Abstract
The FecA outer membrane protein of Escherichia coli functions as a transporter of ferric citrate and as a signal receiver and signal transmitter for transcription initiation of the fec transport genes. Three FecA regions for which functional roles have been predicted from the crystal structures were mutagenized: (i) loops 7 and 8, which move upon binding of ferric citrate and close the entrance to the ferric citrate binding site; (ii) the dinuclear ferric citrate binding site; and (iii) the interface between the globular domain and the β-barrel. Deletion of loops 7 and 8 abolished FecA transport and induction activities. Deletion of loops 3 and 11 also inactivated FecA, whereas deletion of loops 9 and 10 largely retained FecA activities. The replacement of arginine residue R365 or R380 and glutamine Q570, which are predicted to serve as binding sites for the negatively charged dinuclear ferric citrate, with alanine resulted in inactive FecA, whereas the binding site mutant R438A retained approximately 50% of the FecA induction and transport activities. Residues R150, E541, and E587, conserved among energy-coupled outer membrane transporters, are predicted to form salt bridges between the globular domain and the β-barrel and to contribute to the fixation of the globular domain inside the β-barrel. Mutations E541A and E541R affected FecA induction and transport activity slightly, whereas mutations E587A and E587R more strongly reduced FecA activity. The double mutations R150A E541R and R150A E587R nearly abolished FecA activity. Apparently, the salt bridges are less important than the individual functions these residues seem to have for FecA activity. Comparison of the properties of the FecA, FhuA, FepA, and BtuB transporters indicates that although they have very similar crystal structures, the details of their functional mechanisms differ.
FecA serves as a transport protein for ferric citrate across the outer membrane of Escherichia coli and as a signal receiving and signal transduction protein (4, 24, 28). Binding of ferric citrate to FecA induces transcription of the ferric citrate transport genes fecABCDE (4, 5, 7). Transport of ferric citrate is not required for induction. The N terminus of FecA is located in the periplasm and interacts with the FecR signal-transducing protein across the cytoplasmic membrane (17). Deletion of the N terminus abolishes induction, but transport is fully retained (28). FecR interacts with the FecI sigma factor in the cytoplasm and controls transcription initiation of the fec transport genes (18, 43). Comparisons with genome sequences of other bacteria reveal homologs of FecIRA whose encoding genes are arranged in the same order as fecIRA of E. coli (7). Particularly abundant are fecIRA homologs in Pseudomonas aeruginosa (46), Pseudomonas putida (34), Nitrosomonas europaea, and Bacteroides thetaiotaomicron, for which 10, 11, 15, and 23 fecIRA gene clusters, respectively, are predicted (V. Braun and S. Mahren, unpublished results). The E. coli fecIRA type of transmembrane transcriptional control thus seems to represent a paradigm of a large number of similar transcriptional control devices in many gram-negative bacteria.
The crystal structure of FecA is similar to the crystal structures of FhuA (20, 33), FepA (8), and BtuB (12, 13). They all consist of a β-barrel composed of 22 antiparallel β-strands that form a channel closed by the N-terminal globular domain. Only FecA contains, in addition to the globular domain located inside the β-barrel, a periplasmic 79-residue N-terminal extension through which it interacts with FecR for fec transport gene transcription initiation (Fig. 1). Moreover, it is the only transporter for which the crystal structure revealed a strong movement of surface loops 7 and 8 upon binding of the substrate, dinuclear ferric citrate. It is thought that this movement closes the entrance to the ferric citrate binding site and thus prevents escape of ferric citrate into the medium when it is released from the binding site during transport through the β-barrel into the periplasm. Interestingly, when FecA is incubated with iron-free citrate, two citrate molecules bind to the binding site of dinuclear ferric citrate but do not induce movement of loops 7 and 8 (48). Not all citrate binding sites are identical to the ferric citrate binding site, and the configuration of the two citrate molecules differs from that of ferric citrate. Citrate alone does not induce synthesis of the ferric citrate transport system (25), as confirmed by measuring transcription of a fecA-lacZ reporter gene (V. Braun and C. Herrmann, unpublished results).
FIG. 1.
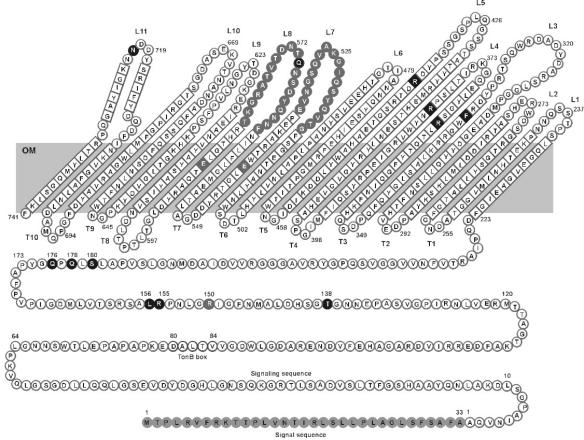
Topology of the FecA polypeptide chain across the outer membrane of E. coli. The outer membrane (OM) transmembrane antiparallel β-strands of the unfolded β-barrel are connected at the cell surface by loops 1 to 11 (L1 to L11). The residues of the globular domain, the β-barrel, and the loops that are predicted to bind dinuclear ferric citrate (21) are shaded black. Residues R150, E541, and E581 at the interface between the globular domain and the β-barrel are shaded grey. The signal sequence released upon secretion of FecA across the cytoplasmic membrane and the TonB box are indicated. The globular cork domain (residues 80 to 221) that is inserted in the β-barrel is shown as linear sequence.
Since closure of the entry cavity upon binding of the substrate is seen only in the crystal of FecA (21) but not in the crystals of FhuA (20, 33) and BtuB (12, 13), whether loops 7 and 8 are essential for FecA activity was studied. It was conceivable that deletion of these loops would reduce, but not abolish, transport. Furthermore, the signaling activity of FecA requires TonB, ExbB, and ExbD, as does the transport activity. Whether signaling has the same FecA structural requirements as transport, in particular with regard to loops 7 and 8, was addressed. Loops 7 and 8 were deleted to determine whether they are essential for ferric citrate induction and transport. For comparison, other loops were deleted, and the activities of the FecA deletion derivatives were tested. Moreover, amino acids in the predicted ferric citrate binding site, composed of residues from the globular domain and the β-barrel, were replaced by alanine. The basic amino acids lysine (K) in loop 7 and arginine (R) in loop 8 were replaced by alanine to determine whether they form primary ferric citrate binding sites that facilitate entry of ferric citrate into the tight binding site inside FecA.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used are listed in Table 1. Cells were grown at 37°C in tryptone-yeast extract and nutrient broth (NB) (Difco Laboratories) and supplemented, as indicated, with 1 mM citrate and where required with ampicillin (40 μg/ml) or chloramphenicol (20 μg/ml).
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| Strains | ||
| AB2847 | aroB malT tsx thi | 6 |
| IS1031 | fecA aroB thi malT tsx fepA lac::Tn10 | 24 |
| AA93 | F− Δfec araD139 ΔlacU169 rpsL150 relA1 flbB5301 deoCI ptsF25 rbsR thi aroB | 38 |
| Plasmids | ||
| pT7-7 | ori ColE1 phage T7 gene promoter Ampr | 44 |
| pMON37 | fecBCDE on pT7-7 | 39 |
| pASA29 | pASA32 with SphI and BsiWI restriction sites | This study |
| pASA30 | pT7-7 fecA Δ313-328 | This study |
| pASA31 | pT7-7 fecA Δ423-433 | This study |
| pASA32 | pT7-7 fecA Δ517-533 | This study |
| pASA33 | pT7-7 fecA Δ519-533 V518G | This study |
| pASA34 | pT7-7 fecA Δ563-577 F562P | This study |
| pASA35 | pT7-7 fecA Δ568-577 D567P | This study |
| pASA36 | pT7-7 fecA Δ616-630 | This study |
| pASA37 | pT7-7 fecA Δ662-673 | This study |
| pASA38 | pT7-7 fecA Δ713-727 | This study |
| pASA39 | pT7-7 fecA Δ517-533 Δ563-577 F562P | This study |
| pASA40 | pT7-7 fecA T138A | This study |
| pASA41 | pT7-7 fecA R365A | This study |
| pASA42 | pT7-7 fecA R380A | This study |
| pASA43 | pT7-7 fecA R438A | This study |
| pASA44 | pT7-7 fecA Q570A | This study |
| pASA45 | pT7-7 fecA D573A | This study |
| pASA46 | pT7-7 fecA K525A | This study |
| pASA47 | pT7-7 fecA R578A | This study |
| pASA48 | pT7-7 fecA E541A | This study |
| pASA49 | pT7-7 fecA E541R | This study |
| pASA50 | pT7-7 fecA E587A | This study |
| pASA51 | pT7-7 fecA E587R | This study |
| pASA52 | pT7-7 fecA R150A E541R | This study |
| pASA53 | pT7-7 fecA R150A E587R | This study |
| pT7-6 | ori ColE1 phage T7 gene promoter, Ampr | 44 |
| pHK763 | pT7-6 fhuA | 27 |
| pASA54 | pT7-7 fecA Δ516-535 replaced by fhuA 243-273 | This study |
| pASA55 | pT7-7 fecA Δ516-535 replaced by fhuA 318-339 | This study |
| pASA56 | pT7-7 fecA Δ516-535 replaced by fhuA 394-419 | This study |
| pASA57 | pT7-7 fecA Δ516-535 replaced by fhuA 502-515 | This study |
| pHSG576 | pSC101 derivative, Cmr | 45 |
| pMMO203 | pHSG576 fecIR | 38 |
| pMMO1034 | ori Col E1 AmprfecA-lacZ | 38 |
| pASA58 | pMMO203 fecA Δ313-328 | This study |
| pASA59 | pMMO203 fecA Δ423-433 | This study |
| pASA60 | pMMO203 fecA Δ517-533 | This study |
| pASA61 | pMMO203 fecA Δ519-533 V518G | This study |
| pASA62 | pMMO203 fecA Δ563-577 F562P | This study |
| pASA63 | pMMO203 fecA Δ568-577 D567P | This study |
| pASA64 | pMMO203 fecA Δ616-630 | This study |
| pASA65 | pMMO203 fecA Δ662-673 | This study |
| pASA66 | pMMO203 fecA Δ713-727 | This study |
| pASA67 | pMMO203 fecA Δ517-533 Δ563-577 F562P | This study |
| pASA68 | pMMO203 fecA T138A | This study |
| pASA69 | pMMO203 fecA R365A | This study |
| pASA70 | pMMO203 fecA R380A | This study |
| pASA71 | pMMO203 fecA R438A | This study |
| pASA72 | pMMO203 fecA Q570A | This study |
| pASA73 | pMMO203 fecA D573A | This study |
| pASA74 | pMMO203 fecA K525A | This study |
| pASA75 | pMMO203 fecA R578A | This study |
| pASA76 | pMMO203 fecA E541A | This study |
| pASA77 | pMMO203 fecA E541R | This study |
| pASA78 | pMMO203 fecA E587A | This study |
| pASA79 | pMMO203 fecA E587R | This study |
| pASA80 | pMMO203 fecA R150A E541R | This study |
| pASA81 | pMMO203 fecA R150A E587R | This study |
| pASA82 | pMMO203 fecA Δ516-535 replaced by fhuA 243-273 | This study |
| pASA83 | pMMO203 fecA Δ516-535 replaced by fhuA 318-339 | This study |
| pASA84 | pMMO203 fecA Δ516-535 replaced by fhuA 394-419 | This study |
| pASA85 | pMMO203 fecA Δ516-535 replaced by fhuA 502-515 | This study |
Construction of plasmids.
Plasmids were constructed by PCR amplification of DNA fragments with appropriate primer pairs, the sequences of which are available upon request. E. coli K-12 strain AB2847 was used as a PCR template, unless indicated otherwise.
To construct plasmids pASA30 to pASA37, two fecA fragments lacking the codons for the amino acids to be deleted were amplified by PCR. Introduced restriction sites were used to ligate the fragments to form mutant fecA genes, which were then PCR amplified. The PCR products were digested with EcoRI and BamHI, which flank fecA, and cloned into vector pT7-7 cleaved with EcoRI and BamHI.
The same procedure was used to construct pASA39, with pASA34 as a template. Plasmid pASA29 was constructed by introducing an SphI restriction site and a BsiWI restriction site before and after the deleted amino acids of loop 7, respectively, in plasmid pASA32. pASA38 was constructed by replacing the EcoRI/BamHI fragment of vector pT7-7 with the EcoRI/BamHI fecA PCR fragment encoding a FecA protein with amino acids of loop 11 deleted.
To construct plasmids pASA40 to pASA53, the QuikChange site-directed mutagenesis kit (Stratagene Europe, Amsterdam, The Netherlands) was used to introduce point mutations. pASA49 and pASA51 were used for construction of pASA52 and pASA53 by PCR.
To obtain plasmids pASA54 to pASA57, SphI/BsiWI fhuA fragments were amplified from plasmid pHK763 and cloned into pASA29 cleaved with SphI and BsiWI.
Plasmids pASA58 to pASA85 were constructed by cloning fecA excised with EcoRI and BamHI from plasmids pASA30 to pASA57 into plasmid pMMO203 cleaved with EcoRI and BamHI.
The sequences of all constructed fecA derivatives were confirmed by nucleotide sequencing.
Recombinant DNA techniques.
Standard techniques (41) or the protocols of suppliers were used for the isolation of plasmid DNA, PCR, digestion with restriction endonucleases, ligation, transformation, and agarose gel electrophoresis. DNA was sequenced commercially using the dideoxy chain-termination method.
Transport assays.
Quantitative transport of 55Fe3+ citrate by plasmid-encoded FecA was determined in freshly transformed E. coli K-12 strain IS1031 fecA and with E. coli AA93 Δfec, which in addition to the various fecA genes was transformed with pMON37 fecBCDE. Cells were grown overnight at 37°C in NB medium and used to inoculate 7-ml NB medium supplemented with 0.4% glucose and 1 mM sodium citrate. The culture was incubated for 3 h to an optical density at 578 nm (OD578) of 0.5 to 1 to fully induce the transport system. After 1.5 h, 50 μM dipyridyl was added to complex free iron(II) ions in the medium and to induce the fec transport genes. Cells were suspended to an OD578 of 0.5 in 5 ml of transport medium (2.07 g NaH2PO4 · H2O, 0.68 g of KH2PO4, 0.66 g of (NH4)2SO4, 47 mg of MgCl2, 22 mg of CaCl2, 1 g of glucose per liter, adjusted to pH 6.9). Sodium nitrilotriacetate (50 μl, 10 mM) was added, and the mixture was incubated for 5 min at 37°C to remove free iron. After the addition of 50 μl of radioactive ferric citrate (10 μM 55Fe3+, 1 M sodium citrate [pH 6.8]), 0.7-ml samples were taken after 1, 6, 11, 16, 21, and 26 min of incubation at 37°C and filtered through cellulose nitrate filters (pore size, 0.45 μm; Sartorius AG, Göttingen, Germany); the filters were then washed twice with 5 ml of 0.1 M LiCl. The filters were dried, and the radioactivity was determined by liquid scintillation counting.
Iron citrate binding assays.
Cells of Δfec E. coli AA93 were freshly transformed with the plasmids to be tested and grown overnight at 37°C in NB medium. fecA transcription was induced by growing cells first in 7 ml of NB medium supplemented with 0.4% glucose and 1 mM sodium citrate. After 1.5 h, 50 μM dipyridyl was added to complex free iron(II) ions in the medium. Cells were suspended to an OD578 of 0.5 in 5 ml of transport medium as described in the transport assays. After addition of 50 μl of the 55Fe3+ citrate mix (10 μM 55Fe3+, 1 M sodium citrate [pH 6.8]), 0.7-ml samples were taken after 1, 6, 11, 16, and 21 min of incubation at 37°C. After 21 min, a 100-fold surplus of nonradioactive ferric citrate (1 mM) was added to chase radiolabeled iron that has not entered the cytoplasm. Two chase samples were measured. Probes were filtered through cellulose nitrate filters (pore size, 0.45 μm; Sartorius AG) and washed twice with 5 ml of 0.1 M LiCl, the filters were dried, and the radioactivity was determined by liquid scintillation counting.
Protein analytical methods and Western blotting.
E. coli AA93 transformed with one of the various plasmids was grown in NB medium with or without 1 mM sodium citrate at 37°C. Cells were harvested by centrifugation at an OD578 of 1.
Outer membranes were prepared by lysing cells with lysozyme-EDTA, followed by solubilization of the cytoplasmic membrane with 0.2% Triton X-100 and differential centrifugation (23). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (11% polyacrylamide) (32) and then transferred to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany) for Western blot analysis to estimate the amount of wild-type FecA and mutant FecA. Blots were blocked in 5% bovine serum albumin in TNT buffer (20 mM Tris-HCl, 500 mM NaCl, 0.05% Tween 20 [pH 7.5]), probed with polyclonal anti-FecA antibodies (diluted 1:20,000) overnight, and then washed with TNT buffer and incubated with anti-rabbit immunoglobulin G conjugated with alkaline phosphatase (Sigma, München, Germany). The blots were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (Serva, Heidelberg, Germany).
Determination of β-galactosidase activity.
β-Galactosidase activity was measured according to published methods (22, 35). To determine the induction level, cells were grown in NB medium with or without 1 mM sodium citrate.
RESULTS
Activity of loop deletion mutants.
Loops 3, 5, 7, 8, 9, 10, and 11 were each deleted such that at least three amino acid residues remained to connect the β-strands with a turn in the periplasm. In loop 7 and 8 two different deletion mutants were constructed. Two fragments in loop 7 were excised that differed by two residues in length, and two fragments in loop 8 were excised that differed by five residues in length, to examine whether the size of the turns remaining affected integration into the outer membrane and FecA activity. The small loops L1, L2, L4, and L6 were not shortened. The fecA wild-type and mutant genes were each cloned on the low-copy-number plasmid pHSG576 and introduced into E. coli AA93 ΔfecIRABCDE bytransformation. To measure fecA transcription, strain AA93 contained a plasmid-encoded fecA-lacZ reporter gene. Ferric citrate did not induce fecA transcription of the loop 7 and loop 8 deletion mutants and the loop 7/8 double mutant (Table 2). The loop 3 and loop 11 deletion mutants were also inactive. The loop 5 mutant showed low activity, and the loop 9 and 10 mutants displayed an intermediate level of activity (Table 2).
TABLE 2.
Properties of FecA mutants
| Mutation | β-Galactosidase activity [Miller units (%)]a |
55Fe3+ transport rate (%)b
|
|
|---|---|---|---|
| Medium copy | Low copy | ||
| None (vector) | 12 (0.7) | 11 | 10 |
| None (wild type) | 1,898 (100) | 100 | 100 |
| ΔL3 (Δ313-328) | 15 (0.8) | 10 | ND |
| ΔL5 (Δ423-433) | 136 (7) | 23 | 16 |
| ΔL71 (Δ517-533) | 16 (0.9) | 7 | ND |
| ΔL72 (Δ519-533) | 15 (0.8) | 6 | ND |
| ΔL81 (Δ563-577) | 15 (0.8) | 7 | ND |
| ΔL82 (Δ568-577) | 15 (0.8) | 8 | ND |
| ΔL9 (Δ616-630) | 695 (37) | 74 | 76 |
| ΔL10 (Δ662-673) | 952 (50) | 48 | 33 |
| ΔL11 (Δ713-727) | 23 (1.2) | 16 | 8 |
| ΔL7ΔL8 (Δ517-533 Δ563-577) | 8 (0.4) | 9 | ND |
| T138A | 1,198 (63) | 46 | 58 |
| R365A | 28 (1.5) | 8 | ND |
| R380A | 15 (0.8) | 8 | ND |
| R438A | 797 (42) | 53 | 73 |
| Q570A | 13 (0.7) | 11 | ND |
| D573A | 1,177 (62) | 97 | 40 |
| K525A | 1,895 (100) | 94 | 126 |
| R578A | 1,556 (82) | 53 | 82 |
| E541A | 1,708 (90) | 112 | 81 |
| E541R | 1,594 (84) | 91 | 72 |
| E587A | 721 (38) | 85 | 87 |
| E587R | 626 (33) | 50 | 47 |
| R150A E541R | 285 (15) | 27 | 42 |
| R150A E587R | 76 (4) | 25 | 29 |
Transcription induction of fecA was measured in E. coli AA93 (Δfec pMMO1034 fecA-lacZ) grown in NB containing 1 mM citrate. The values are the averages of three independent experiments. The uninduced values (cells grown in NB lacking citrate) were between 8 (0.4%) and 18 (1%) Miller units.
Transport was determined in E. coli IS1031 (fecA) and in E. coli AA93. The transport rate was calculated by subtracting the values after a 1-min incubation from the values after a 16-min incubation; the values given are relative to the wild-type rate (100%). The values are the averages of two independent experiments.
Citrate-mediated 55Fe3+ transport was determined for E. coli IS1031, which carries a chromosomally encoded FecA R413H mutation that renders cells inactive for citrate-mediated iron transport and exhibits no polar effect on the downstream fecBCDE genes (24, 47). IS1031 transformants carried the fecA genes on the medium-copy-number plasmid pT7-7. IS1031 transformed with the wild-type fecA gene transported iron (Table 2). IS1031 synthesizing the loop 7 or loop 8 FecA deletion protein did not transport iron (Table 2). The loop 3 and loop 11 mutants were also inactive in citrate-mediated iron transport. The loop 5, 9, and 10 mutants displayed 23, 74, and 48% of the fecA wild-type transport rate, respectively.
In addition, 55Fe3+ transport into E. coli AA93, which was the strain used for the induction experiments, was determined. AA93 was transformed with the fecA wild-type and mutant genes on the low-copy-number plasmid pMMO203 which were used for the induction experiments. The level of transport could thus be directly compared with the level of induction. For transport across the cytoplasmic membrane, AA93 was transformed with plasmid pMON37 carrying the fecBCDE wild-type genes. Transport of 55Fe3+ into AA93 was determined with those fecA mutant genes, which displayed intermediary transport rates in IS1031. Related to the transport rate of the fecA wild-type transformant, the fecA mutant transformants showed rates comparable with those obtained with the IS1031 transformants (Table 2). The plasmid-encoded fecABCDE transport genes in AA93 conferred transport rates which were comparable with those of the chromosomally encoded fecBCDE transport genes in IS1031 complemented by the fecA mutant genes. The similar results obtained with AA93 and IS1031 indicated that gene copy numbers did not strongly influence the mutant values related to wild-type FecA and that recombination between chromosomal and plasmid-encoded fecA in IS1031 played no role. Since the studied mutations were in the β-barrel domain and IS1031 is also mutated in the β-barrel domain, no reconstitution of active FecA could occur of the kind observed with FhuA, where wild-type cork of one FhuA molecule could complement the missing cork of a cosynthesized FhuA β-barrel (3). In fact, such a reconstitution does not occur with FecA (V. Braun, unpublished results).
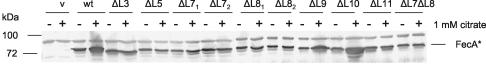
Since the loop 3, 7, 8, and 11 mutants did not induce fecA-lacZ transcription, transport inactivity could be caused by the lack of transport proteins. Induction was determined with the mutant genes cloned on the low-copy-number plasmid pHSG576, whereas transport was determined with the mutant genes cloned on the medium-copy-number plasmid pT7-7. The presence of the mutant FecA proteins in E. coli IS1031 carrying the pT7-7 derivatives, used for the transport assays, was determined by SDS-PAGE. Western blotting with anti-FecA antiserum revealed that all the mutants synthesized the FecA protein in amounts sufficient to mediate transport (Fig. 2). Transformants that synthesized wild-type FecA or mutant FecA with a deletion in loop 9 or 10 produced more FecA protein when grown in ferric-citrate-containing medium than in citrate-free medium (Fig. 2). No ferric-citrate-dependent increase of FecA was observed in the loop 3, 5, 7, 8, and 11 mutants and the loop 7/8 double mutant (Fig. 2). E. coli IS1031 carrying the pT7-7 vector synthesized chromosomally encoded mutant FecA; the amount of FecA did not increase when ferric citrate was present in the medium, as was found previously (47). The results indicated that the lack of citrate-mediated iron transport was not caused by the lack of FecA protein.
FIG. 2.
Anti-FecA Western blot of outer membranes of E. coli IS1031 (fecA) transformed with pT7-7 carrying wild-type fecA or one of a number of mutant fecA genes encoding FecA loop deletion proteins. +, induction with 1 mM citrate; −, uninduced; v, vector; wt, wild-type FecA. The sizes of the marker proteins are indicated on the left in kilodaltons.
The FecA loop 7 cannot be replaced by FhuA loops.
To examine whether loop 7 must assume a certain structure to fulfill its function, residues 516 to 535 of FecA were replaced by residues 502 to 515 (loop 7), residues 243 to 273 (loop 3), residues 318 to 339 (loop 4), or residues 394 to 419 (loop 5) of FhuA. None of the mutants showed induction and transport activity. SDS-PAGE revealed wild-type amounts of uninduced FecA mutant proteins (data not shown). Apparently, movement of loop 7 upon binding of ferric citrate and/or closure of the entry to the binding site depends on a certain loop structure.
Activity of ferric-citrate-binding-site mutants.
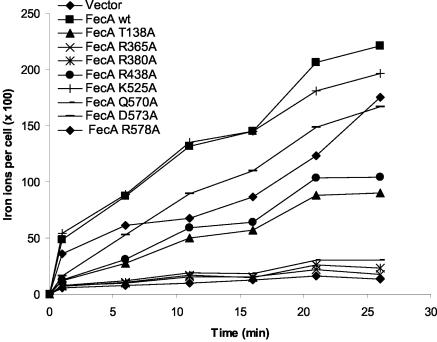
In FecA, residues T138, R365, R380, R438, and Q570 are in closest proximity, 3.0, 3.0, 2.6, 3.3, and 2.5 Å, respectively, to bound dinuclear ferric citrate (21, 48; A. D. Ferguson, personal communication). These residues were individually replaced by alanine, and ferric-citrate-mediated induction of fecA-lacZ transcription and citrate-mediated iron transport were determined. FecA(R365A), FecA(R380A), and FecA(Q570A) expressed in E. coli IS1031 were induction and transport inactive, whereas FecA(T138A) and FecA(R438A) displayed approximately half-maximal induction and transport activities (Table 2). Of particular importance are the positively charged R residues for binding of the negatively charged dinuclear ferric citrate. In addition, residue K525 of loop 7 or residue D573 or R578 of loop 8 was replaced by alanine to determine whether these residues play a role in initial adsorption of ferric citrate to FecA, from which ferric citrate could enter the tight binding site within FecA. We postulated that access to the final binding site might be facilitated when ferric citrate first binds to the loops and then is transferred to the final binding site by the movement of loops 7 and 8. FecA(K525A) and FecA(R578A) showed high induction and transport rates, whereas FecA(D573A) displayed a reduced induction but a high transport rate (Table 2; Fig. 3). Binding of ferric citrate to FecA(D573A) was decreased, as revealed by the low 1-min value (Fig. 3), which reflects binding and initial transport. Since the distance of D573 to dinuclear ferric citrate is 3.5 Å, D573 might contribute to the binding of dinuclear ferric citrate; the mutation D573A might reduce binding and therefore induction.
FIG. 3.
Transport of 55Fe3+ citrate into E. coli IS1031 (fecA) transformed with pT7-7 carrying wild-type fecA or one of a number of mutant fecA genes. The mutant fecA genes encode FecA derivatives mutated in the ferric citrate binding site.
Binding of radiolabeled 55Fe3+ citrate could not reliably be determined. Initial binding (1-min value) to Δfec E. coli AA93 transformed with wild-type fecA was low at 600 ions per cell, which increased to 1,000 ions after 20 min. Chase with a surplus of unlabeled ferric citrate reduced binding to only 600 ions per cell. Mutant K525A gave wild-type values, mutants T138A and D573A gave 400 ions per cell, and the other mutants gave below 200 ions per cell, which was also obtained with AA93 transformed with the vector. It is possible that binding of ferric citrate is not strong enough to withstand washing of the filters with 0.1 M LiCl. In addition, the molecular form of ferric citrate in the assay is not clearly defined, since it polymerizes into numerous molecular forms (40). The results give no indication that closing of loops 7 and 8 prevent release of ferric citrate from the FecA binding site. Under similar conditions, 20,000 iron ions supplied as ferrichrome complex are bound to FhuA, and after chase 2,000 ions remain bound to the cells.
Of those FecA mutants that transported iron in strain IS1031, transport was also determined in strain AA93 to test correlation of transport with induction in the same strain. Qualitatively the results obtained were similar to the results obtained with strain IS1031, but quantitative differences were noted (Table 2). With half of the mutants the level of induction correlated somewhat better for AA93 than for IS1031.
Activity of globular domain and β-barrel contact-site mutants.
The four crystal structures of TonB-dependent outer membrane transporters indicate strictly conserved residues at the interface between the globular domain and the β-barrel. In FecA, these residues are R150 and R196 in the globular domain and E541 and E587 in the β-barrel, which are close enough and oriented such that they form salt bridges (R150 to E541 and E587 and R196 to E587). R150, E541, and E587 were each mutated, and induction and transport activities of the mutant proteins were determined. Induction and transport by FecA(E541A) and FecA(E541R) were close to that of wild-type FecA (Table 2), which indicated that the salt bridge to R150 was dispensable and that even repulsion of R150 by R541 did not impair FecA structure and function. In contrast, induction by FecA(E587A) and FecA(E587R) was reduced to one-third of that of wild-type FecA. Transport was also reduced with FecA(E587A) and even more strongly reduced with FecA(E587R) (Table 2). Induction by the double mutants FecA(R150A E541R) and FecA(R150A E587R) was abolished or nearly abolished, and transport was strongly reduced (Table 2). Outer membrane fractions of cells expressing FecA(E541A) or FecA(E541R) contained reduced amounts of ferric-citrate-induced FecA, and the double mutants contained only uninduced amounts (Fig. 4). The data suggested that the conserved interface residues play a role in FecA structure and function and not just for fixation of the globular domain inside the β-barrel.
FIG. 4.
Anti-FecA Western blot of outer membranes of E. coli IS1031 (fecA) transformed with pT7-7 carrying wild-type fecA or one of a number of mutant fecA genes encoding FecA derivatives mutated in the interface between the globular domain and the β-barrel. +, induction with 1 mM citrate; −, uninduced; v, vector; wt, wild-type FecA. The sizes of the marker proteins are indicated on the left in kilodaltons.
DISCUSSION
Crystal structures provide a precise but static view of a protein in the form in which it crystallizes. Postulations derived from the crystal structure as to how a protein might function have to be tested by studying appropriate mutants and by using biophysical methods that monitor the dynamics of a protein. In this study, three FecA regions with predictable functional roles were selected for further study: (i) loops 7 and 8, (ii) the dinuclear ferric citrate binding site, and (iii) the interface between the globular domain and the β-barrel.
Deletion of loops 7 and 8 abolished induction and transport, which showed that both loops are essential for both FecA activities and suggested that movement of the two loops, as observed in the crystal structures, is important. The difference in size of loop 7 (FecA 18, FhuA 14, and FepA 38 residues) and loop 8 (FecA 20, FhuA 7, and FepA 13 residues) suggest that loops 7 and 8 do not function the same way in all transporters. Loop 7 and 8 deletion mutants of FepA display no ferric enterobactin binding and transport (37). FecA and FepA are similar in that deletion of loop 7 or 8 abolishes all activities; in contrast, FhuA loop 7 or 8 deletion mutants retain all activities (15). FecA is similar to FhuA in that deletion of loop 3 or 11 abolishes all activities; in contrast, a loop 3 deletion mutant of FepA retains fully ferric enterobactin transport and sensitivity to colicin B (37). Comparison of the mutant phenotypes clearly indicates that each loop has different roles in the three transporters.
Surface loops in BtuB have still other properties and functions. In the BtuB crystal, the extracellular loops 2, 3, and 4 are disordered. Upon binding of two calcium ions, loop 2 becomes ordered and loop 3 becomes partially ordered. Upon additional binding of cyanocobalamin, loops 3 and 4 become fully ordered (12, 13). BtuB tolerates duplication of large parts of the sequence to a surprising degree, e.g., duplications extending from β-strand 1 into loop 2, loop 3 to β-strand 6, or β-strand 10 to β-strand 12. Such duplication mutants display 64 to 100% of the wild-type level of vitamin B12 transport activity (31). In FhuA, insertions of 4 to 16 heterologous residues in loops 4, 5, 7, or 10 result in derivatives that support growth on ferrichrome as the sole iron source and sensitivity to the FhuA ligands albomycin, colicin M, microcin J25, and the phages T1, T5, φ80, and UC1 (30, 36). The loops, therefore, display a complex behavior. On one hand, they are highly flexible and show large changes in position upon binding of substrate and in response to TonB and energy input (26); on the other hand, they assume rather rigid structures, e.g., loops 3, 5, and 11 in FhuA form a sturdy protuberance extending approximately 35 Å above the membrane surface (33). Multiple interactions between loops occur, and these interactions might change during transport.
Only the crystal structures of FecA reveal closure of loops 7 and 8 upon binding of dinuclear ferric citrate (21). No such movement is seen in the crystal structures of FhuA (20, 33). However, ferrichrome-induced fluorescence quenching of fluorescein-labeled FhuA at residue D336C indicates movement of loop 4 or movement of neighboring loops such that the environment of the label changes (2). The crystal structure of FepA does not reveal the entire loops 4, 5, and 8 and bound ferric enterobactin (8). However, spectroscopic and cross-linking studies have disclosed movement of loops during ferric enterobactin transport (10, 26, 29, 42).
Biphasic binding kinetics and mutant analyses point to there being two ferric enterobactin binding sites on FepA (9, 10). It is conceivable that ferric enterobactin first binds reversibly to peripheral residues, followed by firm binding inside FepA. With such a model in mind, potential initial binding sites in loops 7 and 8 of FecA were replaced. These are the only positively charged residues—K525 in loop 7 and R578 in loop 8—that could initially bind the negatively charged dinuclear ferric citrate. The induction and transport activities of FecA(K525A) were similar to or even higher than those of wild-type FecA; the activities of FecA(R578A) were diminished. The level of transport reduction suggests that R578 serves as an initial ferric citrate binding site or that the replaced A alters the structure of loop 8 such that it affects movement of loop 8.
Of the binding site mutants studied, R365A, R380A, and Q570A most strongly reduced citrate-mediated iron transport. These residues are the residues closest to dinuclear ferric citrate. The negatively charged dinuclear ferric citrate is probably most strongly bound by the positively charged R365 and R380 residues, suggesting decreased binding of ferric citrate to the R365A and R380A mutants. However, weak binding does not necessarily lead to slow transport, as examples of FhuA (15) and FepA (37) demonstrate. Replacement of R81, which is highly conserved in FhuA homologues, leads to a very strong reduction in binding but fully retained transport (14).
The FecA crystal structure predicts salt bridges of R150 with E541 and E587 and of R196 with E587. Disruption of the salt bridges in the E541A mutant and even repulsion in the E541R mutant had no strong effects on FecA-mediated induction and ferric citrate transport. Apparently, the salt bridge between R150 and E541 is not essential for FecA structure and function. The E587A and E587R mutants displayed less induction, and transport was more strongly reduced in the E587R mutant than in the E587A mutant. Strong reduction of induction and transport was observed in the R150A E541R and R150A E587R double mutants. These results partially differ from those obtained with FhuA (16)—disruption of the salt bridges of R93 to E522 and E571 and of R133 to E571 and repulsion between these residues have either no effects or only small effects on FhuA transport and receptor activities. However, in contrast to FecA, the FhuA(E522R) mutant is inactive and the FhuA(E571R) mutant is nearly inactive, even in the double mutant FhuA(R93E E522R), in which a salt bridge could be formed. R is not tolerated at the E sites, but E is not required, since E-to-A replacements yield FhuA wild-type activities. In FhuA, 60 hydrogen bonds and 7 salt bridges (33) either directly or through intermediate water molecules (19) are predicted to contribute to the fixation of the globular domain to the β-barrel; and the situation is similar for FecA. Disruption of one or two such bridges might not be sufficient to affect the structures to a degree that functions are seriously reduced. There must be other reasons in addition to fixation of the globular domain in the β-barrel to explain why these residues are highly conserved in TonB-dependent transporters and why E587 is important in FecA and E522 and E571 cannot be replaced by R without loss of FhuA activity. A similar conclusion can be drawn from results obtained with FepA in which R75, R126, E511, and E567 constitute the polar interaction sites between the globular domain and the β-barrel. The R75L, R75P, and R126H derivatives resulting from random mutation are strongly reduced in ferric enterobactin transport (1), the site-directed mutant R75Q displays a 10-fold increased Km and a threefold lower Vmax than wild-type FepA, the E511Q mutant has wild-type activities, and the E567A mutant has a Vmax of 17% of that of wild-type FepA, but the E567A E511Q double mutant has 32% of the wild-type level of FepA transport activity (11). The distinct phenotypes of the mutants suggest that they affect the functions of the transporters, such as binding of the substrate, release of the substrate, opening of the β-barrel channel, and restoration of the closed state, in a complex manner.
Acknowledgments
We thank Andrew D. Ferguson for advice and Karen A. Brune for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (BR330/9-1) and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Barnard, T. J., M. E. Watson, Jr., and M. A. McIntosh. 2001. Mutations in the Escherichia coli receptor FepA reveal residues involved in ligand binding and transport. Mol. Microbiol. 41:527-536. [DOI] [PubMed] [Google Scholar]
- 2.Bös, C., D. Lorenzen, and V. Braun. 1998. Specific in vivo labeling of cell surface-exposed protein loops: reactive cysteines in the predicted gating loop mark a ferrichrome binding site and a ligand-induced conformational change of the Escherichia coli FhuA protein. J. Bacteriol. 180:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, M., H. Killmann, and V. Braun. 2003. In vivo reconstitution of the FhuA transport protein of Escherichia coli K-12. J. Bacteriol. 185:5508-5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun, V. 1997. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch. Microbiol. 167:325-331. [DOI] [PubMed] [Google Scholar]
- 5.Braun, V. 2003. Iron uptake by Escherichia coli. Front. Biosci. 8:1409-1421. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V., R. E. W. Hancock, K. Hantke, and A. Hartmann. 1976. Functional organization of the outer membrane of Escherichia coli: phage and colicin receptors as components of iron uptake systems. J. Supramol. Struct. 5:37-58. [DOI] [PubMed] [Google Scholar]
- 7.Braun, V., S. Mahren, and M. Ogierman. 2003. Regulation of the FecI-type ECF sigma factor by transmembrane signalling. Curr. Opinion. 6:173-180. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 9.Cao, Z., Z. Qi, C. Sprencel, S. M. C. Newton, and P. E. Klebba. 2000. Aromatic components of two ferric enterobactin binding sites in Escherichia coli FepA. Mol. Microbiol. 37:1306-1317. [DOI] [PubMed] [Google Scholar]
- 10.Cao, Z., P. Warfel, S. M. C. Newton, and P. E. Klebba. 2003. Spectroscopic observations of ferric enterobactin transport. J. Biol. Chem. 278:1022-1028. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty, R., E. Lembke, Z. Cao, P. E. Klebba, and D. van der Helm. 2003. Identification and mutational studies of conserved amino acids in the outer membrane receptor protein FepA, which affect transport but not binding of ferric enterobactin in Escherichia coli. BioMetals 16:507-518. [DOI] [PubMed] [Google Scholar]
- 12.Chimento, D. P., A. K. Mohanty, R. J. Kadner, and M. C. Wiener. 2003. Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat. Struct. Biol. 10:394-401. [DOI] [PubMed] [Google Scholar]
- 13.Chimento, D. P., R. Kadner, and M. Wiener. 2003. The Escherichia coli outer membrane transporter BtuB: structural analysis of the calcium and substrate binding, and identification of orthologous transporters by sequence/structure conservation. J. Mol. Biol. 332:999-1014. [DOI] [PubMed] [Google Scholar]
- 14.Endriß, F. 2000. Die Funktion des Aminosäurerests Arg-81 von FhuA aus E. coli K-12 für die Aufnahme und Bindung der FhuA spezifischen Liganden. Diploma thesis. Universität Tübingen, Tübingen, Germany.
- 15.Endriß, F., and V. Braun. 2004. Loop deletions indicate regions important for FhuA transport and receptor functions in Escherichia coli. J. Bacteriol. 186:4818-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endriß, F., M. Braun, H. Killmann, and V. Braun. 2003. Mutant analysis of the Escherichia coli FhuA protein reveals sites of FhuA activity. J. Bacteriol. 185:4683-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enz, S., H. Brand, C. Orellana, S. Mahren, and V. Braun. 2003. Sites of interaction between the FecA and FecR signal transduction proteins of ferric citrate transport in Escherichia coli K-12. J. Bacteriol. 185:3745-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enz, S., S. Mahren, U. H. Stroeher, and V. Braun. 2000. Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J. Bacteriol. 182:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faraldo-Gómez, J. D., and M. S. P. Sansom. 2003. Acquisition of siderophores in gram-negative bacteria. Nat. Rev. Mol. Cell Biol. 4:105-116. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 22.Giacomini, A., B. Corich, F. J. Ollero, A. Squartini, and M. P. Nuti. 1992. Experimental conditions may affect reproducibility of the β-galactosidase assay. FEMS Microbiol. Lett. 100:87-90. [DOI] [PubMed] [Google Scholar]
- 23.Hantke, K. 1981. Regulation of the ferric iron transport in Escherichia coli K-12: isolation of a constitutive mutant. Mol. Gen. Genet. 182:288-292. [DOI] [PubMed] [Google Scholar]
- 24.Härle, C., I. Kim, A. Angerer, and V. Braun. 1995. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 14:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussein, S., K. Hantke, and V. Braun. 1981. Citrate-dependent iron transport system in Escherichia coli K-12. Eur. J. Biochem. 117:431-437. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, X., M. A. Payne, Z. Cao, S. B. Foster, J. B. Feix, S. M. C. Newton, and P. E. Klebba. 1997. Ligand specific opening of a gated-porin channel in the outer membrane of living bacteria. Science 276:1261-1264. [DOI] [PubMed] [Google Scholar]
- 27.Killmann, H., R. Benz, and V. Braun. 1993. Conversion of the FhuA transport protein into a diffusion channel through the outer membrane of Escherichia coli. EMBO J. 12:3007-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, I., A. Stiefel, S. Plantör, A. Angerer, and V. Braun. 1997. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol. Microbiol. 23:333-344. [DOI] [PubMed] [Google Scholar]
- 29.Klebba, P. E. 2003. Three paradoxes of ferric enterobactin uptake. Front. Biosc. 8:1422-1436. [DOI] [PubMed] [Google Scholar]
- 30.Koebnik, R., and V. Braun. 1993. Insertion derivatives containing segments of up to 16 amino acids identify surface- and periplasm-exposed regions of the FhuA outer membrane receptor of Escherichia coli K-12. J. Bacteriol. 175:826-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köster, W., A. Gudmundsdottir, M. D. Lundrigan, A. Seiffert, and R. J. Kadner. 1991. Deletions or duplications in the BtuB protein affect its level in the outer membrane of Escherichia coli. J. Bacteriol. 173:5639-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Locher, K. P., B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771-778. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Bueno, M. A., R. Tobes, M. Rey, and J.-L. Ramos. 2002. Detection of multiple extracytoplasmic function (ECF) sigma factors in the genome of Pseudomonas putida KT2440 and their counterparts in Pseudomonas aeruginosa PA01. Environ. Microbiol. 4:842-855. [DOI] [PubMed] [Google Scholar]
- 35.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Moeck, G. S., B. S. F. Bazzaz, M. F. Gras, T. S. Ravi, M. J. Ratcliffe, and J. W. Coulton. 1994. Genetic insertion and exposure of a reporter epitope in the ferrichrome-iron receptor of Escherichia coli K-12. J. Bacteriol. 176:4250-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton, S. M. C., J. D. Igo, D. C. Scott, and P. E. Klebba. 1999. Effect of loop deletions on the binding and transport of ferric enterobactin by FepA. Mol. Microbiol. 32:1153-1165. [DOI] [PubMed] [Google Scholar]
- 38.Ochs, M., S. Veitinger, K. InSook, D. Welz, A. Angerer, and V. Braun. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: FecR is required for transcription activation by FecI. Mol. Microbiol. 15:119-132. [DOI] [PubMed] [Google Scholar]
- 39.Ogierman, M., and V. Braun. 2003. Interactions between the outer membrane ferric citrate transporter FecA and TonB: studies of the FecA TonB box. J. Bacteriol. 185:1870-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierre, J. L., and I. Gautier-Luneau. 2000. Iron and citric acid: a fuzzy chemistry of ubiquitous biological relevance. BioMetals 13:91-96. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Scott, D. C., S. M. C. Newton, and P. E. Klebba. 2002. Surface loop motion in FepA. J. Bacteriol. 184:4906-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stiefel, A., S. Mahren, M. Ochs, P. T. Schindler, S. Enz, and V. Braun. 2001. Control of the ferric citrate transport system of Escherichia coli: mutations in region 2.1 of the FecI extracytoplasmic-function sigma factor suppress mutations in the FecR transmembrane regulatory protein. J. Bacteriol. 183:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeshita, S., S. Masahiro, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63-74. [DOI] [PubMed] [Google Scholar]
- 46.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 47.Wagegg, W., and V. Braun. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein FecA. J. Bacteriol. 145:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue, W. W., S. Grizot, and S. K. Buchanan. 2003. Structural evidence for iron-free citrate and ferric citrate binding to the TonB-dependent outer membrane transporter FecA. J. Mol. Biol. 332:353-368. [DOI] [PubMed] [Google Scholar]