml phytohemagglutinin–P (PHA–P, Micro lab) and 15% fetal calf serum (Gibco). The culture vials were then tightly capped to avoid loss CO2 of and after gently mixing, culture tubes were incubated at 37°C in dark and colchicine was added 2 h prior to harvesting for arresting the cells at metaphase stage.
Harvesting of the cultures
After appropriate durations, the cultures were taken out from the incubator and their contents, after gentle shaking, were transferred to a centrifuge tube, the cells were spun down by centrifugation for 10 min, at 1200 rpm. Pellets were saved by discarding the supernatant. Hypotonic treatment (0.075 M KCl) was given for 10–12 min at 37°C and the cells were recollected by centrifugation. The cell pellet was suspended in 5 ml freshly prepared chilled fixative (3:1; methanol: acetic acid), which was added drop by drop with a Pasteur pipette with continuous shaking to avoid formation of clots. In order to ensure the proper fixation, the cells were kept suspended in the fixative for a minimum period of 1 h but preferably overnight. Two or three changes with fresh fixative were given before preparing the slides.
Slide preparation and staining
After giving final washing in the fixative, the cells were re-suspended in 0.2 ml of fresh fixative. Two or three drops of cell preparation were dropped on clean, grease free, prechilled and wet microscopic slides and air dried. One-day-old slides were stained with Giemsa (Sigma) for 15 min and rinsed in 95% alcohol and finally in absolute alcohol for proper differentiation, after air-drying these slides were dipped in xylene for 5 min before mounting in dibutyl phthalate xylene (DPX).
Analysis of the cells
In order to avoid the bias in scoring of the chromosomal anomalies before and after treatment of different test chemicals all slides were coded prior to scoring. A total of 300 well-spread metaphase were analyze for each concentration of the test chemicals and for each time duration to analyzed various chromosome and chromatid type aberrations by using the method as described by Evans.[15]
Sister chromatid exchange analysis
Sister chromatid exchange is a sensitive rapid and objective method of observing reciprocal exchange between sisters chromatid. This method depends upon the phenomenon of 5-bromo-2-deoxyuridine (BrdU) incorporation into DNA in place of thymidine. After two rounds of cell division, the chromatids were labeled with BrdU and consequently differentially stained with Hoechst stain. The BrdU incorporation quenches the fluorescence of 33258 Hoechst. Therefore, the light energy is absorbed but not emitted by such dyes, which results in the reduced staining of chromatid with Giemsa.[14]
Labeling of chromosomes with 5-bromo-2-deoxyuridine
Sister chromatid exchange analysis was carried out following the standard procedure of Latt and Wohlleb.[16] The cells in the culture were exposed to nucleoside, BrdU (Sigma) after 24 h of culture initiation at the final concentration of 2 μg/ml. The culture vials were tightly capped and covered with aluminum foil to avoid light exposure and incubated at 37°C for another 48 h in the dark.
Slide preparation and staining
After 2 h of colchicines treatment, the cultures were harvested and processed following the same procedure as desired for the CA analysis. For the differential staining of SCEs the methods of Latt et al.[17] with slight modifications were followed. 1-day-old slides were dipped in 0.5 μg/ml of 33258 Hoechst stain (Sigma) dissolved in double distilled water in horizontal coupling jar. The slides were then put in a flat glass dish with the layer of cells facing upwards. These were covered by thick layer (2–3 cm) of phosphate buffer (pH 6.8) and exposed to ultraviolet lamp (15W, 254 μm, Philips) from a distance of 10 to 15 cm for 30–45 min. The slides were taken out from the buffer, washed twice in double distilled water and air dried. These were then incubated in 2X SSC (0.3 M NaCl, 0.03M sodium citrate, pH 7.0) at 65°C in water bath for 90 min using vertical couplin jars. The slides were taken out and rinsed in distilled water. The air-dried slides were then stained with Giemsa for 20 min and rinsed in 90% alcohol, followed by rinsing in absolute alcohol. The dried slides were dipped in xylene for 5 min and mounted in DPX.
Analysis of the cells
All slides were coded prior to scoring so as to avoid any ambiguity. Around 50 metaphases (25 metaphases/donor) with differentially stained chromatid were scored for each test chemical treatment in the absence of S9 mix and 50 metaphases were scored for each treatment in the presence of S9 mix. The interstitial exchanges between two sister chromatid were scored as two exchange, and the terminal exchanges were scored as a single exchange. Student's t-test was applied for calculating the significance of difference between the treated and the controls.
Cell cycle kinetics analysis
The cells undergoing first (M1) second (M2) and third (M3) divisions were detected by studying the BrdU labeled differentially stained chromosomes, following the method of Crossen and Morgan.[18] The cells with both the chromatids being darkly stained were scored as M1 cells, those with one dark and one lightly stained chromatid as M2 cells and those having mixture of both the differentially stained and uniformly stained chromatids were scored as M3 metaphase. Around 100 well-spread metaphase were scored for each concentration and each treatment durations from each donor in the absence as well as in the presence of S9 mix. The RI was calculated according to the formula of Tice et al.[19] as given below. The deviation from the controls was determined by using Chi-square (χ2) test.
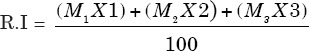
Statistical analysis
2 × 3 Chi-square test (χ2) for homogeneity test of variance was used to analyses the cell growth kinetics exchange with the normal control. The level of significance was tested from standard statistical tables of Fisher and Yates.[20]
Student two-tailed t-test was used for calculating the statistical significance in SCE and CA by comparing the effect induced by different test chemicals with the respective control.
The statistical significance was calculated from Fisher and Yates table at (n1+n2−2) degree of freedom at 0.05% level of significance.
RESULTS
In vivo effects
In this study, the albino mice were exposed till 16 h of treatments and found that the percentage of aberrant cells were 9.9, 8.7, 8.2, 7.3 and 6.4, respectively at five different concentrations of Alstonia extract respectively against 11.0% of aberrant cells induced by MMS in positive control. Fragments types of aberrations were most prominent, followed by breaks and gaps, whereas exchanges were almost negligible. In terms of percentage reduction in the frequencies of aberrant cells, the observed values are 10.0, 20.90, 25.45, 33.63 and 41.87 against five different concentrations of Alstonia extract respectively. The maximum effect of Alstonia extract was 41.87% at the fifth concentration of the extract [Table 1C and Figure 1].
Table 1c.
Effect of with alcoholic extracts of A. scholaris on the frequency of cells with chromosome aberrations induced by MMS
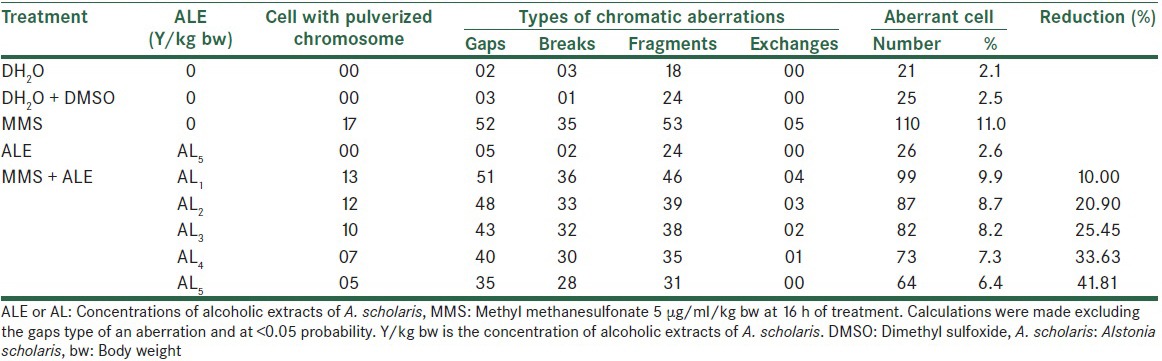
Figure 1.
In vivo effect of alcoholic extracts of Alstonia scholaris at 16 h
The effect on the total number of frequencies per thousand cells was 182, 147, 138, 125 and 105 at five consecutive concentrations of Alstonia extract against 209 when treated with MMS alone. The normal values were 25 for distilled water treatment and 28 and 28 for DMSO and Alstonia extract only treatment [Table 2]. When the treatment durations were increased to 24 h, the effects were still following the same trend, showing increasing values. The values are 11.5%, 11.0%, 10.3%, 9.5% and 8.7% for five concentrations of Alstonia extract respectively against 13.5% of MMS treatment only. The values of normal are 3.0%, 3.0%, and 2.8% respectively for pure water, DMSO and Alstonia extract only. The fifth concentration only shows a noticeable effect on the percentage reductions of aberrant cells [Table 3 and Figure 2].
Table 2.
Effect of with alcoholic extracts of A. scholaris on the total number and types of frequency of cells with chromosome aberrations induced by MMS
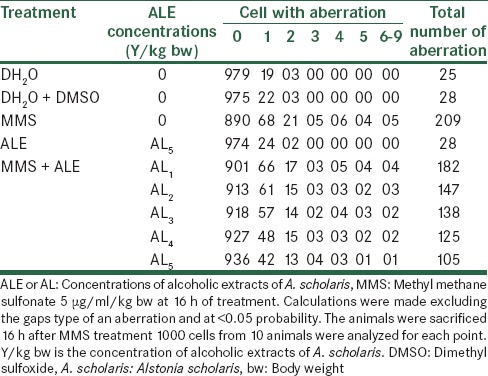
Table 3.
Effect of with alcoholic extracts of A. scholaris on the frequency of cells with chromosome aberrations induced by MMS
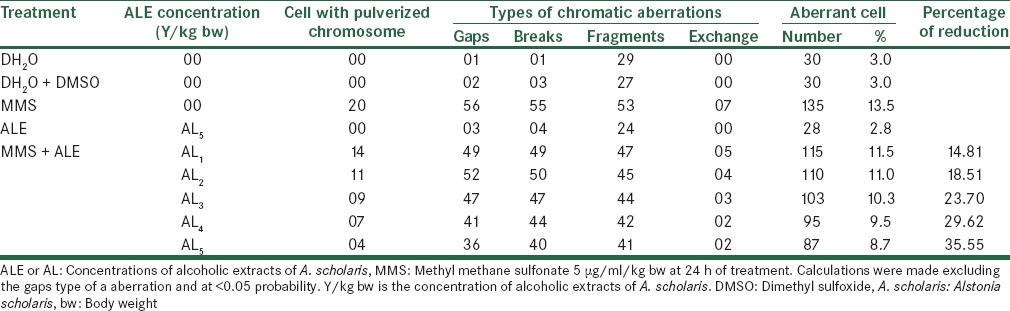
Figure 2.
In vivo effect of alcoholic extracts of Alstonia scholaris at 24 h
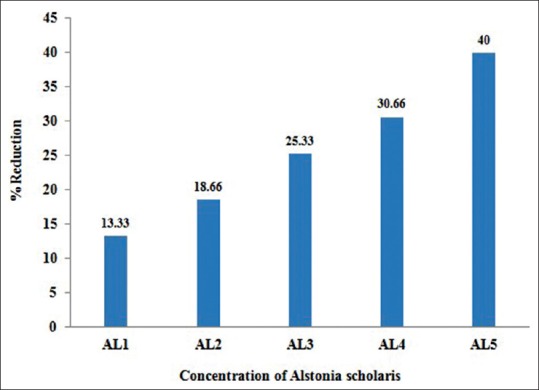
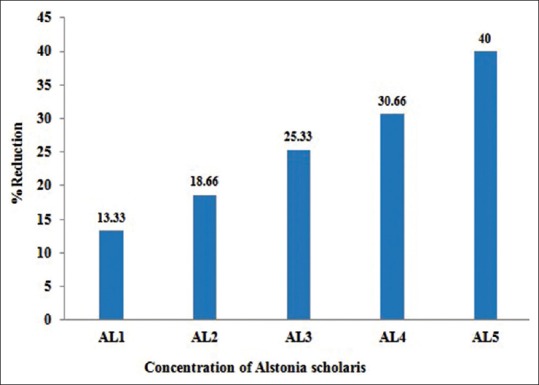
Effect of Alstonia extract on the frequency of aberrations per cell and total aberrations were also not so much promising. The total aberrations per thousand cells were 219, 194, 179, 152 and 141 for Alstonia extract along with MMS against 265 due to MMS only as positive control [Table 4]. At 32 h exposure, the percent aberrant cells observed were 15.0% for MMS alone, and 13.0%, 12.2%, 11.2%, 10.4%, 9.0% for five different concentrations of Alstonia extract along with MMS, whereas the values for normal control was 2.3, whereas for DMSO and Alstonia extract alone, the values were 2.0% and 2.6% respectively. In terms of the effects on the percent reduction in aberrant cells, the range was from 13.33%, 18.66%, 25.33%, and 30.66% to 40.00% respectively. These values show a significant effect of Alstonia extract on the number and percentage of aberrant cells. It also shows almost dose-dependent relationship. More chromosomal exchange types of aberrations were seen in contrast to the previous two durations of treatment [Table 5 and Figure 3].
Table 4.
Effect of with alcoholic extracts of A. scholaris on the total number and types of frequency of cells with chromosome aberrations induced by MMS
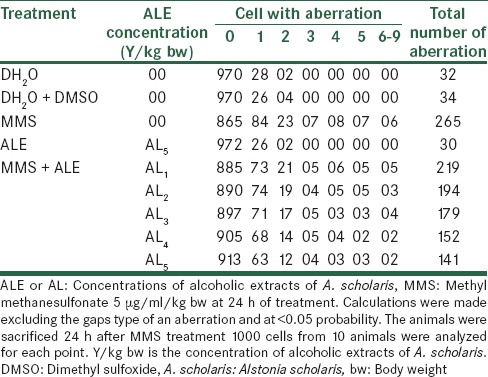
Table 5.
Effect of with alcoholic extracts of A. scholaris on the frequency of cells with chromosome aberrations induced by MMS
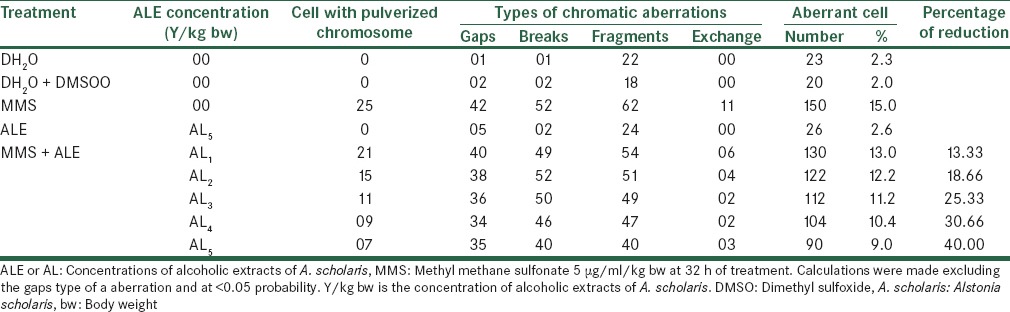
Figure 3.
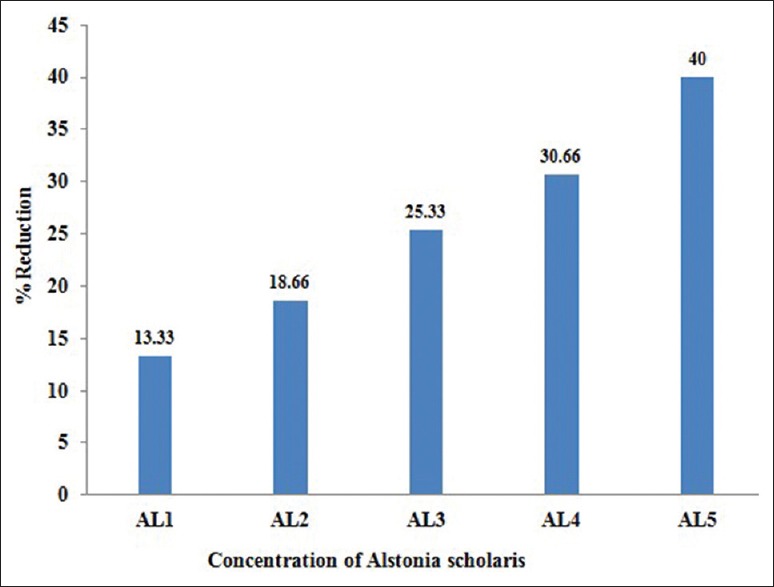
In vivo effect of alcoholic extracts of Alstonia scholaris at 32 h
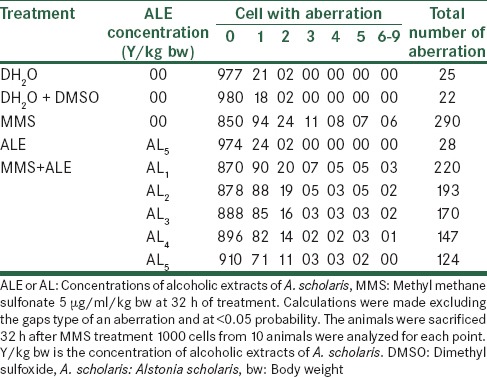
The total aberrant chromosomal frequencies per thousand cells recorded were 290 for MMS and about 220, 193, 170, 147 and 124 for Alstonia extract along with MMS for five different concentrations of Alstonia extract. These frequencies show the effects of Alstonia extract in significantly reducing the total aberrations as well as aberrations per cell as shown in Table 6.
Table 6.
Effect of with alcoholic extracts of A. scholaris on the total number and types of frequency of cells with chromosome aberrations induced by MMS
In vitro effects
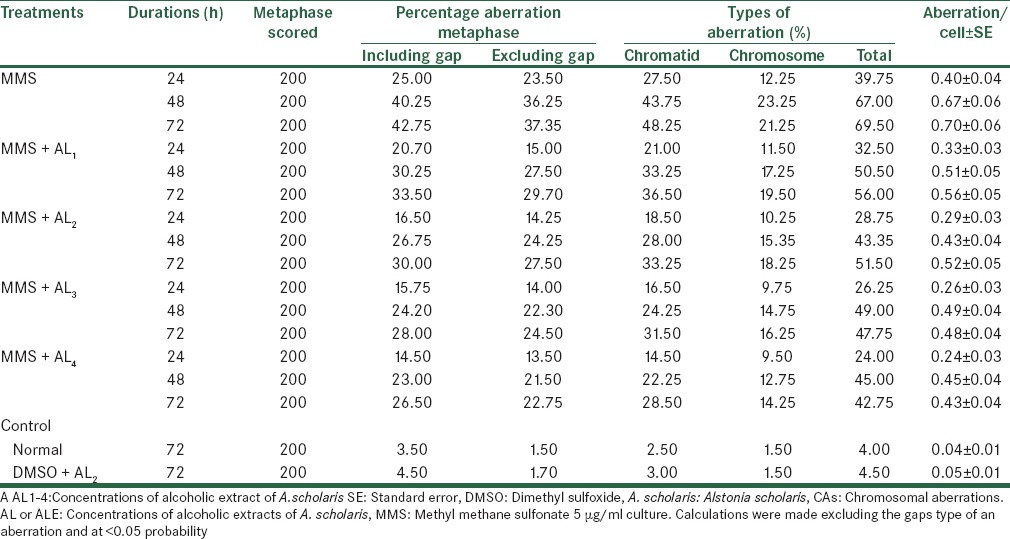
Treatment with MMS results in clastogenic abnormalities as observed in percent metaphase aberration, types of aberrations and aberration per cell viz., 39.75%, 67.00%, 69.50% and 0.40, 0.67 and 0.70 aberration per cell, whereas the control the normal and the DMSO plus Alstonia extract values are 04.00, 04.50 per cell at single standard dosage and for three various durations are 24, 48 and 72 h. Alstonia extract bring down aberrations from 39.75% to 32.50%, 28.75%, 26.25% and 24.00% with four consecutive dosages of Alstonia extract at 24 h of duration, whereas at 48 h, it is lowered from 67.00% to 50.50%, 43.35%, 49.00% and 45.00% respectively by first to fourth concentrations of Alstonia extract. Similar trend was noticed when the treatment durations were increased to 72 h. These values show linear increasing trend with dosages, but it does not depend on dose-durations. The maximum percentage reductions in the aberrations were 39.62 for 24 h, which were 32.83 and 38.48 for 48 and 72 h respectively [Table 7].
Table 7.
Analysis of CAs after treatment with MMS along with alcoholic extracts of A. scholaris in vitro in the absence of −S9 mix
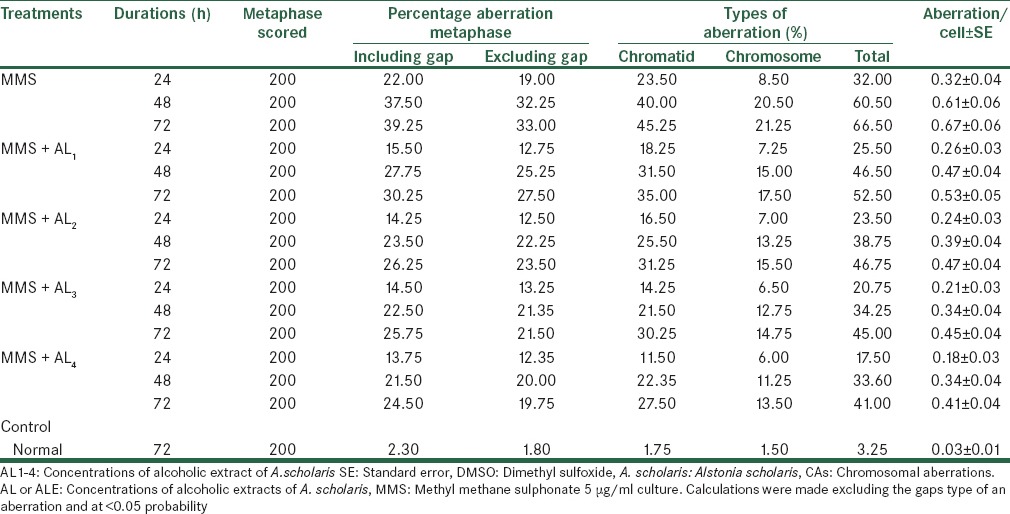
When culture was setup along with metabolic activation system (+S9 mix), the effect of MMS increased. Similarly, the effects of Alstonia extract also lowered the clastogenic activity of MMS. These values show linearly increasing trend with doses [Table 8]. The maximum effective percentage reductions were 45.31%, 44.46%, and 38.34% for 24, 48 and 72 h respectively. The highest reduction on clastogeny of cells was noticed at 24 h durations; though the other values were also statistically significant.
Table 8.
Analysis of CAs after treatment with MMS along with alcoholic extracts of A. scholaris in vitro in the presence of +S9 mix
The experiment were conducted for SCEs assay [Tables 9 and 10], the reduction was evident both in the absence as well as in the presence of metabolic activation; there being a lowering of the mean range and the total SCEs and SCE per cell from 07.70 to 04.30 and from 7.20 to 04.20. For conducting SCE assay, only 48 h of cultures were done, and 50 metaphases were scored for counting the number of exchanges.
Table 9.
Analysis of SCE after treatment with MMS along with alcoholic extracts of A. scholaris in vitro, in the absence of −S9 mix
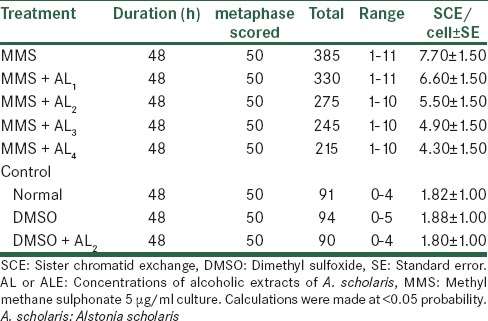
Table 10.
Analysis of SCE after treatment with MMS along with alcoholic extracts of A. scholaris in vitro, in the presence of +S9 mix
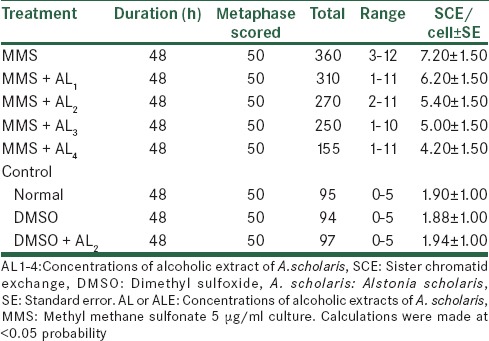
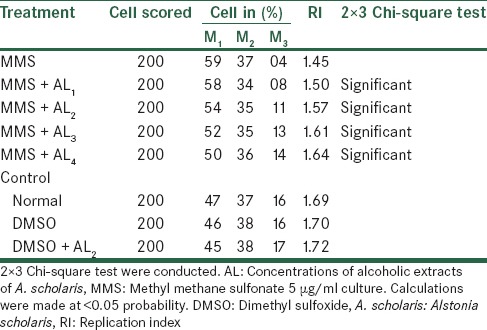
The effects of Alstonia extract on RI [Tables 11 and 12] show an elevated level when compared with the MMS treatment that is, rising from 1.45 to 1.58, though still lower than the normal level of 1.69. The effect, after treatment with metabolic activation system shows to elevated from 1.45 to 1.64 that is, being much effective than one without metabolic activation system. Therefore, we observed that Alstonia extract has potent anti-clastogenic activities in these experiments.
Table 11.
Analysis of cell cycle kinetics after treatment with MMS along with alcoholic extracts of A. scholaris in vitro, in the absence of −S9 mix
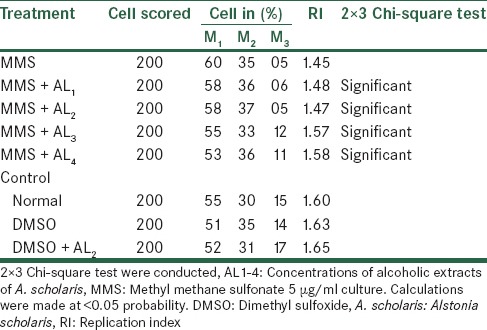
Table 12.
Analysis of cell cycle kinetics after treatment with MMS along with alcoholic extracts of A. scholaris in vitro, in the presence of +S9 mix
DISCUSSION
Cancer cells are “immortal” that is, they have lost their growth restraining mechanisms and so multiply out of control. This results from alteration of cellular DNA or genetic material, which can be an inherited defect. It was found that free radical damage is the cause of these genetic mutations. When DNA or genetic material is involved in free radical reactions, mutations or genetic alteration can result. Free radical chain reactions are stopped by the action of antioxidants. In our experiment, the protective effects of alcoholic extracts of A. scholaris may be due to this reason that is, quenching the free radicals that were generated due to mutagen and carcinogen.
The anticancer effect of various doses of an alkaloid fraction of A. scholaris (ASERS), was studied in vitro in cultured human neoplastic cell lines (HeLa, HepG2, HL60, KB and MCF-7) and in Ehrlich ascites carcinoma-bearing mice.[21] Treatment of HeLa cells with 25 g/ml ASERS resulted in a time-dependent increase in the antineoplastic activity and the greatest activity was observed when the cells were exposed to ASERS for 24 h.[21] However, exposure of cells to ASERS for 4 h resulted in 25% viable cells and hence this time interval was considered to be the optimum time for treatment and further studies were carried out using this time.[21] Treatment of various cells with ASERS resulted in a concentration-dependent decline in the viable cells and a nadir was reached at 200 g/ml in the entire cell lines studied, we too have noticed similar trend in our in vitro experiment. The inhibitory concentration 50% was found to be 5.53, 25, 11.16, 10 and 29.76 g/ml for HeLa, HePG2, HL60, KB and MCF-7 cells, respectively. Similarly, administration of ASERS, once daily for nine consecutive days to the tumor-bearing mice caused a dose-dependent remission of the tumor up to 240 mg/kg bw, where the greatest antitumor effect was observed. Since 240 mg/kg ASERS showed toxic manifestations, the next lower dose of 210 mg/kg was considered as the best effective dose, in which 20% of the animals survived up to 120 days posttumor cell inoculation as against no survivors in the saline-treated control group.[21]
The chemomodulatory activity of A. scholaris extract was studied in combination with berberine hydrochloride (BCL), a topoisomerase inhibitor, in Ehrlich ascites carcinoma-bearing mice. The best effect was observed when 180 mg/kg of A. scholaris extract (ASE) was combined with 6 or 8 mg/kg of BCL, where an increase in the antineoplastic activity was reported.[9]
The possible chemopreventive and anti-oxidative properties of this medicinal plant on two-stage process of skin carcinogenesis induced by a single application of 7,12-dimethyabenz (a) anthracene (100 lg/100 ll acetone), whereas 2 weeks later, these are promoted by repeated application of croton oil (1% increase in incidence, tumor yield, tumor burden and cumulative number of papillomas). These changes are found to be higher in the carcinogen treated control (without ASE treatment) as compared to experimental animals (ASE treated). Furthermore, a significant increase in reduced glutathione, superoxide dismutase, and catalase level but decrease in lipid peroxidation was measured in ASE administered experimental groups than the carcinogen treated controls.[22]
Chemopreventive agents can be targeted by intervention at the initiation, promotion, or progression stage of multistage carcinogenesis.[23,24] The intervention of cancer at the promotion stage, however, seems to be the most appropriate and practical. The major reason for that is, relates to the fact that tumor promotion is a reversible event at least in early stages and requires repeated and prolonged exposure of a promoting agent.[25]
The hydroalcoholic extract of A. scholaris protected against benzo(a) pyrene-induced forestomach carcinoma in the female mice when the extract is added to drinking water at doses of 1, 2 and 4 mg/ml for 2 weeks before the treatment, during the treatment and 2 weeks after the carcinogen exposure. These doses reduced tumor multiplicity by 21.43, 28.57 and 50%, respectively. The greatest protection was afforded by the highest dose, which reduced tumor incidence by 6.67%.[26] Tumor multiplicity incidence was significantly reduced (91.93% with extract vs. 100% in benzo(a) pyrene-treated mice) by 4 mg/ml dose that was added to the drinking water during the postinitiation period, starting at 48 h after the last dose of benzo(a) pyrene (posttreatment) these continued for 8 weeks. These findings were corroborated by the observation that micronuclei frequency reached the lowest point at 4 mg/ml of the extract. The extract was able to inhibit benzo(a) pyrene-induced mutagenic changes as the frequency of splenocytes bearing one micronucleus and also cells, which bear multiple micronuclei were reduced by the administration of the extract.[26] In our in vitro experiments, we observe the enhancement of replication indices that support the above finding.
The anticancer properties of this medicinal plant was evaluated and the tumor incidence, tumor yield, tumor burden and cumulative number of papillomas were found to be higher in the carcinogen treated control compared to animals treated with A. scholaris extract. Furthermore, a significant increase in reduced glutathione, superoxide dismutase, and catalase level but decrease in lipid peroxidation was observed in ASE administered experimental groups than the carcinogen with control-treated. This study demonstrated the chemopreventive potential of A. scholaris bark extract in 7,12-dimethylbenz(a) anthracene-induced skin tumor genesis in albino mice.[27] The aqueous extract at 50 mg/kg bw induced the cellular immune response while at 100 mg/kg bw inhibited the delayed type of hypersensitivity reaction.[7]
An 85% of ethanolic bark extract of A. scholaris showed antitumor and radiation sensitizing activity against a mouse transplantable tumor and is cytotoxic to human tumor cell lines.[28] The ethanolic extract of A. scholaris was also found to decrease the malondialdehyde level that prevented lipid peroxidation.[29] Other reports also suggested the presence of nitric oxide scavenging activity in case of A. scholaris.[9] It was observed the ethanolic extract of Alternanthera sessilis is a free radical inhibitor and scavenger acting possibly as a primary antioxidant, an observation, which can be correlated with studies reported by Mau et al.[30]
Besides flavonoids and phenolic compounds, some of the alkaloids, saponins and triterpenoids are also reported to possess antioxidant activity.[31] The presence of flavonoids, alkaloids, and triterpenoids in the alcoholic extract of A. scholaris has already been reported.[32] The results of the present phytochemical investigation further add to these conclusions.
CONCLUSION
Alcoholic extracts of A. scholaris reduces the total aberrant cells ranges from 10.0% to 41.84% and among them it reduces total frequencies of aberration ranges from 220 to 124 against 290 aberrations causes due to MMS in vivo. The same trends were observed in the in vitro experiments that is, it reduces CAs 39.62%, 32.83%, and 38.48% ± standard error (SE) at 24, 48, and 72 h of exposure respectively; but when experiments were carried out in the presence of liver S9 fraction, these values were 45.31%, 44.46%, and 38.34% ± SE respectively at <0.05 level, likewise it also reduces SCE from 7.70 to 4.20 ± SE per cell and enhances RI from 1.45 to 1.64.
Alcoholic extracts of A. scholaris significantly reduces the number of aberrant cells and frequency of aberration per cell at each concentration and duration of exposure in vivo; similarly it reduces CAs and SCE and enhances RI in vitro both of which were statistically significant at <0.05 level.
Financial support and sponsorship
UGC Major Research project (F. No. 42-500/SR (2013), New Delhi.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Balachandran P, Govindarajan R. Cancer – An ayurvedic perspective. Pharmacol Res. 2005;51:19–30. doi: 10.1016/j.phrs.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Bradstreet K. Australia: Woodland Publishing; 1997. Herbs for Detoxification; pp. 10–3. [Google Scholar]
- 3.Nair CK, Divyasree P, Gopakumar G. Ethnomedicinal plants to fight neoplastic diseases. In: Debprasad C, editor. Ethnomedicine: A Source of Complementary Therapeutics. Trivandrum: Publisher Research Signpost; 2010. p. 661. [Google Scholar]
- 4.Dey A. Alstonia scholaris R.Br. (Apocynaceae): Phytochemistry and pharmacology: A concise review. J Appl Pharm Sci. 2011;01:51–7. [Google Scholar]
- 5.Joshi SG. New Delhi: Oxford and IBH Publishing Co. Pvt. Ltd; 2000. Medicinal Plants. [Google Scholar]
- 6.Arulmozhi S, Mazumder PM, Sathiya NL, Prasad A, Thakurdesai P. In vitro antioxidant and free radical scavenging activity of fractions from Alstonia scholaris Linn. R. Br. Int J Pharm Technol Res. 2010;2:18–25. [Google Scholar]
- 7.Iwo MI, Soemardji AA, Retnoningrum DS, Sukrasno UUM. Immunostimulating effect of pule (Alstonia scholaris L. R.Br. Apocynaceae) bark extracts. Clin Hemorheol Microcirc. 2000;23:177–83. [PubMed] [Google Scholar]
- 8.Saraswathi V, Mathuram V, Subramanian S, Govindasamy S. Modulation of the impaired drug metabolism in sarcoma-180-bearing mice by echitamine chloride. Cancer Biochem Biophys. 1999;17:79–88. [PubMed] [Google Scholar]
- 9.Jagetia GC, Baliga MS. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: A preliminary study. J Med Food. 2004;7:343–8. doi: 10.1089/jmf.2004.7.343. [DOI] [PubMed] [Google Scholar]
- 10.Es-Safi NE, Khlifi S, Kerhoas L, Kollmann A, El Abbouyi A, Ducrot PH. Antioxidant constituents of the aerial parts of Globularia alypum growing in Morocco. J Nat Prod. 2005;68:1293–6. doi: 10.1021/np0501233. [DOI] [PubMed] [Google Scholar]
- 11.Harish R, Shivanandappa T. Antioxidant activity and hepatoprotective potential of Phyllanthus niruri. Food Chem. 2006;95:180–5. [Google Scholar]
- 12.Channa S, Dar A, Ahmed S, Atta-ur-Rahman Evaluation of Alstonia scholaris leaves for broncho-vasodilatory activity. J Ethnopharmacol. 2005;97:469–76. doi: 10.1016/j.jep.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Baliga MS. Alstoni scholaris Linn R Br in the treatment and prevention of cancer: Past, present, and future. Integr Cancer Ther. 2010;9:261–9. doi: 10.1177/1534735410376068. [DOI] [PubMed] [Google Scholar]
- 14.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 15.Evans HJ. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. In: Kiley BJ, Legator M, Nichols W, Ramel C, editors. Handbook of Mutegenicity Test Procedures. Amsterdam: Elsevier; 1984. pp. 40–427. [Google Scholar]
- 16.Latt SA, Wohlleb JC. Optical studies of the interaction of 33258 Hoechst with DNA, chromatin, and metaphase chromosomes. Chromosoma. 1975;52:297–316. doi: 10.1007/BF00364015. [DOI] [PubMed] [Google Scholar]
- 17.Latt SA, Allen J, Bloom SE, Carrano A, Falke E, Kram D, et al. Sister-chromatid exchanges: A report of the GENE-TOX program. Mutat Res. 1981;87:17–62. doi: 10.1016/0165-1110(81)90003-8. [DOI] [PubMed] [Google Scholar]
- 18.Crossen PE, Morgan WF. Analysis of human lymphocyte cell cycle time in culture measured by sister chromatid differential staining. Exp Cell Res. 1977;104:453–7. doi: 10.1016/0014-4827(77)90116-1. [DOI] [PubMed] [Google Scholar]
- 19.Tice R, Schneider EL, Rary JM. The utilization of bromodeoxyuridine incorporation into DNA for the analysis of cellular kinetics. Exp Cell Res. 1976;102:232–6. doi: 10.1016/0014-4827(76)90037-9. [DOI] [PubMed] [Google Scholar]
- 20.Fisher A, Yates F. 6th ed. New York: Hafner Pub. Co; 1963. Statistical Tables for Biological Agricultural and Medical Research. [Google Scholar]
- 21.Jagetia GC, Baliga MS. Evaluation of anticancer activity of the alkaloid fraction of Alstonia scholaris in vitro and in vivo. J Med Food. 2006;20:103–9. doi: 10.1002/ptr.1810. [DOI] [PubMed] [Google Scholar]
- 22.Jahan S, Chaudhary R, Goyal PK. Anticancer activity of an Indian medicinal plant, Alstonia scholaris, on skin carcinogenesis in mice. Integr Cancer Ther. 2009;8:273–9. doi: 10.1177/1534735409343590. [DOI] [PubMed] [Google Scholar]
- 23.Morse MA, Stoner GD. Cancer chemoprevention: Principles and prospects. Carcinogenesis. 1993;14:1737–46. doi: 10.1093/carcin/14.9.1737. [DOI] [PubMed] [Google Scholar]
- 24.Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem Suppl. 1995;22:169–80. doi: 10.1002/jcb.240590822. [DOI] [PubMed] [Google Scholar]
- 25.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 26.Jagetia GC, Baliga MS. Treatment with Alstonia scholaris enhances radiosensitivity in vitro and in vivo. J Med Food. 2003;18:917–29. doi: 10.1089/108497803322702888. [DOI] [PubMed] [Google Scholar]
- 27.Swafiya J, Ranu C, Pradeepkumar G. Anticancer activity of an Indian medicinal plant, Alstonia scholaris on skin carcinogenesis in mice. Integr Cancer Ther. 2010;9:261–9. doi: 10.1177/1534735409343590. [DOI] [PubMed] [Google Scholar]
- 28.Baliga MS, Jagetia GC, Ulloor JN, Baliga MP, Venkatesh P, Reddy R, et al. The evaluation of the acute toxicity and long term safety of hydroalcoholic extract of Sapthaparna (Alstonia scholaris) in mice and rats. Toxicol Lett. 2004;151:317–26. doi: 10.1016/j.toxlet.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Arulmozhi S, Rasal VP, Sathiyanarayanan L, Ashok P. Screening of Alstonia scholaris Linn. R Br. for wound healing activity. Orient Pharm Exp Med. 2007;7:254–60. [Google Scholar]
- 30.Mau JL, Chao GR, Wu KT. Antioxidant properties of methanolic extracts from several ear mushrooms. J Agric Food Chem. 2001;49:5461–7. doi: 10.1021/jf010637h. [DOI] [PubMed] [Google Scholar]
- 31.Rai S, Wahile A, Mukherjee K, Saha BP, Mukherjee PK. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J Ethnopharmacol. 2006;104:322–7. doi: 10.1016/j.jep.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Khan MR, Omoloso AD, Kihara M. Antibacterial activity of Alstonia scholaris and Leea tetramera. Fitoterapia. 2003;74:736–40. doi: 10.1016/s0367-326x(03)00192-8. [DOI] [PubMed] [Google Scholar]


