Abstract
Objective
Obstructive sleep apnea (OSA); is a leading sleep disorder that is disproportionately more prevalent in minority populations and is a major risk factor for cardiovascular disease (CVD) morbidity and mortality. OSA is associated with many chronic conditions including hypertension, diabetes, and obesity, all of which disproportionately burden blacks (i.e., peoples of African American, Caribbean, or African descent).
Methods
This article will review studies conducted in the U.S. that examined sleep screenings and adherence to treatment for obstructive sleep apnea among blacks. In addition, we provide guidelines for implementing a practical framework to increase OSA screening and management among blacks.
Results
Several studies have documented racial/ethnic disparities in adherence to treatment for obstructive sleep apnea. However, despite its public health significance, there is a paucity of studies addressing these disparities. Further, there is a lack of health programs and policies to increase screening and treatment of OSA among blacks and other minority populations. A practical framework to increase the number of blacks who are screened for OSA and treated appropriately is warranted. Such a framework is timely and is of major importance, as early identification of OSA in this high-risk population could potentially lead to early treatment and prevention of CVD, thereby reducing racial and ethnic disparities in sleep-related CVD morbidity and mortality.
Keywords: obstructive sleep apnea, positive airway pressure, black, community-based, minorities, African American
1. Introduction
Published reports indicate far worse health outcomes for blacks (i.e., peoples of African American, Caribbean, or African descent) than any other racial/ethnic groups in the United States [1–3]. Blacks experience disproportionately higher mortality rates in all leading causes of death; 40% of blacks die prematurely from cardiovascular disease (CVD) compared with 21% of whites [4]. Coronary heart disease mortality among blacks is estimated to be 73% higher than among age-matched whites [5, 6]. Plausibly, mortality rates are higher among blacks because their diseases are detected at a more advanced stage and are often complicated by co-existing conditions [5].
Obstructive Sleep Apnea (OSA) constitutes an important public health challenge that is responsible for cardiovascular mortality and all-cause mortality [7, 8]. Evidence suggests it is an ignored chronic condition among blacks [9–14]. OSA is a strong CVD risk factor, especially when associated with hypertension [14], the most important contributor to the ethnic mortality gap between blacks and whites [15]. OSA is also associated with obesity and diabetes [16–19], two highly prevalent diseases among blacks. Untreated OSA leads to cardiovascular morbidity [10–14], automobile accidents [12, 20, 21], cognitive deficits [22, 23], excessive daytime sleepiness [24] excess mortality [11, 25, 26] and psychosocial sequelae [27, 28]. Fortunately, OSA treatment with positive airway pressure (PAP) may reduce risk for CVD, as one prospective study has shown that PAP treatment reduces CVD risk by 64% among patients with mild to moderate OSA [29]. A recent study found that CPAP was more effective in reducing CVD markers than the control group and sleep education with supplemental oxygen among patients 45–74 years of age. The primary outcome measure was 24-hour mean arterial blood pressure [30]. OSA is amenable to improvement through tailored behavioral interventions that combine education, social support, and Positive Airway Pressure (PAP) therapy, which may be particularly important in managing OSA among blacks.
Effective behavioral interventions are needed to increase the number of blacks receiving adequate OSA screening and treatment. However, researchers and practitioners lack practical guides for the implementation of such studies at the community level. This paper provides a review of the literature to identify studies conducted in the U.S. that examined sleep screenings and adherence to treatment for obstructive sleep apnea among blacks. We also provide a practical community-oriented framework to increase the number of blacks who are screened for OSA and treated appropriately. Such a framework is timely and is of major importance, as early identification of OSA in this high-risk population could potentially lead to early treatment and prevention of CVD, thereby reducing racial and ethnic disparities in sleep-related CVD morbidity and mortality.
2. Review of the Literature
Racial/ethnic disparities in sleep apnea
Observational studies suggest that OSA is disproportionately more prevalent among blacks compared with other racial and ethnic groups. A case-control family study comparing 225 blacks and 622 whites showed 31% of blacks vs. 10% of whites had OSA [9]. Blacks usually present with OSA at a younger age, and OSA is more severe in older blacks [9, 31]. A home sleep study performed among community-dwelling adults [32] showed that blacks were 2.5 times more likely to have an apnea hypopnea index of 30 or higher compared with whites [31]. A cohort study of 5,301 participants showed that blacks had a significant burden of OSA risk based on a modified version of the Berlin Sleep Questionnaire [33]. However, the authors note that objective measures are needed to determine whether the findings support an actual OSA diagnosis. Some have argued for a genetic basis examining candidate genes to influence sleep apnea [34]. One study found two separate SNPs to influence the development of OSA among blacks compared with whites [35].
Racial/ethnic disparities in sleep apnea screening
We searched PubMed database from 2000 to the present to identify studies to enhance our understanding of OSA screening and adherence to continuous positive airway pressure. Although OSA is a public health threat that disproportionately affects blacks [9], no systematic study has documented adherence to recommended OSA screening and treatment at the community level, where most blacks could be reached to address health-related issues. Evidence from a retrospective chart review we conducted at a hospital-based sleep clinic showed that only 38% of patients adhered to physician’s recommendations for a sleep evaluation [36]. Of the patients that underwent a polysomnography, 91% received a positive diagnosis of OSA [36]. These findings are important, given evidence suggesting that blacks are less likely to adhere to OSA treatment [37–39].
Traditionally, screening for OSA has required a nocturnal polysomnographic study, measuring sleep architecture, airflow and ventilatory effort, peripheral oxygen saturation, electrocardiogram, body position, and periodic limb movement [40]. Although no single factor has been identified to contribute to poor OSA screening rates among blacks, a broad array of issues encompassing access to health care, health care delivery and individual behavior and attitudes toward treatment and care are plausible explanatory factors [3, 41]. On balance, we should note that generally detection and treatment of OSA have been problematic, albeit more challenging in minority populations [41]. National estimates indicate that only 25% of people with symptoms of OSA sought a medical examination from 2005–2008 [42]. Moreover, it is estimated that it can take up to 10 weeks for individuals with an OSA referral to undergo a polysomnographic screening [41]. It is believed that the number of accredited sleep centers is far below what is needed, and few of those sleep centers are located in sleep disparity communities. Evidently, with the increasing availability of in-home sleep testing devices, as well as the demand to use in-home sleep testing to reduce healthcare costs [43], access to diagnosis of OSA is more readily available, but may not have adequate penetration in minority communities. Moreover, although these tools are useful, some patients with OSA still require in-lab titration study [44]. To our knowledge, there is only one study that examined the feasibility of in-home sleep testing in 75 urban blacks. The authors concluded that in-home sleep testing is feasible [45], but this study did not assess adherence to treatment or other outcomes and was a feasibility study, limiting definitive conclusions. As such, it remains unclear whether in-home sleep testing will address disparities in access to adequate sleep care. Similarly, with the expansion of Medicaid through the Patient Protection and Affordable Care Act, it is plausible that more blacks will benefit and could have increased access to important diagnostic testing, including sleep screenings. However, access to health insurance alone is not sufficient to encourage the uptake of OSA screening [46].
A few widely used and validated screening questionnaires are available to assess OSA risk including the Berlin [47] and the STOP-Bang [48]. Another validated measure is the Apnea Risk Evaluation System (ARES™) questionnaire [49], which includes questions on sleep patterns, daytime functioning, and the Epworth sleepiness scale. The ARES™ questionnaire can be self-administered [49], and has been used by our research group in non-clinical settings. Conceivably, all of these screening questionnaires can be easily scored, and transformed to an electronic data capture system for research studies. In doing so, providers must consider other factors including feasibility and efficiency of administering these questionnaires.
Given the paucity of data on OSA screening and current attitudes toward sleep apnea, in our previous work [50], we conducted five focus groups (see Table 1 for focus group guidelines) in order to delineate further some of the barriers preventing or delaying adequate sleep care among blacks. Focus groups are recommended when there is little known about a particular phenomenon of interest [51]. We found that a major barrier to sleep apnea screening is related to the environment itself, as is routinely required in current clinical practice (i.e. sleeping in a hospital overnight) [50] Other barriers included a lack of information about OSA and mistrust of the healthcare system [50, 52]. Large-scale studies are needed to corroborate these findings and test effectiveness of innovative interventions to address such concerns.
Table 1.
Required elements of focus groups for cultural and linguistic tailoring of sleep health information to blacks
| Elements of Focus Groups | |
|---|---|
| Format | Group session |
| Size | 8–12 per session |
| Length | 1.5 to 2 hours |
| # of sessions | 3–4 |
| Participants | Similar characteristics |
| Forms of data | Conversation |
| Data collection | Transcription |
| Moderator | Uses semi-structured interview guide |
| Analysis | Identify trends and patterns in sleep perceptions, attitudes, and beliefs |
Racial/ethnic disparities in adherence to PAP treatment
We searched PubMed database from 2000 to the present to identify studies relating to adherence to continuous positive airway pressure. We also used the Cochrane Database of Systematic Reviews. The concepts included continuous positive airway pressure, or auto-PAP, adherence or compliance. The search was restricted to the English language, and to adults at least 18 years of age or older. We reviewed studies that included an intervention whereby the primary outcome measure was machine usage. A total of 91 articles were reviewed, 86 of which were excluded because they did not report information on the race/ethnicity of the study patients, and one study was excluded because it focused on Chinese patients. The study findings regarding CPAP usage were mixed. The studies included education and motivational interviewing, social support, and one study focused on positive vs. negative message framing in CPAP usage. These studies did not reveal ethnic differences in CPAP adherence, but one study noted that perhaps one of the limitations in their study was failing to address some of the cultural norms and practices as it relates to the sleep behavior that may be pertinent to blacks. Available studies on PAP adherence among blacks indicate that blacks use PAP far less than their white counterparts [53]. One published abstract suggested that blacks used PAP treatment 2.07 hours (95% CI: 0.57–3.57) less than did whites [39]. A few studies have shown that black race predicts PAP adherence [37, 53–55]. These studies vary in sample size, covariates controlled for, and methodological design. Clearly race/ethnicity alone is not sufficient to explain the poor adherence rate among blacks, as in other chronic disease conditions there are a multitude of factors that explain adherence rates. In any case, these studies shed light on the role of race/ethnicity on PAP adherence and provide an important opportunity for future research that incorporates larger sample sizes of blacks and mixed-method designs to understand better the role of race/ethnicity in PAP adherence.
Although the underlying mechanisms for the disparities in PAP adherence are not well understood [53, 56–59], these findings indicate that there is a need for culturally tailored interventions that engage and empower blacks to manage better their OSA condition. However, a major limitation of available intervention studies on PAP adherence is that they are conducted in homogenous groups [60], so it is unclear if the current studies are generalizable to individuals belonging to cultural groups, particularly blacks.
3. Framework to increase access to OSA management and screening among blacks
To address challenges of under-diagnosis and suboptimal treatment of OSA among blacks, we propose a practical community-oriented framework that includes strategies guiding development of effective interventions to engage communities in the research process and ensure OSA interventions are relevant and culturally appropriate. Thus, the goal of this framework is to: 1) increase engagement of blacks to participate in OSA screening and treatment as well as sleep-related research and 2) ultimately improve their cardiovascular health.
It is critically important that tailored interventions make implicit the importance of the community, as we have observed that failure to engage communities in the research process results in protocols and programs that are ineffective or incompatible with community needs. Community is broadly defined to include populations in neighborhoods and organizations, including health care settings. Although community engagement in the development and implementation of interventions can be a complex process and may appear to be beyond the scope of routine clinical practice, it remains an important component of sleep research and interventions intended to advance our understanding of factors that need to be addressed to eliminate sleep-health disparities [61]. We have developed this framework based on our previous community-engaged research (see section 3.2) undergirded by a synthesis of relevant literature, particularly in community-based participatory research [62] with a focus on blacks (see Table 2).
Table 2.
Proposed guidelines for implementing a sleep apnea program based on evidence from our community-engaged research
| Guidelines to Implement a Community-Based Sleep Apnea Program |
|---|
|
Access to a community-based sleep disorders program with culturally concordant staff
We focus our academic resources and utilize our community-based infrastructure for interventions aimed at understanding and reducing disparities in cardio-metabolic diseases including OSA. Thus, the innovative aspect of our framework is the availability of a Sleep Disorders Center in the community, rather than at an academic facility. Community-based Sleep Disorder Centers facilitate access to sleep care, dissemination of sleep-heart health information, and early detection of OSA and other sleep disorders. Another unique characteristic of our program is the hiring of culturally concordant staff at the center. To our knowledge, there are no published studies that have evaluated whether academic sleep centers have documented greater adherence to OSA screening relative to community-based sleep centers. Our decision to locate the Sleep Disorders Center in the community was motivated by multiple meetings with various stakeholders arguing in favor of a community-based center (see Table 2). Indeed, results of our focus groups with blacks at risk for OSA indicated that many black patients would not undergo sleep screening at a hospital-based sleep center because of negative beliefs associated with hospital procedures [50].
Assessing patients’ illness perspectives, attitudes, and beliefs through mixed-methods formative research
We identified community-driven health priorities and developed our framework through a deliberative process of self-assessment and consultation with various community stakeholders. Together, we identified: 1) diseases and conditions that are a high priority among blacks, including those that are highly prevalent, through a Delphi survey; 2) availability of scientific expertise at the academic university and existing partnerships with black-owned barbershops, beauty salons, and predominantly black churches; 3) diseases that are amenable to improvement through behavioral health intervention; 4) barriers to healthful practices through focus groups of community members; and 5) approaches that are responsive to community needs and that leverage our strengths and resources. This formative work was crucial to ensure that we developed strategies that are relevant to the community priorities and needs, thus potentiating our likelihood for success.
These guidelines could provide practitioners with important, but simple processes to engage minorities in recognizing risk factors for OSA, encouraging referrals for OSA screening, and fostering adherence to treatment recommendations. In doing so, we hope that these steps would be useful in addressing sleep-related cardiovascular health disparities. Below, we provide findings from one study using the aforementioned framework (see Table 2).
Preliminary results of our community-engaged pilot OSA intervention using the proposed framework
We conducted a community-engaged pilot study with black volunteers recruited from community sites including barbershops and beauty salons to assess OSA risk and related symptoms. Ninety-eight blacks (age range: 23–68 years) provided demographic and medical data. Participants were screened using the ARES™ questionnaire [49]. We found that overall the rate of OSA symptoms in that sample was: snoring (40%), excessive daytime sleepiness (36%), and difficulty maintaining sleep (30%). Sixty percent reported either falling asleep while watching TV (42%) or while driving (21%), 35% reported a history of hypertension, and 15% a history of CVD; mean body mass index was 30.14 ± 7.32 kg/m2. As seen in Figure 1, a significant number of blacks (39%) were at high OSA risk, highlighting the need for tailored interventions.
Figure 1.
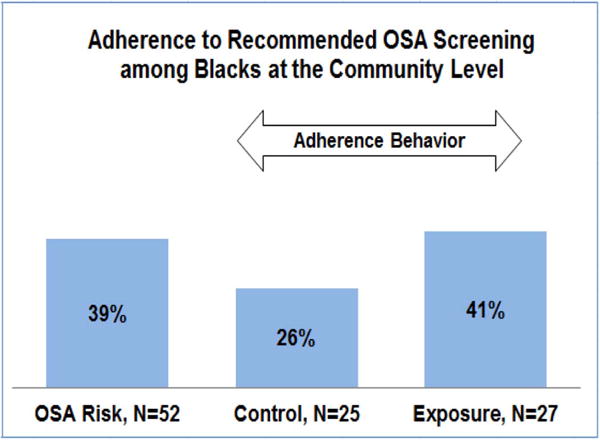
Results of a pilot OSA intervention in Brooklyn, NY
Multi-level approach to increase awareness and treatment of OSA at the community level
As seen in Figure 2, our interventions examine both individual- and contextual-level mediators of reasoned actions toward decisions to engage in available sleep services. It recognizes the interplay of biological and behavioral factors, as well as the influence of contextual factors in determining health behavior of blacks. Our research revealed unique contextual factors influencing underreport of sleep problems among blacks, which may influence low participation in sleep services [63]. We also found several determinants of insufficient sleep among blacks, including working >40hrs [OR=1.72], care-giving to family/friends [OR=1.23], and lack of emotional support [OR=1.21] [63]. We surmise that early detection of OSA is impacted by a number of individual and contextual level factors. Therefore, a contextual approach that includes understanding of sociocultural barriers to OSA screening is needed.
Figure 2.
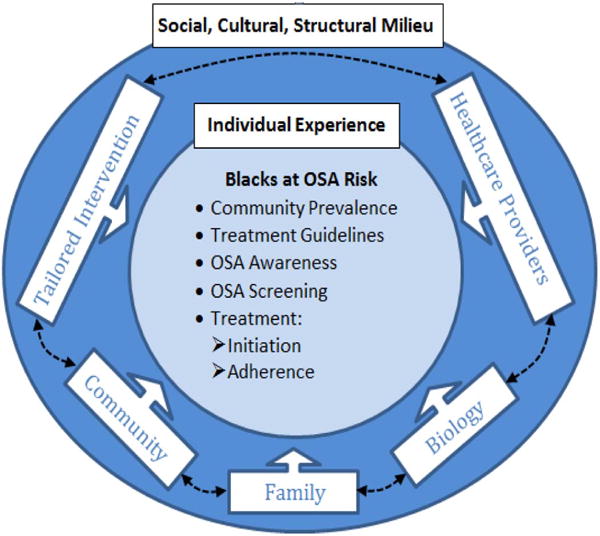
Proposed multi-level approach to increase awareness and treatment of OSA at the community level.
Preliminary results of a tailored behavioral health intervention
Using the multi-level intervention paradigm, we conducted a pilot study assessing effectiveness of an adapted model [64] that was culturally and linguistically tailored to enhance adherence to recommended OSA screening and treatment of blacks in the primary-care setting [65]. The intervention focused on motivating and empowering blacks to make informed decisions about OSA screening thus, activating them in the process of their own health care. The intervention was also tailored to individual needs based on their readiness to change using the Prochaska’s stage-of-change model [66] as well as feelings and ways of reasoning. Tailoring is an iterative process in which a Health Educator considers individual’s incentives facilitating screening and barriers limiting the desired behavior (e.g., OSA screening and adherence to PAP treatment). Thus, the trained Health Educator attempted to maximize incentives to overcome identified barriers.
Analysis of intervention data (ages 23–68 years) showed that 26% of blacks in the control condition, receiving only sleep literature, agreed to undergo OSA screening; this compares to 41% of those exposed to the tailored behavioral sleep health interventions (Unpublished work) (see Figure 1). This represents a 15% increase in the likelihood that blacks exposed to tailored interventions would undergo sleep screening, which if verified would have an important public health impact. Future studies should determine the effectiveness of such a model in increasing adherence to recommended OSA screening and treatment in a large sample of at at-risk blacks or other minority groups and explore potential mediators of intervention effectiveness.
3. Conclusions
We recognize that blacks are not a homogenous group and that these recommendations may not apply to all black individuals, families, and communities. However, our goal is to provide a framework for which public health practitioners and researchers can build upon to customize interventions and health messages around healthful sleep practices, screening and treatment that are community-focused and tailored to the needs of different populations. We have found that this multi-level framework works well in elucidating the socio-cultural context that might support or hinder OSA screening and treatment among black communities in New York City and should be empirically tested to ascertain whether or not it would make a difference in other communities. Such an approach would provide adequate evidence for the proposed framework. The field could benefit from other alternative frameworks that may be translatable to other underserved, minority populations. Finally, the field would be well served by developing and implementing more innovative behavioral health models that are culturally and linguistically tailored to address needs of at-risk populations, which heretofore have not benefited from evidence-based PAP treatment.
Highlights.
We propose guidelines for a practical framework to encourage screening and treatment of obstructive sleep apnea among blacks
Community-based health promotion could engage blacks in the health care system
Community-based multi-level interventions could be used to reduce and eliminate sleep-related cardiovascular health disparities
Acknowledgments
This work was supported by funding from the National Institutes of Health (R01MD004113, R25HL105444, R01MD007716, R01HL095799).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no conflict of interest and have signed the ICMJE Uniform Disclosure Form.
References
- 1.Anonymous. A Report on Gender, Racial, and Ethnic Health Disparities in Cambridge. 2009. (Men’s Health Report). [Google Scholar]
- 2.Williams DR. The health of men: structured inequalities and opportunities. Am J Public Health. 2008;98(9 Suppl):S150–7. doi: 10.2105/ajph.98.supplement_1.s150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smedley BD, Stith AY, Nelson AR. In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (with CD) Smedley BD, Stith AY, Nelson AR, editors. The National Academies Press; 2003. [PubMed] [Google Scholar]
- 4.Prevention, C.f.D.C.a. Health disparities experienced by black or African Americans–United States. MMWR Morb Mortal Wkly Rep. 2005;54(1):1–3. [PubMed] [Google Scholar]
- 5.Palaniappan L, Wang Y, Fortmann SP. Coronary heart disease mortality for six ethnic groups in California, 1990–2000. Ann Epidemiol. 2004;14(7):499–506. doi: 10.1016/j.annepidem.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Traven ND, et al. Coronary heart disease mortality and sudden death among the 35–44-year age group in Allegheny County, Pennsylvania. Ann Epidemiol. 1996;6(2):130–6. doi: 10.1016/1047-2797(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 7.Research, N.C.o.S.D. and U.S.D.o.H.a.H. Services, Wake Up America: A National Sleep Alert: Report of the National Commission on Sleep Disorders Research. The Commission; 2008. [Google Scholar]
- 8.Ge X, et al. Is Obstructive Sleep Apnea Associated with Cardiovascular and All-Cause Mortality? Plos One. 2013;8(7) doi: 10.1371/journal.pone.0069432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redline S, et al. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155(1):186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 10.Kiely JL, McNicholas WT. Cardiovascular risk factors in patients with obstructive sleep apnoea syndrome. European Respiratory Journal. 2000;16(1):128–133. doi: 10.1034/j.1399-3003.2000.16a23.x. [DOI] [PubMed] [Google Scholar]
- 11.Wright J, et al. Health effects of obstructive sleep apnoea and the effectiveness of continuous positive airways pressure: A systematic review of the research evidence. British Medical Journal. 1997;314(7084):851–860. doi: 10.1136/bmj.314.7084.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Findley L, Weiss J, Jabour E. Drivers with untreated sleep apnea. A cause of death and serious injury. Arch Intern Med. 1991;151(7):1451–2. [PubMed] [Google Scholar]
- 13.Newman AB, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors - The Sleep Heart Health Study. American Journal of Epidemiology. 2001;154(1):50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 14.Somers VK, et al. Sleep apnea and cardiovascular disease - An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Journal of the American College of Cardiology. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg DS, Oksenberg A, Iaina A. Sleep-related breathing disorders as a major cause of essential hypertension: fact or fiction? Curr Opin Nephrol Hypertens. 1998;7(4):353–7. doi: 10.1097/00041552-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher EC. The relationship between systemic hypertension and obstructive sleep apnea: facts and theory. Am J Med. 1995;98(2):118–28. doi: 10.1016/S0002-9343(99)80395-7. [DOI] [PubMed] [Google Scholar]
- 17.Grunstein R, et al. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17(9):533–40. [PubMed] [Google Scholar]
- 18.Ip MS, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 19.Punjabi NM, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiol Neurobiol. 2003;136(2–3):167–78. doi: 10.1016/s1569-9048(03)00079-x. [DOI] [PubMed] [Google Scholar]
- 20.Stoohs R, et al. Traffic accidents in commercial long-haul truck drivers: the influence of sleep-disordered breathing and obesity. Sleep. 1994;17(7):619–23. [PubMed] [Google Scholar]
- 21.Sassani A, et al. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27(3):453–458. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 22.El-Ad B, Lavie P. Effect of sleep apnea on cognition and mood. Int Rev Psychiatry. 2005;17(4):277–82. doi: 10.1080/09540260500104508. [DOI] [PubMed] [Google Scholar]
- 23.Karkoulias K, et al. The impact of obstructive sleep apnea syndrome severity on physical performance and mental health. The use of SF-36 questionnaire in sleep apnea. European Review for Medical and Pharmacological Sciences. 2013;17(4):531–536. [PubMed] [Google Scholar]
- 24.Guilleminault C, et al. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104(3):781–7. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 25.Lavie P, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep. 1995;18(3):149–57. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, et al. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: A meta-analysis of prospective cohort studies. International Journal of Cardiology. 2013;169(3):207–214. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 27.McFadyen TA, et al. Controlled, prospective trial of psychosocial function before and after continuous positive airway pressure therapy. European Respiratory Journal. 2001;18(6):996–1002. doi: 10.1183/09031936.01.00209301. [DOI] [PubMed] [Google Scholar]
- 28.Brown WD. The psychosocial aspects of obstructive sleep apnea. Seminars in Respiratory and Critical Care Medicine. 2005;26(1):33–43. doi: 10.1055/s-2005-864199. [DOI] [PubMed] [Google Scholar]
- 29.Buchner NJ, et al. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176(12):1274–80. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb DJ, et al. CPAP versus Oxygen in Obstructive Sleep Apnea. New England Journal of Medicine. 2014;370(24):2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, et al. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1946–9. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 32.Blazer DG, Hays JC, Foley DJ. SLEEP COMPLAINTS IN OLDER ADULTS - A RACIAL COMPARISON. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 1995;50(5):M280–M284. doi: 10.1093/gerona/50a.5.m280. [DOI] [PubMed] [Google Scholar]
- 33.Fueloep T, et al. Sleep-disordered breathing symptoms among African-Americans in the Jackson Heart Study. Sleep Medicine. 2012;13(8):1039–1049. doi: 10.1016/j.sleep.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel SR, et al. Association of genetic loci with sleep apnea in European Americans and African-Americans: the Candidate Gene Association Resource (CARe) PLoS One. 2012;7(11):e48836. doi: 10.1371/journal.pone.0048836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larkin EK, et al. A candidate gene study of obstructive sleep apnea in European Americans and African Americans. Am J Respir Crit Care Med. 2010;182(7):947–53. doi: 10.1164/rccm.201002-0192OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jean-Louis G, et al. Evaluation of sleep apnea in a sample of black patients. J Clin Sleep Med: J Clin Sleep Med. 2008:421–5. [PMC free article] [PubMed] [Google Scholar]
- 37.Billings ME, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34(12):1653–8. doi: 10.5665/sleep.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace DM, et al. The association of age, insomnia, and self-efficacy with continuous positive airway pressure adherence in black, white, and Hispanic U.S. Veterans. J Clin Sleep Med. 2013;9(9):885–95. doi: 10.5664/jcsm.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawyer A, et al. P395A A prospective cohort study examining race as a predictor of adherence to continuous positive airway pressure therapy in obstructive sleep apnea. Sleep Medicine 2006 [Google Scholar]
- 40.Littner M, et al. The American Academy of Sleep Medicine (Practice Parameters) Sleep. 2002;25(2) doi: 10.1093/sleep/25.2.143. [DOI] [PubMed] [Google Scholar]
- 41.Colten H, Altevogt A, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. National Academies Press; Washington, DC: 2006. p. 404. [PubMed] [Google Scholar]
- 42.People H. Healthy People 2020. 2010 [cited 2014. [Google Scholar]
- 43.Kirsch DB. PRO: Sliding into Home: Portable Sleep Testing Is Effective for Diagnosis of Obstructive Sleep Apnea. Journal of Clinical Sleep Medicine. 2013;9(1):5–7. doi: 10.5664/jcsm.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collop N, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 45.Garg N, et al. Home-based Diagnosis of Obstructive Sleep Apnea in an Urban Population. Journal of Clinical Sleep Medicine. 2014;10(8):879–885. doi: 10.5664/jcsm.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McBean A, Gornick M. Differences by race in the rates of procedures performed in hospitals for Medicare beneficiaries. Health Care Financ Rev. 1994;15:77–90. [PMC free article] [PubMed] [Google Scholar]
- 47.Netzer NC, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of Internal Medicine. 1999;131(7):485–+. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 48.Chung F, et al. STOP questionnaire - A tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 49.Levendowski DJ, et al. Prevalence of probable obstructive sleep apnea risk and severity in a population of dental patients. Sleep Breath. 2008;12(4):303–9. doi: 10.1007/s11325-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 50.Shaw R, et al. Beliefs and attitudes toward obstructive sleep apnea evaluation and treatment among blacks. J Natl Med Assoc. 2012;104(11–12):510–9. doi: 10.1016/s0027-9684(15)30217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patton M. Qualitative Research and Evaluation Methods. 3. Thousand Oaks: Sage; 2002. [Google Scholar]
- 52.Williams N, et al. I put the machine on and I sleep like a baby…” a culturally and linguistically tailored telephone-behavioral intervention to increase adherence to sleep apnea recommendations among blacks with metabolic syndrome. Sleep. 2014:A59. [Google Scholar]
- 53.Means MK, Edinger JD, Husain AM. CPAP compliance in sleep apnea patients with and without laboratory CPAP titration. Sleep & breathing = Schlaf & Atmung. 2004;8(1):7–14. doi: 10.1007/s11325-004-0007-5. [DOI] [PubMed] [Google Scholar]
- 54.Pamidi S, et al. The Impact of Sleep Consultation Prior to a Diagnostic Polysomnogram on Continuous Positive Airway Pressure Adherence. Chest. 2012;141(1):51–57. doi: 10.1378/chest.11-0709. [DOI] [PubMed] [Google Scholar]
- 55.Guralnick AS, et al. CPAP Adherence in Patients with Newly Diagnosed Obstructive Sleep Apnea prior to Elective Surgery. Journal of Clinical Sleep Medicine. 2012;8(5):501–506. doi: 10.5664/jcsm.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balachandran JS, et al. A Brief Survey of Patients’ First Impression after CPAP Titration Predicts Future CPAP Adherence: A Pilot Study. Journal of Clinical Sleep Medicine. 2013;9(3):199–205. doi: 10.5664/jcsm.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Billings ME, et al. Is the Relationship between Race and Continuous Positive Airway Pressure Adherence Mediated by Sleep Duration? Sleep. 2013;36(2):221–227. doi: 10.5665/sleep.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Budhiraja R, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–4. [PubMed] [Google Scholar]
- 59.Platt AB, et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32(6):799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ball EM, Banks MB. Determinants of compliance with nasal continuous positive airway pressure treatment applied in a community setting. Sleep Med. 2001;2(3):195–205. doi: 10.1016/s1389-9457(01)00086-7. [DOI] [PubMed] [Google Scholar]
- 61.Zerhouni EA. A New Vision for the National Institutes of Health. J Biomed Biotechnol. 2003;2003(3):159–160. doi: 10.1155/S1110724303306023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Israel BA, et al. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 63.Bachmann MR, et al. SOCIAL DETERMINANTS OF SHORT SLEEP AMONG BLACK AND WHITE AMERICANS. Sleep. 2011;34:A261–A261. [Google Scholar]
- 64.Zizi F, et al. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Current diabetes reports. 2010;10(1):43–7. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams N, et al. Telephone-delivered behavioral intervention among blacks with sleep apnea and metabolic syndrome: study protocol for a randomized controlled trial. Trials. 2014:225. doi: 10.1186/1745-6215-15-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]


