Abstract
Background:
Hypercholesterolemia is one of the most important risk factors for atherosclerosis and subsequent cardiovascular diseases.
Objective:
The present work was aimed to study the efficacy of curcuminoid fraction from Curcuma xanthorrhiza and its curcuminoid cider in reducing blood cholesterol level and four genes related to oxidative stress, including cluster of differentiation 44 (CD44), intercellular adhesion molecule 1 (ICAM-1), inducible nitric oxide synthase (iNOS), and lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in high-cholesterol fed rats in vivo.
Materials and Methods:
Twenty-four male Sprague-Dawley rats were divided into six groups, namely normal group diet, high-cholesterol diet (HCD) 2%, HCD + 100 mg/kg b.w. curcuminoid fraction, HCD + 300 mg/kg b.w. curcuminoid fraction, HCD + cider 1% v/v, and HCD + curcuminoid cider 2% v/v for 4 weeks. Total cholesterol levels were measured at day 1, 14, and 28. Vascular tissues and organs from lung and liver were collected for RNA extraction, followed by quantitative analysis using real-time polymerase chain reaction (PCR).
Results:
Our results demonstrated that among all the treatment groups, curcuminoid cider at 2% v/v significantly lowered total cholesterol level compared to those of positive control. Real-time PCR data showed both curcuminoid fractions (100 and 300 mg/kg) and curcuminoid cider (1 and 2% v/v) inhibited the gene expression of CD44, ICAM-1, iNOS, and LOX-1, indicating their hypocholesterolemic effects via attenuating genes related to oxidative stress in rats in vivo.
Conclusion:
Oral administration of curcuminoid fraction and its cider product may exert potential inhibitory effects on oxidative stress related-genes for preventing hypercholesterolemia-induced atherosclerosis in vivo.
SUMMARY
Curcuminoid and its cider significantly inhibited the gene expression of CD44, ICAM-1, iNOS, and LOX-1 in rats in vivo
Curcuminoid and its cider suppressed oxidative stress-related genes inducing formation of atherosclerosis
Curcuminoid and its cider may offer cardioprotective effect for preventing hypercholesterolemia-induced atherosclerosis
Abbreviations Used: ROS: Reactive oxygen species, NO: Nitric oxide, NOS: NO synthase, NADPH: Nicotinamide adenine dinucleotide phosphate, CD44: Cluster of differentiation 44, ICAM-1: Intercellular adhesion molecule 1, iNOS: inducible NOS, LOX-1: lectin-like oxidized LDL receptor-1, HMG-CoA: 3-hydroxy-3-methylglutaryl-coenzyme A, 5-HMF: 5-hydroxymethylfurfural, HCD: High-cholesterol diet
Keywords: Atherosclerosis, curcuminoid cider, curcuminoid fraction, genes related to oxidative stress, hypercholesterolemia
INTRODUCTION
Hypercholesterolemia is one of the metabolic disorders characterized by high serum levels of low-density lipoprotein (LDL) cholesterol and blood cholesterol. As a result, cholesterol level and triglycerides metabolism affect oxidative stress mediators and promote the production of reactive oxygen species (ROS) by various mechanisms, which leads to increased lipid peroxidation.[1] Oxidative stress is an imbalance condition between oxidants and antioxidants in favor of the oxidants, potentially leading to cell damages. Atherosclerosis is a condition of high oxidative stress characterized by lipid and protein oxidation in the vascular walls. Overproduction of ROS under pathophysiologic conditions forms an integral part of the development of cardiovascular disease and in particular atherosclerosis. Several characteristics, such as endothelial dysfunction and the loss of nitric oxide (NO) activity, may occur in the development of atherosclerosis and determine further vascular complications.
ROS belongs to the major mediator of signaling pathways that underlie vascular inflammation in atherogenesis, starting from the initiation of fatty streak development, through lesion progression, to ultimate plaque rupture. Several studies demonstrated that ROS released from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, myeloperoxidase, xanthine oxidase, lipoxygenase, NO synthase (NOS), and heightened ROS production from dysfunctional mitochondrial respiratory chain plays a key role in atherosclerosis and other vascular diseases.[2] Curcuminoids and sesquiterpenoids as major compounds found in the rhizomes of Curcuma longa (turmeric) and Curcuma xanthorrhiza (temulawak) have been widely used as dietary spice and folk medicine for reducing swelling and symptoms of osteoarthritis and rheumatoid arthritis such as pain and inflammation. It is reported that curcuminoids are known to have anti-oxidative, anti-carcinogenic, and anti-inflammatory activities.[3] This study was aimed to examine the efficacy of curcuminoid fraction from C. xanthorrhiza and curcuminoid cider products on modulating lipid profiles and inhibiting the expression of several genes related to oxidative stress inducing atherosclerotic plaque formation in rats in vivo.
MATERIALS AND METHODS
Preparation of curcuminoid extract from Curcuma xanthorrhiza
One kilogram of fresh plant materials from C. xanthorrhiza was purchased from a local market in Bogor, Indonesia. Fresh rhizomes were cleaned, sliced, and freeze-dried for 3 days. Afterward, the dried rhizomes were blended and filtered to obtain the same size particle powder. The powder of C. xanthorrhiza was macerated with 80% ethanol (1:4, w/v) at room temperature for 2 days. The supernatant was combined and concentrated under reduced pressure at 45°C using a rotary vacuum evaporator. The crude extract was then freeze-dried until a constant weight was obtained to afford the maceration extract.
Preparation of curcuminoid cider from Curcuma xanthorrhiza
Curcuminoid fraction from C. xanthorrhiza was dissolved in hot water with final concentration of 1% and 2% (w/v). Acetobacter xylinum culture was added to curcuminoid solution with ratios of 2:1 and 4:1, sugar of 2.5% was added to provide nutrients for the culture growth as well as providing taste and aroma to the cider product. Furthermore, the solution was filtered and incubated for 14 days at 30°C. The cider was further pasteurized at 50–60°C for 20 min and then filtered to obtain the functional food product curcuminoid cider. Cider products were tested for its efficacy in vivo.
Identification of chemical compounds in curcuminoid fraction and curcuminoid cider
Curcuminoid extract and curcuminoid cider were analyzed using pyrolysis gas chromatography mass spectrometry (Py-GC/MS). Py-GC/MS QP2010 (Shimadzu, Duisburg, Germany) was carried out to analyze the chemical compounds found in each sample. Sample was injected to the capillary column (Phase Rtx-5MS) with a film thickness of 60 m × 0.25 mm ID. Pyrolysis temperature was set to 280°C. Helium was used as the carrier gas.[4]
Preparation of animals
The experiment was conducted on 24 male Sprague-Dawley rats weighing approximately 140 g and adapted to the laboratory environment until stable condition for 1 week. The animals were divided into six groups: Negative control (K−) was fed a standard diet; the remaining five received hypercholesterolemic diets [Table 1] for 2 weeks. The rats were maintained in individual cages, under controlled temperature, humidity and illumination conditions, with water and diet ad libitum for 2 weeks. Body weight, total blood cholesterol, LDL, and high-density lipoprotein (HDL) level were recorded weekly. At the end of 4 weeks, the animals were sacrificed. Blood was collected through the retro-orbital puncture, and tissue organs (lung, vascular, and liver) were collected in RNA later solution. These organs were sectioned for quantitative real-time polymerase chain reaction (qRT-PCR). The protocol was approved by the Animal Care and Use Committee of the Faculty of Veterinary, Bogor Agricultural University, Bogor, Indonesia.
Table 1.
Treatment of the group rats diets

Determination of genes related to oxidative stress using quantitative real-time polymerase chain reaction
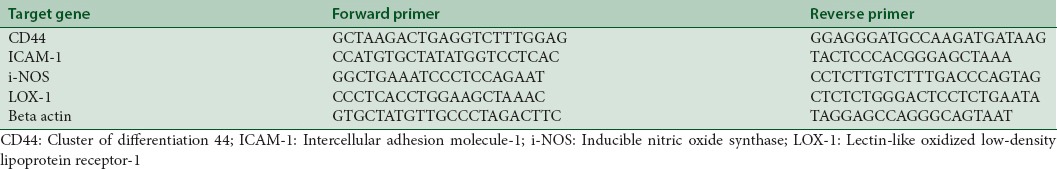
Analysis of qRT-PCR was used to quantify the expressions of four genes related to oxidative stress, including cluster of differentiation 44 (CD44), intercellular adhesion molecule 1 (ICAM-1), inducible NOS (iNOS), and lectin-like oxidized LDL receptor-1 (LOX-1).[5] RNA was isolated from the rats’ tissue organs (heart, vascular, and liver) using TRIzol® reagent (Life Technologies, California, US) according to the manufacturer's protocol. cDNA was synthesized using iScript One-Step RT-PCR Kit with SYBR® Green and KAPA SYBR® FAST qPCR Kit for analysis by RT-PCR with beta-actin as internal control. Primer sequences for RT-PCR were listed in Table 2.
Table 2.
Oligonucleotide primer for quantitative real-time polymerase chain reaction
Statistical analysis
The duplicate experiments were performed throughout this study. All data are presented as the mean ± standard deviation. The significant difference between control and treated groups was statistically analyzed by the t-test (P ≤ 0.05).
RESULTS
Identification of chemical compounds in curcuminoid fraction and curcuminoid cider
The curcuminoid fraction obtained was 11.5 g from 1 kg C. xanthorrhiza rhizome. The Py-GC/MS chromatogram of the rhizome extract of C. xanthorrhiza is shown in Figure 1. Twenty-five peaks were obtained; all chemical constituents were characterized and identified [Table 3]. The components were grouped into main classes: Sesquiterpenoid (67.85%), phenolic (7.73%), alkene (7.66%), monoterpene (5.35%), benzene (2.63%), ester (0.97%), diterpene (0.55%), fatty acid (2.14%), benzyhydrol (2.18%), and others (2.94%).
Figure 1.
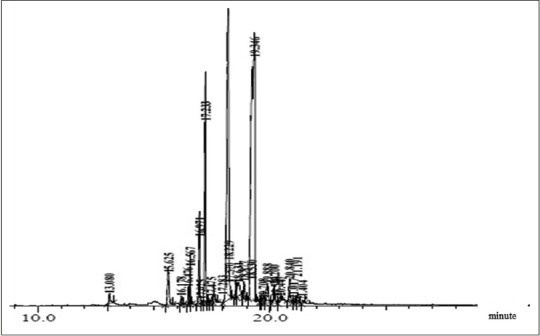
Pyrolysis gas chromatography mass spectrometry chromatogram of curcuminoid fraction from Curcuma xanthorrhiza
Table 3.
Identification of chemical compounds in curcuminoid fraction from Curcuma xanthorrhiza

Furthermore, GC-MS chromatogram of the curcumonoid cider is shown in Figure 2. Forty peaks were obtained; all the chemical compounds were characterized and identified [Table 4]. Chromatogram demonstrated that components were grouped into main classes: Furfural derivatives (25.75%), cycloalkanes (21.8%), organic acids (17.63%), benzopyrones (10.39%), benzene (4.41%), phenolics (4%), and others (16.05%).
Figure 2.
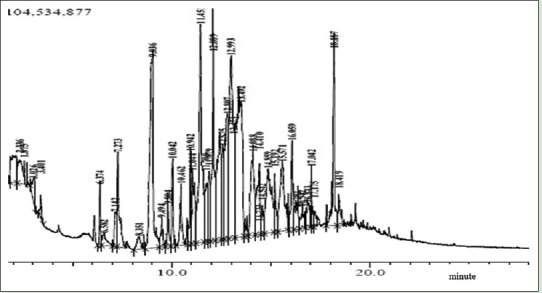
Pyrolysis gas chromatography mass spectrometry chromatogram of curcuminoid cider
Table 4.
Identification of chemical compounds in curcuminoid cider
Body weight and lipid profiling in rats
After 2 weeks of high-cholesterol diet (HCD) treatment, five groups showed higher body weight in relation to negative control. Serum levels of cholesterol increased in all the experimental groups after 2 weeks and decreased after 2 weeks administration of curcuminoid fraction and cider [Figure 3]. Interestingly, our results showed that there were no significant effects on LDL and HDL values after treatment with both samples. Then, curcuminoid fraction and its cider only affected cholesterol total, but not LDL and HDL values.
Figure 3.
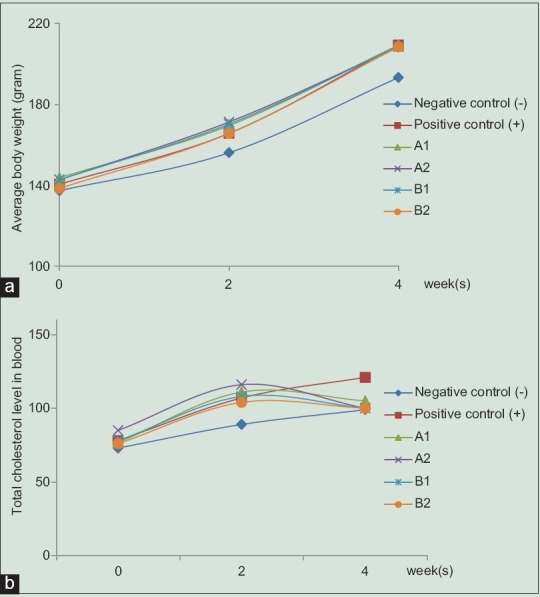
Diagram of average body weights (a) and total cholesterol level in blood (b). Six groups of male Sprague-Dawley rats were divided into: normal group diet (negative control), high-cholesterol diet 2% (positive control), high-cholesterol diet + 1% v/v cider (A1), high-cholesterol diet + 2% v/v cider (A2), high-cholesterol diet + 100 mg/kg b.w. (B1), and high-cholesterol diet + 300 mg/kg b.w. (B2) at 8 weeks of age (0 week), and body weight gain after 2 weeks (high-cholesterol) and 4 weeks (after treatment). Data were shown as mean ± standard deviation from duplicate experiments
Determination of genes related to oxidative stress using quantitative real-time polymerase chain reaction
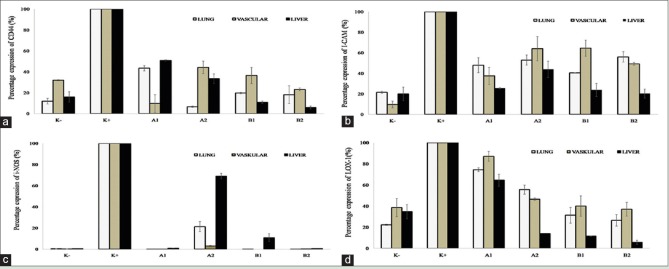
The expression of CD44, ICAM-1, iNOS, and LOX-1 mRNAs in various rat organs (lung, vascular, and liver) showed different patterns after treated with curcuminoid fraction and cider product. At all concentrations, curcuminoid fraction and cider significantly inhibited >50% of gene expression of CD44, ICAM-1, iNOS, and LOX-1 [Figure 4], indicating that both samples had potential effect to reduce oxidative stress-related genes inducing the formation of atherosclerotic plaque.
Figure 4.
Percentage expression of cluster of differentiation 44 (a), intercellular adhesion molecule-1 (b), inducible nitric oxide synthase (c), lectin-like oxidized low-density lipoprotein receptor (d), and genes in various rat organs (lung, vascular, and liver). Six groups of male Sprague-Dawley rats were divided into: normal group diet (negative control), high-cholesterol diet 2% (positive control), high-cholesterol diet + 1% v/v cider (A1), high-cholesterol diet + 2% v/v cider (A2), high-cholesterol diet + 100 mg/kg b.w. (B1), and high-cholesterol diet + 300 mg/kg b.w. (B2). Data were shown as mean ± standard deviation from duplicate experiments.P < 0.05 against positive control
DISCUSSION
Spices not only make our food palatable, but also give beneficial pharmacological effects when used in judicious quantities. Even though they cannot totally displace the medications, they may be used in conjunction with medications to have better therapeutic potential to minimize the drug dose with little or no toxic effects as compared to synthetic chemicals and thereby be most cost effective. Many studies showed that the usage of alternative herbal medications such as curcumin have antihypercholesterolemic effects in Triton WR1339-induced hypercholesterolemia. Triton WR1339 (Tyloxapol) is widely used to induce hypercholesterolemia in animal models.[6] It is noted that the hypocholesterolemic effect of curcumin was attributed to its stimulatory effect on hepatic cholesterol-7α-hydroxylase enzyme, an enzyme that regulates cholesterol catabolism.[7]
Curcumin was also reported to modulate 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase activity that lead to the decrease in the levels of serum and liver cholesterol, triglycerides, and free fatty acid.[8] Moreover, a study by Ramírez-Tortosa et al. also described about hypocholesterolemic mechanism of turmeric extract in rabbits fed by a HCD as an experimental atherosclerotic model.[9] The mechanism was assumed through increased cholesterol excretion in the gall bladder together with decreased saturation of biliary cholesterol and increased fat excretion in the feces. Therefore, oral administration of turmeric extract significantly inhibited LDL oxidation and exerted hypocholesterolemic potential in rabbits.
Our data showed that Py-GC/MS analysis of curcuminoid fraction from C. xanthorrhiza rhizome revealed the presence of 25 components [Figure 1 and Table 3]. Major compound was identified as sesquiterpenoids (~67.85%). This study is in linear with a study by He et al. which reported that sesquiterpenoids are the major constituents of turmeric oil from C. longa.[10] Curcuminoids are primarily accumulated in the rhizomes of C. longa. The contents of curcuminoids in C. longa rhizomes vary often with varieties, locations, sources, and cultivation conditions. To date, at least 235 compounds, primarily terpenoids compounds and phenolics, have been identified from the species, including 22 diarylheptanoids and diarylheptanoids, eight phenylpropenes and other phenolic compounds, 68 monoterpenes, 109 sesquiterpenes, five diterpenes, three triterpenoids, four sterols, two alkaloids, and 14 other compounds.
Curcuminoids and essential oils are the major bioactive ingredients with various bioactivities in vitro and in vivo bioassays. Dried turmeric rhizomes usually yield 1.5–5% essential oils, which are dominated by sesquiterpenes and are responsible for its aromatic taste and smell.[11] In terms of its bioefficacy, curcumin regulates the expression of genes related to energy metabolic process and fat deposition, thus decreasing the quantity of intra-cellular lipids. In adipose tissues (body fat), curcumin suppresses angiogenesis, which is essential for tissue growth and together with the effects on lipid metabolic process in adipocytes. Curcumin as an anti-obesity agent lowers body fat and reduces weight.[12]
Furthermore, our results also demonstrated that curcuminoid cider product consisted of 40 chemical compounds [Figure 2 and Table 4], and the major substances from the cider were identified as 3-methylcoumarin (10.39%) and 5-hydroxymethylfurfural (5-HMF; 8.65%). Both compounds have been widely reported for contributing to the aroma of fermented beverages.[13] Coumarin is a fragrant organic chemical compound in the benzopyrone chemical class. Many coumarins and their derivatives have the special ability to scavenge ROS and show lipid lowering potentials. It has been known that coumarins also exert dual ability to scavenge ROS and modulate HMG-CoA reductase in HepG2 cell line via ROS-induced dysregulation of enzyme activity.[14]
The 5-HMF, a product of the famous Maillard reaction, is mainly generated by acid-catalyzed thermal dehydration of fructose and identified as a flavoring substance in a wide variety of heat-processed products. It has been reported that 5-HMF has favorable biological effects, such as antioxidant activity, anti-hypoxia, and inhibition of sickling of red blood cells.[15,16,17] Mechanism of hypoxic injury contains oxidative stress process and 5-HMF can protect hepatocytes against damages induced by H2O2 in vitro.[18,19] Other compounds such as furfural derivative (furan) as a potential carcinogen can be induced by heat from sugars, ascorbic acid, and fatty acids during cider fermentation process. Furan levels tend to be greater in foods that are heat processed in cans or jars, since the furan that forms cannot escape from the closed container.[20]
Initial body weight of the animals was approximately 140 g for all groups. After 2 weeks of diet treatment, body weight increased more rapidly in high-cholesterol fed diet groups compared to normal diet groups [Figure 3a]. As a consequence, the percentage of weight gain increased compared with that in control negative group (K−) and the total blood cholesterol level in the HCD group was significantly higher. After 2 weeks administration of curcuminoid fraction and cider, total cholesterol levels from all animals fed with the treatment group (A1, A2, B1, and B2) were found to be effectively decreased [Figure 3b]. Oral administration of 2% curcuminoid cider (A2) caused significant elevation in reducing total cholesterol level in blood. Unfortunately, lipid profiles showed that administration of curcuminoid fraction and its cider at various doses did not affect significant change in decreasing LDL and increasing HDL compared to those of control group (data not shown). Previous study reported that the deposition of cholesterol in the liver decreased with the increasing dosage of curcumin in the diet. Deposition was maximum with dietary cholesterol and no curcumin and minimum when diets included curcumin.[21] Signs with any tendency for the total cholesterol level to rise due to faulty dieting or defective metabolism can be reversed by the ingestion of curcuminoid fraction.
In this study, we quantified whether the treatment of curcuminoid fraction and its cider modulated the expression of several genes related to stress oxidative (CD44, ICAM-1, iNOS, and LOX-1) in various rat organs using qRT-PCR [Figure 4]. Our results showed that among all genes, the expression of CD44 mRNA after sample treatments (curcuminoid fraction and cider) in lung, vascular, and liver exerted fluctuative results [Figure 4a]. Overall, both curcuminoid fraction and cider may offer significant protection against stress oxidative effects via attenuating CD44, ICAM-1, iNOS, and LOX-1 genes expression [Figure 4a–d]. Previous study described that curcumin was known as a downregulator of stem cell marker CD44 in colon cancer and suppressed the increased intensity of ICAM-1 expression in the diabetic rats.[22,23] At low concentration, curcumin inhibited NO production by suppressing the expression of iNOS mRNA and protein induction in macrophages.[24]
Our results showed that curcuminoid fraction at 300 mg/kg body weight (B2) reduced the expression of CD44, ICAM-1, and iNOS mRNAs in the concentration-independent manner [Figure 4a–c]. CD44 is expressed on both inflammatory and vascular cells and it mediates the adhesion of T-lymphocytes to endothelium and smooth muscle cells (SMCs), release of inflammatory mediators from macrophages and T-lymphocytes, and proliferation of vascular SMCs. The principal ligand of CD44 is hyaluronan, a major glycosaminoglycan constituent of extracellular matrix, which is upregulated within atherosclerotic lesions.[25]
ICAM-1 is one of the immunoglobulin superfamilies of endothelial adhesion molecules, participates in leukocyte adhesion to the endothelium and plays an important role in all stages of atherosclerosis.[26] ICAM-1 and most of the other adhesion molecules can be regulated by cytokines. ICAM-1 was the most prominent adhesion molecule in lesion-prone sites; its expression appeared to be independent of plasma cholesterol levels. NO is an important biological mediator in the living organism that is synthesized from L-arginine using NADPH and molecular oxygen. NO is a short-lived free radical and a very small compound that diffuses freely within cells from its site of formation to its site of action. However, the overproduction of NO which is catalyzed by iNOS, a soluble enzyme and active in its dimeric form, is cytotoxic and lead cell to oxidative stress condition.[27]
Treatment of curcuminoid fraction and cider also significantly reduced the expression of LOX-1 genes in a concentration-dependent manner. Several studies showed that curcumin inhibited LOX-1 and suppressed the inflammatory response in vitro and in vivo.[28] LOX-1 expression is highly correlated with atherosclerotic plaque instability.[24] In strong contrast, deletion of LOX-1 reduced atherogenesis in LDLR knockout mice, fed with a HCD.[29] Furthermore, human genetic studies link LOX-1 polymorphisms to cardiovascular disease susceptibility.[30]
CONCLUSION
Oral administration of curcuminoid fraction from C. xanthorrhiza and curcuminoid cider exerted promising hypocholesterolemic effects through inhibition of genes related to oxidative stress (CD44, ICAM-1, iNOS, and LOX) in rats in vivo. Therefore, curcuminoid fraction and its cider may offer cardioprotective effect for preventing hypercholesterolemia-induced atherosclerosis in vivo.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Yanti
Yanti, obtained her PhD from Department of Biotechnology, Yonsei University (Korea). She is a lecturer at Food Technology Program, Faculty of Biotechnology, Atma Jaya Catholic University (Indonesia). Her research is focused on natural products and their molecular mechanisms for treatment of inflammatory-related diseases.
Acknowledgment
This project was supported by Competency Grant from General Directorate of Higher Education, Ministry of Education and Culture, Republic of Indonesia (2014–2015; Contract No. 0094/E5.1/PE/2015).
REFERENCES
- 1.Otunola GA, Oloyede OB, Oladiji AT, Afolayan AJ. Selected spices and their combination modulate hypercholesterolemia-induced oxidative stress in experimental rats. Biol Res. 2014;47:5. doi: 10.1186/0717-6287-47-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonomini F, Tengattini S, Fabiano A, Bianchi R, Rezzani R. Atherosclerosis and oxidative stress. Histol Histopathol. 2008;23:381–90. doi: 10.14670/HH-23.381. [DOI] [PubMed] [Google Scholar]
- 3.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 4.Nandagopal S, Kumar AG, Dhanalaksmi DP, Prakash P. Bio-prospecting the antibacterial and anticancer activities of silver nanoparticles synthesized using Terminalia chebula seed extract. Int J Pharm Pharm Sci. 2014;6:368–73. [Google Scholar]
- 5.Yanti, Oh HI, Anggakusuma, Hwang JK. Effects of panduratin A isolated from Kaempferia pandurata ROXB. on the expression of matrix metalloproteinase-9 by Porphyromonas gingivalis supernatant-induced KB cells. Biol Pharm Bull. 2009;32:110–5. doi: 10.1248/bpb.32.110. [DOI] [PubMed] [Google Scholar]
- 6.Bertges LC, Souza JB, Cardoso VA. Hyperlipidemia induced by triton WR1339 (Tyloxapol) in wistar rats. Braz J Med Sci Health. 2011;1:32–4. [Google Scholar]
- 7.Babu PS, Srinivasan K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol Cell Biochem. 1997;166:169–75. doi: 10.1023/a:1006819605211. [DOI] [PubMed] [Google Scholar]
- 8.Murugan P, Pari L. Effect of tetrahydrocurcumin on lipid peroxidation and lipids in streptozotocin-nicotinamide-induced diabetic rats. Basic Clin Pharmacol Toxicol. 2006;99:122–7. doi: 10.1111/j.1742-7843.2006.pto_447.x. [DOI] [PubMed] [Google Scholar]
- 9.Ramírez-Tortosa MC, Mesa MD, Aguilera MC, Quiles JL, Baró L, Ramirez-Tortosa CL, et al. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371–8. doi: 10.1016/s0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 10.He XG, Lin LZ, Lian LZ, Lindenmaier M. Liquid chromatography – Electrospray mass spectrometric analysis of curcuminoids and sesquiterpenoids in turmeric (Curcuma longa) J Chromatogr A. 1998;818:127–32. [Google Scholar]
- 11.Li S, Yuan W, Deng G, Wang P, Yang P, Aggrawal BB. Chemical composition and product quality control of turmeric (Curcuma longa L.) Pharm Crops. 2011;2:28–54. [Google Scholar]
- 12.González-Castejón M, Rodriguez-Casado A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol Res. 2011;64:438–55. doi: 10.1016/j.phrs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Zhou F, Dziugan P, Yao Y, Zhang J, Zhaolin LV, et al. Development of organic acids and volatiles compounds in cider during malolactic fermentation. Czech J Food Sci. 2014;32:69–76. [Google Scholar]
- 14.Trapani L, Segatto M, Simeoni V, Balducci V, Dhawan A, Parmar VS, et al. Short- and long-term regulation of 3-hydroxy 3-methylglutaryl coenzyme A reductase by a 4-methylcoumarin. Biochimie. 2011;93:1165–71. doi: 10.1016/j.biochi.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L, Chen J, Su J, Li L, Hu S, Li B, et al. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J Agric Food Chem. 2013;61:10604–11. doi: 10.1021/jf403098y. [DOI] [PubMed] [Google Scholar]
- 16.Li MM, Wu LY, Zhao T, Xiong L, Huang X, Liu ZH, et al. The protective role of 5-HMF against hypoxic injury. Cell Stress Chaperones. 2011;16:267–73. doi: 10.1007/s12192-010-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell RD, Swet JH, Kennedy KL, Huynh TT, McKillop IH, Evans SL. Resveratrol attenuates hypoxic injury in a primary hepatocyte model of hemorrhagic shock and resuscitation. J Trauma Acute Care Surg. 2014;76:409–17. doi: 10.1097/TA.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 18.Janzowski C, Glaab V, Samimi E, Schlatter J, Eisenbrand G. 5-hydroxymethylfurfural: Assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem Toxicol. 2000;38:801–9. doi: 10.1016/s0278-6915(00)00070-3. [DOI] [PubMed] [Google Scholar]
- 19.Ding X, Wang MY, Yao YX, Li GY, Cai BC. Protective effect of 5-hydroxymethylfurfural derived from processed fructus corni on human hepatocyte LO2 injured by hydrogen peroxide and its mechanism. J Ethnopharmacol. 2010;128:373–6. doi: 10.1016/j.jep.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Fan X, Huang L, Sokorai KJ. Factors affecting thermally induced furan formation. J Agric Food Chem. 2008;56:9490–4. doi: 10.1021/jf801612c. [DOI] [PubMed] [Google Scholar]
- 21.Rao DS, Sekhara NC, Satyanarayana MN, Srinivasan M. Effect of curcumin on serum and liver cholesterol levels in the rat. J Nutr. 1970;100:1307–15. doi: 10.1093/jn/100.11.1307. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl Oncol. 2009;2:321–8. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang Y, Belkin R, et al. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J Clin Invest. 2001;108:1031–40. doi: 10.1172/JCI12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fotis L, Agrogiannis G, Vlachos IS, Pantopoulou A, Margoni A, Kostaki M, et al. Intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 at the early stages of atherosclerosis in a rat model. In Vivo. 2012;26:243–50. [PubMed] [Google Scholar]
- 26.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–53. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Kang Q, Chen A. Curcumin eliminates oxidized LDL roles in activating hepatic stellate cells by suppressing gene expression of lectin-like oxidized LDL receptor-1. Lab Invest. 2009;89:1275–90. doi: 10.1038/labinvest.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishino S, Mukai T, Kume N, Asano D, Ogawa M, Kuge Y, et al. Lectin-like oxidized LDL receptor-1 (LOX-1) expression is associated with atherosclerotic plaque instability – Analysis in hypercholesterolemic rabbits. Atherosclerosis. 2007;195:48–56. doi: 10.1016/j.atherosclerosis.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100:1634–42. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 30.Mango R, Biocca S, del Vecchio F, Clementi F, Sangiuolo F, Amati F, et al. In vivo and in vitro studies support that a new splicing isoform of OLR1 gene is protective against acute myocardial infarction. Circ Res. 2005;97:152–8. doi: 10.1161/01.RES.0000174563.62625.8e. [DOI] [PubMed] [Google Scholar]