Abstract
Cerebral palsy (CP) has significant impact on both patients and society but therapy is limited. Human umbilical cord blood cells (HUCBC), containing various stem and progenitor cells, have been used to treat various brain genetic conditions. In small animal experiments, HUCBC have improved outcomes after hypoxic-ischemic injury. Clinical trials using HUCBC are underway testing feasibility, safety and efficacy for neonatal injury as well as CP. We tested HUCBC therapy in a validated rabbit model of CP after acute changes secondary to hypoxic-ischemic (H-I) injury had subsided. Following uterine ischemia at 70% gestation, we infused HUCBC to newborn rabbit kits with either mild or severe neurobehavioral changes. Infusion of high dose HUCBC, 5x106 cells, dramatically altered the natural history of the injury alleviating the abnormal phenotype including posture, righting reflex, locomotion, tone, and dystonia. Half the high dose showed lesser but still significant improvement. The swimming test however showed that joint function did not restore to naïve control function in either group. Tracing HUCBCs with either MRI biomarkers or PCR for human DNA found little penetration of HUCBC in the newborn brain in the immediate newborn period, suggesting that the beneficial effects were not due to cellular integration or direct proliferative effects but rather to paracrine signaling. This is the first study to show that HUCBC improve motor performance in a dose-dependent manner perhaps by improving compensatory repair processes.
Introduction
Cerebral palsy (CP) has a very high index of disease burden resulting in life-long neurologic consequences to the patient, care-takers, and social institutions. One of the major pathogenetic causes of CP is hypoxia-ischemia (H-I) in the antenatal period [1–2]. Hypoxia-ischemia at the maternal, placental or fetal level often results in neonatal encephalopathy or newborn hypoxic-ischemic encephalopathy (HIE). At the present time, the only treatment available for HIE is hypothermia initiated within 6 hours of birth. Hypothermia offers only 11% reduction in risk of death or disability, from 58% to 47% [3]. Further, there are considerable, unresolved safety concerns around cooling preterm newborns [4]. Part of the reason for the modest effect in term babies is that the precise timing of initial insult is often unclear. Promising add-on treatments such as erythropoietin and Xenon are now in phase 2 clinical trials. After the child manifests CP there are no available curative therapies.
There have been excellent reviews exploring potential use of progenitor or stem cells as a therapy for H-I [5–6]. It is postulated that they might replace lost neurons, protect endogenous host cells and promote their growth and differentiation, as well as modulate the host immune response, all of which may decrease disability after H-I. Human umbilical cord blood cells (HUCBC) has been shown to be beneficial in numerous preclinical studies using models of newborn rodent H-I [7–10] except one, which used a lower dose than the others and which showed no benefit [11].
So far >35,000 allogeneic transplants using cryopreserved and publicly banked HUCBC have been performed worldwide over the past 20 years. At Duke University alone, >1500 have been performed, of which >300 were performed in infants and children using autologous cells [12]. Currently, in allogeneic transplantation, the minimal effective dose after myeloablative therapy to re-establish hematopoiesis is 25 million nucleated cells/kg of recipient body weight. Doses up to 800 million cells per kg have been given safely to infants in the first month of life. Minimally effective doses of cord blood cells in the autologous setting have not been established and are likely to vary depending on the clinical indication. We have recently reported the safety and feasibility of collection, preparation, and infusion of fresh autologous HUCBC for use in infants with HIE. Cell doses of 1–5×107 cells/kg/dose were given intravenously for up to 4 doses [13].
We tested the use of HUCBC in the model of CP we’ve developed. We infused intravenous HUCBC postnatally into our newborn rabbit kits following antenatal hypoxia-ischemia at 70% gestation [14] and showed significant improvement in motor function. The initial dose we used for rabbits was comparable to the standard dose of HUCBC transfusions for humans on a per kg basis, 5×106 cells/single dose.
Results
HUCBC improves motor outcome across severity injury groups
A detailed neurobehavioral examination at P1 (E32) revealed that there was initially no significant difference in motor deficits scores between the treatment and control groups either in the Severe or Mild Groups (see Table 1), indicating that the groups most likely were equivalent at study entry at P0 (E31). Conversely, one could infer that there was no significant improvement in 1 day after treatment.
Table 1.
Results of neurobehavioral examination at P1. Test scores were not different between treatment groups in the Severe and in the Mild groups (ANOVA). Scores based on ordinal scoring from 0 to 4 in neurobehavioral test battery (24).
| Severe P1 | Mild P1 | |||||
|---|---|---|---|---|---|---|
| Saline | Media | HUCBC | Saline | Media | HUCBC | |
| n=5 | n=5 | n=7 | n=3 | n=5 | n=8 | |
| Posture | 1.4±0.2 | 1.7±0.2 | 1.5±0.1 | 2.5±0.0 | 2.6±0.1 | 2.4±0.1 |
| Righting | 1.4±0.2 | 1.7±0.2 | 1.6±0.1 | 2.5±0.0 | 2.6±0.1 | 2.3±0.1 |
| Tone | 3.5±0.4 | 2.8±0.5 | 3.4±0.3 | 1.0±0.0 | 0.8±0.1 | 0.9±0.1 |
| Locomotion | 1.2±0.4 | 1.7±0.2 | 1.6±0.2 | 2.5±0.0 | 2.5±0.0 | 2.3±0.1 |
| Dystonia | 1.8±0.3 | 1.9±0.4 | 2.3±0.1 | 1.5±0.3 | 0.9±0.2 | 0.9±0.1 |
The differences in motor outcome measures between saline and media groups were tested in repeated measures analysis of variance. No difference was found between the groups with respect to the interaction term time*treatment in any outcome measure, given α=0.05. Saline and media groups were combined for the further analysis. The effect of HUCBC (“Treatment”) on motor outcome measures was tested using repeated measures analysis of variance with time (“Time”), taking into consideration the outcomes at P1,P5 and P11 as repeated measure (n=15) and compared to a new control group containing both saline and media groups (n=18). The initial severity of motor deficits, Mild or Severe, was added to the model as a covariate “Severity” (n=17 severe, 16 mild). Using multivariate statistics Wilks' Lambda, the interaction term “Time*Treatment” was found significantly related to Locomotion, Tone, Posture, and Righting outcome measures (p-values <.0001 for all outcomes) thus affirming statistically significant effects of the HUCBC on changes in motor outcome between P1, P5, and P11 time points. The interaction term “Time*Severity” was also significantly associated with the outcome measure of Tone (p-value 0.0091), indicating that effect of the treatment was different between Mild and Severe groups. Therefore the changes in motor scores from P1 to P5 and P11 for the Mild and Severe groups are presented separately on Fig.1 and Fig. 2. Absolute values of motor outcome variables between ages, severity of initial injury and treatment groups are presented in Supplementary Table 1.
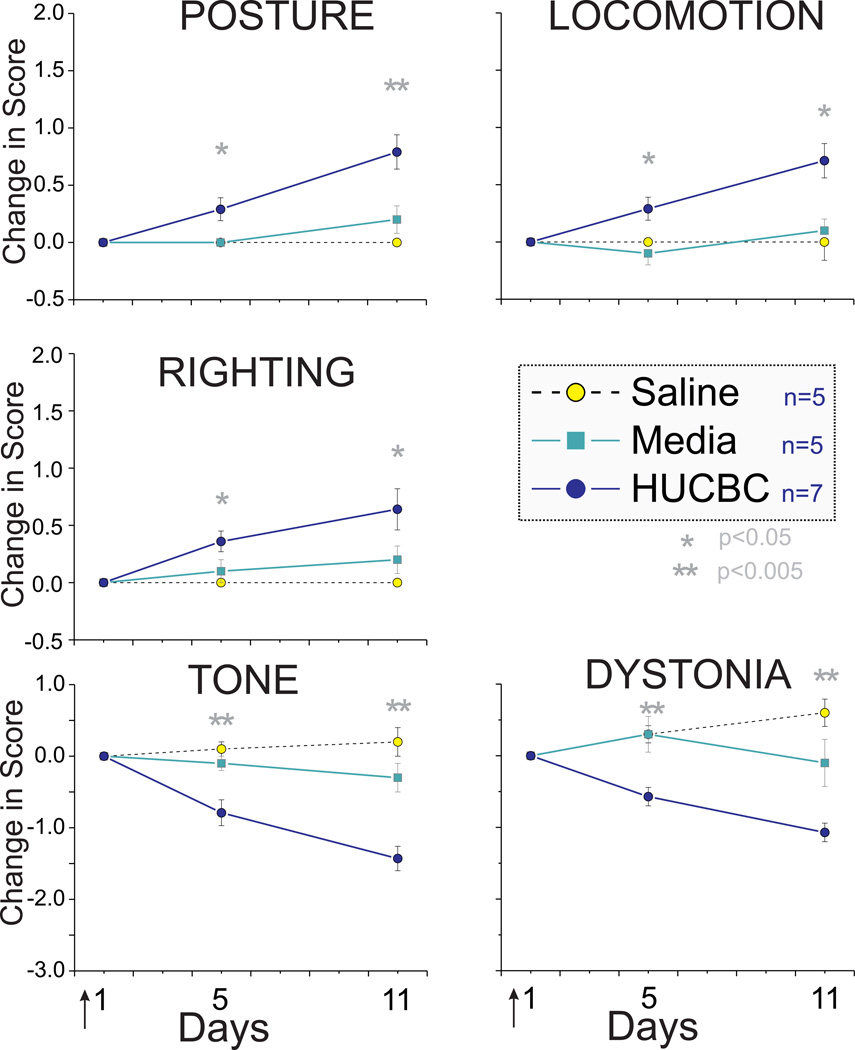
Fig. 1.
HUCBC administration improved neurobehavioral scores at day 5 and day 11 in Severe Group compared to saline and media. * p< 0.05, **p< 0.01, ANOVA
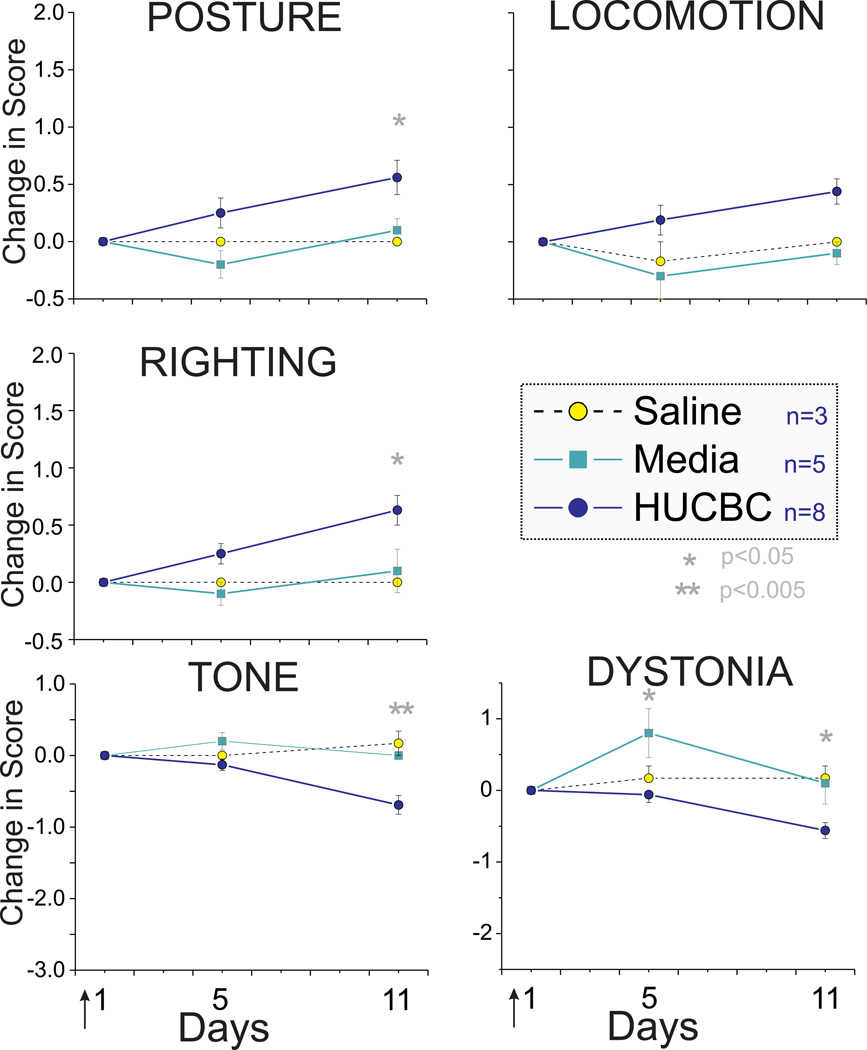
Fig. 2.
HUCBC administration improved neurobehavioral scores at day 5 and day 11 in the Mild group. * p< 0.05, **p< 0.01, ANOVA
High Dose HUCBC Infusion Improves Outcome in Survivors
Following High Dose HUCBC infusion (see Methods), the Severe group survivors showed significant improvement in motor function from postnatal day 1 to 11 (P1 to P11) in all categories of neurobehavioral testing (Fig 1). There was increase in scores of posture, locomotion and righting with a concomitant decrease in hypertonia and dystonia scores. Mild group survivors also showed improvement of motor deficits (Fig 2). The absolute change in scores was smaller in this group but the initial impairment was also mild.
Examination of the survival curves revealed that there was a trend to increased mortality, although not statistically significant , following High Dose HUCBC infusion relative to saline and media control groups (p = 0.070, χ2 test). The initially higher mortality rate in HUCBC group was comparable to control groups after reduction of infusion rate. There was no difference in weight gain in survivors.
Low Dose HUCBC Infusion Causes No Mortality But Effect is Less
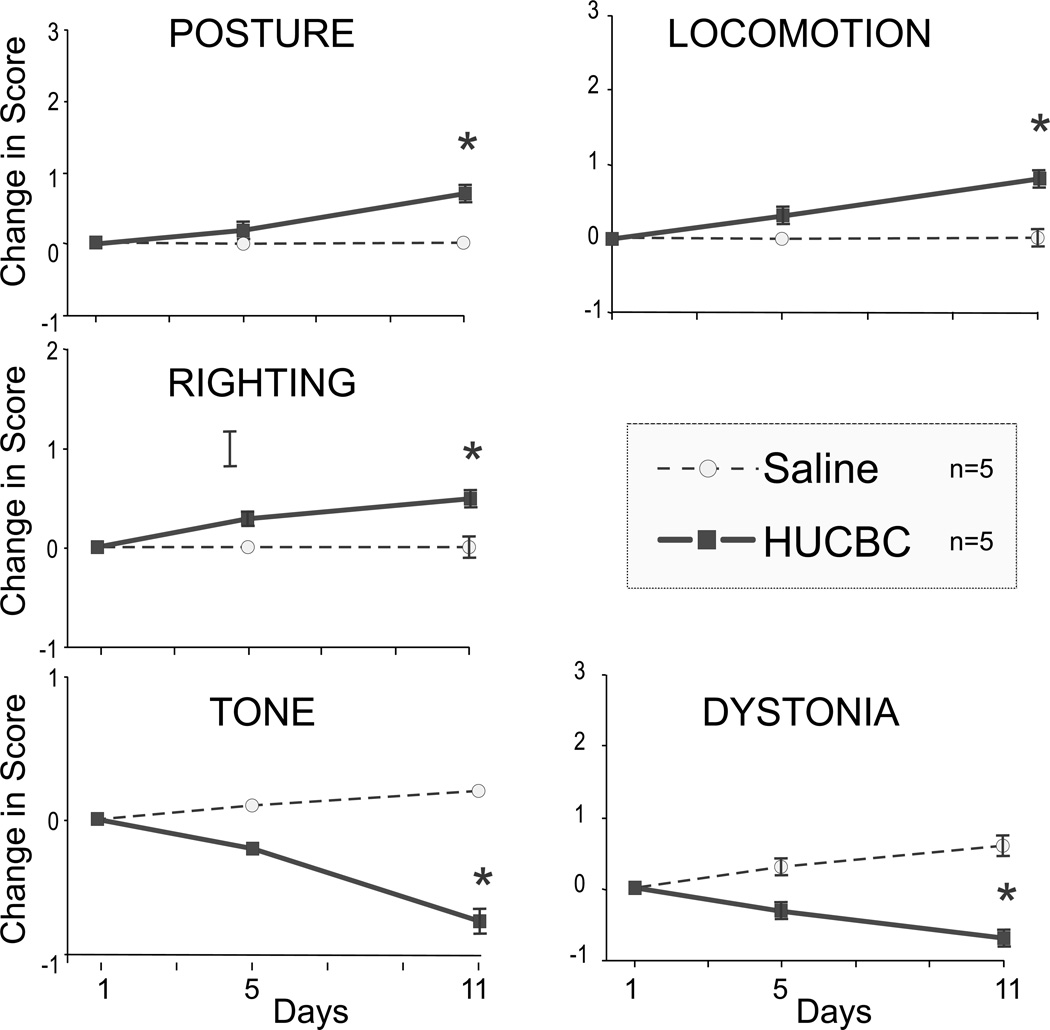
We then hypothesized that a lower dose HUCBC cell infusion will result in no increase in mortality while retaining the capacity to improve neurobehavioral outcome. In the Severe group, Low Dose HUCBC or saline infusion resulted in no mortality. Again, there was significant (but milder than noted in the High Dose group) improvement in tone, posture, righting reflex, locomotion and dystonia score from P1 to P11 (n=5, repeated measures ANOVA, p<0.05 in all 5 measures) (Fig 3). There was also no difference in weight gain in the survivors.
Fig. 3.
Low Dose HUCBC administration improved neurobehavioral scores at day 5 and day 11. n=5, p< 0.05 in all 5 measures, ANOVA
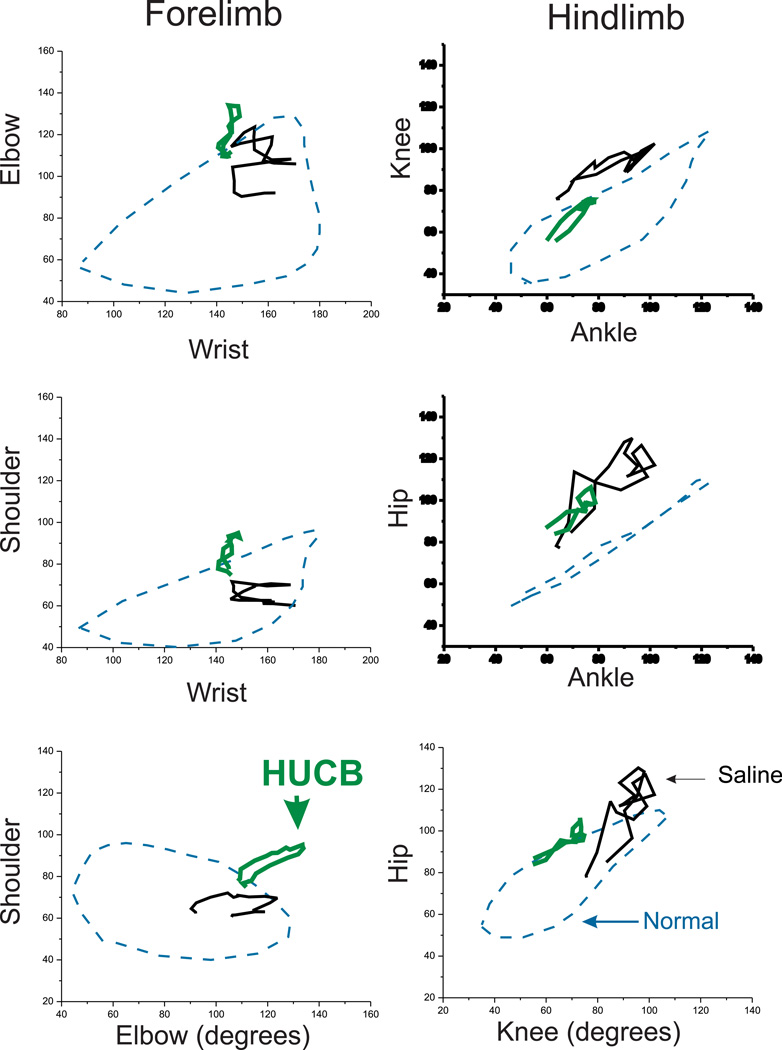
Forced swimming test [15] allows the detection of lack of coordination between upper and lower joints, mild motor deficits, and assessing recovery with HUCBC with two-joint analysis, thus providing some insight into sites of injury. We also wanted to know whether the neurobehavioral improvement was due to a restoration to the control state or to some other compensatory state.
Functional deficits of joint movements can be discovered using graphs obtained from two-joint analysis. As shown in Figure 4, the Low Dose HUCBC did not restore joint function to the control state but joint movements in this group had a different pattern from that of saline treated kits, suggesting involvement of compensatory mechanisms in adaptation of muscle tone, range of motion and joint movement pattern in HUCBC treated group. The range of motion and median angle did not change with HUCBC treatment (Supplementary Figure 1).
Fig. 4.
Two-joint analysis of P11 kits. Saline with H-I (mean, black, n=9) had distinctive deficits both in fore and hind limbs; compared to control (blue dashed, n=18) and Low Dose HUCBC treated kits (green, n=4) following E22 H-I.
Absence of MRI Evidence of Intraparenchymal Entry of HUCBC into Newborn Brain
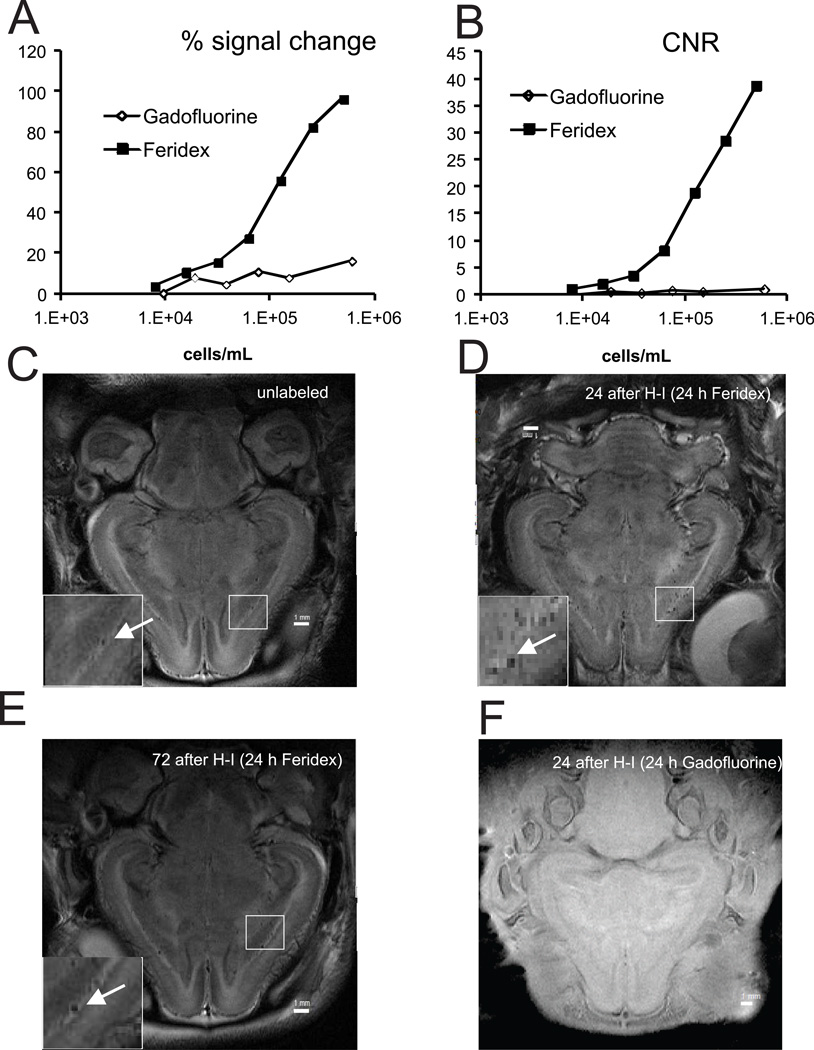
Cells labeled with Feridex provided larger signal difference (Fig. 5A) and contrast to noise ratio (Fig. 5B) on T2-weighted images than Gadofluorine on T1-weighted imaging. Contrast to noise ratio (CNR) for Feridex label was 2.05 with signal to noise ratio (SNR) being 129 to detect 15,600 cells/mL in region of interest (ROI). With a resolution of 0.08x0.1x1 mm in voxel used for in-vivo imaging this would theoretically provide detection of 1.23 Feridex labelled cells in a ROI 3 by 3 voxel size. This calculation was done with CNR relative to agarose gel phantom, which has similar T2 properties to newborn rabbit gray matter. Detection level in a real brain requires much large CNR due to high signal inhomogeneity of brain tissue.
Fig. 5.
Cells were labeled with T2-shortening superparamagnetic iron oxide MRI contrast (Feridex) or T1- shortening contrast Gadofluorine M. Signal change (A) and contrast-to-noise ratio (B) determined on T2-weighted images for Feridex and T1-weighted images for Gadofluorine on agarose gel phantom with serial cell dilutions on 4.7T.
Detection of intravenously infused HUCBC on MRI in vivo. Rabbit kits were imaged on 4.7T magnet 24 hours after infusion of media (C, T2-weighted) or Feridex labeled cells (D, T2-weighted) 24 hours or 72 hours (E) after E29 H-I. F.- T1-weighted image of kit infused with Gadofluorine labeled cells 24 hours after E29 H-I. 2.5x106 HUCBC cells were delivered by a PHD 2000 programmable pump. (Harvard Apparatus Holliston, MA).
No clear evidence of the HUCBC penetrating brain parenchyma was found on T2-weighted images in kits infused with labeled HUCBC with slower infusion rates at 24 (Fig. 5D) and 72 hours after H-I and imaged 24 hours later (Fig. 5E). Nor were hyper-intensities suggestive of presence of labeled cells found on T1-weighted images (Fig. 5F). Blood vessels penetrating white and gray matter were conspicuous on T2-weighted images (Fig. 5C-E, arrows) as black punctate hypo-intensities. Notably, the vessels were darker in images of kits infused with labeled cells then infused with unlabeled cells (Fig. 5C-E, arrows). The difference of CNR between these two images relative to gray matter was 0.38. Assuming the same CNR for labeled cells as obtained in phantom (Fig. 5B) and blood volume of 6 mL in E32 rabbit kits, these numbers imply the presence of 4.11x103 HUBC in the circulatory system 24 hours after infusion of 2.5x106 cells.
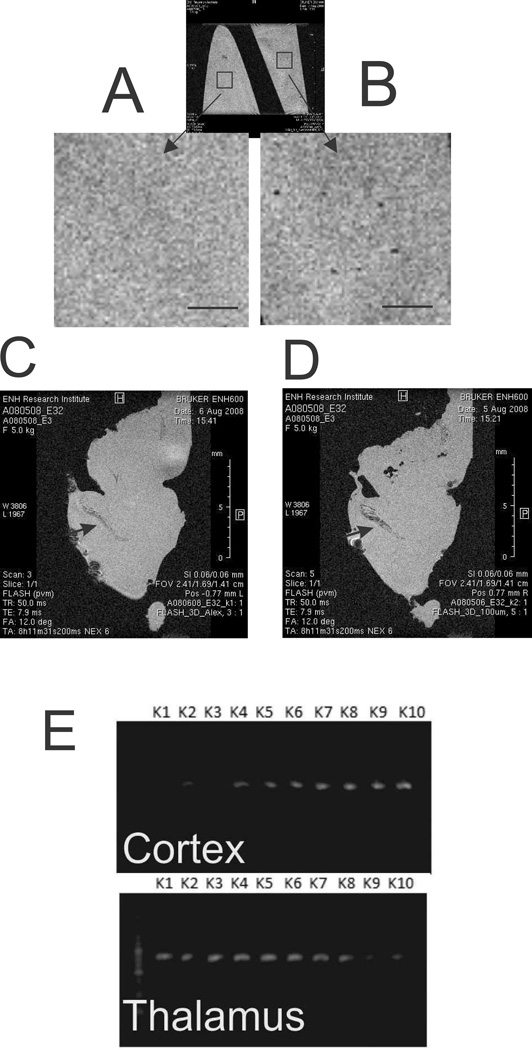
To confirm that there was no extravasation of the labeled cells, the brains from the kits that had been imaged in vivo were transcardially perfused to remove blood, and subsequently fixed and imaged overnight with high resolution using a 14.1T magnet with 50 µm isotropic resolution. Feridex labeled single cell detection was possible at this resolution, as confirmed by imaging a gel phantom prepared from Feridex labeled cell suspension with the same imaging parameters (Fig. 6A,B). When imaged together punctate signal voids were clearly visible on the phantom with labeled cells (Fig. 6B) but none in the phantom without cells (Fig. 6A). No image signatures of the labeled cells were detected in the parenchyma of the brain sample with Feridex labeled HUCBC infusion (Fig. 6D), as compared with the sample with unlabeled cell infusion (Fig. 6C). Choroid plexus was darker in the brain sample where labeled cells were infused than in the brain with non-labeled cell infusion (Fig.6C.D, arrows). This may indicate that some HUCBC may be lodged in this highly vascularized region, but no cell propagation to parenchyma was noticed.
Fig 7.
Detection of intravenously infused HUCBC ex vivo. High resolution imaging with isotopic 50µm voxel resolution on 14.1T magnet. Agarose phantoms without (A) and with Feridex labeled (B) cells. Rectangle ROIs placed on phantoms are shown with high magnification. Note punctate hypo-intensities in panel B, indicating labeled cells. Perfusion fixed brain of E32 kit infused with media (C) or Feridex labeled cells (D). Arrows point to choroid plexus on C and D. Scale bar 1 mm. E .PCR for human DNA showing most of cortex and thalamus samples having some small amounts of human DNA.
Possible Entry of HUCBC into Newborn Brain
There are several possibilities that may explain the MRI results such as the following: HUCBC did not enter the brain in appreciable numbers to be detected on MRI using our techniques, loss of the MRI label with cell division, or the labeling procedure changed the properties of HUCBC and they lost the ability to enter brain parenchyma and reach sites of injury. We next tested for sub-acute evidence of human cells in the rabbit newborn brain parenchyma by detecting human DNA in P5 kits. PCR reactions targeting specific human gene sequence were performed. In the 10 brain samples we tested, 8 cortex and 9 thalamus samples showed positive results for human DNA with none for the saline group (Fig. 6E).
Discussion
This is the first study to test whether HUCBC infusion after the discovery of neurobehavioral deficits following antenatal H-I would ameliorate the clinical deficits cause by H-I injury. The results suggest that there may be improvements in locomotion following HUCBC (see Video) over a moderate period of time, considering that rabbit-days are shorter than human-days in the rate of development. High Dose HUCBC may provide a larger degree of amelioration of neurobehavioral deficits, although this was not definitely proven as the experiments were designed to compare different doses. There was a definite improvement over the saline treated group in both phases of the study, first with High Dose and later with Low Dose.
In contrast to usual drug development, there has been a paucity of animal studies in this field. The present study’s translational implications reside in a narrow time period following an antenatal H-I event. The antenatal insult in this study was 9 days before delivery and the infusion of HUCBC. An approximate corollary in humans would be if H-I occurred at 24–28 weeks gestation based on oligodendrocyte development in rabbits [16] and HUCBC were given at term birth. If one looks at the evolution of injury after H-I, broadly there are three time epochs. The first is the ‘Acute’ epoch where cell injury occurs with the onset of primary and secondary energy failure [17]. Cytotoxic edema, cell death and apoptosis result from severe H-I in the first few days. Hypothermia as a rescue therapy is given at this time to prevent further deterioration [18].
The second time epoch can be called the “Chronic” epoch that spans the next few days to months and involves a period of cell repair and recovery. It may also involve secondary inflammatory processes, some of them damaging. This is the time epoch that the HUCBC were given in the present study. Mention must be made of two adjunct therapies with hypothermia that are now in clinical trials, erythropoietin and xenon. The elucidation of mechanism of action of HUCBC runs into the same problems that have faced the mechanism of action of erythropoietin, a circulating hormone. Erythropoietin does not cross the blood brain barrier and is also a blood borne growth factor. Erythropoietin as a drug therapy is now being tested in clinical trials despite not knowing its exact mechanism of action. For both term and preterm infants, erythropoietin involves the modification of ‘Acute’ injury initially in most animal studies [19] and extend into the ‘Chronic’ epoch in some animal and human studies [20] and the proposed ongoing clinical trials. Erythropoietin is being tested in newborn babies and needs to be given in repeated doses [21–22]. Xenon is another adjunct therapy with hypothermia now being tested for term HIE in clinical trials [23]. The cost of xenon is somewhat prohibitive at the present time.
The third time epoch is the ‘Late’ epoch that starts with the definitive diagnosis of cerebral palsy at about 12–24 months of age. Damaging processes have become quiescent and development adaptations result in the final clinical phenotype. Autologous HUCBC is presently in clinical trials for this time period for CP patients [24–25]. The two actively enrolling studies are at Duke and Georgia Regents University (see clinicaltrials.gov).
All the previous animal studies using HUCBC have been performed in the ‘Acute’ Epoch and employ the Vannucci model of unilateral carotid artery ligation followed by a period of moderate hypoxia on postnatal day 7 in rats or mice. Meier et al reported reduced “spastic paresis” when 107 HUCBC were given intraperitoneally 24 h after H-I in P7 rats [8]. Cells migrated to the injured side, but the cells had not differentiated 21 days after infusion. This group also showed no effect on the size of the lesion, but significantly preserved somatosensory function on the side of the lesion [26]. Notably, they found reduced hyperexcitabilty, and restoration of the cortical maps and receptive fields in the somatosensory cortex. This strongly suggests that the benefit was mediated by improved function of the surviving brain tissue. This group also showed faster resolution of inflammation and astrocytic activation with HUCBC [27]. Similarly, Yasuhara et al found that intravenous infusion of 15,000 HUCBCs, with or without co-treatment with mannitol, 7 days after H-I in P7 rats was associated with significant improvement in motor asymmetry and coordination 7 and 14 days later [9]. Only a few cells were found in the damaged area and the authors suggest a paracrine effect as the levels of neural growth factor, glial cell line derived neurotrophic factor and brain derived neurotrophic factor were increased 3 days after treatment, but only when cells were combined with mannitol to increase blood brain barrier permeability and not with cells alone. In contrast, the same group reported that intra-hippocampal injection of 200,000 marrow-derived multipotent progenitor cells from Athersys, Inc. (Cleveland, Ohio) did not improve rotarod testing and only improved motor asymmetry 14 days later [28]. Consistent with these findings, Pimentel-Coelho et al found that intraperitoneal injection of HUCBCs in P7 rats 3 h after H-I was associated with only trivial numbers of HUCBCs in the lesion in contrast with slight improvements in sensorimotor reflexes 4 days after H-I but not different at 2 and 7 days [7]. The improved outcomes were associated with reduced caspase-3 cleavage around the lesion site and an overall reduction in activated microglia and macrophages consistent with anti-apoptotic and anti-inflammatory actions. In contrast, de Paula et el initially reported, eight deaths and no improvement in infarct volume or in spatial memory 3 weeks after1×107 mononuclear cells were given intravenously 24 h after H-I in P7 rats [11]. Subsequently, the same group reported dose-response findings on infarction by showing reduced infarction volume with 107 and 108 HUCBCs, but not 106 HUCBCs. Improvement in the Morris water maze for spatial memory was only found with a dose of 108 HUCBCs [29].
The various stem cells present in HUCBC include hematopoietic stem cells (CD34), unrestricted somatic stem cells (USSCs), umbilical cord-stem cells (UC-SCs), and very small embryonic-like (VSEL) stem cells, all of which contribute to the high proliferative and self-renewing capacity. HUCBC have been shown to differentiate in vitro under the right conditions to neurons [30], oligodendrocytes [31–32] and astrocytes [33]. They also contain precursors of mesenchymal stromal cells [34]. Autologous HUCBC’s have the major advantage of not requiring immunosuppression [12, 35], but this is balanced by the hematogenous origin of the cells, and thus less clear commitment to neural differentiation.
Given the example of hypothermia [18] and erythropoietin [36], where the therapy was tested in large animal models before implementation in humans, the Study Group for Therapies for CP recommended in the recent review that fresh autologous HUCBC is of sufficiently low risk to justify early safety and feasibility studies but more testing should be done in animals larger and more complex than rodents [5]. Compared to rodents, rabbits are perinatal brain developers especially concerning motor development, similar to humans. Most human perinatal H-I is global in origin, similar to the present model. The timing of the antenatal insult is similar to an insult to the extreme premature human newborn, considering the maturation of oligodendrocytes [16].
Our results showed that IV infusion of HUCBC may not necessarily lead to HUCBC entry into brain tissue in appreciable numbers, since MRI demonstrated only cells in the vascular system and DNA studies showed only a very small number in the brain. With the limitation of employed MRI methods we cannot exclude possibility of small HUCBC engraftment, not detectable by MRI, as reported in some studies [37], but the amount of such engrafted cells is likely small. Dilution of iron label due to cell division is probably not a reason for the absence of signal as this has been shown to be a factor only several days after infusion [38]. The small numbers reaching brain probably are due the trapping of most HUCBC in the lungs on a first pass through the lungs [39] and additionally in the kidneys, and spleen [40].
The rate of intravascular infusion of HUCBC may be a factor as too rapid an infusion may be deleterious, reflected in the trend in increase of mortality in the beginning of the study. Mortality probably occurred from the complication of pulmonary emboli that are known to form after intravenous injection of human mesenchymal stem cells in mice [41]. It is also possible that rapid infusion caused pulmonary edema due to sudden fluid overload. When we slowed down the injection speed to 1ml/5 minutes in the second phase, there was no more mortality.
Among the various possible neuroprotective mechanisms, paracrine effects [42] such as anti-inflammatory and growth factor-effect [43] have been suggested as possible candidates, although the evidence is scanty. There is a suggestion that following intraperitoneal injection in the Vannucci model in rodents, HUCBC home in on the damaged cortex, and this targeting depends upon a chemokine stromal derived factor [43]. Following intravenous injection in a similar model with concurrent cyclosporine treatment, progenitor neurons and microglia were increased in the brain, but there was not much change in brain chemokines [42]. Chemokines were shown to be increased in vitro in HUCBC [42] but the direct link between these chemokines originating from HUCBC and beneficial effects has not been shown, and is worthy of further study.
The entry into brain may not be totally necessary for a neuroprotective effect. The endothelial cells in the neurovascular cells can play a major role in protecting neurons [44]. The speculation is that possibly the HUCBC modifies the neurovascular cells to secrete more stimulatory and neurotrophic factors. Although human DNA PCR results did not exactly correlate with neurobehavioral improvement, the presence of human DNA would suggest the possibility of that even a few HUCBC may also directly stimulate endogenous proliferation of stem cells or progenitor cells.
Significant improvement in motor scores with HUCBC treatment was observed as the primary outcome of the study. The findings of the swimming test have to be taken into context as it was designed to discover mild locomotor deficits in the absence of hypertonia, as a test mitigating the effect of gravity. We also use the swimming test to give us a clue about the regional nature of the injury in the brain, whether it was affecting the fore-limb or hind-limb joints. Thus, e.g., the swimming test can tell us whether an intervention caused the animal to adopt a motor strategy similar to control animals or something different. An intervention such as HUCBC that does not achieve control curves is not necessarily deemed unsuccessful. It is known that human patients with CP adapt their internal locomotor models [45] in order to be able to ambulate even with quite severe restrictions in range of motion and hypertonia. The key issue is if an intervention can restore the ability to walk, which was the case with HUCBC for CP rabbits. A decrease in hypertonia and increase in range of motion which was observed in our study, would certainly improve motor abilities in CP rabbits. Furthermore, a decrease in hypertonia would greatly facilitate rehabilitation therapy and ability for motor learning. Also, the video clearly shows the rabbits at P5 and P11 walking even with postural deficits. This is observed in humans to some extent where there can be severe postural changes in the peripheral joints but the ability to walk is still retained or restored after an insult.
The study raises several translational research questions. HUCBC from each delivery may be different in potency. Screening for effectiveness of HUCBC could be first tested in rabbits before use in humans. There are three mitigating factors that allow us to give xenogeneic HUCBC in rabbit newborns. First, the experiments were meant to be proof-of-concept studies justifying the use of autologous HUCBC in human newborn infant. Second, the newborn immunological response is known to be not developed as well as in adults [46–47]. This is true for rabbits and rodents as well [48]. Third, all the previous studies were done in newborn rodents without much response. Although we did not specifically study the immunological response, we expect an absence of a major immunological reaction.
Presently clinical trials are only using autologous HUCBC. There is presently an ongoing clinical trial of autologous HUCBC and hypothermia for term HIE and preliminary studies show that it is safe and may be promising [13]. Allogeneic cells have been tested in one randomized trial, along with erythropoietin, and this study had promising results (35). Ultimately a future question clinically would be feasibility of allogeneic infusions because it would expand access to therapy with HUCBC in individuals with CP. However, before we reach that stage, fundamental issues about the mechanism of action of HUCBC must be addressed such as whether HUCBC acts by 1) paracrine, 2) growth factors, or 3) stimulation of endogenous neurogenesis or if engraftment or tissue integration is needed. The clinical protocols for use of allogeneic cells in human infants with HIE would need careful study and optimization.
In conclusion, our study adds supportive evidence for use of HUCBC in neonatal hypoxic-ischemic brain injury. Further studies testing various dosing and timing strategies, infusions before, during or after initiation of hypothermia, and further investigating mechanisms are needed.
Materials and Methods
The Institutional Animal Care and Use Committee of NorthShore University HealthSystem Research Institute approved all experimental procedures with animals.
Collection of HUCBC
Fresh, HUCBC, donated to the Carolinas Cord Blood Bank (CCBB) by mothers delivering normal, term infants but not qualifying for banking for transplantation, was obtained from the CCBB after processing. Cord blood was collected aseptically into cord blood collection bags (Pall, Medsep, Covina, California) containing 35 mL of citrate phosphate dextrose anticoagulant and obtained from deliveries of mothers [49]. HUCBC were transported at room temperature in validated shippers to the CCBB processing laboratory at Duke. The collected cells were reduced in volume and RBC count after 20–30 minute incubation with 6% hetastarch using the Sepax 1 automated processing system (Biosafe, Geneva, Switzerland). Total nucleated cell counts pre- and post-processing, post-processing CD34+ cell content, colony forming units, sterility, and viability were assessed. HUCBC were concentrated into appropriate volumes for injection then shipped overnight at 4oC to NorthShore University HealthSystem where they were injected the next day to kits. After arrival at NorthShore, an aliquot was taken for flow cytometry for a cell count before infusion to newborn kits.
Antenatal Hypoxia-Ischemia
The surgical procedure has been described in detail previously [14, 50]. In vivo global H-I of fetuses was induced by sustained 40-min uterine ischemia at 22 days gestation (70% term, E22) in timed pregnant New Zealand white rabbits (Myrtle’s Rabbits, Thompson Station, TN). Briefly, dams were anesthetized with intravenous fentanyl (75 µg/kg/hr) and droperidol (3.75 mg/kg/hr), followed by spinal anesthesia using 0.75% bupivicaine. A balloon catheter was introduced into the left femoral artery and advanced into the descending aorta to above the uterine arteries. Body core temperature was monitored with a rectal temperature probe. The balloon was inflated for 40 minutes causing uterine ischemia and subsequent global fetal H-I. At the end of period of H-I, the balloon was deflated, resulting in uterine reperfusion and a reperfusion-reoxygenation period for the fetal brains. The catheter was removed, femoral artery repaired, and the dam was allowed to recover. The dams next underwent C-section at E31 just before term delivery (term 31.5 days). C-section was done to ensure that the HUCBC were given at the proper time for all newborn kits.
Abbreviated Neurobehavioral Examination
After birth each newborn kit was subjected to an abbreviated neurobehavioral examination investigating locomotor function and muscle tone based on the previous published protocols [50]. The newborn kits were classified as ‘Severe’ if they had either hypertonia or postural changes or obvious locomotor deficits, or ‘Mild’ if they had just mild deficit(s). The kits that were not affected were not included in the study. The division into mild and severe was performed for purposes of avoiding bias and avoiding chance imbalance in populations in treated group with HUCBC.
Infusion of HUCBC
After assignment to Mild and Severe groups, infusion of HUCBC or saline or media, was randomly performed. This was performed in two phases. In the first phase, each of the Severe and Mild groups were independently randomized to receive 1.0 ml volume containing either 1) 5x106 HUCBC cells, or 2) media that was used to transport HUCBC, or 3) saline. The solutions were given by intravenous injection via the external jugular or anterior abdominal vein using a 26 G butterfly needle or Abbocath T.I.V intravenous catheter 26Gx3/4" (Abbott Laboratories, IL).. The solutions were infused at ~4 hours of birth on E31 (postnatal day 0, P0). The duration of infusion was 1–2 min. For convenience sake, the HUCBC treatment was labeled as High Dose.
In the second phase following analysis of results of phase one, each of the Severe and Mild groups were again independently randomized to receive either 1) 2.5x106 HUCBC cells or 2) saline, 1 ml volume. The solutions were infused at ~4 hours of birth on E31 (P0). The duration of infusion was 5 min and delivered by a PHD 2000 programmable pump (Harvard Apparatus Holliston, MA). This HUCBC treatment was labeled as Low Dose.
Detailed Neurobehavior Examination
At E32 (P1), P5 and P11, we performed a neurobehavioral battery of tests on newborn kits, which assessed both motor and sensory function, as both are often affected in CP. Primary end points included: 1) Tone: Scoring used a modified Ashworth scale; 2) Posture: evaluations of standing posture and trunk; 3) Locomotion: ability to walk and jump; 4) Righting: trunk and limb movement and 5) Dystonia score: assessing involuntary movements by adapting a clinical classification [51]. All tests were videotaped and scored by two investigators masked to group identity. P11 was chosen as a significant developmental milestone since it corresponds with eye opening and substantial increase in mobility and motor abilities to near adult levels.
Joint function and limb movement were assessed by a forced swimming test [52], which allows the detection of lack of coordination between upper and lower joints, mild motor deficits, and assessing recovery with two-joint analysis. The maximum and minimum angles were calculated to define maximal extension and flexion known to be affected in CP and rabbits [52]. The mean and median angles, a measure of the mid joint motion, reflected differences between extensor and flexor tone that could change the midpoint of joint motion.
MRI estimation of brain entry
As proof of principle, we assessed whether HUCBC enters newborn kit brain and whether we could detect it non-invasively by MRI. Dams at E22 were subjected to 40 min uterine ischemia. We added another E29 H-I protocol to maximize chances of HUCBC entering a freshly injured brain, with the differences from the E22 protocol being that at E29, dams were subjected to 32 min uterine ischemia and C-section was performed at E30. The duration of ischemia at E29 was decreased to make the potency of the insult equivalent to the one at E22 (as advanced gestation fetuses were more susceptible to length of insult, [53]).
Cell labeling for MRI tracking
HUCBC cells were labeled with two compounds: Gadofluorine M (Shering, Germany), that provide positive image contrast (signal enhancement on T1-weighted images), and Feridex, (Feridex, Berlex Imaging, Wayne, NJ, USA, 11.2 mg Fe/ml) that provide negative image contrast (signal drop on T2-weighted images) [54]. 100 µL of 250 mM Gadofluorine stock was dissolved in 1 mL final volume of media and incubated at 37°C/5% CO2 for 45 minutes. Cells were washed and incubated in six-well plates, 5×107 cells in 5 ml culture medium with 25 mM Gadofluorine or 25 mM of Feridex for 24 h at 37°C. Cells were washed three times by sedimentation (5 min, 210×g, 25°C). Unlabeled cells were prepared by incubation without labels with same cell concentrations as above. Cells were re-suspended in 1 mL of media in 1.5 mL tubes. Incubation time and label concentration for labeling were optimized in a separate set of experiments.
In vivo MR imaging
The dams underwent C-section at E30 and newborn kits were infused intravenously with 5x106 HUCBC labeled either with Gadofluorine, Feridex or unlabeled cells. Rabbit kits were imaged 24 hours (n=3 per group) or 72 hours (n=2 per group) after HUCBC infusion.
Rabbit kits were sedated with i.m. injection of a mixture of Ketamine (35 mg/kg), Xylazine (5 mg/kg), and Acepromazine (1.0 mg/kg). Animals were placed supine in a cradle heated with water blanket at 35oC and imaged in a 4.7 T Bruker Biospec system (Bruker, Billerica, MA) with 30 mm single loop receiver only coil. A 70 mm-volume coil was used for excitation. High resolution T2-weighted (TR/TE/NEX 5200/31/5) and T1-weighted images (TR/TE/NEX 500/9/12) were obtained in axial and coronal planes using RARE sequence, matrix 256x256, in plane resolution 0.08x0.11 mm, 1 mm slice thickness. FLASH T2*-weighted images (TR/TE/NEX 1000/10/5) were also obtained with the same geometry as above.
Evaluating minimum visibility threshold
Gel phantoms were prepared by suspending labeled cells in 2% agarose in serial dilutions up to 1:256 from initial concentration of 1.2–2 x106 cells/mL. Phantoms were imaged in a 4.7 T Bruker Biospec system (Bruker, Billerica, MA) with 30 mm single loop receiver only coil with the same imaging sequences and resolution as above for in-vivo imaging. Signal to noise and contrast to noise (against gel phantom without cells) values were obtained for each cell concentration in T1-weighted imaged for Gadofluorine labeled cells and T2-weighted imaged for Feridex labeled cells.
High field MR imaging
E32 rabbit kits with HUCBC injection 24 hours after 32 min H-I at E29, that were used in in-vivo imaging experiments, were transcardially perfused with PBS, followed by 4% paraformaldehyde solution. Samples were post-fixed in the fixative solution for 1 week and rehydrated in PBS 24 hours before imaging. A gel phantom was prepared by suspension of Feridex labeled HUCBC in 2% agarose with final concentration 3.65×103 cells/mL and 0.5 mL in a centrifuge tube. Fixed brain specimens and gel phantoms were imaged in 14.1 vertical Bruker magnet using 20 mm volume probe and 3D FLASH sequence (TR/TE/FA/NEX 50/23/12/6) providing T1/T2 contrast with isotropic resolution 50 µm and imaging time 10 hours 38 min.
Estimating for Human DNA entry into newborn brain
To assess whether there was any long term bio-distribution or engraftment of HUCBC into newborn brain, we next devised a high sensitivity method to detect human DNA entry in newborn brain. Rabbit brains were obtained after transcardial perfusion with saline, and total DNA was extracted. We used a polymerase chain reaction (PCR) method using 30 cycles of PCR amplification based on a published method [55]. DNA was isolated from several rabbit kit brains at P18 using Allprep DNA/RNA kit (Qiagen™). The DNA was PCR amplified using primers derived from a portion of the human mitochondrial cytochrome b region, which shows no homology to other animal species [55]. These primers showed no cross reactivity to rabbit brain samples and we were able to identify as little as 40 human cells in 50 mg rabbit brain tissue. This sensitivity was established by adding series diluted HUCBCs into 50 mg rabbit brain tissue.
Statistical analysis
The effect of HUCBC treatment was analyzed by repeated measures two-way analysis of variance (GLM procedure in SAS statistical software).Data was presented as means± standard error of means. Level of statistical significance α was set as 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Lei Yu for his assistance in human DNA experiments, and Sylvia Honda Takada for help in immunohistochemistry. Funding: NIH NS043285, NS051402, NS081936 (S.T.), HD057307 (M.D.) and awards from Reaching for the Stars, Jean and George Brumley, Jr. Neonatal-Perinatal Research Institute (C.M.C., R.N.G.) and Associate Board of NorthShore University HealthSystem (S.T.).
References
- 1.Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, Pemberton PJ, Stanley FJ. Antepartum risk factors for newborn encephalopathy: The western australian case-control study. British Medical Journal. 1998;317:1549–1553. doi: 10.1136/bmj.317.7172.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, Pemberton PJ, Stanley FJ. Intrapartum risk factors for newborn encephalopathy: The western australian case-control study. BMJ. 1998;317:1554–1558. doi: 10.1136/bmj.317.7172.1554. [comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunn AJ, Bennet L. Brain cooling for preterm infants. Clin Perinatol. 2008;35:735–748. doi: 10.1016/j.clp.2008.07.012. vi-vii. [DOI] [PubMed] [Google Scholar]
- 5.Bennet L, Tan S, Van den Heuij L, Derrick M, Groenendaal F, van Bel F, Juul S, Back SA, Northington F, Robertson NJ, Mallard C, Gunn AJ. Cell therapy for neonatal hypoxia-ischemia and cerebral palsy. Ann Neurol. 2012;71:589–600. doi: 10.1002/ana.22670. [DOI] [PubMed] [Google Scholar]
- 6.Liao Y, Cotten M, Tan S, Kurtzberg J, Cairo MS. Rescuing the neonatal brain from hypoxic injury with autologous cord blood. Bone marrow transplantation. 2013;48:890–900. doi: 10.1038/bmt.2012.169. [DOI] [PubMed] [Google Scholar]
- 7.Pimentel-Coelho PM, Magalhaes ES, Lopes LM, deAzevedo LC, Santiago MF, Mendez-Otero R. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: Functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2010;19:351–358. doi: 10.1089/scd.2009.0049. [DOI] [PubMed] [Google Scholar]
- 8.Meier C, Middelanis J, Wasielewski B, Neuhoff S, Roth-Haerer A, Gantert M, Dinse HR, Dermietzel R, Jensen A. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatric Research. 2006;59:244–249. doi: 10.1203/01.pdr.0000197309.08852.f5. [DOI] [PubMed] [Google Scholar]
- 9.Yasuhara T, Hara K, Maki M, Xu L, Yu G, Ali MM, Masuda T, Yu SJ, Bae EK, Hayashi T, Matsukawa N, Kaneko Y, Kuzmin-Nichols N, Ellovitch S, Cruz EL, Klasko SK, Sanberg CD, Sanberg PR, Borlongan CV. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14:914–921. doi: 10.1111/j.1582-4934.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 11.de Paula S, Vitola AS, Greggio S, de PD, Mello PB, Lubianca JM, Xavier LL, Fiori HH, Dacosta JC. Hemispheric brain injury and behavioral deficits induced by severe neonatal hypoxia-ischemia in rats are not attenuated by intravenous administration of human umbilical cord blood cells. PediatrRes. 2009;65:631–635. doi: 10.1203/PDR.0b013e31819ed5c8. [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Allison J, McLaughlin C, Sledge L, Waters-Pick B, Wease S, Kurtzberg J. Differences in quality between privately and publicly banked umbilical cord blood units: A pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion. 2010;50:1980–1987. doi: 10.1111/j.1537-2995.2010.02720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, Fisher KA, Gustafson KE, Waters-Pick B, Swamy GK, Rattray B, Tan S, Kurtzberg J. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr. 2013 doi: 10.1016/j.jpeds.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derrick M, Drobyshevsky A, Ji X, Tan S. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- 15.Derrick M, Drobyshevsky A, Ji X, Chen L, Yang Y, Ji H, Silverman RB, Tan S. Hypoxia-ischemia causes persistent movement deficits in a perinatal rabbit model of cerebral palsy: Assessed by a new swim test. Int J Dev Neurosci. 2009;27:549–557. doi: 10.1016/j.ijdevneu.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buser JR, Segovia KN, Dean JM, Nelson K, Beardsley D, Gong X, Luo NL, Ren J, Wan Y, Riddle A, McClure MM, Ji X, Derrick M, Hohimer AR, Back SA, Tan S. Timing of appearance of late oligodendrocyte progenitors coincides with enhanced susceptibility of preterm rabbit cerebral white matter to hypoxia-ischemia. J Cereb Blood Flow Metab. 2010;30:1053–1065. doi: 10.1038/jcbfm.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, Peebles D, Wylezinska M, Owen-Reece H, Kirkbride V. Delayed ("Secondary") cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: Continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. PediatrRes. 1994;36:699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. JClinInvest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazur M, Miller RH, Robinson S. Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic-ischemic injury. Journal of neurosurgery Pediatrics. 2010;6:206–221. doi: 10.3171/2010.5.PEDS1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010;67:657–666. doi: 10.1002/ana.21977. [DOI] [PubMed] [Google Scholar]
- 21.Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, Chang T, Durand DJ, Song D, Bonifacio SL, Gonzalez FF, Glass HC, Juul SE. Erythropoietin for neuroprotection in neonatal encephalopathy: Safety and pharmacokinetics. Pediatrics. 2012;130:683–691. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benders MJ, van der Aa NE, Roks M, van Straaten HL, Isgum I, Viergever MA, Groenendaal F, de Vries LS, van Bel F. Feasibility and safety of erythropoietin for neuroprotection after perinatal arterial ischemic stroke. J Pediatr. 2014;164:481–486. doi: 10.1016/j.jpeds.2013.10.084. e481-482. [DOI] [PubMed] [Google Scholar]
- 23.Dingley J, Tooley J, Liu X, Scull-Brown E, Elstad M, Chakkarapani E, Sabir H, Thoresen M. Xenon ventilation during therapeutic hypothermia in neonatal encephalopathy: A feasibility study. Pediatrics. 2014 doi: 10.1542/peds.2013-0787. [DOI] [PubMed] [Google Scholar]
- 24.Bae SH, Lee HS, Kang MS, Strupp BJ, Chopp M, Moon J. The levels of pro-inflammatory factors are significantly decreased in cerebral palsy patients following an allogeneic umbilical cord blood cell transplant. International journal of stem cells. 2012;5:31–38. doi: 10.15283/ijsc.2012.5.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, Jang SJ, Kim SH, Oh D, Kim MK, Kim SS, Kim M. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: A double-blind, randomized, placebo-controlled trial. Stem Cells. 2013;31:581–591. doi: 10.1002/stem.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geissler M, Dinse HR, Neuhoff S, Kreikemeier K, Meier C. Human umbilical cord blood cells restore brain damage induced changes in rat somatosensory cortex. PloS one. 2011;6:e20194. doi: 10.1371/journal.pone.0020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasielewski B, Jensen A, Roth-Harer A, Dermietzel R, Meier C. Neuroglial activation and cx43 expression are reduced upon transplantation of human umbilical cord blood cells after perinatal hypoxic-ischemic injury. Brain Res. 2012;1487:39–53. doi: 10.1016/j.brainres.2012.05.066. [DOI] [PubMed] [Google Scholar]
- 28.Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, Deans RJ, Hess DC, Carroll JE, Borlongan CV. Behavioral and histological characterization of intrahippocampal grafts of human bone marrow-derived multipotent progenitor cells in neonatal rats with hypoxic-ischemic injury. Cell Transplant. 2006;15:231–238. doi: 10.3727/000000006783982034. [DOI] [PubMed] [Google Scholar]
- 29.Paula SD, Greggio S, Marinowic DR, Machado DC, Dacosta JC. The dose-response effect of acute intravenous transplantation of human umbilical cord blood cells on brain damage and spatial memory deficits in neonatal hypoxia-ischemia. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Ramos JR, Song S, Kamath SG, Zigova T, Willing A, Cardozo-Pelaez F, Stedeford T, Chopp M, Sanberg PR. Expression of neural markers in human umbilical cord blood. Exp Neurol. 2001;171:109–115. doi: 10.1006/exnr.2001.7748. [DOI] [PubMed] [Google Scholar]
- 31.Tracy E, Aldrink J, Panosian J, Beam D, Thacker J, Reese M, Kurtzberg J. Isolation of oligodendrocytelike cells from human umbilical cord blood. Cytotherapy. 2008;10:518–525. doi: 10.1080/14653240802154586. [DOI] [PubMed] [Google Scholar]
- 32.Tracy ET, Zhang CY, Gentry T, Shoulars KW, Kurtzberg J. Isolation and expansion of oligodendrocyte progenitor cells from cryopreserved human umbilical cord blood. Cytotherapy. 2011;13:722–729. doi: 10.3109/14653249.2011.553592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buzanska L, Machaj EK, Zablocka B, Pojda Z, Domanska-Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci. 2002;115:2131–2138. doi: 10.1242/jcs.115.10.2131. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan G, Pappas A, Shankaran S, Laptook AR, Walsh M, McDonald SA, Ehrenkranz RA, Tyson JE, Goldberg RN, Bara R, Higgins RD, Das A, Munoz B. Effect of inborn vs Outborn delivery on neurodevelopmental outcomes in infants with hypoxic-ischemic encephalopathy: Secondary analyses of the nichd whole-body cooling trial. Pediatr Res. 2012;72:414–419. doi: 10.1038/pr.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile krabbe's disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 36.Juul SE, Aylward E, Richards T, McPherson RJ, Kuratani J, Burbacher TM. Prenatal cord clamping in newborn macaca nemestrina: A model of perinatal asphyxia. Dev Neurosci. 2007;29:311–320. doi: 10.1159/000105472. [DOI] [PubMed] [Google Scholar]
- 37.Tajiri N, Acosta SA, Shahaduzzaman M, Ishikawa H, Shinozuka K, Pabon M, Hernandez-Ontiveros D, Kim DW, Metcalf C, Staples M, Dailey T, Vasconcellos J, Franyuti G, Gould L, Patel N, Cooper D, Kaneko Y, Borlongan CV, Bickford PC. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: Cell graft biodistribution and soluble factors in young and aged rats. The Journal of Neuroscience. 2014;34:313–326. doi: 10.1523/JNEUROSCI.2425-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnitsky S, Watson DJ, Walton RM, Pickup S, Bulte JWM, Wolfe JH, Poptani H. In vivo and ex vivo mri detection of localized and disseminated neural stem cell grafts in the mouse brain. Neuroimage. 2005;26:744–754. doi: 10.1016/j.neuroimage.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 39.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS., Jr Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borlongan CV, Hadman M, Davis Sanberg C, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 41.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hmscs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein tsg-6. Cell stem cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bae SH, Kong TH, Lee HS, Kim KS, Hong KS, Chopp M, Kang MS, Moon J. Long-lasting paracrine effects of human cord blood cells on damaged neocortex in an animal model of cerebral palsy. Cell transplantation. 2012;21:2497–2515. doi: 10.3727/096368912X640457. [DOI] [PubMed] [Google Scholar]
- 43.Rosenkranz K, Meier C. Umbilical cord blood cell transplantation after brain ischemia--from recovery of function to cellular mechanisms. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2011;193:371–379. doi: 10.1016/j.aanat.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci U S A. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iosa M, Zoccolillo L, Montesi M, Morelli D, Paolucci S, Fusco A. The brain’s sense of walking: A study on the intertwine between locomotor imagery and internal locomotor models in healthy adults, typically developing children and children with cerebral palsy. Frontiers in Human Neuroscience. 2014:8. doi: 10.3389/fnhum.2014.00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ygberg S, Nilsson A. The developing immune system - from foetus to toddler. Acta Paediatr. 2012;101:120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 47.Nikonova MF, Maiskii IN, Pokrovskaia TA. [comparative study of the immune response of newborn and adult animals to the administration of tumor extracts] Biull Eksp Biol Med. 1975;79:85–88. [PubMed] [Google Scholar]
- 48.Smith RT, Bridges RA. Immunological unresponsiveness in rabbits produced by neonatal injection of defined antigens. J Exp Med. 1958;108:227–250. doi: 10.1084/jem.108.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurtzberg J, Lyerly AD, Sugarman J. Untying the gordian knot: Policies, practices, and ethical issues related to banking of umbilical cord blood. J Clin Invest. 2005;115:2592–2597. doi: 10.1172/JCI26690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, Gladson CL, Beardsley DJ, Murdoch G, Back SA, Tan S. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: A model for human cerebral palsy? J Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T. Rating scales for dystonia: A multicentre assessment. Mov Disord. 2003;18:303–312. doi: 10.1002/mds.10377. [DOI] [PubMed] [Google Scholar]
- 52.Derrick M, Drobyshevsky A, Ji X, Chen L, Yang Y, Ji H, Silverman RB, Tan S. Hypoxia-ischemia causes persistent movement deficits in a perinatal rabbit model of cerebral palsy: Assessed by a new swim test. Int J Dev Neurosci. 2009;27:549–557. doi: 10.1016/j.ijdevneu.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drobyshevsky A, Derrick M, Luo K, Zhang LQ, Wu YN, Takada SH, Yu L, Tan S. Near-term fetal hypoxia-ischemia in rabbits: Mri can predict muscle tone abnormalities and deep brain injury. Stroke. 2012;43:2757–2763. doi: 10.1161/STROKEAHA.112.653857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henning TD, Saborowski O, Golovko D, Boddington S, Bauer JS, Fu Y, Meier R, Pietsch H, Sennino B, McDonald DM, Daldrup-Link HE. Cell labeling with the positive mr contrast agent gadofluorine m. Eur Radiol. 2007;17:1226–1234. doi: 10.1007/s00330-006-0522-9. [DOI] [PubMed] [Google Scholar]
- 55.Matsuda H, Seo Y, Kakizaki E, Kozawa S, Muraoka E, Yukawa N. Identification of DNA of human origin based on amplification of human-specific mitochondrial cytochrome b region. Forensic SciInt. 2005;152:109–114. doi: 10.1016/j.forsciint.2004.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.