Abstract
Background
Papua New Guinea exhibits a complex malaria epidemiology due to diversity in malaria parasites, mosquito vectors, human hosts, and their natural environment. Heterogeneities in transmission and burden of malaria at various scales are likely to affect the success of malaria control interventions, and vice-versa. This manuscript assesses changes in malaria prevalence, incidence and transmission in sentinel sites following the first national distribution of long-lasting insecticidal nets (LLINs).
Methods
Before and after the distribution of LLINs, data collection in six purposively selected sentinel sites included clinical surveillance in the local health facility, household surveys and entomological surveys. Not all activities were carried out in all sites. Mosquitoes were collected by human landing catches. Diagnosis of malaria infection in humans was done by rapid diagnostic test, light microscopy and PCR for species confirmation.
Results
Following the roll-out of LLINs, the average monthly malaria incidence rate dropped from 13/1,000 population to 2/1,000 (incidence rate ratio = 0.12; 95 % CI: 0.09–0.17; P < 0.001). The average population prevalence of malaria decreased from 15.7 % pre-LLIN to 4.8 % post-LLIN (adjusted odds ratio = 0.26; 95 % CI: 0.20–0.33; P < 0.001). In general, reductions in incidence and prevalence were more pronounced in infections with P. falciparum than with P. vivax. Additional morbidity indicators (anaemia, splenomegaly, self-reported fever) showed a decreasing trend in most sites. Mean Anopheles man biting rates decreased from 83 bites/person/night pre-LLIN to 31 post-LLIN (P = 0.008). Anopheles species composition differed between sites but everywhere diversity was lower post-LLIN. In two sites, post-LLIN P. vivax infections in anophelines had decreased but P. falciparum infections had increased despite the opposite observation in humans.
Conclusions
LLIN distribution had distinct effects on P. falciparum and P. vivax. Higher resilience of P. vivax may be attributed to relapses from hypnozoites and other biological characteristics favouring the transmission of P. vivax. The effect on vector species composition varied by location which is likely to impact on the effectiveness of LLINs. In-depth and longer-term epidemiological and entomological investigations are required to understand when and where residual transmission occurs and whether observed changes are sustained.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1635-x) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Insecticide treated nets, Anopheles, Papua New Guinea, Anopheles punctulatus
Background
Papua New Guinea (PNG) exhibits a diverse and complex malaria epidemiology [1]. Four Plasmodium species (P. falciparum, P. vivax, P. ovale and P. malariae) are endemic and more than ten Anopheles species filling different ecological niches have been incriminated in the parasite’s transmission [2]. Historical malaria control measures in the 1960s and 1970s included indoor residual spraying of insecticides, mass drug administration as well as environmental management in certain areas resulting in substantial initial reductions in malaria in many locations [3, 4]. The cessation of the spraying program in the 1980s coinciding with the decentralization of responsibilities for malaria control as well as emerging resistance of the malaria parasites to commonly used drugs [5, 6] led to a subsequent resurgence, particularly of P. falciparum malaria, across most parts of PNG [7].
In 2004, the Government of Papua New Guinea re-intensified its malaria control efforts with the financial support of a round 3 grant from the Global Fund to Fight AIDS, Tuberculosis and Malaria. The central component of this program was the first country-wide free distribution of long-lasting insecticidal mosquito nets (LLIN) [8]. Insecticide-treated nets had been shown to reduce the incidence and prevalence of falciparum-malaria in children in PNG as early as 1985 [9]. Yet, while these results were later reflected in policy documents [10], little effort was made in practice to scale up mosquito net use. Coverage with mosquito nets, particularly insecticide-treated nets, therefore remained patchy and low in most parts of the country until 2004 [11–13]. The Global Fund supported program that subsequently facilitated a single round of LLIN distribution resulted in an increase in ownership and use of bednets, particularly LLINs. A national household survey after the distribution found 80 % of all households owning a mosquito net of any type and 65 % owning a LLIN, while 33 % of people reported to sleep under a LLIN [8]. In six sentinel surveillance sites established by the Papua New Guinea Institute of Medical Research (PNGIMR), average LLIN ownership increased from 9 % before the distribution to 89 % thereafter and LLIN use rose from 6 to 55 % [8].
This manuscript assesses malaria morbidity and transmission indicators in sentinel sites before and after the LLIN distribution. The study was part of the evaluation of the Global Fund round 3 malaria grant.
Methods
Study sites
Sentinel site locations were selected purposively in 2008 in places which were yet to be covered with the LLIN campaign. The sites were located in the Momase and Highlands regions of the main island of PNG (Fig. 1) and consisted of three to four randomly selected villages in the catchment area of a sentinel health facility. Five sites were located in Momase: Dreikikir in the hills on the edges of the Sepik River basin (altitude 200–400 m), Finschhafen on the Morobe coastline of the Huon peninsula (altitude 0–50 m), Sausi in the Ramu River valley (altitude 100–250 m), Mumeng at an intermediate altitude (450–1,550 m) along the road to Bulolo and Wau and Yapsie/Yapsiei near the Indonesian border along a tributary of the Sepik River (altitude 150–250 m). Tabibuga in the Jimi Valley was the only site in the Highlands (altitude 1,350–1,500 m).
Fig. 1.
Location of sentinel sites used for (1) clinical surveillance, (2) household surveys, and (3) entomological surveys. Major rivers and lake in blue
Data collection
Data were collected prior to the LLIN campaign (10/2008–08/2009) and one year later after LLINs had been distributed (10/2009–08/2010). Data collection included clinical surveillance in the local health centre in three sites (Dreikikir, Mumeng and Sausi) over a period of approximately two months pre- and post-LLIN, coinciding roughly with the main malaria transmission season. A household survey in two to three randomly selected villages in the health centre’s catchment area was carried out in five sites (Finschhafen, Mumeng, Sausi, Tabibuga and Yapsie) and entomological surveys were conducted in five sites (Dreikikir, Finschhafen, Mumeng, Sausi and Yapsie). For operational reasons not all activities could be carried out in all sites (Additional file 1: Table S1).
Clinical surveillance
For the clinical surveillance, all patients attending the health facility were screened for a history of fever within the previous three days. A research nurse collected a finger-prick blood sample from all consenting fever patients and malaria was diagnosed by rapid diagnostic test (RDT; ICT Malaria Combo, ICT Diagnostics, South Africa). From the same blood sample, thick and thin blood films were prepared on one slide and haemoglobin (Hb) levels were measured using a portable analyser (HemoCue Hb 201+, HemoCue AB, Sweden). Anaemia and severe anaemia were defined in accordance with World Health Organization definitions applying age group-specific cut-offs and altitude adjustment [14]. A dry blood spot was prepared on Whatman 3MM filter paper (GE Healthcare), whenever possible, and stored in individual plastic zip-bags with desiccant silica gel. Axillary temperature was measured with an electronic thermometer, and in patients between 2 and 9 years of age the spleen was palpated and graded according to Hackett [15]. Demographic details of the patient were recorded in a paper form. The subsequent clinical assessment, final diagnosis and treatment were then taken over by the health facility’s clinician following standard procedures and results were recorded on the patient’s study form.
Household survey
For the household survey, three (in the case of Yapsie four) villages were randomly selected from the sentinel health facility’s catchment area. In each village, 30 to 35 households were randomly sampled from a list of all households. A structured questionnaire about the coverage with malaria control interventions was administered to the heads of sampled households. A finger-prick blood sample was collected from household members above five months of age for preparing a microscopy slide, a dry blood spot on filter paper, and for measuring Hb levels as described above. Symptomatic individuals were diagnosed on the spot using a malaria RDT and positive cases were treated according to standard treatment guidelines [16]. Axillary temperature was measured with an electronic thermometer. Details of the household survey methodology have been described in more details elsewhere [8, 17].
Entomological surveys
Entomological surveys were conducted in two villages per site. Mosquitoes were collected by outdoor human landing catch from 6 households per village prior to the LLIN distribution and again 12 months later. The number of person-nights per collection ranged from 16 to 48. Collectors worked in pairs with one member collecting from 18:00 to 24:00 h and the second collecting from 24:00 to 06:00 h. Mosquitoes were stored per collection hour and identified to morphological species before storage on silica gel. Lysates from whole mosquitoes were screened for P. falciparum, P. vivax 210 and P. vivax 247 circumsporozoite proteins by enzyme-linked immunosorbent assay [18]. DNA was extracted from a portion of the lysate using DNEasy blood and tissue kit (QIAGEN, Maryland, USA). Mosquitoes that were morphologically identified as members of the An. punctulatus group were confirmed to species by polymerase chain reaction (PCR) restriction length polymorphism of the ITS2 region [2].
Laboratory procedures
Thin blood smears were first fixed with methanol and thick and thin smears stained with Giemsa and read by light microscopy independently by two PNGIMR microscopists. Discordant reads were confirmed by a senior microscopist. The number of parasites was counted until reaching 200 white blood cells and a slide was declared negative only after reading a minimum of 200 thick film fields. Molecular diagnosis was used to complement missing second reads and to disambiguate discordant species read results (clinical surveillance n = 127; household survey n = 87). DNA was extracted from filter papers using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, USA) or the Favorgen 96-Well Genomic DNA Kit (Favorgen Biotech Corp., Taiwan) following the manufacturer’s protocols. The molecular assay was a semi-quantitative post-PCR, ligase detection reaction/fluorescent microsphere assay described in more detail elsewhere [19–21].
Data analysis
Data were double-entered into a Microsoft FoxPro or Microsoft Access database at PNGIMR Goroka and analysed with Stata (StataCorp, USA) software. Differences in binary and categorical variables between study sites were assessed by Chi-square test and logistic regression, differences in continuous variables by t-test and linear regression and a non-parametric test for median age. Multivariable logistic and linear regression models assessing changes in malaria prevalence, parasite density and morbidity indicators were adjusted for age group and study site, anaemia furthermore for sex. In the clinical surveillance samples, primary diagnosis of malaria was based on the result of the RDT as performed in routine clinical practice while species-results were based on light microscopic/PCR diagnosis, which comprised only a sub-set of patients in the case of Mumeng post-LLIN. For the calculation of incidence rates, a stable population denominator was used for both years in the absence of available village-level growth rates.
Results
Study sample
Clinical surveillance in three sites included 1,325 fever patients pre-LLIN and 680 post-LLIN. The household surveys in five sites included blood samples from 1,967 individuals pre-LLIN and 1,986 individuals post-LLIN. Pre-LLIN and post-LLIN samples had comparable age distributions in all sites with the exception of the Mumeng clinical surveillance sample (P = 0.02). Sample details including LLIN coverage by site are provided in Additional file 2: Table S2. Entomological surveys collected 15,481 anophelines pre-LLIN and 6,066 anophelines post-LLIN. Due to the high density of mosquitoes collected in Sausi, only 34 % (n = 5,036) of the pre-LLIN collection in this site and 43 % (n = 2,558) of the post-LLIN collection were screened for Plasmodium spp. infection.
Malaria incidence and morbidity in health facilities
Across the three sentinel health facilities (Dreikikir, Mumeng, Sausi), the average proportion of fever patients with a positive RDT decreased from 57.3 % (95 % CI: 54.6–60.0) pre-LLIN to 17.9 % (95 % CI: 15.1–21.0) post-LLIN resulting in a 87 % drop in the pooled crude monthly incidence rate from 13/1,000 population to 2/1,000 (incidence rate ratio IRR = 0.12; 95 % CI: 0.09–0.17; P < 0.001) (Table 1).
Table 1.
Crude monthly malaria incidence rate (IR) in three sentinel health facilities. Diagnosis by RDT
| Site | Patients | Pre-LLIN | Post-LLIN | Change | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | RDT+/ Month | IR/1,000 | n | RDT+/ Month | IR/1,000 | /1,000 | % | ||
| Dreikikir | 8,300 | 377 | 178 | 21.5 | 290 | 27 | 3.3 | -18.2 | -84.7 |
| Mumeng | 17,000 | 469 | 102 | 6.0 | 215 | 11 | 0.6 | -5.4 | -90.0 |
| Sausi | 6,700 | 458 | 122 | 18.2 | 172 | 12 | 1.8 | -16.4 | -90.1 |
| Overall | 32,000 | 1,304 | 402 | 12.6 | 677 | 50 | 1.6 | -11.0 | -87.3 |
Based on PCR-corrected light microscopy, the reduction was more pronounced in the proportion of fever cases infected with P. falciparum (46.8 to 9.1 %; adjusted odds ratio AOR = 0.10; 95 % CI: 0.07–0.14; P < 0.001) than with P. vivax (12.7 to 6.9 %; AOR = 0.59; 95 % CI: 0.40–0.85; P < 0.001). Reductions in P. vivax infections were statistically significant only in children below 5 years of age in two sites (Mumeng AOR = 0.09, 95 % CI: 0.01–0.64; P = 0.017; Sausi AOR = 0.36; 95 % CI: 0.15–0.91) but not in older age groups (all sites AOR = 0.62; 95 % CI: 0.34–1.14; P = 0.122). In Dreikikir, there was no significant reduction in P. vivax in any age group (AOR = 0.87; 95 % CI: 0.51–1.48; P = 0.6). The decrease in infections with P. malariae was substantial but due to low numbers of cases not statistically significant (2.1 to 0.3 %; AOR = 0.24; 95 % CI: 0.06–1.04; P = 0.057).
The parasite species composition in the febrile patient sample changed in Dreikikir (but not in the other sites) from a clear dominance of P. falciparum over P. vivax (67.9 vs 12.9 %, Fisher's exact test, P < 0.001) to equal proportions of the two species (7.9 vs 8.6 %, P = 0.88). Pre-LLIN, P. falciparum infection prevalence peaked in fever patients aged 5–9 years, and P. vivax in 1–4 year olds. Post-LLIN, the P. falciparum peak had shifted to later age groups, while for P. vivax infections a clear peak could not be identified due to the low number of positive patients. Changes in test-positivity and species composition by site are shown in Fig. 2.
Fig. 2.
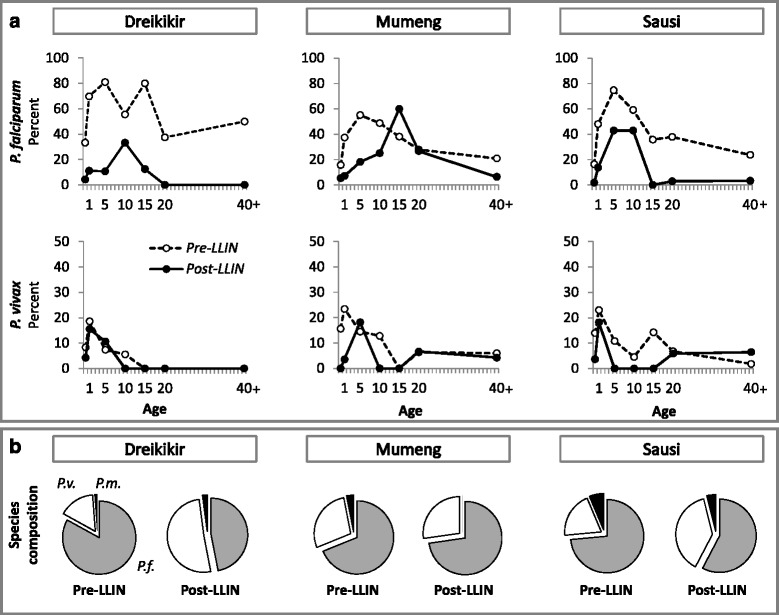
a Prevalence of malaria in fever patients by site and age group before (dotted line) and after (solid line) LLIN distribution. b Species distribution by site. Abbreviations: P.f., P. falciparum (grey), P.v., P. vivax (white), P.m., P. malariae (black)
In two sites (Mumeng and Sausi), anaemia and splenomegaly were assessed in the fever patients. A statistically significant reduction was found in anaemia in Mumeng (52.9 to 40.7 %; AOR = 0.6; 95 % CI: 0.5–0.9; P = 0.015), and in splenomegaly (all grades) in both sites (pooled 36.9 to 12.9 %; AOR = 0.3; 95 % CI: 0.1–0.5; P < 0.001). Reductions in anaemia in Sausi and of severe anaemia in both sites were not statistically significant (Fig. 3). Details by site are provided as supplementary material (Additional file 3: Table S3).
Fig. 3.
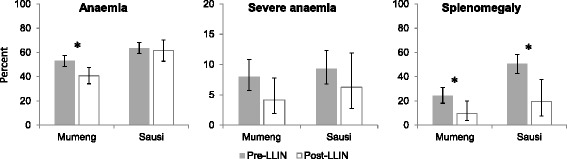
Indicators of morbidity in fever patients before and after LLIN distribution. Error bars show 95 % confidence intervals. *P < 0.05
Malaria prevalence and morbidity in the general population
Across five sentinel sites, the average prevalence of malaria by light microscopy in the general population of the health facility catchment areas decreased from 15.7 % pre-LLIN to 4.8 % post-LLIN (AOR = 0.26; 95 % CI: 0.20–0.33; P < 0.001). The largest reduction was observed in Yapsie, the site with the highest pre-LLIN prevalence of both P. falciparum (18.9 %) and P. vivax (11.8 %). Across all sites, the reductions were more pronounced in infections with P. falciparum than with P. vivax (Table 2). The changes in P. vivax infections were only statistically significant in two sites (Finschhafen and Yapsie) (Table 2). In three sites (Finschhafen, Tabibuga and Yapsie), there was a change from P. falciparum dominance to P. vivax dominance, which reached statistical significance (P = 0.005) only in Tabibuga (Fig. 4). There was no statistically significant difference in the observed reductions between age groups or sex (Mantel Haenszel test of homogeneity of odds ratios, all P > 0.1).
Table 2.
Malaria prevalence in the general population before and after LLIN distribution
| Site | Pre-LLIN | Post-LLIN | ||||
|---|---|---|---|---|---|---|
| n | % (95 % CI) | n | % (95 % CI) | AOR | P-value | |
| Any species | ||||||
| Finschhafen | 455 | 9.9 (7.3–13.0) | 442 | 2.5 (1.2–4.4) | 0.16 (0.07–0.34) | < 0.001 |
| Mumeng | 290 | 10.0 (6.8–14.0) | 462 | 7.8 (5.5–10.6) | 0.62 (0.36–1.07) | 0.086 |
| Sausi | 337 | 9.2 (6.3–12.8) | 422 | 4.7 (2.9–7.2) | 0.48 (0.26–0.88) | 0.017 |
| Tabibuga | 325 | 9.8 (6.8–13.6) | 341 | 3.2 (1.6–5.7) | 0.25 (0.12–0.52) | < 0.001 |
| Yapsie | 560 | 30.5 (26.7–34.5) | 319 | 5.6 (3.4–8.8) | 0.10 (0.06–0.18) | < 0.001 |
| Overall | 1,967 | 15.7 (14.1–17.3) | 1,986 | 4.8 (3.9–5.9) | 0.26 (0.20–0.33) | < 0.001 |
| P. falciparum | ||||||
| Finschhafen | 455 | 6.2 (4.1–8.8) | 442 | 0.7 (0.1–2.0) | 0.10 (0.03–0.33) | < 0.001 |
| Mumeng | 290 | 8.6 (5.7–12.5) | 462 | 5.4 (3.5–7.9) | 0.49 (0.27–0.91) | 0.023 |
| Sausi | 337 | 5.9 (3.7–9.0) | 422 | 2.6 (1.3–4.6) | 0.46 (0.21–0.98) | 0.044 |
| Tabibuga | 325 | 6.2 (3.8–9.3) | 341 | 0.6 (0.1–2.1) | 0.08 (0.02–0.34) | 0.001 |
| Yapsie | 560 | 18.9 (15.8–22.4) | 319 | 2.5 (1.1–4.9) | 0.12 (0.05–0.24) | < 0.001 |
| Overall | 1,967 | 10.1 (8.8–11.5) | 1,986 | 2.5 (1.8–3.2) | 0.23 (0.16–0.32) | < 0.001 |
| P. vivax | ||||||
| Finschhafen | 455 | 4.2 (2.5–6.4) | 442 | 1.6 (0.6–3.2) | 0.25 (0.09–0.68) | 0.007 |
| Mumeng | 290 | 3.8 (1.9–6.7) | 462 | 2.2 (1.0–3.9) | 0.44 (0.18–1.07) | 0.071 |
| Sausi | 337 | 3.6 (1.9–6.1) | 422 | 2.6 (1.3–4.6) | 0.67 (0.28–1.58) | 0.357 |
| Tabibuga | 325 | 2.8 (1.3–5.2) | 341 | 2.6 (1.2–5.0) | 0.84 (0.32–2.20) | 0.728 |
| Yapsie | 560 | 11.8 (9.2–14.7) | 319 | 3.1 (1.5–5.7) | 0.18 (0.08–0.39) | < 0.001 |
| Overall | 1,967 | 5.9 (4.9–7.1) | 1,986 | 2.4 (1.7–3.1) | 0.38 (0.26–0.55) | < 0.001 |
| P. malariae | ||||||
| Finschhafen | 455 | 0.4 (0.1–1.6) | 442 | 0.2 (0–1.3) | 0.51 (0.05–5.68) | 0.587 |
| Mumeng | 290 | 0 (0–1.3) | 462 | 0.2 (0–1.2) | na | |
| Sausi | 337 | 0.3 (0–1.6) | 422 | 0 (0–0.9) | na | |
| Tabibuga | 325 | 1.5 (0.5–3.6) | 341 | 0 (0–1.1) | na | |
| Yapsie | 560 | 2.7 (1.5–4.4) | 319 | 0 (0–1.1) | na | |
| Overall | 1,967 | 1.2 (0.7–1.7) | 1,986 | 0.1 (0–0.4) | 0.09 (0.02–0.36) a | 0.001 |
a unadjusted odds ratio
na, not available
Fig. 4.
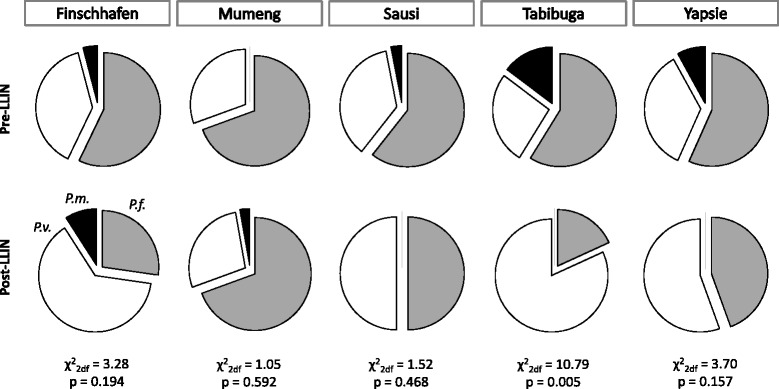
Plasmodium species composition in infected community members before (top) and after (bottom) LLIN distribution. Abbreviations: P.f., P. falciparum (grey), P.v., P. vivax (white), P.m., P. malariae (black)
Axillary temperature, self-reported fever and anaemia were assessed as additional morbidity indicators in the general population in four sites (Finschhafen, Mumeng, Sausi, Tabibuga). Acute fever, defined as axillary temperature of > 37.5 °C, was measured in 2.3 % (95 % CI: 1.6–3.2) of individuals pre-LLIN and 1.9 % (95 % CI: 1.3–2.7) post-LLIN (AOR = 0.72; 95 % CI: 0.43–1.20; P = 0.208). Of those infected with malaria parasites, 12.9 % (95 % CI: 7.7–19.8) had an acute fever pre-LLIN and 8.1 % (95 % CI: 3.0–16.8) post-LLIN (AOR = 0.30; 95 % CI: 0.09–0.95; P = 0.041). Self-reported recent febrile illness (previous 2 days) was reported more frequently, by 14.3 % (95 % CI: 12.5–16.3) pre-LLIN and 7.9 % (95 % CI: 6.6–9.3) post-LLIN (AOR = 0.5; 95 % CI: 0.4–0.7; P < 0.001). A reduction was also noted in anaemia from 68.3 % (95 % CI: 65.6–70.9) to 50.1 % (95 % CI: 47.6–52.6) (AOR = 0.4; 95 % CI: 0.4–0.5; P < 0.001), and in severe anaemia from 4.9 % (95 % CI: 3.8–6.2) to 2.4 % (95 % CI: 1.7–3.3) (AOR 0.5; 95 % CI: 0.3–0.7; P < 0.001). Not all reductions reached statistical significance in all sites, partly due to small sample sizes (Fig. 5). Details by site are provided as supplementary material (Additional file 4: Table S4).
Fig. 5.
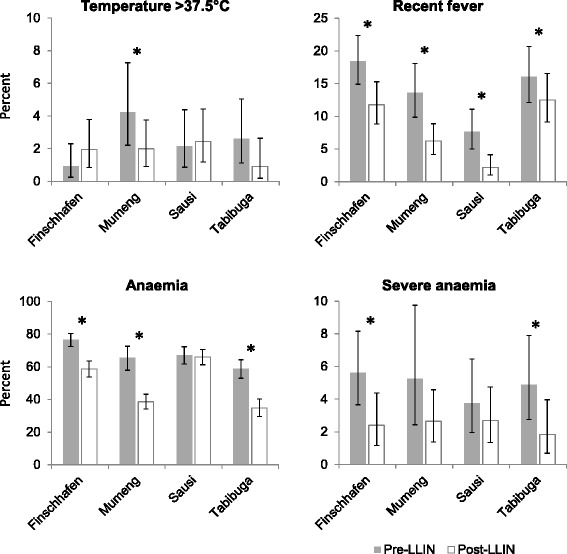
Indicators of morbidity in the general population before and after LLIN distribution. Error bars show 95 % confidence intervals. *P < 0.05
Malaria transmission
Mean Anopheles man biting rates (MBR) across five sites decreased from 83 (95 % CI: 48– 117) bites/person/night pre-LLIN to 31 (95 % CI: 15–47) bites/person/night post-LLIN (t(141) = 2.69, P = 0.008). The highest mean pre-LLIN MBR (361/person/night) was found in Sausi and dominated by An. farauti 4 (Fig. 6). Reductions were greatest in biting rates of An. koliensis (none collected post-LLIN), An. punctulatus (-93.3 %) and An. farauti (s.s.) (formerly An. farauti 1; -76.2 %), while An. farauti 4 (-48.1 %), present in large numbers in Sausi, and An. longirostris (-6.3 %) appeared least affected. An. hinesorum and An. farauti 5 were mainly found post-LLIN. Diversity in species composition was lower after the LLIN distribution and in all sites except Yapsie, the species dominant pre-LLIN remained dominant post-LLIN (Fig. 6).
Fig. 6.
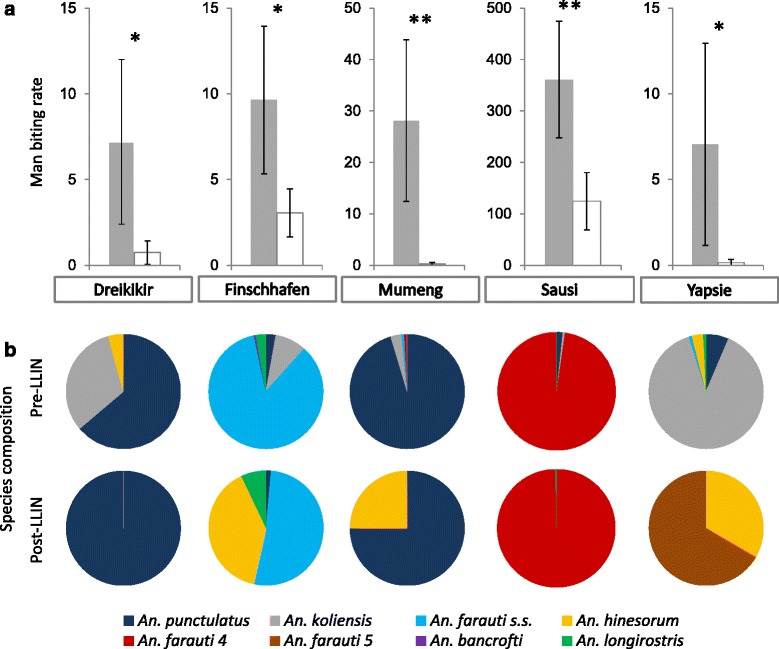
a Mean nightly Anopheles man biting rate (MBR; bites/person/night) before (grey) and after (white) LLIN distribution. b Anopheles species composition before and after LLIN distribution. Error bars show 95 % confidence intervals. *P < 0.05; **P < 0.001
Prior to LLIN roll-out, the majority of infected mosquitoes in three sites (Finschhafen, Mumeng and Sausi) carried P. vivax sporozoites, while in two sites (Dreikikir and Yapsie) P. falciparum infections were dominant. Sporozoite prevalence was lowest in Sausi, where the MBR was highest. Post-LLIN, no infected mosquitoes were found in Dreikikir, Mumeng and Yapsie. In the remaining sites, pre-LLIN infection of mosquitoes and monthly entomological inoculation rate (EIR) were higher for P. vivax than for P. falciparum. Post-LLIN, P. vivax infections had decreased, but P. falciparum infections had increased in both sites (Table 3).
Table 3.
Anopheles nightly man biting rate and malaria transmission before and after LLIN distribution
| Pre-LLIN | Post-LLIN | Change | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Prevalence of infection (%) | Monthly EIR | Prevalence of infection (%) | Monthly EIR | MBR | |||||||||
| An (n) | An MBR | P.f. | P.v. | P.f. | P.v. | An (n) | An MBR | P.f. | P.v. | P.f. | P.v. | % | ||
| Dreikikir | 144 | 7.2 | 3.4 | 1.7 | 7.3 | 3.7 | 12 | 0.8 | 0 | 0 | -7 | -90 | ||
| Finschhafen | 212 | 9.6 | 0.5 | 2.7 | 1.6 | 7.9 | 67 | 3.0 | 3.2 | 0 | 2.9 | 0.0 | -7 | -68 |
| Mumeng | 562 | 28.1 | 2.2 | 3.7 | 18.9 | 31.5 | 5 | 0.3 | 0 | 0 | -28 | -99 | ||
| Yapsie | 127 | 7.1 | 2.4 | 0.8 | 5.0 | 1.7 | 3 | 0.2 | 0 | 0 | -7 | -98 | ||
| Sausi | 14,436 | 360.9 | 0.02 | 0.2 | 2.2 | 23.7 | 5,979 | 124.6 | 0.2 | 0.04 | 5.8 | 1.5 | -236 | -65 |
Abbreviations: An Anopheles, P.f. Plasmodium falciparum, P.v., P vivax, MBR man biting rate (bites/person/night), EIR entomological inoculation rate
Discussion
The roll-out of LLINs in 2009 was followed by substantial reductions in key indicators of malaria morbidity and transmission in six sites in PNG within less than one year. Average LLIN use in these sites had increased from 6 to 55 %, and 89 % of households in the sites owned at least one LLIN after the distribution [8]. The LLINs were new and efficacious [22] and there was no indication of resistance to pyrethroids in PNG, even though data at that time was limited [23]. The prevalence of Plasmodium spp. infection in the general population dropped significantly over the same period mainly due to a 76 % reduction in infections with P. falciparum (AOR = 0.23, P < 0.001). The prevalence of blood-stage P. vivax parasitaemia decreased less prominently (-60 %) reaching statistical significance in two of five study sites (Finschhafen and Yapsie). A significant reduction was also observed in the incidence of clinical malaria cases in three sites, primarily as a result of an 81 % drop in the proportion of P. falciparum-infected febrile patients (AOR = 0.1, P < 0.001). The proportion of cases infected with P. vivax decreased less across all age groups (-46 %), and statistically significantly only in children under 5 years of age. In the Dreikikir site, no significant decrease in the proportion of P. vivax cases was detected in any age group. These findings confirm results from a cohort study in young children conducted near Dreikikir which found that LLIN use was associated with a reduction in both P. falciparum infections and clinical episodes, while it only reduced P. vivax infections but not clinical episodes [24].
A relative increase in P. vivax over P. falciparum following implementation of malaria control measures confirms previous observations both from PNG [7, 9, 20, 25] and elsewhere [26, 27]. The shift in species composition was observed in both asymptomatic community members and febrile patients although not to the same degree in all sites. The differential impact of interventions on the two parasites species is likely a result of the parasite’s [28] and potentially the vector’s biology [29].
The ability of P. vivax to relapse from long-lasting liver stages and the higher probability of gametocytaemia are most likely key factors. A recent study confirmed that relapses from P. vivax hypnozoites contribute 80 % of the burden of malaria infection and clinical episodes in PNG [30]. In tropical strains, such as the South-West Pacific Chesson strains, relapses occur rapidly and frequently [31]. Although the majority of hypnozoites may therefore activate within 12 months of an initial infection, hypnozoites can survive in the human liver for up to 3–5 years. As a consequence, P. vivax blood-stage infections detected post-LLIN roll-out are not only due to new infections from mosquito bites but also due to relapses from hypnozoites established both pre- and post-LLIN [32]. Gametocytaemia was previously found to be driven by asexual blood stage parasitaemia, with a 10-fold increase in parasite density leading to a 1.8-fold and 3.3-fold increase in the odds of carrying P. falciparum and P. vivax gametocytes, respectively [33]. Reducing the chance of P. vivax transmission would therefore require a stronger reduction of P. vivax parasitaemia. Also, P. vivax gametocytes appear early in an infection, when asexual densities are still very low [34] and almost all P. vivax infections are thus thought to be gametocyte positive [35] hence increasing the probability of transmission. Previous studies in PNG further found evidence of earlier biting Anopheles mosquitoes being more likely infected with P. vivax than P. falciparum [29, 36], thus increasing the relative probability of P. vivax transmission before people retire to bed. This is further aggravated by the shorter extrinsic incubation period for P. vivax. As LLINs reduce the biting density as well as the lifespan of mosquitoes, an equal reduction in lifespan is more likely to interrupt P. falciparum transmission [37]. As a consequence of the above, particularly indoor vector control interventions are likely to have less impact on P. vivax than P. falciparum gametocytaemia and hence on transmission. However, once the pre-LLIN hypnozoite reservoir is exhausted, P. vivax prevalence can be expected to drop in line with reductions in the P. vivax EIR.
The entomological results indicate a significant impact of LLINs on transmission but a varying effect by species and study site. In the site with the largest number of mosquitoes pre- and post-LLIN (Sausi, An. farauti four in both rounds), only the P. vivax EIR was found to decrease after LLIN distribution, while the P. falciparum EIR increased. The same was observed in Finschhafen, yet with a small number of mosquitoes post-LLIN (n = 67). In the other sites the mosquito sample size post-LLIN was too limited to draw strong conclusion. However, more comprehensive entomological data published by Reimer et al. [38] confirmed the parasite species shift in Anopheles infections from P. vivax pre-LLIN to P. falciparum one to two years post-LLIN in Madang province and Dreikikir. The different baseline species composition reported by the two studies for Dreikikir is likely related to the low number of infected mosquitoes (two P. falciparum, one P. vivax) caught pre-LLIN in this study. At the same time, a comparison of results from remote Yapsie(i) area, where a 1986 survey in three villages had found 0.4–1.5 % of anophelines (mostly An. koliensis) to be infected with P. falciparum and 0–1.7 % with P. vivax [39], confirms the short term impact of LLINs on both species in areas with well-established transmission. Interestingly in that site, neither in 1986 nor in 2009 infected were mosquitoes collected in the central Yapsie station.
Further in-depth entomological and epidemiological investigations over longer periods of time are needed to clarify the relationship between infections in mosquitoes and in humans. Sub-microscopic infections in humans may explain part of the differences in parasite species detected in humans and mosquitoes [30, 40]. A better understanding is urgently required of when and where (at both macro- and micro-levels) human-mosquito contacts result in malaria transmission in order to target both vector control and vector surveillance. It remains unclear how well current entomological monitoring practices capture actual transmission in space and time. A longer-term follow-up will be required to measure the full impact of LLINs on P. vivax prevalence, incidence, and transmission.
Few additional morbidity indicators were assessed in this study, all of which showed a reduction in frequency (even though not all reached statistical significance) suggesting generally positive health developments. Splenomegaly, a syndrome closely associated with chronic malaria in PNG [41, 42], was less frequent among malaria patients in two sites following LLIN distribution. The trend in anaemia differed between sites. This study confirmed previous findings that anaemia is highly prevalent in some parts of PNG [17, 43, 44] with pre-LLIN prevalence of mild to moderate anaemia between 59 and 77 %. The prevalence of anaemia decreased in the general population (and in fever patients in Mumeng) post-LLIN. No statistically significant decrease was found in Sausi, interestingly the site in which parasite density in malaria patients remained higher than in the two other sites with clinical surveillance. However, the aetiology of anaemia is known to be multi-factorial and a causal link between LLIN and anaemia is likely to be confounded by a number of other contributing factors. Manning et al. [44] for example identified parvovirus B19 infection, P. falciparum infection, vitamin A deficiency, wasting, and incomplete vaccination as primary risk factors for severe anaemia in PNG. As expected, the vast majority of infections were asymptomatic both pre- and post-LLIN.
This study was not without limitations. Due to financial and operational constraints not all data collection components (clinical surveillance, household survey, entomology) could be implemented in all study sites, thus limiting the extent to which trends in these indicators can be compared. Household survey and entomology survey were cross sectional in nature and the clinical surveillance limited to approximately three months each round. While this approach effectively disregards seasonality, pre-LLIN and post-LLIN assessments were implemented during the same time of the year in an effort to maximize comparability. Differences in weather patterns potentially impacting on entomological indicators could not be reliably investigated due to a lack of detailed weather records (U.S. National Centers for Environmental Information; http://www.ncdc.noaa.gov/), but rainfall data from Madang airport presented by Reimer et al. [38] indicates no abnormalities in the seasonal fluctuations over the study period. However, as the comparison of different entomology datasets from Dreikikir shows, small differences in data collection may alter results and care should therefore be taken when making inferences particularly when measurements are based on small numbers of samples, in this specific case number of mosquitoes. The design of this study does not allow establishing a causal link between the LLIN and the observed health effects. However, to the best of our knowledge no other important health interventions were implemented in the study areas over the course of the two data collection rounds making it highly probable that much of the observed reductions in malaria morbidity and transmission is linked to this single intervention.
Conclusions
The distribution of LLIN had distinct effects on P. falciparum and P. vivax. Higher resilience of P. vivax may be attributed to relapses from hypnozoites and other biological characteristics favouring the transmission of P. vivax. The effect on vector species composition varied by location which is likely to impact on the effectiveness of LLINs. The relationship between prevalence, incidence and transmission is a function of several factors, including many that could not be investigated in the frame of this study. The findings of this analysis however clearly show the complexity of these relationships in PNG. Heterogeneity in the impact of LLINs across different sites is obvious from the data presented in this article. Care should therefore be taken when extrapolating findings generated in a particular site in PNG to the entire country. In the past, most in-depth malaria studies in PNG have been conducted in East Sepik (Maprik area) and Madang provinces (Madang North coast area). Gathering data from a larger number of locations over multiple years therefore appears essential to improve our understanding of the malaria epidemiology in PNG particularly as some of the observed effect, such as the shift to P. vivax as the most prevalent Plasmodium spp. infection, may be transient.
Abbreviations
AOR, adjusted odds ratio; CI, confidence interval; DNA, Deoxyribonucleic acid; EIR, entomological inoculation rate; Hb, haemoglobin; IR, Incidence rate; IRR, incidence rate ratio; LLIN, long-lasting insecticidal net; MBR, Man biting rate; PCR, Polymerase chain reaction; PNG, Papua New Guinea; PNGIMR, Papua New Guinea Institute of Medical Research; RDT, rapid diagnostic test.
Acknowledgements
We thank all study participants who agreed to take part in the various study components and the authorities and organizations that hosted our study, including the Health Offices of East Sepik, Morobe, Madang, Western Highlands and Sandaun Provinces and the Evangelical Brotherhood Church Health Services. We acknowledge the support of the National Department of Health and the efforts of our field, laboratory and data teams at PNGIMR in Goroka and Madang.
Funding
This study was funded through a Round 3 malaria grant from the Global Fund to Fight AIDS, Tuberculosis and Malaria.
Availability of data and material
Not applicable.
Authors’ contributions
MWH and LJR developed the study protocol and survey tools, supervised the data collection, analysed the data and wrote the manuscript. GG and GK contributed to the development of survey tools, led the data collection, participated in data analysis and writing of the manuscript. CB was in charge of the molecular diagnosis. LM reviewed the study protocol, supported the data collection and revised the manuscript. IM and PMS conceived the overall project and supported data collection, analysis and manuscript writing. All authors (except GG, deceased) read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical clearance for all described study components was granted by the Papua New Guinea Medical Research Advisory Committee (MRAC No. 07.30). Participation in the study was voluntary and based on verbal informed consent.
Additional files
Data collection periods by study site. (DOCX 18 kb)
Description of study population by site. (XLSX 15 kb)
Morbidity indicators in two sentinel health facilities before and after LLIN distribution. (DOCX 15 kb)
Morbidity indicators in the general population before and after LLIN distribution. (DOCX 19 kb)
Footnotes
Gibson Gideon is deceased
Contributor Information
Manuel W. Hetzel, Email: manuel.hetzel@unibas.ch
Lisa J. Reimer, Email: Lisa.Reimer@lstmed.ac.uk
Gussy Koimbu, Email: gkoimbu@hotmail.com.
Céline Barnadas, Email: celine.barnadas@gmail.com.
Leo Makita, Email: leo.makita@gmail.com.
Peter M. Siba, Email: peter.siba@pngimr.org.pg
Ivo Mueller, Email: ivomueller@fastmail.fm.
References
- 1.Müller I, Bockarie M, Alpers M, Smith T. The epidemiology of malaria in Papua New Guinea. Trends Parasitol. 2003;19(6):253–259. doi: 10.1016/S1471-4922(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 2.Cooper RD, Waterson DGE, Frances SP, Beebe NW, Pluess B, Sweeney AW. Malaria vectors of Papua New Guinea. Int J Parasitol. 2009;39(13):1495–1501. doi: 10.1016/j.ijpara.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Parkinson AD. Malaria in Papua New Guinea 1973. PNG Med J. 1974;17(1):8–16. [Google Scholar]
- 4.Spencer T, Spencer M, Venters D. Malaria vectors in Papua New Guinea. PNG Med J. 1974;17(1):22–30. [Google Scholar]
- 5.Grimmond TR, Donovan KO, Riley I. Chloroquine resistant malaria in Papua New Guinea. PNG Med J. 1976;19:184–185. [PubMed] [Google Scholar]
- 6.Marfurt J, Mueller I, Sie A, Maku P, Goroti M, Reeder JC, et al. Low efficacy of amodiaquine or chloroquine plus sulfadoxine-pyrimethamine against Plasmodium falciparum and P. vivax malaria in Papua New Guinea. Am J Trop Med Hyg. 2007;77(5):947–954. [PubMed] [Google Scholar]
- 7.Mueller I, Tulloch J, Marfurt J, Hide R, Reeder JC. Malaria control in Papua New Guinea results in complex epidemiological changes. PNG Med J. 2005;48(3–4):151–157. [PubMed] [Google Scholar]
- 8.Hetzel MW, Gideon G, Lote N, Makita L, Siba PM, Mueller I. Ownership and usage of mosquito nets after four years of large-scale free distribution in Papua New Guinea. Malar J. 2012;11(1):192. doi: 10.1186/1475-2875-11-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graves PM, Brabin BJ, Charlwood JD, Burkot TR, Cattani JA, Ginny M, et al. Reduction in incidence and prevalence of Plasmodium falciparum in under-5-year-old children by permethrin impregnation of mosquito nets. Bull World Health Organ. 1987;65(6):869–877. [PMC free article] [PubMed] [Google Scholar]
- 10.Papua New Guinea Department of Health. Papua New Guinea National Health Plan 1991–1995. Port Moresby: Papua New Guinea Department of Health; 1991.
- 11.Genton B, Hii J, Al Yaman F, Paru R, Beck HP, Ginny M, et al. The use of untreated bednets and malaria infection, morbidity and immunity. Ann Trop Med Parasitol. 1994;88(3):263–270. doi: 10.1080/00034983.1994.11812866. [DOI] [PubMed] [Google Scholar]
- 12.Hii JL, Smith T, Vounatsou P, Alexander N, Mai A, Ibam E, Alpers MP. Area effects of bednet use in a malaria-endemic area in Papua New Guinea. Trans R Soc Trop Med Hyg. 2001;95(1):7–13. doi: 10.1016/S0035-9203(01)90315-3. [DOI] [PubMed] [Google Scholar]
- 13.Betuela I, Maraga S, Hetzel MW, Tandrapah T, Sie A, Yala S, et al. Epidemiology of malaria in the Papua New Guinean highlands. Trop Med Int Health. 2012;17(10):1181–1191. doi: 10.1111/j.1365-3156.2012.03062.x. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. WHO/NMH/NHD/MNM/11.1. Geneva, Switzerland: World Health Organization; 2011.
- 15.Hackett LW. Spleen measurement in malaria. J Natl Malar Soc. 1944;3(2):121–133. [Google Scholar]
- 16.Papua New Guinea Department of Health . National Malaria Treatment Policy. Port Moresby: Papua New Guinea Department of Health; 2009. [Google Scholar]
- 17.Hetzel MW, Morris H, Tarongka N, Barnadas C, Pulford J, Makita L, et al. Prevalence of malaria across Papua New Guinea after initial roll-out of insecticide-treated mosquito nets. Trop Med Int Health. 2015;20:1745–1755. doi: 10.1111/tmi.12616. [DOI] [PubMed] [Google Scholar]
- 18.Wirtz RA, Burkot TR, Graves PM, Andre RG. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol. 1987;24(4):433–437. doi: 10.1093/jmedent/24.4.433. [DOI] [PubMed] [Google Scholar]
- 19.McNamara DT, Thomson JM, Kasehagen LJ, Zimmerman PA. Development of a multiplex PCR-ligase detection reaction assay for diagnosis of infection by the four parasite species causing malaria in humans. J Clin Microbiol. 2004;42(6):2403–2410. doi: 10.1128/JCM.42.6.2403-2410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasehagen LJ, Mueller I, McNamara DT, Bockarie MJ, Kiniboro B, Rare L, et al. Changing patterns of Plasmodium blood-stage infections in the Wosera region of Papua New Guinea monitored by light microscopy and high throughput PCR diagnosis. Am J Trop Med Hyg. 2006;75(4):588–596. [PMC free article] [PubMed] [Google Scholar]
- 21.Barnadas C, Kent D, Timinao L, Iga J, Gray LR, Siba P, et al. A new high-throughput method for simultaneous detection of drug resistance associated mutations in Plasmodium vivax dhfr, dhps and mdr1 genes. Malar J. 2011;10:282. doi: 10.1186/1475-2875-10-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katusele M, Gideon G, Thomsen EK, Siba PM, Hetzel MW, Reimer LJ. Long-lasting insecticidal nets remain efficacious after five years of use in Papua New Guinea. PNG Med J. 2014;57(1–4):86–93. [PubMed] [Google Scholar]
- 23.Keven JB, Henry-Halldin CN, Thomsen EK, Mueller I, Siba PM, Zimmerman PA, Reimer LJ. Pyrethroid susceptibility in natural populations of the Anopheles punctulatus group (Diptera: Culicidae) in Papua New Guinea. Am J Trop Med Hyg. 2010;83(6):1259–1261. doi: 10.4269/ajtmh.2010.10-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koepfli C, Colborn KL, Kiniboro B, Lin E, Speed TP, Siba PM, et al. A high force of Plasmodium vivax blood-stage infection drives the rapid acquisition of immunity in Papua New Guinean children. PLoS Negl Trop Dis. 2013;7(9):e2403. doi: 10.1371/journal.pntd.0002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewers WH, Jeffrey WT. Parasites of man in Niugini. Milton, Queensland: Jacaranda Press; 1971. [Google Scholar]
- 26.Chaves SS, Rodrigues LC. An initial examination of the epidemiology of malaria in the state of Roraima, in the Brazilian Amazon basin. Rev Inst Med Trop Sao Paulo. 2000;42(5):269–275. doi: 10.1590/S0036-46652000000500006. [DOI] [PubMed] [Google Scholar]
- 27.Luxemburger C, Perea WA, Delmas G, Pruja C, Pecoul B, Moren A. Permethrin-impregnated bed nets for the prevention of malaria in schoolchildren on the Thai-Burmese border. Trans R Soc Trop Med Hyg. 1994;88(2):155–159. doi: 10.1016/0035-9203(94)90273-9. [DOI] [PubMed] [Google Scholar]
- 28.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9(9):555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 29.Bockarie MJ, Alexander N, Bockarie F, Ibam E, Barnish G, Alpers M. The late biting habit of parous Anopheles mosquitoes and pre-bedtime exposure of humans to infective female mosquitoes. Trans R Soc Trop Med Hyg. 1996;90(1):23–25. doi: 10.1016/S0035-9203(96)90465-4. [DOI] [PubMed] [Google Scholar]
- 30.Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, et al. Strategies for Understanding and Reducing the Plasmodium vivax and Plasmodium ovale Hypnozoite Reservoir in Papua New Guinean Children: A Randomised Placebo-Controlled Trial and Mathematical Model. PLoS Med. 2015;12(10):e1001891. doi: 10.1371/journal.pmed.1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White NJ, Imwong M. Relapse. Adv Parasitol. 2012;80:113–150. doi: 10.1016/B978-0-12-397900-1.00002-5. [DOI] [PubMed] [Google Scholar]
- 32.Betuela I, Rosanas-Urgell A, Kiniboro B, Stanisic DI, Samol L, de Lazzari E, et al. Relapses contribute significantly to the risk of Plasmodium vivax infection and disease in Papua New Guinean children 1–5 years of age. J Infect Dis. 2012;206(11):1771–1780. doi: 10.1093/infdis/jis580. [DOI] [PubMed] [Google Scholar]
- 33.Koepfli C, Robinson LJ, Rarau P, Salib M, Sambale N, Wampfler R, et al. Blood-stage parasitaemia and age determine Plasmodium falciparum and P. vivax gametocytaemia in Papua New Guinea. PLoS One. 2014;10(5):e0126747. doi: 10.1371/journal.pone.0126747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarthy JS, Griffin PM, Sekuloski S, Bright AT, Rockett R, Looke D, et al. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis. 2013;208(10):1688–1694. doi: 10.1093/infdis/jit394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbosa S, Gozze AB, Lima NF, Batista CL, Bastos Mda S, Nicolete VC, et al. Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis. 2014;8(8):e3109. doi: 10.1371/journal.pntd.0003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bockarie M, Dagoro H. Are insecticide-treated bednets more protective against Plasmodium falciparum than Plasmodium vivax-infected mosquitoes? Malar J. 2006;5(1):15. doi: 10.1186/1475-2875-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curtis CF, Maxwell CA, Magesa SM, Rwegoshora RT, Wilkes TJ. Insecticide-treated bed-nets for malaria mosquito control. J Am Mosq Control Assoc. 2006;22(3):501–506. doi: 10.2987/8756-971X(2006)22[501:IBFMMC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Reimer LJ, Thomsen EK, Koimbu G, Keven JB, Mueller I, Siba PM, et al. Malaria transmission dynamics surrounding the first nationwide long-lasting insecticidal net distribution in Papua New Guinea. Malar J. 2016;15:25. doi: 10.1186/s12936-015-1067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attenborough RD, Burkot TR, Gardner DS. Altitude and the risk of bites from mosquitoes infected with malaria and filariasis among the Mianmin people of Papua New Guinea. Trans R Soc Trop Med Hyg. 1997;91(1):8–10. doi: 10.1016/S0035-9203(97)90373-4. [DOI] [PubMed] [Google Scholar]
- 40.Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol. 2014;30(4):183–190. doi: 10.1016/j.pt.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crane GG, Pryor DS. Malaria and the tropical splenomegaly syndrome in New Guinea. Trans R Soc Trop Med Hyg. 1971;65(3):315–324. doi: 10.1016/0035-9203(71)90006-X. [DOI] [PubMed] [Google Scholar]
- 42.Leoni S, Buonfrate D, Angheben A, Gobbi F, Bisoffi Z. The hyper-reactive malarial splenomegaly: a systematic review of the literature. Malar J. 2015;14:185. doi: 10.1186/s12936-015-0694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senn N, Riddell M, Omena M, Siba P, Reeder JC, Clements CJ, Morgan C. Measles in Papua New Guinea: an age-specific serological survey. Vaccine. 2010;28(7):1819–1823. doi: 10.1016/j.vaccine.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Manning L, Laman M, Rosanas-Urgell A, Michon P, Aipit S, Bona C, et al. Severe anemia in Papua New Guinean children from a malaria-endemic area: a case–control etiologic study. PLoS Negl Trop Dis. 2012;6(12):e1972. doi: 10.1371/journal.pntd.0001972. [DOI] [PMC free article] [PubMed] [Google Scholar]


