INTRODUCTION
Osteonecrosis of the jaw (ONJ) was defined as exposed, necrotic bone in the maxillofacial region for at least 8 weeks in patients receiving an antiresorptive medication for primary or metastatic bone cancer, osteoporosis, or Paget’s disease, without history of radiation therapy to the jaws [1, 2]. Recently, the AAOMS revised the definition to include exposed bone, or bone that can be probed through an intraoral or extraoral fistula in patients on antiresorptive or antiangiogenic medications [3]. The addition of “probed bone” to the case definition is of clinical significance since frank exposed bone is not always seen, even though it is notably necrotic and radiographically similar.
The staging of the disease is based on severity of symptoms and extent of clinical and radiographic findings [3]. The 2009 and 2014 AAOMS position papers outline the disease stages including Stage 0, where there is no frank bone exposure [2, 3]. Chronic exposed, necrotic bone, inflammation, swelling, pain and radiographic changes are some of the more common clinical findings. ONJ can present as subtle, commonly overlooked Stage 0, as exposed bone without any pain or signs of infection (Stage I), as exposed bone with associated infection, pain, swelling (Stage II), or as extensive disease that forms in large segments of the maxilla or mandible with extraoral fistulae, involvement of vital structures, or pathologic fracture (Stage III).
Treatment strategies range from conservative local wound care to aggressive resective surgery of all necrotic bone. Conservative strategies include systemic antibiotics, oral antibacterial rinse, and debridement of loose necrotic bone that no longer has soft tissue coverage. Recent literature demonstrates that disease prevention with dental exams and treatment before initiating antiresorptive therapy is the most effective method to decrease ONJ incidence [4]. In the conservative management of patients with active ONJ, the treatment goal is focused on preventing disease progression rather than reversal of the process [4–7]. Any procedures that remove soft tissue and/or expose bone, including extractions, are generally avoided when a conservative treatment plan is followed. More invasive treatment strategies may include local curettage and debridement, en bloc resection, flap advancement and resective surgery [8–10].
PROPOSED HYPOTHESES OF MRONJ PATHOPHYSIOLOGY
The first ONJ cases were reported in 2003 and 2004, and although significant progress has been made in our understanding of the disease, much more work needs to be done to completely explain its pathophysiology [11, 12]. Many hypotheses have been proposed, which have sparked empirically-based treatment modalities. Since it is unlikely that one single hypothesis can explain the pathophysiology of ONJ, as it is indeed multifactorial, it is also unlikely that one treatment modality will be successful in all patients. Moreover, since ONJ is a relatively newly described disease entity, as more clinical and preclinical evidence becomes available, it is apparent that our hypotheses and treatment approaches will need to be continuously modified.
HYPOTHESIS 1: BONE REMODELING INHIBITION
Osteoclast activity is tightly regulated by RANK/RANKL/OPG signaling, where an increase in RANKL or decrease in OPG lead to increased bone resorption. In cancer states, tumor cells release growth factors or cytokines, which in turn stimulate osteoblast RANKL release, causing increased bone resorption, and subsequently increased tumor cell presence and growth [13]. Because of their direct effects on osteoclasts, antiresorptives significantly decrease skeletal-related complications, relieve severe bone pain, and correct hypercalcemia in patients with malignant diseases [14–18].
BPs have direct effects on osteoclasts to significantly attenuate bone remodeling [19, 20] and decrease skeletal-related complications in patients with malignant diseases or osteoporosis [14, 15, 20]. Osteoclast differentiation and function play vital roles in bone healing and remodeling at all skeletal sites, but osteonecrosis of the jaws only occurs in alveolar bone of the maxilla and mandible [21]. Alveolar bone may demonstrate an increased remodeling rate as compared to other bones in the axial or appendicular skeleton, which may explain the ONJ predilection in the jaws [22, 23]. However, other studies have failed to confirm differences in bone turnover between the mandible and femur by bone scintigraphy, while the maxilla did show increased bone turnover; administration of BP or denosumab did not change the turnover rate of any bones [24]. Interestingly in mice, fluorescent-labeled BPs demonstrate preferential accumulation in sites of tooth extraction or dental disease, where bone turnover is increased. This is why increased uptake may predispose such sites to higher BP doses and increase susceptibility to BP effects. Although this may not demonstrate a general increase in bone turnover in the jaws, it does show a localized increase in potentially future ONJ sites [25]. The increased bone resorption in the setting of dental disease, coupled with the thin overlying mucosa and a direct pathway through the periodontal ligament with the external environment, make the jaws a suitable breeding ground for ONJ to develop.
Since the primary mechanism of BPs and denosumab is to inhibit osteoclast function by different mechanisms, it is not surprising that altered bone remodeling is the leading hypothesis for ONJ development [26–29]. Importantly, the prevalence of ONJ in patients receiving denosumab and BPs is not significantly different [30–32]. Moreover, animal studies demonstrate a similar rate of periosteal bone deposition, histologic necrosis, and bone exposure when rodents with periodontal or periapical disease or tooth extractions are treated with zoledronate as compared to RANKL inhibitors [21, 33–35]. These human and animal studies highlight the central role of bone remodeling suppression. To combat the effects of bone turnover suppression, withdrawing antiresorptive medications before tooth extraction of surgical procedures is often advocated to potentially reduce the risk of ONJ [3, 36–38]. However, no controlled studies confirm the reduction or reversal of ONJ after a “drug holiday.” Only one clinical report demonstrates a 40% resolution after discontinuing denosumab and 30% after discontinuing ZA [31].
ONJ prevalence in patients treated with BP or denosumab appears similar [39, 40]. BPs bind to exposed hydroxyapatite and incorporate into the bone matrix, where they are retained with a half-life of many years [41–43]. With the advent of denosumab, which does not incorporate into the bone matrix, the half-life is significantly shorter at 32 days maximum [44, 45] and rapid reversibility of its antiresorptive effects [46]. Interestingly, our recent animal study demonstrates faster normalization of TRACP-5b levels after discontinuation of RANKL inhibitor OPG-Fc, a surrogate to denosumab, as compared to zoledronic acid [47]. In addition, radiographic and histologic indices of ONJ returned to levels of control animals after withdrawal of OPG-FC, whereas ZA-treated mice still demonstrated ONJ features [47]. If these data can be validated in controlled clinical studies, they may support the rationale for drug holidays in the management of ONJ patients. They may also demonstrate that discontinuing denosumab vs. bisphosphonate therapy prior to surgical intervention offers faster recovery of normal bone homeostasis.
Another factor that points to the central role of osteoclastic bone resorption in ONJ pathophysiology is the effect of parathyroid hormone (PTH). Initial case reports in osteoporotic patients and animal studies simulating osteoporosis demonstrate the improved healing of extraction sockets and ONJ lesions with administration of parathyroid hormone, possibly due to its ability to improve bone homeostasis, by directly stimulating osteoblastic function and indirectly increasing osteoclastic bone resorption [48–51].
HYPOTHESIS 2: INFLAMMATION & INFECTION
The fact that only 0.8–12% of patients on systemic antiresorptives for malignant disease develop ONJ [3, 52–56], although this may be underestimated [57, 58], points to additional inciting factors beside antiresorptives that contribute to ONJ. Valuable information can be gained from patients with ONJ and their coexisting risk factors. Tooth extraction is generally the most common inciting event associated with ONJ, but teeth in adults are almost always extracted because they have periapical or periodontal infections or inflammation [3, 57, 59, 60]. Animal models of inflammation and infection have been developed to parallel the clinical presentation of ONJ with associated dental pathology, and have consistently shown that both inflammation/infection and a systemic antiresorptive are sufficient for ONJ development [21, 33, 34, 61–65].
Inflammation/infection has been thought to play a role in ONJ, often occuring after extraction of teeth with advanced dental disease or around teeth with periodontal or periapical infection [3, 57, 60, 66]. In multiple myeloma and metastatic cancer patients, aggressive dental hygiene therapy reduces the incidence of ONJ [67, 68]. Further evaluation of histologic specimens detect bacteria on the exposed bone, including Actinomyces species [69, 70]. However, the question remains. Did the bacteria induce the infection and exposed bone, or did the exposed bone develop a bacterial biofilm? Recent studies have shed light on the complexity of biofilm, which include fungi and viruses in addition to the bacterial species [71, 72]. These multiorganism biofilms present challenges to therapy, and may require complicated strategies to eradicate the infection [73–75].
HYPOTHESIS 3: ANGIOGENESIS INHIBITION
Angiogenesis involves the formation of new blood vessels, and necrosis of bones such as the femur are usually of vascular etiology [76]. Bone becomes necrotic without adequate blood supply, as do most tissues, even in pathologic processes. Anti-angiogenic therapies are now widely utilized to inhibit tumor invasion and metastases, targeting vascular signaling molecules such as vascular endothelial growth factor (VEGF) [77]. Zoledronic acid is a known agent that reduces circulating VEGF levels in cancer patients in vivo and reduces angiogenesis in vitro [78–80]. Zoledronic acid inhibits proliferation and interferes with adhesion and migration of human endothelial cells [4, 78], which is thought to interrupt tumor invasion and metastases [4, 80]. In addition, all BPs, especially nitrogen-containing BPs, induce a statistically significant decrease in microvessel density in vivo [81].
Recently, new antiangiogenic therapies such as tyrosine kinase inhibitors and anti-VEGF monoclonal antibodies are associated with ONJ development [82–84]. For these reasons, the new AAOMS guidelines have recognized antiangiogenics as a contributing factor and modified the disease name to medication related ONJ (MRONJ) [85]. Moreover, the prevalence of ONJ is highest in patients with mulitiple myeloma, which is thought to be caused by concomitant antiangiogenic medications and steroids [86, 87]. Even though there is some evidence that antiangiogenesis is involved in the ONJ disease process, histopathologic studies have shown normal vasculature in post-mortem specimens [36]. Most importantly, denosumab has not been associated with antiangiogenesis [88]. Therefore, although unlikely to be central in the development of ONJ, antiangiogenesis is thought to be a significant contributor to the disease process.
HYPOTHESIS 4: SOFT TISSUE TOXICITY
An early hypothesis in ONJ pathophysiology was a direct soft tissue toxicity of BPs [89]. BP exposure, especially nitrogen-containing BPs, induces apoptosis or decreased proliferation of cervical, prostate, and oral epithelial cells in vitro [81, 89–93]. In vitro studies also demonstrate that nitrogen containing BPs localize to epithelial tissue, as well as bone [94]. In addition, oral alendronate is associated with esophageal irritation, requiring special precautions for patients during administration [95]. However, this hypothesis has become less likely due to the lack of soft tissue toxicity reported with denosumab.
HYPOTHESIS 5: – INNATE OR ACQUIRED IMMUNITY DYSFUNCTION
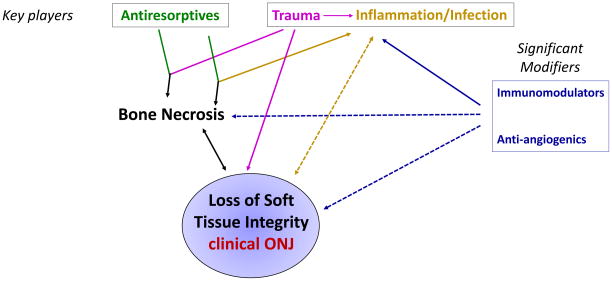
A continued debate exists about the effect of alterned immunity on ONJ development. Tumor pathogenesis is often associated with an impaired immune function [96], and animal studies have implicated immune deficiency in the development of ONJ, while infusion of mesenchymal stem cells or T-regulatory cells prevents and alleviates ONJ-like lesions [97]. In addition, the highest prevalence of ONJ in patients with multiple myeloma, who receive steroids and anti-angiogenics as part of their chemotherapy regimen further points to a role of immune dysfunction in ONJ pathogenesis [87]. Additionally, in many animal models of ONJ, incidence and severity of disease increases with the presence of chemotherapy or steroids [6, 49, 63, 97, 98]. In patients on oral BPs, steroids are also a risk factor for ONJ [3, 60]. This points to the potential significant contribution of immunomodulators in the pathophysiology of the disease (Fig. 1).
Figure 1.
The potential synergy of multiple pathways of ONJ
CONCLUSION
ONJ is a multifactorial disease in patients with primary or metastatic bone malignancy or osteoporosis undergoing systemic antiresorptive therapy, where the pathophysiology has not yet been fully determined. Human and animal studies point to a combination of mechanisms, interacting with each other to increase the development and severity of the disease. The complexity of ONJ and the potential synergy of multiple pathways is depicted on the schematic diagram of Figure 1. Histologic bone necrosis is at the center of the disease process. Strong evidence points to antiresorptives in combination with trauma and/or inflammation/infection as key factors that are necessary and sufficient for ONJ development. Antiresorptives alone do not cause bone necrosis, but when combined with trauma such as a tooth extraction or inflammation/infection from periodontal or periapical disease, bone necrosis can occur. Necrotic bone in turn can lead to loss of soft tissue integrity, or clinical ONJ. Surgical intervention also results in direct disruption of soft tisseus and further complicates the disease. Case reports of exposed, necrotic bone with loss of soft tissue integrity have been associated with trauma or infection alone. Bone exposure propagates infection and inflammation in a positive feedback loop increase disease severity or extent. Further loss of soft tissue integrity leads to continued bone necrosis, and the cycle perpetuates.
Imunomodulators such as steroids or chemotherapy, as well as immunocompromised states from disease such as diabetes, and antiangiogenics are significant modifiers that may increase disease prevalence or severity when combined with inflammation/infection or trauma in the presence of antiresorptives. Again, reported cases of ONJ associated with steroids, chemotherapy, or anti-angiogenics alone are fewer in number. Bone necrosis does not always lead to bone exposure, which is why Stage 0 ONJ has received much attention in recent years. Unless loss of soft tissue integrity occurs, frank bone exposure or Stage I, II, or III clinical ONJ is not diagnosed. Preliminary reports demonstrate that approximately Stage 0 ONJ often progresses to clinical bone exposure [99, 100]. However, it is unclear what determines progression in those patients. We anticipate that continued preclinical and clinical studies will shed light on the key players and significant modifiers in the development, severity, and progression, and resolution of ONJ. Understanding pathophysiologic mechanisms of ONJ will help explore targeted treatment interventions to reduce development and improve management of patients with established disease.
KEY POINTS.
ONJ is a multifactorial disease in patients with primary or metastatic bone malignancy or osteoporosis undergoing systemic antiresorptive therapy, where the pathophysiology has not yet been fully determined
The staging of Osteonecrosis of the jaw (ONJ) is based on severity of symptoms and extent of clinical and radiographic findings.
Treatment strategies range from conservative local wound care to aggressive resective surgery of all necrotic bone
The first ONJ cases were reported in 2003 and 2004, and although significant progress has been made in our understanding of the disease, much more work needs to be done to completely explain its pathophysiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, Al Dabagh R, Davison KS, Kendler DL, Sandor GK, Josse RG, Bhandari M, El Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J International Task Force on Osteonecrosis of the J. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30:3. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B American Association of O Maxillofacial S. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67:2. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw-2014 Update. J Oral Maxillofac Surg. 2014;72:1938. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 4.McLeod NM, Brennan PA, Ruggiero SL. Bisphosphonate osteonecrosis of the jaw: a historical and contemporary review. Surgeon. 2012;10:36. doi: 10.1016/j.surge.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Williamson RA. Surgical management of bisphosphonate induced osteonecrosis of the jaws. Int J Oral Maxillofac Surg. 2010;39:251. doi: 10.1016/j.ijom.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Bi Y, Gao Y, Ehirchiou D, Cao C, Kikuiri T, Le A, Shi S, Zhang L. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol. 2010;177:280. doi: 10.2353/ajpath.2010.090592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E American Society for B Mineral R. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 8.Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:85. doi: 10.1016/j.joms.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Carlson ER, Fleisher KE, Ruggiero SL. Metastatic Cancer Identified in Osteonecrosis Specimens of the Jaws in Patients Receiving Intravenous Bisphosphonate Medications. J Oral Maxillofac Surg. 2013 doi: 10.1016/j.joms.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Spinelli G, Torresetti M, Lazzeri D, Zhang YX, Arcuri F, Agostini T, Grassetti L. Microsurgical reconstruction after bisphosphonate-related osteonecrosis of the jaw: our experience with fibula free flap. J Craniofac Surg. 2014;25:788. doi: 10.1097/SCS.0000000000000833. [DOI] [PubMed] [Google Scholar]
- 11.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 12.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Baud’huin M, Duplomb L, Ruiz Velasco C, Fortun Y, Heymann D, Padrines M. Key roles of the OPG-RANK-RANKL system in bone oncology. Expert Rev Anticancer Ther. 2007;7:221. doi: 10.1586/14737140.7.2.221. [DOI] [PubMed] [Google Scholar]
- 14.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, San Martin J, Dansey R. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 15.Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, Cook R. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 16.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 17.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 18.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S, von Moos R, Willenbacher W, Woll PJ, Wang J, Jiang Q, Jun S, Dansey R, Yeh H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 19.Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67:61. doi: 10.1016/j.joms.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Rodan GA, Reszka AA. Bisphosphonate mechanism of action. Curr Mol Med. 2002;2:571. doi: 10.2174/1566524023362104. [DOI] [PubMed] [Google Scholar]
- 21.Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res. 2011;26:1871. doi: 10.1002/jbmr.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinwald S, Burr D. Review of nonprimate, large animal models for osteoporosis research. J Bone Miner Res. 2008;23:1353. doi: 10.1359/JBMR.080516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huja SS, Fernandez SA, Hill KJ, Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1243. doi: 10.1002/ar.a.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ristow O, Gerngross C, Schwaiger M, Hohlweg-Majert B, Kehl V, Jansen H, Hahnefeld L, Koerdt S, Otto S, Pautke C. Effect of antiresorptive drugs on bony turnover in the jaw: denosumab compared with bisphosphonates. Br J Oral Maxillofac Surg. 2014;52:308. doi: 10.1016/j.bjoms.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Cheong S, Sun S, Kang B, Bezouglaia O, Elashoff D, McKenna CE, Aghaloo TL, Tetradis S. Bisphosphonate uptake in areas of tooth extraction or periapical disease. J Oral Maxillofac Surg. 2014;72:2461. doi: 10.1016/j.joms.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimmel DB. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86:1022. doi: 10.1177/154405910708601102. [DOI] [PubMed] [Google Scholar]
- 27.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Takeyama S, Sakai Y, Yamada S, Shinoda H. Current topics in pharmacological research on bone metabolism: inhibitory effects of bisphosphonates on the differentiation and activity of osteoclasts. J Pharmacol Sci. 2006;100:189. doi: 10.1254/jphs.fmj05004x2. [DOI] [PubMed] [Google Scholar]
- 29.Ito M, Amizuka N, Nakajima T, Ozawa H. Ultrastructural and cytochemical studies on cell death of osteoclasts induced by bisphosphonate treatment. Bone. 1999;25:447. doi: 10.1016/s8756-3282(99)00197-0. [DOI] [PubMed] [Google Scholar]
- 30.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, Miller K, Sieber P, Karsh L, Damiao R, Tammela TL, Egerdie B, Van Poppel H, Chin J, Morote J, Gomez-Veiga F, Borkowski T, Ye Z, Kupic A, Dansey R, Goessl C. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, Diel IJ, Takahashi S, Shore N, Henry DH, Barrios CH, Facon T, Senecal F, Fizazi K, Zhou L, Daniels A, Carriere P, Dansey R. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 32.Dranitsaris G, Hatzimichael E. Interpreting results from oncology clinical trials: a comparison of denosumab to zoledronic acid for the prevention of skeletal-related events in cancer patients. Support Care Cancer. 2012;20:1353. doi: 10.1007/s00520-012-1461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghaloo TL, Cheong S, Bezouglaia O, Kostenuik P, Atti E, Dry SM, Pirih FQ, Tetradis S. RANK-L inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang B, Cheong S, Chaichanasakul T, Bezouglaia O, Atti E, Dry SM, Pirih FQ, Aghaloo TL, Tetradis S. Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J Bone Miner Res. 2013;28:1631. doi: 10.1002/jbmr.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams DW, Lee C, Kim T, Yagita H, Wu H, Park S, Yang P, Liu H, Shi S, Shin KH, Kang MK, Park NH, Kim RH. Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-kappaB ligand antibody in mice. Am J Pathol. 2014;184:3084. doi: 10.1016/j.ajpath.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, Migliorati CA, Ristic H American Dental Association Council on Scientific Affairs Expert Panel on Antiresorptive A. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142:1243. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 37.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 38.Damm DD, Jones DM. Bisphosphonate-related osteonecrosis of the jaws: a potential alternative to drug holidays. Gen Dent. 2013;61:33. [PubMed] [Google Scholar]
- 39.Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, Richardson GE, Siena S, Maroto P, Clemens M, Bilynskyy B, Charu V, Beuzeboc P, Rader M, Viniegra M, Saad F, Ke C, Braun A, Jun S. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Sinningen K, Tsourdi E, Rauner M, Rachner TD, Hamann C, Hofbauer LC. Skeletal and extraskeletal actions of denosumab. Endocrine. 2012;42:52. doi: 10.1007/s12020-012-9696-x. [DOI] [PubMed] [Google Scholar]
- 41.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 42.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinoda H, Adamek G, Felix R, Fleisch H, Schenk R, Hagan P. Structure-activity relationships of various bisphosphonates. Calcif Tissue Int. 1983;35:87. doi: 10.1007/BF02405012. [DOI] [PubMed] [Google Scholar]
- 44.Lewiecki EM. Denosumab: an investigational drug for the management of postmenopausal osteoporosis. Biologics. 2008;2:645. doi: 10.2147/btt.s2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewiecki EM. Denosumab update. Curr Opin Rheumatol. 2009;21:369. doi: 10.1097/BOR.0b013e32832ca41c. [DOI] [PubMed] [Google Scholar]
- 46.Silva I, Branco JC. Denosumab: recent update in postmenopausal osteoporosis. Acta Reumatol Port. 2012;37:302. [PubMed] [Google Scholar]
- 47.de Molon RS, Shimamoto H, Bezouglaia O, Pirih FQ, Dry SM, Kostenuik P, Boyce RW, Dwyer D, Aghaloo TL, Tetradis S. OPG-Fc but not Zoledronic Acid Discontinuation Reverses Osteonecrosis of the Jaws (ONJ) in Mice. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuroshima S, Kovacic BL, Kozloff KM, McCauley LK, Yamashita J. Intra-oral PTH administration promotes tooth extraction socket healing. J Dent Res. 2013;92:553. doi: 10.1177/0022034513487558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuroshima S, Entezami P, McCauley LK, Yamashita J. Early effects of parathyroid hormone on bisphosphonate/steroid-associated compromised osseous wound healing. Osteoporos Int. 2014;25:1141. doi: 10.1007/s00198-013-2570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dayisoylu EH, Senel FC, Ungor C, Tosun E, Cankaya M, Ersoz S, Taskesen F. The effects of adjunctive parathyroid hormone injection on bisphosphonate-related osteonecrosis of the jaws: an animal study. Int J Oral Maxillofac Surg. 2013;42:1475. doi: 10.1016/j.ijom.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Duong LT, Rodan GA. Regulation of osteoclast formation and function. Rev Endocr Metab Disord. 2001;2:95. doi: 10.1023/a:1010063225902. [DOI] [PubMed] [Google Scholar]
- 52.Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitriou C, Terpos E, Dimopoulos MA. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23:8580. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 53.Berenson JR, Hillner BE, Kyle RA, Anderson K, Lipton A, Yee GC, Biermann JS American Society of Clinical Oncology Bisphosphonates Expert P. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002;20:3719. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 54.Wang EP, Kaban LB, Strewler GJ, Raje N, Troulis MJ. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxillofac Surg. 2007;65:1328. doi: 10.1016/j.joms.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Zavras AI, Zhu S. Bisphosphonates are associated with increased risk for jaw surgery in medical claims data: is it osteonecrosis? J Oral Maxillofac Surg. 2006;64:917. doi: 10.1016/j.joms.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Jadu F, Lee L, Pharoah M, Reece D, Wang L. A retrospective study assessing the incidence, risk factors and comorbidities of pamidronate-related necrosis of the jaws in multiple myeloma patients. Ann Oncol. 2007;18:2015. doi: 10.1093/annonc/mdm370. [DOI] [PubMed] [Google Scholar]
- 57.Boonyapakorn T, Schirmer I, Reichart PA, Sturm I, Massenkeil G. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol. 2008;44:857. doi: 10.1016/j.oraloncology.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 58.Walter C, Al-Nawas B, Frickhofen N, Gamm H, Beck J, Reinsch L, Blum C, Grotz KA, Wagner W. Prevalence of bisphosphonate associated osteonecrosis of the jaws in multiple myeloma patients. Head Face Med. 2010;6:11. doi: 10.1186/1746-160X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marx R. Oral and intravenous bisphosphonate induced osteonecrosis of the jaws: history, etiology, prevention, and treatment. 2. 2007. [Google Scholar]
- 60.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Aguirre JI, Akhter MP, Kimmel DB, Pingel JE, Williams A, Jorgensen M, Kesavalu L, Wronski TJ. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J Bone Miner Res. 2012;27:2130. doi: 10.1002/jbmr.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice cat. II. The effects of a diphosphonate on the periodontium. J Periodontal Res. 1981;16:441. doi: 10.1111/j.1600-0765.1981.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Jornet P, Camacho-Alonso F, Martinez-Canovas A, Molina-Minano F, Gomez-Garcia F, Vicente-Ortega V. Perioperative antibiotic regimen in rats treated with pamidronate plus dexamethasone and subjected to dental extraction: a study of the changes in the jaws. J Oral Maxillofac Surg. 2011;69:2488. doi: 10.1016/j.joms.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 64.Mawardi H, Treister N, Richardson P, Anderson K, Munshi N, Faiella RA, Woo SB. Sinus tracts--an early sign of bisphosphonate-associated osteonecrosis of the jaws? J Oral Maxillofac Surg. 2009;67:593. doi: 10.1016/j.joms.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 65.de Molon RS, Cheong S, Bezouglaia O, Dry SM, Pirih F, Cirelli JA, Aghaloo TL, Tetradis S. Spontaneous osteonecrosis of the jaws in the maxilla of mice on antiresorptive treatment: a novel ONJ mouse model. Bone. 2014;68:11. doi: 10.1016/j.bone.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ficarra G, Beninati F, Rubino I, Vannucchi A, Longo G, Tonelli P, Pini Prato G. Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. J Clin Periodontol. 2005;32:1123. doi: 10.1111/j.1600-051X.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- 67.Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G, Bareggi C, Ascani L, Cislaghi E. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20:137. doi: 10.1093/annonc/mdn526. [DOI] [PubMed] [Google Scholar]
- 68.Dimopoulos MA, Kastritis E, Bamia C, Melakopoulos I, Gika D, Roussou M, Migkou M, Eleftherakis-Papaiakovou E, Christoulas D, Terpos E, Bamias A. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20:117. doi: 10.1093/annonc/mdn554. [DOI] [PubMed] [Google Scholar]
- 69.Hansen T, Kunkel M, Weber A, James Kirkpatrick C. Osteonecrosis of the jaws in patients treated with bisphosphonates - histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 2006;35:155. doi: 10.1111/j.1600-0714.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 70.Sedghizadeh PP, Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW. Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. J Oral Maxillofac Surg. 2008;66:767. doi: 10.1016/j.joms.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 71.Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW, Sedghizadeh PP. The role of microbial biofilms in osteonecrosis of the jaw associated with bisphosphonate therapy. Curr Osteoporos Rep. 2010;8:40. doi: 10.1007/s11914-010-0008-1. [DOI] [PubMed] [Google Scholar]
- 72.Pushalkar S, Li X, Kurago Z, Ramanathapuram LV, Matsumura S, Fleisher KE, Glickman R, Yan W, Li Y, Saxena D. Oral microbiota and host innate immune response in bisphosphonate-related osteonecrosis of the jaw. Int J Oral Sci. 2014;6:219. doi: 10.1038/ijos.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sedghizadeh PP, Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J Am Dent Assoc. 2009;140:1259. doi: 10.14219/jada.archive.2009.0049. [DOI] [PubMed] [Google Scholar]
- 74.Kos M, Junka A, Smutnicka D, Bartoszewicz M, Kurzynowski T, Gluza K. Pamidronate enhances bacterial adhesion to bone hydroxyapatite. Another puzzle in the pathology of bisphosphonate-related osteonecrosis of the jaw? J Oral Maxillofac Surg. 2013;71:1010. doi: 10.1016/j.joms.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Wanger G, Gorby Y, El-Naggar MY, Yuzvinsky TD, Schaudinn C, Gorur A, Sedghizadeh PP. Electrically conductive bacterial nanowires in bisphosphonate-related osteonecrosis of the jaw biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:71. doi: 10.1016/j.oooo.2012.08.446. [DOI] [PubMed] [Google Scholar]
- 76.Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014;22:455. doi: 10.5435/JAAOS-22-07-455. [DOI] [PubMed] [Google Scholar]
- 77.Gacche RN, Meshram RJ. Angiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapy. Biochim Biophys Acta. 2014;1846:161. doi: 10.1016/j.bbcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Wood J, Bonjean K, Ruetz S, Bellahcene A, Devy L, Foidart JM, Castronovo V, Green JR. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 79.Bezzi M, Hasmim M, Bieler G, Dormond O, Ruegg C. Zoledronate sensitizes endothelial cells to tumor necrosis factor-induced programmed cell death: evidence for the suppression of sustained activation of focal adhesion kinase and protein kinase B/Akt. J Biol Chem. 2003;278:43603. doi: 10.1074/jbc.M308114200. [DOI] [PubMed] [Google Scholar]
- 80.Santini D, Vincenzi B, Dicuonzo G, Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L, Tirindelli MC, Altomare V, Tocchini M, Bonsignori M, Tonini G. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 2003;9:2893. [PubMed] [Google Scholar]
- 81.Pabst AM, Ziebart T, Ackermann M, Konerding MA, Walter C. Bisphosphonates’ antiangiogenic potency in the development of bisphosphonate-associated osteonecrosis of the jaws: influence on microvessel sprouting in an in vivo 3D Matrigel assay. Clin Oral Investig. 2014;18:1015. doi: 10.1007/s00784-013-1060-x. [DOI] [PubMed] [Google Scholar]
- 82.Guarneri V, Miles D, Robert N, Dieras V, Glaspy J, Smith I, Thomssen C, Biganzoli L, Taran T, Conte P. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat. 2010;122:181. doi: 10.1007/s10549-010-0866-3. [DOI] [PubMed] [Google Scholar]
- 83.Koch FP, Walter C, Hansen T, Jager E, Wagner W. Osteonecrosis of the jaw related to sunitinib. Oral Maxillofac Surg. 2011;15:63. doi: 10.1007/s10006-010-0224-y. [DOI] [PubMed] [Google Scholar]
- 84.Santos-Silva AR, Belizario Rosa GA, Castro G, Junior, Dias RB, Prado Ribeiro AC, Brandao TB. Osteonecrosis of the mandible associated with bevacizumab therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:e32. doi: 10.1016/j.oooo.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F American Association of O, Maxillofacial S. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014;72:1938. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 86.Van den Wyngaert T, Huizing MT, Vermorken JB. Bisphosphonates and osteonecrosis of the jaw: cause and effect or a post hoc fallacy? Ann Oncol. 2006;17:1197. doi: 10.1093/annonc/mdl294. [DOI] [PubMed] [Google Scholar]
- 87.Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136:1117. doi: 10.1007/s00432-010-0907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Christodoulou C, Pervena A, Klouvas G, Galani E, Falagas ME, Tsakalos G, Visvikis A, Nikolakopoulou A, Acholos V, Karapanagiotidis G, Batziou E, Skarlos DV. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76:209. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 89.Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41:318. doi: 10.1016/j.bone.2007.04.196. [DOI] [PubMed] [Google Scholar]
- 90.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 91.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montague R, Hart CA, George NJ, Ramani VA, Brown MD, Clarke NW. Differential inhibition of invasion and proliferation by bisphosphonates: anti-metastatic potential of Zoledronic acid in prostate cancer. Eur Urol. 2004;46:389. doi: 10.1016/j.eururo.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 93.Landesberg R, Cozin M, Cremers S, Woo V, Kousteni S, Sinha S, Garrett-Sinha L, Raghavan S. Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg. 2008;66:839. doi: 10.1016/j.joms.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bae S, Sun S, Aghaloo T, Oh JE, McKenna CE, Kang MK, Shin KH, Tetradis S, Park NH, Kim RH. Development of oral osteomucosal tissue constructs in vitro and localization of fluorescently-labeled bisphosphonates to hard and soft tissue. Int J Mol Med. 2014;34:559. doi: 10.3892/ijmm.2014.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 96.Kabilova TO, Kovtonyuk LV, Zonov EV, Ryabchikova EI, Popova NA, Nikolin VP, Kaledin VI, Zenkova MA, Vlassov VV, Chernolovskaya EL. Immunotherapy of hepatocellular carcinoma with small double-stranded RNA. BMC Cancer. 2014;14:338. doi: 10.1186/1471-2407-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kikuiri T, Kim I, Yamaza T, Akiyama K, Zhang Q, Li Y, Chen C, Chen W, Wang S, Le AD, Shi S. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res. 2010;25:1668. doi: 10.1002/jbmr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ali-Erdem M, Burak-Cankaya A, Cemil-Isler S, Demircan S, Soluk M, Kasapoglu C, Korhan-Oral C. Extraction socket healing in rats treated with bisphosphonate: animal model for bisphosphonate related osteonecrosis of jaws in multiple myeloma patients. Med Oral Patol Oral Cir Bucal. 2011;16:e879. doi: 10.4317/medoral.17150. [DOI] [PubMed] [Google Scholar]
- 99.Fedele S, Porter SR, D’Aiuto F, Aljohani S, Vescovi P, Manfredi M, Arduino PG, Broccoletti R, Musciotto A, Di Fede O, Lazarovici TS, Campisi G, Yarom N. Nonexposed variant of bisphosphonate-associated osteonecrosis of the jaw: a case series. Am J Med. 2010;123:1060. doi: 10.1016/j.amjmed.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 100.Patel S, Choyee S, Uyanne J, Nguyen AL, Lee P, Sedghizadeh PP, Kumar SK, Lytle J, Shi S, Le AD. Non-exposed bisphosphonate-related osteonecrosis of the jaw: a critical assessment of current definition, staging, and treatment guidelines. Oral Dis. 2012;18:625. doi: 10.1111/j.1601-0825.2012.01911.x. [DOI] [PubMed] [Google Scholar]