Abstract
Background:
Curculigo orchioides Gaertn (Kali musli; Family: Hypoxidaceae) is an endangered medicinal plant used for many medicinal purposes such as impotency, aphrodisiac, tonic, jaundice, and skin ailments. Its hepatoprotective, antioxidant, and anti-cancerous potential have also been evaluated by many scientists.
Objective:
The objective of this study is to enhance the curculigoside content in tissue culture of C. orchioides.
Materials and Methods:
The present study deals with the enhancement of an active compound of C. orchioides by incorporating various concentration of phenylalanine (Phe), tyrosine, (20, 40, 60, and 80 mg/100 ml), chromium (Cr) and nickel (Ni) (1, 2, 3, 4, and 5 ppm) into Zenk media in controlled and aseptic conditions.
Results:
Plant secondary metabolites are unique sources for pharmaceuticals, food additives, flavors, and industrially important biochemicals. Accumulation of such metabolites often occurs in plants subjected to stresses including various elicitors or signal molecules. A significantly remarkable enhancement in all induced samples was noted. Curculigoside content was maximum in the 6-week-old tissue induced with 3 ppm of Cr (7.63%) followed by 4 weeks tissue of tissue fed with 4 ppm of Ni (5.66%) and 4-week-old tissue fed with tyrosine 7.5 mg/100 ml (2.38%) among all samples used. These results suggest that tyrosine is better enhancer than Phe in the biosynthetic pathway of curculigoside. The presence of curculigoside in all extracts was confirmed by Fourier transform infrared spectroscopy, high-performance thin layer chromatography analysis with standard compound of curculigoside and histology of treated samples.
Conclusion:
This investigation was carried out for the 1st time, and it is a significant step in understanding the biochemistry of curculigoside. The developed protocol will be beneficial for marketing in pharmaceutical industries.
SUMMARY
Curculigo orchioides Gaertn (Kali musli; Family: Hypoxidaceae) is an endangered medicinal plant used for many medicinal purposes such as impotency, aphrodisiac, tonic, jaundice, and skin ailments.
It was observed that dry matter % was maximum in 6-week-old tissue fed with 2.5 mg/100 ml of tyrosine and diminished beyond this concentration among all samples used
The nickel (Ni) and chromium (Cr) stress has enhanced the curculigoside in considerable amount in nontoxic range, in tissue culture of C. orchioides.
Curculigoside content was maximum in 6-week-old tissue induced with 3 ppm of Cr (7.63%; 11-fold enhancement) followed by 4 weeks tissue of tissue fed with 4 ppm of Ni (5.66%) and 4-week-old tissue fed with tyrosine 7.5 mg/100 ml (2.38%) among all samples used. Histological studies confirmed the enhanced production of curculigoside.
Abbreviations Used: Phe: Phenylalanine, PAL: Phenylalanine ammonia-lyase, mM: mille Molar, Cr: Chromium, Ni: Nickel, HPTLC: High-performance thin layer chromatography
Keywords: Curculigo orchioides, curculigoside, enhancement, intermediate compound, medicinal plant, metal stress, phenolics
INTRODUCTION
Curculigo orchioides Gaertn (Kali musli; Family: Hypoxidaceae) is an endangered medicinal plant used for many medicinal purposes such as impotency, aphrodisiac, tonic, jaundice, and skin ailments. Its hepatoprotective, antioxidant, and anti-cancerous potential have been evaluated by many scientists.[1] This tiny herbal plant widely distributed in China, India, Malaya, Japan, and Australia. In Chinese medicine system, the experimental plant is used for the treatment of many diseases. Its rhizomes have the properties used for warming kidney, invigorating yang, expelling cold, and eliminating dampness.[2] Previous phytochemical investigations of rhizomes (principal active organ) revealed the presence of curculigoside (Phenolic glycoside).[3] In addition, some other major chemical constituents of the experimental plant are cellulose, hemicellulose, and calcium oxalate. Its medicinal values are due to the presence of different secondary metabolites such as triterpenoid, saponins, flavones, and curculigoside. Plant tissue culture has given a new approach to exploit its technology for production of natural products. It provides a new platform to study and explore the field of transcriptomics, proteomics, and biochemistry. The experimental medicinal plant is becoming endangered due to its overexploitation, so there is a requirement to explore biotechnological strategies to increase its number as also to enhance its tuber's quality in a limited period. Some reports related to micro propagation of C. orchioides in tissue culture have been well documented.[4] However, the research regarding the bioenhancement of active principal, i.e., curculigoside (polyphenolics) is not well documented and almost untouched; hence, in the present investigation, an attempt was made to optimize the tissue culture parameters for bioenhancement in the production of chief active principal, curculigoside. The development of appropriate protocol for optimum production of curculigoside is essential for exploiting the tissue culture system and for relevant advantage. Many pharmaceutical industries are engaged with the exploitation of the active principal (curculigoside) of Kali Musli; therefore, the technology development in this area is a revolutionary step to enhance the usefulness of plant.[5] Curculigoside (C22H26O12) is identified as colorless, needle-shaped polyphenolic compound. (C22H26O11), mp 159–161°C. Infrared (IR) (KBr) max cm−1: 3370 (OH), 2922, 1724 (ester), 1598 (aromatic ring). ESI-MS m/z: 484 (M + NH4) +, 489 (M + Na).[6]
The capacity for plant cells, tissue, and organ cultures to produce and accumulate many of the valuable chemical compounds as the parent plant in nature has been recognized almost since the commencement of in vitro technology. Enhancement of polyphenolic compounds has been well documented in many medicinal plants using tissue culture strategies. Chaturvedi et al. 2014[7] have developed an economically feasible technology to optimize the production of curcumin in tissue culture of Curcuma longa. In the same way, Chaturvedi and Chowdhary, in 2013,[8] have observed the enhancement in flavonoids content of Allium cepa, Trachyspermum ammi, Tylophora indica, and Helipterum roseum separately in in vitro condition. Palacio et al., in 2013,[9] have depicted the production of phenolic compounds in in vitro and in vivo studies. Al-Amier et al., in 1999,[10] have screened different clones of lavender for their phenolic production in tissue culture system. Published documents reveal that phenylalanine (Phe), an aromatic amino acid, is the substrate of phenylalanine ammonia-lyase and catalyzes the reductive deamination of L-phe into trans-cinnamic acid as the first step of the biosynthesis of plant phenolic compound.[11] Bemani et al., in 2012,[12] found that Phe up to 3 mM had no significant effect on the growth of hazel cells and at 6 mM, it affected the dry weight adversely. In the search for alternatives for production of desirable medicinal compounds from plants, biotechnological approaches, especially plant tissue cultures, are found to have potential as a supplement to traditional agriculture in the industrial production of bioactive plant metabolites.[13] Plant cell and tissue cultures hold great promise for controlled production of useful secondary metabolites; on demand, they are responsible for its therapeutic effect and are often produced as a result of adaptation to biotic and abiotic stress. Several strategies are being followed to improve yields of secondary metabolites in plant cell cultures,[14] and DUAL culture is one of them. Dual culture system involves biomass production in a medium optimum for cell proliferation followed by transfer of healthy cells to a different medium which is favorable for product yield. This strategy was used by Zenk et al., in 1977,[15] for the production of indole alkaloids by Catharanthus roseus cells. Curculigoside is a significantly valuable bioactive principal of C. orchioides; hence, there is a need to enhance its content to improve tubers quality. In the present investigation, Zenk media was used to enhance the curculigoside in C. orchioides tissue culture. Plant tissue culture has been proven as an efficient mean to enhance the medicinally valuable compounds by manipulating the media components (Chaturvedi et al., 2011, 2011a, 2011b, 2012, 2012a, 2013a, 2014, and 2015).[16,17,18,19,20,21,22,23,24] Keeping in perspective all these facts, the experiment regarding the bioenhancement of curculigoside content in tissue culture of C. orchioides was carried out. This was attempted by feeding the tissue with various concentrations of Phe and tyrosine (Precursor and intermediate compounds) and by giving heavy metal stress (chromium [Cr] and nickel [Ni] stress) to already maintained C. orchioides cultures. An important approach toward enhanced production of curculigoside in tissue culture of C. orchioides has been established.
MATERIALS AND METHODS
Plants were initially obtained from the nursery at Dediapada village of district Dang, Gujarat. The germplasm was maintained at the Greenhouse of Loyola Centre for Research and Development (LCRD), St Xavier's College campus, Ahmedabad, Gujarat, India. C. orchioides is an endangered medicinal plant (IUCN) and grows only for 3 months of the monsoon; therefore, there is a need to grow rapidly and to increase its quality in a limited period using different strategies in tissue culture. The cultures for the present study were taken from already established cultures of C. orchioides in our Research Centre LCRD. Described study depicts the yield enhancement of curculigoside in tissue culture of C. orchioides. The study covers the incorporation of intermediate compounds (in various concentrations) into Zenk media with 20, 40, 60, and 80 mg/100 ml of Phe and 2.5, 5, 7.5, and 10 mg/100 ml of tyrosine separately. The other experiment regarding the addition of various concentrations of Cr and Ni (1, 2, 3, 4, and 5 ppm) separately into Zenk (Zenk 1975) basal media was also carried out, to see its effect on the production of active principle. Five percent sucrose and 0.8% agar were used and 5.6 pH was set before auto cleaving. For stress experiments, potassium dichromate and sodium sulfate were used for preparing stock solutions. After aseptic inoculation, the cultures were incubated for 2, 4, and 6 weeks in 16 h (1000 lux of light) of photoperiod and 8 h of dark period with 70% humidity and 25–27°C temperature. All samples were harvested at the time interval of 2, 4, and 6 weeks separately, dried, and weighed and dry matter % was calculated [Table 1]. The dried powder of different samples was subjected for cold extraction with methanol at room temperature. After 36 h, the extracts were filtered, dried in vacuo, and weighed separately. All extracts were subjected to high-performance thin layer chromatography (HPTLC) analysis and IR spectral studies with standard reference compound of curculigoside.
Table 1.
Effect of nickel and chromium stress on dry matter % in Curculigo orchioides tissue culture
High-performance thin layer chromatography analysis
HPTLC (CAMAG) of different samples was carried out with standard compound of curculigoside. Mobile phase was ethyl acetate: ethanol: water (5:1:5). The scanning was done at 285 nm. Rf = 0.3 as noted. We also have used the other solvent system such as ethyl acetate: n-butanol: water (2:3:5). n-butanol: water (1:1), ethyl acetate: methanol: water (4:1:4), but the separation was observed better in ethyl acetate: ethanol: water (5:1:5). Detection was done by spraying developed plates with reagent mixture (1:1) of 0.02% potassium ferrocyanide and 0.02% Fecl3 solution. Dark blue color was observed. The quantitative estimation was done using peak area mentioned in the given results of HPTLC graphs.
Infrared spectral studies
IR spectral studies of all used samples were carried out with standard compound of curculigoside, using equipment Buck scientific 500.
Histological studies
Histological studies of in vitro treated samples of C. orchioides were carried out by cross sectioning. The samples were stained with safranin previously and afterward spraying reagent of curculigoside (mentioned above).[25] The sections were observed in microscope in ×40 power [Figure 1].
Figure 1.
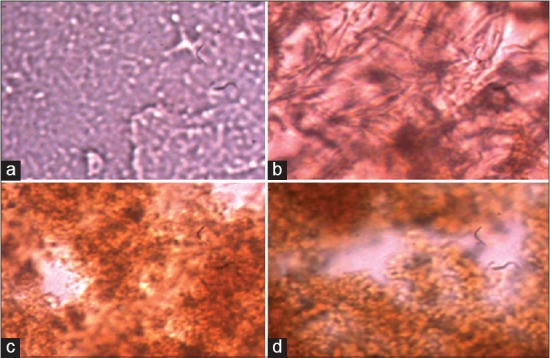
Histological studies of treated tissue of Curculigo orchioides static culture with intermediate and stress giving compounds. (a) Control, (b) tyrosine 7.5 mg/100 ml (4-week-old tissue), (c) nickel 4 ppm (4-week-old tissue), and (d) chromium 3 ppm (6-week-old tissue). The phenolic compound can be determined in the form of black spots. It is observed that black spots are increased according to the enhancement of curculigoside in various treated samples
Statistical analysis
Statistical analysis of obtained data (triplicate) was performed using Microsoft Excel and standard deviation was obtained.
RESULTS AND DISCUSSIONS
Plant secondary metabolites are unique sources for pharmaceuticals, food additives, flavors, and industrially important biochemicals. Accumulation of such metabolites often occurs in plants subjected to stresses including various elicitors or signal molecules. Secondary metabolites play a major role in the adaptation of plants to the environment and in overcoming stress conditions. Environmental factors, namely, temperature, humidity, light intensity, the supply of water, minerals, and CO2 influence the growth of a plant and secondary metabolite production. Drought, high salinity, and freezing temperatures are environmental conditions that cause adverse effects on the growth of plants and the productivity of crops.[26]
The stimulation of phenolic compound biosynthesis was noted in wheat in response to Ni toxicity. Insoluble and soluble phenolic were produced in response to Cd2+.[27] One of the most important groups of secondary metabolites is phenolic compound. They are characterized by at least one aromatic ring having one or more hydroxyl groups and are mainly synthesized from cinnamic acid, which is formed from Phe by the action of L-phenylalanine ammonia-lyase. Plants accumulate ultraviolet (UV)-absorbing flavonoids and other phenolic compounds mostly in vacuoles of epidermal cells, to prevent the penetration of UV-B into the deeper tissues of the plant.[28]
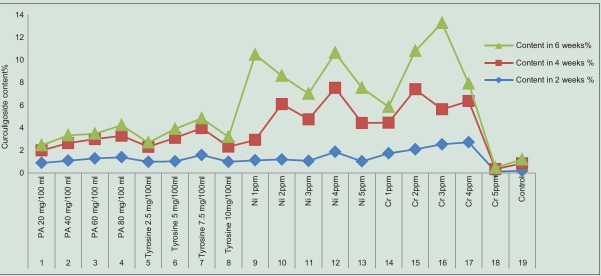
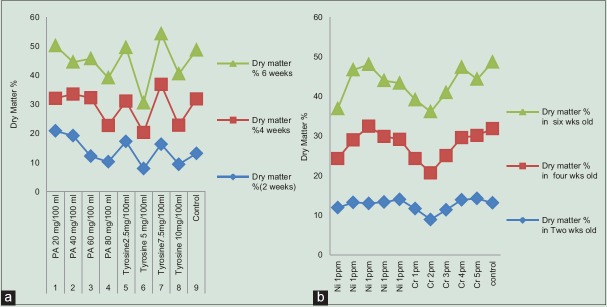
In the present study, we have given treatment of intermediate compounds such as Phe (20, 40, 60, and 80 mg/100 ml) and tyrosine (2.5, 5, 7.5, and 10 mg/100 ml) in various concentrations and also Cr and Ni stress to C. orchioides. It was observed that dry matter % was maximum in 6-week-old tissue fed with 2.5 mg/100 ml of tyrosine and diminished beyond this concentration [Table 2]. Among all samples used, this may be due to lethal effect of higher concentrations of these compounds on growth of tissue. Curculigoside content was maximum in 6-week-old tissue induced with 3 ppm of Cr (7.63%; 11-fold enhancement) followed by 4 weeks tissue of tissue fed with 4 ppm of Ni (5.66%) and 4-week-old tissue fed with tyrosine 7.5 mg/100 ml (2.38%) among all samples used. These results suggest that tyrosine is better precursor than Phe in the biosynthetic pathway of curculigoside [Table 3]. The presence of curculigoside in all extracts was confirmed by histological studies [Figure 1], IR, HPTLC analysis with standard compound of curculigoside [Figures 2 and 3].
Table 2.
The dry matter % of treated samples with various concentrations of precursor compounds in Curculigo orchioides
Table 3.
The effect of precursor compounds and heavy metal stress on production of curculigoside in tissue culture of Curculigo orchioides
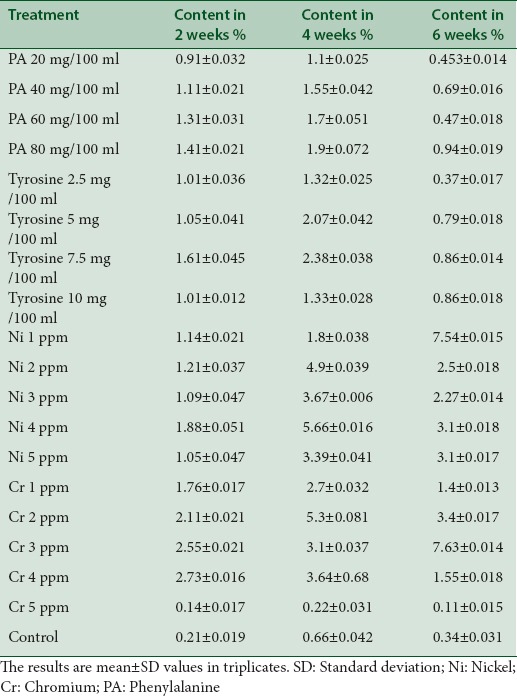
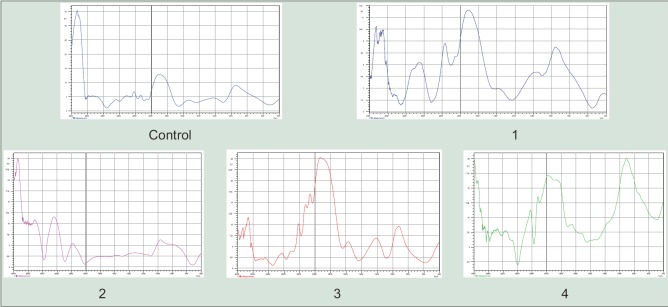
Figure 2.
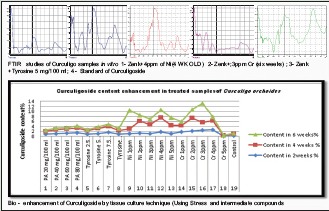
Infrared studies of curculigo samples in vitro 1-Zenk + 4 ppm of nickel (4-week-old) 2- Zenk +; 3 ppm chromium (6 weeks); 3-Zenk + tyrosine 5 mg/100 ml; 4. standard of curculigoside. Graphs showing the similar pattern of peaks in all samples and comparable to standard compound of Curculigo orchioides
Figure 3.
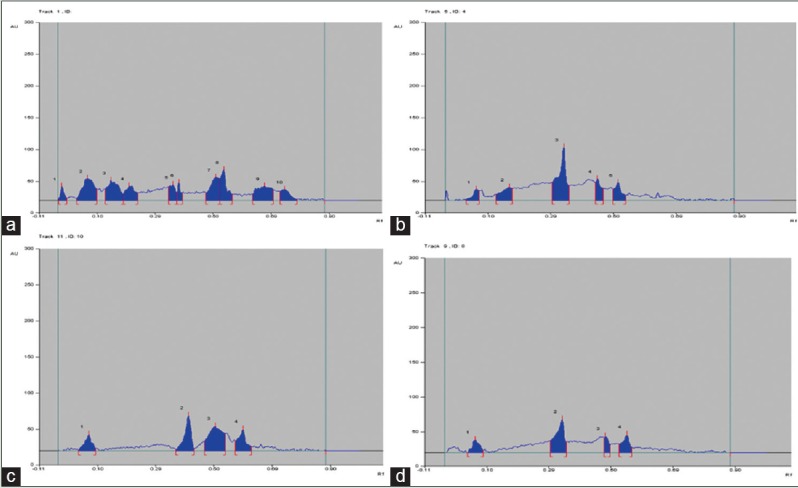
High-performance thin layer chromatography (a) standard, (b) 4-week-old tissue fed with 4 ppm of (nickel), (c) 6-week-old fed with 3 ppm of chromium, and (d) 4-week-old fed with 7.5 MG/100 ml tyrosine. The curculigoside content in all samples were calculated using their peak area
The cytological studies of cross section of treated samples were carried out [Figure 1]. The stained sections were showing the black spot (due to derivatization) in ×40 power, which were seen maximum in 6-week-old tissue fed with 3 ppm/100 ml of Cr followed by 4-week-old tissue fed with 4 ppm of Ni/100 ml and 4-week-old tissue fed with 7.5 mg/100 ml tyrosine in ×40 in microscope [Table 3 and Figure 1].
Infrared spectral studies
IR spectral studies of all extracts with standard compound of curculigoside showing the presence of curculigoside are shown in Table 4.
Table 4.
Infrared spectral studies showing the characteristic peaks of treated samples, corresponded with standard compound of curculigoside

Melato et al., in 2012,[29] have investigated the positive effect of metal stress (Ni and Cr) on production of phenolics in Vetiver grass. Plants may undergo significant morphological and metabolic changes in response to metal uptake. Many of these changes are believed to be adaptive responses to metal stress.[30] Additional consequences of phytotoxicity are enhanced production of reactive oxygen species and oxidative damage of important macromolecules including DNA, protein, lipids, chloroplast pigments, and enzymes.[31] However, enhancement of their metabolism was observed under different environmental factors and stress conditions.[32] An increase in phenolic content is correlated with the increase in enzymatic activity involved in phenolic metabolism,[33] suggesting synthesis of phenolic under metal stress and supported previous findings.
In sensitive species (for example, barley, water spinach, and wheat), chlorosis and necrosis of leaves can appear after plants are treated with Ni at very low concentrations (0.2 mM or 11.74 ppm) for less than a week.[34] Cr compounds are highly toxic to plants and are detrimental to their growth and development. Cr is toxic to most of higher plants at 100 μmol/kg (29.4 ppm) dry weight.[35] Hence, Ni and Cr concentrations that we have used in the present investigation are in nontoxic range for in vitro studies to enhance curculigoside in C. orchioides. Lu et al., 2002[36] have reported a range of the curculigoside content in 6 different samples. They reported that range varied from 0.11% to 0.35%. We have reported 0.2% curculigoside content in our findings, which supports the previous results [Table 3 and Figures 1–5].
Figure 5.
Graphical presentation of bioenhancement of curculigoside, a medicinally valuable phenolic compound by incorporation of intermediate compounds and heavy metal (nickel and chromium) into Zenk medium
Figure 4.
Effect of intermediate compounds (a) and heavy metal stress ((b) chromium and nickel) on dry matter % in Curculigo orchioides tissue culture reveals the higher concentration of treatment is harmful for growth of the tissues
CONCLUSION
The described study is showing the optimization of static media for production of curculigoside in tissue culture of C. orchioides. The Ni and Cr stress have enhanced the curculigoside in considerable amount in nontoxic range, in tissue culture of C. orchioides. Intermediate compounds of phenolics play a major role in the production of curculigoside. The results suggest that tyrosine is better precursor compound than Phe. Static tissue culture of Curculigo orchioides is suitable system to study biosynthetic pathway of phenolic active compound. The developed technology will be useful for pharmaceutical industries. All results support the previous findings.
Financial support and sponsorship
Authors are grateful for giving financial support to LCRD, Xavier's Research Foundation, St Xavier's college campus Ahmadabad
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Pratibha Chaturvedi
Pratibha Chaturvedi, obtained her Ph.D from Rajasthan University. Her main interests in research field are Plant Tissue Culture, Pharmacognosy, and Natural Products. She is working as the Senior Scientist in Loyola Center for Research and Development, Ahmadabad, Gujarat, India. She is positioned as National Professor and Visiting Scientist, Haffkine Institute for Training, Research and Testing, Mumbai, India. She has many Publications and Books from National and International reputed publishers.
Acknowledgment
We are grateful to Xavier's Research Foundation for giving financial support.
REFERENCES
- 1.Soni N, Lal VK, Agrawal S, Verma H. Golden eye grass-a magical remedy by nature. Int J Pharm Sci Res. 2012;3:2407–20. [Google Scholar]
- 2.Liu GW. Beijing: HuaXia Publishing House; 2001. Chinese Herbal Medicine; p. 99. [Google Scholar]
- 3.Li N, Zhao YX, Jia AQ, Liu YQ, Zhou J. Study on the chemical constituents of Curculigo orchioides. Nat Prod Res Dev. 2003;15:208–11. [Google Scholar]
- 4.Babaei N, Abdullah NA, Saleh G, Abdullah TL. An efficient in vitro plantlet regeneration from shoot tip cultures of Curculigo latifolia, a medicinal plant. ScientificWorldJournal 2014. 2014:275028. doi: 10.1155/2014/275028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li N, Zhao YX, Jia A, Liu YQ, Zhou J. Study on the chemical constituents of Curculigo orchioides. Nat Prod Res Dev. 2003;15:208–11. [Google Scholar]
- 6.Fu DX, Lei GQ, Cheng XW, Chen JK, Zhou TS. Curculigoside C, a new phenolic glucoside from rhizomes of Curculigo orchioides. Acta Bot Sin. 2004;46:621–4. [Google Scholar]
- 7.Chaturvedi P, Mukherjee S, Mehta S, Chatterjee P, Chowdhary A. Media standardization for enhancement the content of curcumin in Curcuma longa (zingiberaceae) and protein profile of treated samples in static culture. World J Pharm Pharm. 2014;10:965–75. [Google Scholar]
- 8.Chaturvedi P, Chowdhary A. Enhancement of antioxidant compound in Tylophora indica callus. Adv Appl Sci. 2013;4:325–30. [Google Scholar]
- 9.Palacio L, Cantero JJ, Cusidó RM, Goleniowski ME. Phenolic compound production in relation to differentiation in cell and tissue cultures of Larrea divaricata (Cav.) Plant Sci. 2012;193-194:1–7. doi: 10.1016/j.plantsci.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Al-Amier H, Mansour BM, Toaima N, Korus RA, Shetty K. Tissue culture based screening for selection of high biomass and phenolic producing clonal lines of lavender using pseudomonas and azetidine-2-carboxylate. J Agric Food Chem. 1999;47:2937–43. doi: 10.1021/jf9813889. [DOI] [PubMed] [Google Scholar]
- 11.Kubota NH, Yakushiji N, Nishiyama H, Shimamura K. Phenolic contents and l-phenylalanine ammonia-lyase activity in peach fruit as affected by rootstocks. J Jpn Soc Hort Sci. 2001;70:151–6. [Google Scholar]
- 12.Bemani E, Ghanati F, Rezaei A, Jamshidi M. Effect of phenylalanine on Taxol production and antioxidant activity of extracts of suspension-cultured hazel (Corylus avellana L.) cells. J Nat Med. 2013;67:446–51. doi: 10.1007/s11418-012-0696-1. [DOI] [PubMed] [Google Scholar]
- 13.Rao SR, Ravishankar GA. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol Adv. 2002;20:101–53. doi: 10.1016/s0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 14.Tabata M. Plant Cell Cultures. In: Vasil IK, editor. Cell Culture and Somatic Cell Genetics of Plants. 5. San Diego: Academic Press; 1988. pp. 5–99. [Google Scholar]
- 15.Zenk MH, el-Shagi H, Schulte U. Anthraquinone production by cell suspension cultures of Morinda citrifolia. Planta Med. 1975;Suppl 75:79–81. doi: 10.1055/s-0028-1104768. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi P, Chowdhary A. Enhancement of tylophorin in Tylophora indica callus. J Phytological Res. 2011;24:9–13. [Google Scholar]
- 17.Chaturvedi P, Chowdhary A. Strategies for enhancement of tylophorin in Tylophora indica in vitro. J Phytological Res. 2011;24:191–5. [Google Scholar]
- 18.Chaturvedi P, Chowdhary A, Roy S. Evaluation of anti-influenza activity of Tylophora indica leaves. J Phytological Res. 2011;24:155–9. [Google Scholar]
- 19.Chaturvedi P, Sawant S, Talekar J, Chowhary A. Effect of different sugars on stigmasterol production in callus of Tylophora indica. Int J Pharmacol Phytochem. 2012;2:226–8. [Google Scholar]
- 20.Chaturvedi P, Chowdhary A. Enhancement of antioxidant compound in Tylophora indica callus. Adv Appl Sci Res. 2012;4:325–30. [Google Scholar]
- 21.Chaturvedi P, Khanna P, Chowdhary A. Germany: Lambert Publications; 2012a. In vitro Production of Secondary Metabolites from Some Medicinal Plants. [Google Scholar]
- 22.Chaturvedi P, Khanna P, Chowdhary A. Isolation, identification and characterization rotenoids from Cajanus cajan seeds. Indian Drugs. 2013a;50:15–7. [Google Scholar]
- 23.Chaturvedi P, Chowdhary A. Scientific Study Germany: GRIN Verlag GmbH München; 2014. Tylophora indica: Phytochemical, Biotechnological and Pharmacological Approach a Wide Spectrum Study, Scientific Study. [Google Scholar]
- 24.Chaturvedi P, Chowdhary A. US: OMICS Publications; 2015. Approaches for Antivirals Production in Tissue Culture of Medicinal Plants. [Google Scholar]
- 25.Nema RK, Ramawat KG. Isolation and identification of a new molecule from Curculigo orchioides (Hypoxidaceae) J Chem Pharm Res. 2010;2:610–7. [Google Scholar]
- 26.Ramakrishna A, Ravishankar GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6:1720–31. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schützendübel A, Polle A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot. 2002;53:1351–65. [PubMed] [Google Scholar]
- 28.Kondo N, Kawashima M. Enhancement of tolerance to oxidative stress in cucumber (Cucumis sativus L.) Seedlings by UV-B irradiation: Possible involvement of phenolic compounds and antioxidant enzymes. J Plant Res. 2000;113:311. [Google Scholar]
- 29.Melato FA, Regnier T, McCrindle RI, Mokgalaka NS. Impact of metals on secondary metabolites production and plant morphology in Vetiver grass (Chrysopogon zizanioides) 178 S. Afr J Chem. 2012;65:178–83. [Google Scholar]
- 30.Sharma H. Metal hyperaccumulation in plants: A review. J Environ Sci Technol. 2011;4:118–38. [Google Scholar]
- 31.Schutzendubel A, Polle A. Plant responses to abiotic stresses: Heavy. J Exp Bot. 2002;53:1351–65. [PubMed] [Google Scholar]
- 32.Górecka K, Cvikrová M, Kowalska U, Eder J, Szafranska K, Górecki R, et al. The impact of Cu treatment on phenolic and polyamine levels in plant material regenerated from embryos obtained in anther culture of carrot. Plant Physiol Biochem. 2007;45:54–61. doi: 10.1016/j.plaphy.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Michalak A. Comparative assessment on in vitro antioxidant activities of ethanol extracts of Averrhoa bilimbi, Gymnema sylvestre and Capsicum frutescens. Pol J Environ Stud. 2006;15:523–30. doi: 10.4103/0974-8490.122915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun EJ, Wu FY. Along-vein necrosis as indicator symptom on water spinach caused by nickel in water culture. Bot Bull Acad Sin. 1998;39:255–9. [Google Scholar]
- 35.Diáz J, Bernal A, Pomar F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annum L.) Seedlings in response to copper stress and its relation to lignification. Plant Sci. 2001;161:179. [Google Scholar]
- 36.Lu HW, Zhu BH, Liang YK. Determination of curculigoside in crude medicine Curculigo orchioides by HPLC. Zhongguo Zhong Yao Za Zhi. 2002;27:192–4. [PubMed] [Google Scholar]