Abstract
BACKGROUND
In a trial comparing coronary-artery bypass grafting (CABG) alone with CABG plus mitral-valve repair in patients with moderate ischemic mitral regurgitation, we found no significant difference in the left ventricular end-systolic volume index (LVESVI) or survival after 1 year. Concomitant mitral-valve repair was associated with a reduced prevalence of moderate or severe mitral regurgitation, but patients had more adverse events. We now report 2-year outcomes.
METHODS
We randomly assigned 301 patients to undergo either CABG alone or the combined procedure. Patients were followed for 2 years for clinical and echocardiographic outcomes.
RESULTS
At 2 years, the mean (±SD) LVESVI was 41.2±20.0 ml per square meter of body-surface area in the CABG-alone group and 43.2±20.6 ml per square meter in the combined-procedure group (mean improvement over baseline, −14.1 ml per square meter and −14.6 ml per square meter, respectively). The rate of death was 10.6% in the CABG-alone group and 10.0% in the combined-procedure group (hazard ratio in the combined-procedure group, 0.90; 95% confidence interval, 0.45 to 1.83; P=0.78). There was no significant between-group difference in the rank-based assessment of the LVESVI (including death) at 2 years (z score, 0.38; P = 0.71). The 2-year rate of moderate or severe residual mitral regurgitation was higher in the CABG-alone group than in the combined-procedure group (32.3% vs. 11.2%, P<0.001). Overall rates of hospital readmission and serious adverse events were similar in the two groups, but neurologic events and supraventricular arrhythmias remained more frequent in the combined-procedure group.
CONCLUSIONS
In patients with moderate ischemic mitral regurgitation undergoing CABG, the addition of mitral-valve repair did not lead to significant differences in left ventricular reverse remodeling at 2 years. Mitral-valve repair provided a more durable correction of mitral regurgitation but did not significantly improve survival or reduce overall adverse events or readmissions and was associated with an early hazard of increased neurologic and supraventricular arrhythmias.
Ischemic mitral regurgitation of moderate severity develops in approximately 10% of patients after myocardial infarction.1,2 Mitral regurgitation is caused by the displacement of papillary muscle, leaflet tethering, reduced closing forces, and annular dilatation. Over time, the condition has an adverse effect on the rate of survival free of heart failure.3 Because most patients with ischemic mitral regurgitation have multivessel coronary artery disease requiring revascularization, surgeons have to consider whether to add mitral-valve repair to coronary-artery bypass grafting (CABG).
The appropriate surgical management of moderate ischemic mitral regurgitation at the time of CABG remains controversial. Some experts advocate revascularization alone for moderate ischemic mitral regurgitation, because improvements in regional and global left ventricular function and geometry after CABG can reduce rates of mitral regurgitation.4,5 Others support restrictive mitral annuloplasty repair at the time of CABG to directly reduce the degree of mitral regurgitation, thus preventing further adverse remodeling and decreasing the risk of heart failure.6,7 However, the addition of mitral-valve repair to CABG necessitates open-heart exposure with an increased duration of aortic cross-clamping and cardiopulmonary bypass, which can increase perioperative risk.
The Cardiothoracic Surgical Trials Network (CTSN) addressed this trade-off by conducting a multicenter, randomized trial comparing CABG alone with CABG plus mitral-valve repair (combined procedure) in patients with moderate ischemic mitral regurgitation.8 At 1 year, there was no significant difference in left ventricular reverse remodeling (as measured by the left ventricular end-systolic volume index [LVESVI]) or in rates of survival or major adverse cardiac and cerebrovascular events (MACCE). The combined procedure was associated with a significantly reduced prevalence of moderate or severe mitral regurgitation but a longer hospital stay after surgery, a higher incidence of postoperative supraventricular arrhythmias, and a higher rate of serious neurologic events than was CABG alone. We report here the 2-year outcomes for patients in the trial.
METHODS
TRIAL DESIGN AND OVERSIGHT
The trial design has been described previously.9 The trial was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Patients with moderate ischemic mitral regurgitation were randomly assigned to undergo either CABG alone or CABG plus mitral-valve repair using a restrictive annuloplasty technique. Randomization was stratified according to center and blocked to ensure the equivalence of group size. The trial was conducted at 26 centers with a coordinating center, an event-adjudication committee, and a data and safety monitoring board overseeing trial progress. The institutional review board at each center approved the protocol, and all patients provided written informed consent. The investigators vouch for the accuracy and completeness of the data and for the fidelity of this report to the trial protocol, which is available with the full text of this article at NEJM.org.
PATIENTS AND INTERVENTIONS
We enrolled adults with moderate ischemic mitral regurgitation and multivessel coronary artery disease. We performed preoperative resting trans-thoracic echocardiography to assess the degree of mitral regurgitation using integrative criteria10 verified by an independent core laboratory. Patients with any echocardiographic evidence of structural mitral-valve disease were excluded. (For details on exclusion criteria, see the Supplementary Appendix, available at NEJM.org.)
The protocol mandated the use of an approved rigid or semirigid annuloplasty ring for mitral-valve repair; the ring was downsized from the annulus diameter. CABG was performed with the use of standard techniques and was supported by cardiopulmonary bypass. The protocol specified the use of guideline-directed medical therapy.
TRIAL END POINTS
The original trial’s primary end point was the degree of left ventricular reverse remodeling, as measured by means of the LVESVI on transthoracic echocardiography 1 year after randomization. All patients were followed for 2 years with end points measured at 6, 12, and 24 months. Secondary end points included findings on trans-thoracic echocardiography at other time points, rate of death, MACCE (defined as a composite of death, stroke, subsequent mitral-valve surgery, hospitalization for heart failure, or worsening New York Heart Association [NYHA] class), serious adverse events, degree of postoperative mitral regurgitation, quality of life, and rehospitalization.
We assessed left ventricular regional wall motion on echocardiography at baseline and at 6, 12, and 24 months. The motion of each of 17 wall segments at rest was quantified (with a score of 1 for normal, 2 for hypokinetic, 3 for akinetic, 4 for dyskinetic, and 5 for aneurysmal), and the sum of the wall-motion scores for the myocardial segments was divided by the number of segments to provide a wall-motion index. A modified wall-motion index was calculated for the inferior–posterior–lateral myocardial region on the basis of seven segments that receive their blood supply largely from the right and left circumflex coronary arteries. (Details about the wall-motion score are provided in the Supplementary Appendix.)
STATISTICAL ANALYSIS
The trial was designed to have a power of 90% to detect a difference in the LVESVI of 12 ml per square meter from baseline to 12 months. We assumed a baseline LVESVI of 80 ml per square meter, improvements of 4 ml per square meter in the CABG-alone group and 16 ml per square meter in the combined-procedure group, and an equal risk of death of 10 to 20% in the two groups at 1 year.11,12 The primary null hypothesis was no between-group difference in the LVESVI at 1 year.1 We evaluated the LVESVI at 2 years in an intention-to-treat analysis using a two-tailed Wilcoxon rank-sum test with an alpha level of 0.05, which accommodated nonignorable missing LVESVI outcomes owing to death by assigning deceased patients the worst ranks on the basis of the time of death. We calculated values for missing data for the 2-year LVESVI using multiple imputation and assuming that data were missing at random. Secondary hypotheses were tested at an alpha level of 0.01. We used the log-rank test to compare rates of MACCE and death and to calculate hazard ratios from Cox regression models to quantify relative risks. We used Poisson regression to test group differences in event rates and t-tests to evaluate differences in wall-motion scores between baseline and 2 years. We used chi-square tests to compare NYHA functional status in the two groups. We assessed patients’ quality of life using the Minnesota Living with Heart Failure questionnaire, the Duke Activity Status Index (DASI), European Quality of Life–5 Dimensions (EQ-5D), and the physical and mental subscales of the Medical Outcomes Study 12-Item Short Form General Health Survey (SF-12) and analyzed the results using mixed-effects models.
RESULTS
PATIENTS
A total of 301 patients underwent randomization, 151 to the CABG-alone group and 150 to the combined-procedure group (Fig. S1 in the Supplementary Appendix). The two groups had similar characteristics at baseline (Table S1 in the Supplementary Appendix). The mean (±SD) LVESVI was 54.8±24.9 ml per square meter of body-surface area in the CABG-alone group and 59.6±25.7 ml per square meter in the combined-procedure group. Concomitant procedures were performed in 19% of patients. In the combined-procedure group, the mean annulus diameter was 31.4±5.0 mm and the average ring diameter was 27.9±2.1 mm; 93% of patients received a ring measuring 30 mm or less. Aortic cross-clamp and cardiopulmonary-bypass times were significantly longer in the combined-procedure group than in the CABG-alone group (aortic cross-clamp time, 117.1±35.4 minutes vs. 74.7±36.7 minutes; cardiopulmonary-bypass time, 163.1±54.9 minutes vs. 106.8±49.7 minutes; P<0.001 for both comparisons). Eight patients in the CABG-alone group underwent the combined procedure, and 3 patients in the combined-procedure group underwent CABG alone.
LEFT VENTRICULAR DIMENSIONS AND FUNCTION
At 2 years, the mean LVESVI in surviving patients was 41.2±20.0 ml per square meter in the CABG-alone group and 43.2±20.6 ml per square meter in the combined-procedure group (mean change from baseline, −14.1 ml per square meter and −14.6 ml per square meter, respectively); most of the improvement (−9.4 ml per square meter and −9.3 ml per square meter) occurred during the first year. There was no significant between-group difference in the rank-based assessment of the primary outcome (LVESVI including death) at 2 years (z score, 0.38; P = 0.71). At the same time, the mean left ventricular ejection fraction was 46.1±10.5% in the CABG-alone group and 45.6±10.0% in the combined-procedure group (change from baseline, 5.4±11.7 percentage points and 6.4±11.0 percentage points, respectively).
PERSISTENT OR RECURRENT MITRAL REGURGITATION AND ADDITIONAL INTERVENTIONS
At 2 years, the prevalence of moderate or severe mitral regurgitation was higher in the CABG-alone group than in the combined-procedure group (32.3% vs. 11.2%, P<0.001); only 2% of patients in the CABG-alone group and none in the combined-procedure group had severe mitral regurgitation. During the course of the trial, two patients in the CABG-alone group underwent mitral-valve surgery after the index procedure, and two patients in the combined-procedure group underwent mitral-valve reoperation. The proportion of patients with postoperative moderate mitral regurgitation at any time within 2 years was significantly higher in the CABG-alone group than in the combined-procedure group (43.0% vs. 24.8%, P = 0.004). The proportion of patients with severe mitral regurgitation or mitral-valve reoperation was 11.4% in the CABG-alone group and 3.5% in the combined-procedure group (P = 0.02). In the CABG-alone group, patients who never had moderate or severe persistent mitral regurgitation and who had not undergone a mitral-valve intervention had more reverse remodeling than those who did (LVESVI, 36.3±15.1 and 47.8±20.8, respectively; P=0.001), with corresponding results in the combined-procedure group (LVESVI, 40.9±20.6 vs. 51.7±19.6; P = 0.02).
WALL-MOTION CHANGES AND RECURRENCE OF MITRAL REGURGITATION
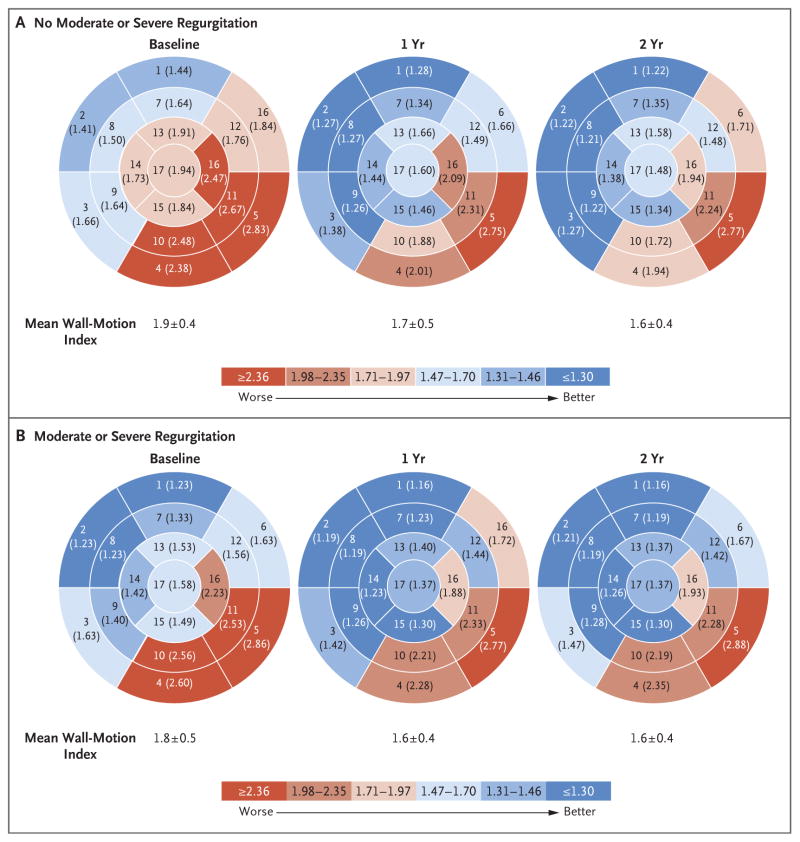
To explore the effect of revascularization on the risk of persistence of mitral regurgitation, we analyzed changes in echocardiographic wall-motion scores, which were stratified according to the recurrence of mitral regurgitation. The percent improvement in the global wall-motion index was larger for patients who were free of moderate or severe mitral regurgitation at 2 years than for those with such mitral regurgitation (16.5±20.1% vs. 7.4±16.7%, P = 0.008) (Fig. 1). The greatest degree of improvement in the overall wall-motion score occurred during the first year after surgery. The percent improvement in the inferior–posterior–lateral regional wall-motion score was greater for patients who were free of moderate or severe mitral regurgitation at 2 years than for those with such mitral regurgitation (18.1±18.9% vs. 7.9±17.5%, P = 0.002).
Figure 1. Wall-Motion Scores at Baseline, 1 Year, and 2 Years, According to the Presence of Postoperative Moderate or Severe Mitral Regurgitation.
Shown are the results of analyses of each of 17 wall segments at rest for patients without moderate or severe mitral regurgitation and those with moderate or severe mitral regurgitation at 2 years after the procedure. Baseline values were recorded before the procedure. The values in parentheses are the mean wall-motion scores at three time points for each segment. Scores on the wall-motion index are as follows: 1, normal; 2, hypokinetic; 3, akinetic; 4, dyskinetic; and 5, aneurysmal. The sum of the wall-motion scores for the myocardial segments was divided by the number of segments to provide a wall-motion index. At 2 years, the relative percent improvement in the global wall-motion index was larger for patients free of moderate or severe mitral regurgitation than for those with mitral regurgitation (16.5±20.1% vs. 7.4±16.7%, P = 0.008). A chart showing the name of each numbered segment is provided in the Supplementary Appendix.
DEATH, ADVERSE EVENTS, AND HOSPITALIZATION
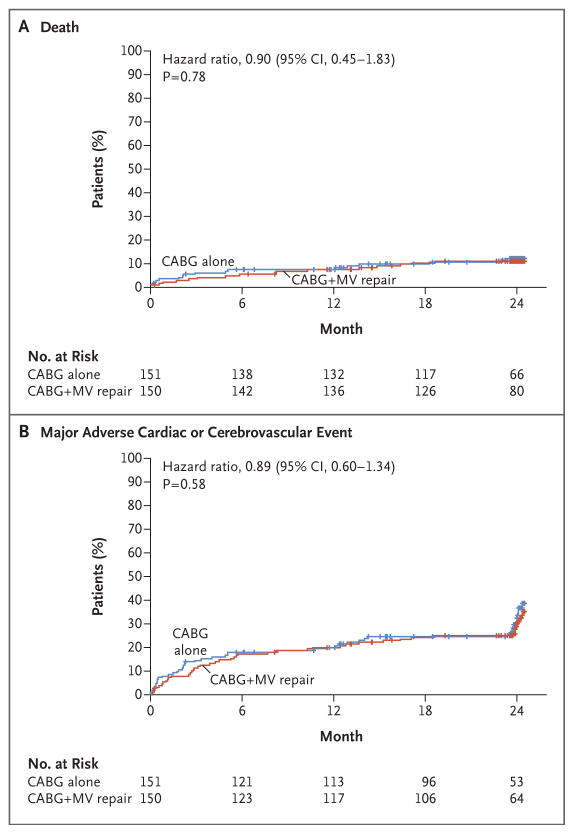
Clinical outcomes at 1 year have been described previously.1 At 2 years, we observed no significant difference in cumulative mortality between the two study groups, with 10.6% for CABG alone and 10.0% for the combined procedure (hazard ratio in the combined-procedure group, 0.90; 95% confidence interval [CI], 0.45 to 1.83; P =0.78) (Fig. 2A). At 2 years, there was no significant between-group difference in the rate of MACCE (hazard ratio, 0.89; 95% CI, 0.60 to 1.34; P = 0.58) (Fig. 2B) or in any of its component events (Table 1).
Figure 2. Rates of Death and Cardiovascular Events.
Shown are the rates of death and a composite of major adverse cardiac and cerebrovascular events (which were defined as death, stroke, subsequent mitral-valve surgery, hospitalization for heart failure, or worsening New York Heart Association class) among patients undergoing either coronary-artery bypass grafting (CABG) or CABG plus mitral-valve (MV) repair. The tick marks show censoring of data.
Table 1.
Clinical End Points, Serious Adverse Events, and Hospitalizations.*
| End Point or Event | From Baseline to 1 Yr | From 1 Yr to 2 Yr† | From Baseline to 2 Yr | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CABG (N = 151) | CABG plus Repair (N = 150) | P Value | CABG (N = 128) | CABG plus Repair (N = 137) | P Value | CABG (N = 151) | CABG plus Repair (N = 150) | P Value | |
| no. of patients (%) | no. of patients (%) | no. of patients (%) | |||||||
|
| |||||||||
| Clinical end point | |||||||||
|
| |||||||||
| Death | 11 (7.3) | 10 (6.7) | 0.83 | 5 (3.9) | 5 (3.6) | 1.0 | 16 (10.6) | 15 (10.0) | 0.86 |
|
| |||||||||
| Stroke | 2 (1.3) | 6 (4.0) | 0.17 | 0 | 0 | NA | 2 (1.3) | 6 (4.0) | 0.17 |
|
| |||||||||
| Increase of ≥1 NYHA class‡ | 9 (7.7) | 12 (9.4) | 0.63 | 14 (14.0) | 12 (10.4) | 0.42 | 14 (14.0) | 12 (10.4) | 0.42 |
|
| |||||||||
| Rehospitalization for heart failure | 20 (13.2) | 22 (14.7) | 0.72 | 6 (4.7) | 6 (4.4) | 0.90 | 22 (14.6) | 26 (17.3) | 0.51 |
|
| |||||||||
| Mitral-valve surgery after index procedure | 0 | 2 (1.3) | 0.25 | 2 (1.6) | 0 | 0.23 | 2 (1.3) | 2 (1.3) | 1.0 |
|
| |||||||||
| LVAD or heart transplantation | 1 (0.7) | 4 (2.7) | 0.21 | 0 | 1 (0.7) | 1.0 | 1 (0.7) | 4 (2.7) | 0.21 |
|
| |||||||||
| Composite MACCE end point | 38 (25.2) | 38 (25.3) | 0.97 | 24 (18.8) | 19 (13.9) | 0.28 | 48 (31.8) | 46 (30.7) | 0.83 |
|
| |||||||||
| no. of events (no./100 patient-yr) | no. of events (no./100 patient-yr) | no. of events (no./100 patient-yr) | |||||||
|
| |||||||||
| Serious adverse events | |||||||||
|
| |||||||||
| Any | 158 (118.9) | 192 (141.1) | 0.11 | 42 (40.0) | 43 (36.0) | 0.63 | 200 (84.0) | 235 (92.0) | 0.35 |
|
| |||||||||
| Heart failure | 30 (22.6) | 31 (22.8) | 0.97 | 9 (8.6) | 9 (7.5) | 0.79 | 39 (16.4) | 40 (15.7) | 0.84 |
|
| |||||||||
| Neurologic dysfunction | |||||||||
|
| |||||||||
| Any | 4 (3.0) | 14 (10.3) | 0.02 | 0 | 0 | NA | 4 (1.7) | 14 (5.5) | 0.02 |
|
| |||||||||
| Stroke | 2 (1.5) | 7 (5.1) | 0.10 | 0 | 0 | NA | 2 (0.8) | 7 (2.7) | 0.11 |
|
| |||||||||
| Myocardial infarction | |||||||||
|
| |||||||||
| Nonperioperative | 1 (0.8) | 1 (0.7) | 0.99 | 1 (1.0) | 0 | 0.32 | 2 (0.8) | 1 (0.4) | 0.53 |
|
| |||||||||
| Perioperative | 1 (0.8) | 0 | 0.32 | 0 | 0 | NA | 1 (0.4) | 0 | 0.32 |
|
| |||||||||
| Renal failure | 4 (3.0) | 4 (2.9) | 0.97 | 3 (2.9) | 2 (1.7) | 0.56 | 7 (2.9) | 6 (2.3) | 0.69 |
|
| |||||||||
| Bleeding | 4 (3.0) | 2 (1.5) | 0.40 | 2 (1.9) | 0 | 0.16 | 6 (2.5) | 2 (0.8) | 0.14 |
|
| |||||||||
| Arrhythmia | |||||||||
|
| |||||||||
| Supraventricular | 11 (8.3) | 24 (17.6) | 0.03 | 0 | 0 | NA | 11 (4.6) | 24 (9.4) | 0.04 |
|
| |||||||||
| Ventricular | 5 (3.8) | 2 (1.5) | 0.25 | 0 | 2 (1.7) | 0.16 | 5 (2.1) | 4 (1.6) | 0.66 |
|
| |||||||||
| Localized infection | 19 (14.3) | 18 (13.2) | 0.81 | 3 (2.9) | 7 (5.9) | 0.28 | 22 (9.2) | 25 (9.8) | 0.84 |
|
| |||||||||
| Endocarditis | 0 | 2 (1.5) | 0.16 | 1 (1.0) | 0 | 0.32 | 1 (0.4) | 2 (0.8) | 0.60 |
|
| |||||||||
| Sepsis | 7 (5.3) | 9 (6.6) | 0.65 | 0 | 0 | NA | 7 (2.9) | 9 (3.5) | 0.72 |
|
| |||||||||
| Respiratory failure | 8 (6.0) | 10 (7.3) | 0.67 | 1 (1.0) | 0 | 0.32 | 9 (3.8) | 10 (3.9) | 0.94 |
|
| |||||||||
| Hospitalization | |||||||||
|
| |||||||||
| Any readmission | 96 (75.2) | 89 (68.6) | 0.54 | 37 (35.4) | 40 (33.8) | 0.83 | 133 (57.3) | 129 (52.0) | 0.43 |
|
| |||||||||
| Readmission for cardiovascular causes | 51 (40.0) | 46 (35.5) | 0.56 | 20 (19.2) | 18 (15.2) | 0.48 | 71 (30.6) | 64 (25.8) | 0.32 |
CABG denotes coronary-artery bypass grafting, LVAD left ventricular assist device, MACCE major adverse cardiac or cerebrovascular event, NA not applicable, and NYHA New York Heart Association.
During the period from 1 year to 2 years, the number of patients includes all those with MACCE, which was defined as death, stroke, subsequent mitral-valve surgery, hospitalization for heart failure, or worsening New York Heart Association class. Excluded from the number of patients who were evaluated were patients who died, withdrew, or were lost to follow-up by 1 year. Several patients had events both before and after 1 year. In the CABG-alone group, 10 of 14 patients with worsening NYHA class had their first event after 1 year, 2 of 6 patients were readmitted to the hospital for heart failure for the first time after 1 year, and 13 of 24 with MACCE had their first event after 1 year. In the combined-procedure group, the numbers of patients were 6 of 12 for worsening NYHA class, 4 of 6 for hospital readmission, and 11 of 19 with MACCE. In the combined-procedure group, 1 patient received an LVAD during year 1 and underwent heart transplantation in year 2.
Changes in the NYHA class were evaluated in 117 patients in the CABG group and in 127 patients in the combined-procedure group at 1 year and in 100 patients and 115 patients, respectively, at 2 years.
In addition, there were no significant differences in the overall rate of serious adverse events between the CABG-alone group and the combined-procedure group (84.0 events per 100 patient-years vs. 92.0 events per 100 patient-years, P = 0.35). During the second year of follow-up, the number of serious adverse events increased by 21.0% in the CABG-alone group and by 18.3% in the combined-procedure group over 1 year. This increase was largely related to infections and heart-failure events, but rates did not differ significantly in the two groups. At 2 years, there were 16.4 serious heart-failure events per 100 patient-years in the CABG-alone group versus 15.7 events per 100 patient-years in the combined-procedure group (P = 0.84). Serious neurologic adverse events, including stroke, transient ischemic attack, and metabolic encephalopathy, were more frequent in the combined-procedure group than in the CABG-alone group (14 events vs. 4 events, P=0.02). All the neurologic events occurred during the first postoperative year, and half of all strokes occurred during the index hospitalization. In 75% of the patients with stroke, the score on the modified Rankin scale was 3 or more, indicating at least moderate disability; 63% of the patients with stroke died. Similarly, there was a higher rate of supraventricular arrhythmias in the combined-procedure group than in the CABG-alone group (24 events vs. 11 events, P = 0.04), but all such events occurred during the first year.
Overall rates of readmission and cardiovascular readmission did not differ significantly in the two study groups. The most common reasons for cardiovascular readmission were heart failure (53%) and placement of an implantable cardioverter–defibrillator or pacemaker (12%).
QUALITY OF LIFE
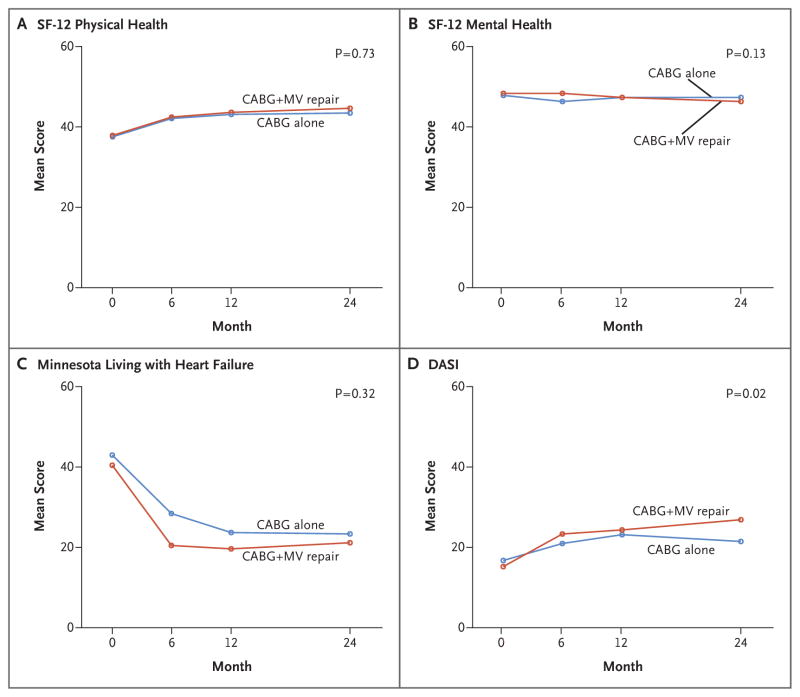
The pattern of change in quality-of-life measures during 2 years of follow-up was similar in the two groups, with the most improvement occurring within 6 months after surgery. There were no significant between-group differences in scores on the Minnesota Living with Heart Failure questionnaire, on the SF-12 physical and mental subscales, or on the EQ-5D. All patients started with a relatively low score for cardiac physical function on the DASI (mean score, 16.8±15.4 in the CABG-alone group and 15.3±14.8 in the combined-procedure group, on a scale of 0 to 58, with higher scores indicating better function). Patients in the two groups had substantial improvement in scores during follow-up, but those in the combined-procedure group had higher DASI scores overall (P = 0.02), suggesting that the patients had an increased ability to undertake tasks with higher metabolic demands (Fig. 3).
Figure 3. Quality-of-Life Scores.
Shown are the mean scores on the Medical Outcomes Study 12-Item Short-Form General Health Survey (SF-12) for physical health (Panel A) and mental health (Panel B) for patients undergoing either CABG alone or CABG plus mitral-valve repair. The SF-12 scale ranges from 0 to 100, with higher scores indicating better health. Panel C shows mean scores on the Minnesota Living with Heart Failure questionnaire; scores can range from 0 to 105, with higher scores indicating a lower quality of life. Panel D shows mean scores on the Duke Activity Status Index (DASI), on which scores range from 0 to 58, with higher scores indicating a better activity level. On these measures of quality of life, the only significant between-group difference was on the DASI (P = 0.02).
DISCUSSION
In this 2-year trial, we evaluated two types of surgical treatment for patients with moderate ischemic mitral regurgitation associated with multivessel coronary artery disease. At baseline, the patients had abnormal regional left ventricular function, which was more pronounced in the inferior–posterior–lateral region, an area that is perfused by the right or left circumflex coronary artery. Overall, surgical revascularization with or without mitral-valve repair typically results in significant improvements in ventricular function, as measured by means of the LVESVI, ejection fraction, and global and regional wall motion. However, at 2 years, patients who underwent CABG alone had a rate of postoperative moderate or severe mitral regurgitation that was three times the rate among patients who underwent both CABG and mitral-valve repair. Independent of treatment group, patients with postoperative mitral regurgitation had significantly less reverse remodeling than those without such mitral regurgitation. Moreover, the absence of postoperative mitral regurgitation was associated with the greatest improvement in global and regional wall-motion scores at 2 years.
Improvement in global and regional wall motion and reverse left ventricular remodeling after revascularization are indicative of viable myocardium. Successful revascularization can also be favorable to mitral-valve function in patients with ischemic mitral regurgitation in relation to the attendant decrease in left ventricular size, increased mitral-valve closing forces, improved papillary-muscle synchrony, and enhanced contractility of subjacent myocardium.13–15 In this trial, improvements in both global and regional wall-motion scores were associated with significantly less moderate or severe mitral regurgitation at 2 years — in other words, a more durable and successful outcome.
These findings imply that many patients who were enrolled in this trial had mitral regurgitation that was caused by reversible ischemia rather than by nonviable scar formation. Therefore, surgical decision making could be improved by identifying which patients are most likely to have an improvement in regional wall motion and global left ventricular function after revascularization. Further investigation is required to determine whether patients with baseline abnormalities in inferior–posterior–lateral wall motion that are considered to be irreversible from infarction (e.g., on the basis of viability testing) would benefit more from mitral-valve repair than from revascularization. Larger trials that use viability testing with cardiac magnetic resonance imaging or positron-emission tomography are needed.4 Studies of measurement of mitral-valve tethering and left ventricular geometry are also under way to address this question. The favorable contribution of beta-blockers and cardiac resynchronization to this approach should also be emphasized.16,17
At 2-year follow-up, the significantly higher frequency of postoperative moderate or severe mitral regurgitation among patients in the CABG-alone group did not translate into a higher risk of death than among those who underwent the combined procedure (10.6% and 10.0%, respectively). Few additional deaths occurred in the second year in the two study groups, and the overall rate of death was consistent with results that have been published previously.18 We also observed no significant differences in the rates of MACCE, overall serious adverse events, or hospital readmission, including cardiovascular readmission. Studies have shown that one of the adverse consequences of persistent mitral regurgitation is worsening heart failure,3 an outcome observed in the CTSN trial involving patients with severe ischemic mitral regurgitation. In that trial, the group that underwent mitral-valve repair had higher rates of persistent mitral regurgitation, heart-failure events, and cardiovascular readmissions than the group that underwent mitral-valve replacement.19 However, among patients with moderate mitral regurgitation in our current trial, we did not find an excess hazard of more postoperative heart-failure events, an outcome that can probably be attributed to the relatively small proportion (1%) of patients who had progression to severe mitral regurgitation at 2 years. The combined procedure involved more complicated surgery, which was associated with longer bypass times and an increased risk of embolization, which may explain the higher rate of serious composite neurologic events in this group. Moreover, the atrial incision that is required for mitral-valve exposure may have predisposed the patients to more supraventricular arrhythmias and an additional risk of thromboembolism.
Measurements of overall quality of life and heart-failure symptoms improved in the two study groups. The change in the DASI score, which captures self-reported exercise capacity, also showed substantial improvement in the two groups during the first 12 months. However, during the second year, the scores for the two groups diverged, which indicated an overall improvement in the mean DASI score for the combined-procedure group. The observed between-group difference at 24 months was 5.3 points, a clinically meaningful improvement that is similar in direction to the increase in peak myocardial oxygen consumption noted on exercise testing after CABG plus mitral-valve repair, as compared with CABG alone, that was reported in a smaller, randomized trial involving patients with moderate mitral regurgitation.7,20,21
The current trial has several limitations. First, the primary end point was an echocardiographic measure of left ventricular remodeling, not a clinical outcome such as MACCE or survival. However, a randomized trial with an end point of death or MACCE at 1 year or 2 years would have required the enrollment of thousands of patients. On the other hand, there is strong evidence of an association between the LVESVI and clinical outcome, including NYHA class and rates of hospitalization and survival.22 Second, although we did not specify preoperative evaluation of myocardial viability, echocardiographic assessment of regional and global left ventricular systolic function can predict the effectiveness of revascularization in specific patient populations.23–25 Lastly, the time horizon for observations was relatively short. Additional events would be captured with longer follow-up in these patient cohorts.
In conclusion, the addition of mitral-valve repair to CABG had no incremental effect on reverse left ventricular remodeling at 2 years. However, patients who underwent CABG alone had a higher prevalence of postoperative moderate or severe mitral regurgitation than did those who underwent the combined procedure, although this difference did not translate into higher rates of death, MACCE, serious adverse events (including heart failure), or readmission during 2 years of follow-up. Patients who underwent CABG plus mitral-valve repair had higher DASI scores at 2 years, indicative of improved exercise capacity. Nevertheless, the addition of mitral-valve repair was associated with longer cross-clamp or bypass times, a longer postoperative length of stay during the index hospitalization, and significantly higher rates of serious neurologic events and supraventricular arrhythmias. Individual treatment decisions require balancing the risks of these adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation. Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral-valve repair.
Supplementary Material
Acknowledgments
(Funded by the National Institutes of Health and Canadian Institutes of Health Research; ClinicalTrials.gov number, NCT00806988.)
Supported by a cooperative agreement (U01 HL088942) funded by the National Heart, Lung, and Blood Institute and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health and the Canadian Institutes of Health Research.
APPENDIX
The authors’ full names and academic degrees are as follows: Robert E. Michler, M.D., Peter K. Smith, M.D., Michael K. Parides, Ph.D., Gorav Ailawadi, M.D., Vinod Thourani, M.D., Alan J. Moskowitz, M.D., Michael A. Acker, M.D., Judy W. Hung, M.D., Helena L. Chang, M.S., Louis P. Perrault, M.D., A. Marc Gillinov, M.D., Michael Argenziano, M.D., Emilia Bagiella, Ph.D., Jessica R. Overbey, M.S., Ellen G. Moquete, R.N., Lopa N. Gupta, M.P.H., Marissa A. Miller, D.V.M., Wendy C. Taddei-Peters, Ph.D., Neal Jeffries, Ph.D., Richard D. Weisel, M.D., Eric A. Rose, M.D., James S. Gammie, M.D., Joseph J. DeRose, Jr., M.D., John D. Puskas, M.D., François Dagenais, M.D., Sandra G. Burks, R.N., Ismail El-Hamamsy, M.D., Ph.D., Carmelo A. Milano, M.D., Pavan Atluri, M.D., Pierre Voisine, M.D., Patrick T. O’Gara, M.D., and Annetine C. Gelijns, Ph.D.
The authors’ affiliations are as follows: the Department of Cardiothoracic and Vascular Surgery, Montefiore Medical Center–Albert Einstein College of Medicine (R.E.M., J.J.D.), the International Center for Health Outcomes and Innovation Research (InCHOIR), Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai (M.K.P., A.J.M., H.L.C., E.B., J.R.O., E.G.M., L.N.G., A.C.G.), the Division of Cardiothoracic Surgery, Department of Surgery, College of Physicians and Surgeons, Columbia University (M.A.), and the Department of Cardiac Surgery, Mount Sinai Health System (E.A.R., J.D.P.) — all in New York; the Division of Cardiovascular and Thoracic Surgery, Department of Surgery, Duke University Medical Center, Durham, NC (P.K.S., C.A.M.); the Division of Thoracic and Cardiovascular Surgery, University of Virginia School of Medicine, Charlottesville (G.A., S.G.B.); the Clinical Research Unit, Division of Cardiothoracic Surgery, Emory University School of Medicine, Atlanta (V.T.); the Department of Surgery, Division of Cardiovascular Surgery, University of Pennsylvania School of Medicine, Philadelphia (M.A.A., P.A.); the Division of Cardiology, Massachusetts General Hospital (J.W.H.), and the Cardiovascular Division, Brigham and Women’s Hospital (P.T.O.) — both in Boston; Montreal Heart Institute, University of Montreal, Montreal (L.P.P., I.E.-H.), Peter Munk Cardiac Centre and Division of Cardiovascular Surgery, Toronto General Hospital, University Health Network and the Division of Cardiac Surgery, University of Toronto, Toronto (R.D.W.), and Institut Universitaire de Cardiologie de Québec, Hôpital Laval, Quebec, QC (F.D., P.V.) — all in Canada; the Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic Foundation, Cleveland (A.M.G.); and the Division of Cardiovascular Sciences (M.A.M., W.C.T.-P.) and Office of Biostatistics Research (N.J.), National Heart, Lung, and Blood Institute, Bethesda, and the Department of Surgery, University of Maryland Medical Center, Baltimore (J.S.G.) — both in Maryland.
Footnotes
References
- 1.Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111:295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 2.Pérez de Isla L, Zamorano J, Quezada M, et al. Functional mitral regurgitation after a first non-ST-segment elevation acute coronary syndrome: contribution to congestive heart failure. Eur Heart J. 2007;28:2866–72. doi: 10.1093/eurheartj/ehm469. [DOI] [PubMed] [Google Scholar]
- 3.Grigioni F, Detaint D, Avierinos JF, Scott C, Tajik J, Enriquez-Sarano M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J Am Coll Cardiol. 2005;45:260–7. doi: 10.1016/j.jacc.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Penicka M, Linkova H, Lang O, et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation. 2009;120:1474–81. doi: 10.1161/CIRCULATIONAHA.108.842104. [DOI] [PubMed] [Google Scholar]
- 5.Roshanali F, Mandegar MH, Yousefnia MA, Alaeddini F, Wann S. Low-dose dobutamine stress echocardiography to predict reversibility of mitral regurgitation with CABG. Echocardiography. 2006;23:31–7. doi: 10.1111/j.1540-8175.2006.00163.x. [DOI] [PubMed] [Google Scholar]
- 6.Fattouch K, Guccione F, Sampognaro R, et al. POINT: efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg. 2009;138:278–85. doi: 10.1016/j.jtcvs.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Chan KM, Punjabi PP, Flather M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation. 2012;126:2502–10. doi: 10.1161/CIRCULATIONAHA.112.143818. [DOI] [PubMed] [Google Scholar]
- 8.Smith PK, Puskas JD, Ascheim DD, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–88. doi: 10.1056/NEJMoa1410490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith PK, Michler RE, Woo YJ, et al. Design, rationale, and initiation of the Surgical Interventions for Moderate Ischemic Mitral Regurgitation Trial: a report from the Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg. 2012;143:111–7. doi: 10.1016/j.jtcvs.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 11.Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: a quantitative clinical study. Circulation. 2000;102:1400–6. doi: 10.1161/01.cir.102.12.1400. [DOI] [PubMed] [Google Scholar]
- 12.Bax JJ, Braun J, Somer ST, et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation. 2004;110(Suppl 1):II103–8. doi: 10.1161/01.CIR.0000138196.06772.4e. [DOI] [PubMed] [Google Scholar]
- 13.Meluzín J, Cerný J, Frélich M, et al. Prognostic value of the amount of dysfunctional but viable myocardium in revascularized patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 1998;32:912–20. doi: 10.1016/s0735-1097(98)00324-6. [DOI] [PubMed] [Google Scholar]
- 14.The Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group. Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable angina. N Engl J Med. 1984;311:1333–9. doi: 10.1056/NEJM198411223112102. [DOI] [PubMed] [Google Scholar]
- 15.Bax JJ, Visser FC, Poldermans D, et al. Time course of functional recovery of stunned and hibernating segments after surgical revascularization. Circulation. 2001;104(Suppl 1):I314–8. doi: 10.1161/hc37t1.094853. [DOI] [PubMed] [Google Scholar]
- 16.Capomolla S, Febo O, Gnemmi M, et al. Beta-blockade therapy in chronic heart failure: diastolic function and mitral regurgitation improvement by carvedilol. Am Heart J. 2000;139:596–608. doi: 10.1016/s0002-8703(00)90036-x. [DOI] [PubMed] [Google Scholar]
- 17.Breithardt OA, Sinha AM, Schwammenthal E, et al. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol. 2003;41:765–70. doi: 10.1016/s0735-1097(02)02937-6. [DOI] [PubMed] [Google Scholar]
- 18.Castleberry AW, Williams JB, Daneshmand MA, et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: a 20-year experience. Circulation. 2014;129:2547–56. doi: 10.1161/CIRCULATIONAHA.113.005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2016;374:344–53. doi: 10.1056/NEJMoa1512913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suri RM, Antiel RM, Burkhart HM, et al. Quality of life after early mitral valve repair using conventional and robotic approaches. Ann Thorac Surg. 2012;93:761–9. doi: 10.1016/j.athoracsur.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 21.Grodin JL, Hammadah M, Fan Y, Hazen SL, Tang WH. Prognostic value of estimating functional capacity with the use of the duke activity status index in stable patients with chronic heart failure. J Card Fail. 2015;21:44–50. doi: 10.1016/j.cardfail.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michler RE, Rouleau JL, Al-Khalidi HR, et al. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg. 2013;146(5):1139–1145. e6. doi: 10.1016/j.jtcvs.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonow RO, Maurer G, Lee KL, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–25. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonow RO, Castelvecchio S, Panza JA, et al. Severity of remodeling, myocardial viability, and survival in ischemic LV dysfunction after surgical revascularization. JACC Cardiovasc Imaging. 2015;8:1121–9. doi: 10.1016/j.jcmg.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundt TM. Surgery for ischemic mitral regurgitation. N Engl J Med. 2014;371:2228–9. doi: 10.1056/NEJMe1412045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.