Abstract
The galls of Terminala chebula (Gaertn.) Retz. (Combretaceae) are used for the treatment of various diseases in folk medicine and has been found to posses anti-inflammatory, anti-bacterial, anti-helmintic, anti-tyrosinase, and anti-aging activities. Considering the ethano-botanical and diverse pharmacological applications of galls of T. chebula, in this study, we investigate the possible toxic effects of different gall extracts of T. chebula by Brine shrimp (Artemia salina) toxicity assay. The cytotoxicity test of leaf gall extracts (petroleum ether, chloroform, ethanol, and aqueous) of T. chebula was evaluated by Brine shrimp (A. salina) toxicity assay, which is based on the ability to kill laboratory cultured Artemia nauplii (animals eggs) and also total content of polyphenols, flavonoids with other qualitative phytochemical analysis of the extract were determined. It was observed that the petroleum ether extract was virtually nontoxic on the shrimps, and exhibited very low toxicity with LC50 value of 4356.76 μg/ml. Furthermore, the chloroform extract exhibited very low toxicity, giving LC50 value of 1462.2 μg/ml. On the other hand, the ethanol extract was very toxic to brine shrimps with LC50 value of 68.64 μg/ml. The ethanol extract had the highest total phenolic and flavonoid content of 136 ± 1.5 mg of gallic acid equivalent/g d.w and 113 ± 1.6 mg of quercetin equivalent/g d.w, respectively. The higher toxicity effect was positively correlated to the high content of total polyphenols/flavonoids in the extract. This significant lethality of different extracts to brine shrimp is an indicative of the presence of potent cytotoxic components which warrants further investigation.
SUMMARY
The present study investigates the toxicity effect of different extracts of galls of T. chebulla, which would serve as an index for formulation of drugs for treatment of various diseases. Presumably, these activities could be attributed in part to the polyphenolic features of the extract, as there was a strong correlation of higher toxic effect with that of high total phenolic and flavonoids content in the ethanolic leaf gall extracts of T. chebula.
Keywords: Assay, brine shrimp assay, cytotoxic, drug, extract, galls, toxicity
INTRODUCTION
Terminala chebula (Gaertn.) Retz. (Combretaceae), is an important medicinal plant native to tropical regions of southern Asia viz., India, Nepal, China, Sri Lanka, Malaysia, Vietnam. It is commonly known as black myrobalan and haritaki, and amply referred to as “King of medicines;” as it has been the component of many formulations for the treatment of various diseases in all the streams of Indian system of medicines such as Ayurveda, Siddha, Unani, and Homeopathy.[1,2] It consists of gall-like excrescences formed by plant insect Dixothrips onerosus (Thysanoptera) on the leaves, petioles, and branches.[3] These galls are commonly known as Karkatshringi, which is an important ayurvedic drug used in preparations of Dasamularista, Cyavanaprasa, and Shringyadi curna and used in the treatment of diseases such as swasa (asthma), yakshma (tuberculosis), ajeerna (indigestion), hydroga (heart diseases), jwara (fevers), and yakrt roga (liver disorders) to mention a few.[1,2] Karkatshringi also finds usage in the treatment of children's ear infections, suppress hemorrhage from gums and also used to suppress bleeding from nose.[4] Hakims consider galls as useful in pulmonary infections, diarrhea, and vomiting.[5] Although the accepted source of Karkatasringi is the galls of Rhus succedanea L., however Pistacia integerrima and T. chebula are also generally used in preparations.[2,6] Gall extracts of T. chebula have been found to posses anti-inflammatory, anti-bacterial, anti-helmintic, anti-tyrosinase, and anti-aging activities.[7,8,9,10,11,12,13] Considering the ethno-botanical and various pharmacological applications of galls of T. chebula, the toxicity aspect of it has to be verified. Since until date, there are no scientific literature in these lines, the aim of this study was to investigate the possible toxic effects of gall extracts of T. chebula by brine shrimp (Artemia salina) toxicity assay, which is based on the ability to kill laboratory cultured Artemia nauplii (animals eggs).[14]
MATERIALS AND METHODS
Chemicals
Potassium dichromate was purchased from SRL Chemicals, India. All other chemical reagents and solvents of analytical grade were purchased from SRL Chemicals, India.
Plant material
The gall induced leaves of T. chebula were purchased from local market of Bengaluru, India. The plant materials were certified and authenticated by Dr. S. Sundara Rajan and the voucher specimen (JU-RUV-52) were deposited at Research centre of vrkshayurveda, Jain University, Bangalore. Further letter of authentication of the plant material was provided by vrkshayurveda centre, Jain University dated May 24, 2014. The galls were cleaned with distilled water, dried and crushed into fine powder by using electric grinder.
Preparation of extracts
The coarsely powdered gall materials were sequentially extracted with ethanol, petroleum ether, chloroform and aqueous solvents in Soxhlet apparatus for 24 h. The extracts were evaporated to dryness under reduced pressure using a Rotavapor (BuchiFlawil, Switzerland) and a portion of the residue was used for the Brine Shrimp Toxicity assays.
Phytochemical analysis
The preliminary qualitative phytochemical analyses of carbohydrates, saponins, alkaloids, flavonoids, fixed oils and fats, phenolic and tannins, glycosides, phytosterols and triterpenoids in the extracts were carried out using the standard methods as described.[13]
Quantitative analysis
Determination of total phenolic content
The total phenolics were determined in the T. chebula leaf gall extracts (ethanol, petroleum ether, chloroform and aqueous) using Folin - Ciocalteau reagent method, employing Gallic acid as standard.[15] Briefly, 200 ml of both methanol and aqueous extracts (2 mg/ml) were made up to 3 ml with distilled water, then mixed thoroughly with 0.5 ml of Folin - Ciocalteu reagent. After mixing for 3 min, 2 ml of 20% (w/v) sodium carbonate was added and allowed to stand for a further 60 min in the dark. The absorbance of the reaction mixtures was measured at 650 nm, and the results were expressed as mg of gallic acid equivalent (GAE)/g of dry weight.
Determination of total flavonoid content
Total flavonoid content of the extracts (ethanol, petroleum ether, chloroform and aqueous) was determined using the aluminum chloride colorimetric method as described by Chang et al.[16] In brief, 50 μl of methanol and aqueous extracts (2 mg/ml) were made up to 1 ml with methanol then mixed with 4 ml of distilled water and subsequently with 0.3 ml of 5% NaNO2 solution. After 5 min of incubation, 0.3 ml of 10% AlCl3 solution was added and then allowed to stand for 6 min, followed by adding 2 ml of 1 M NaOH solution to the mixture. Then water was added to the mixture to bring the final volume to 10 ml and the mixture was allowed to stand for 15 min. The absorbance was measured at 510 nm. Total flavonoid content was calculated as quercetin from a calibration curve. The calibration curve was prepared by preparing quercetin solutions at concentrations 12.5–100 mg/ml in methanol. The result was expressed as mg quercetin equivalent (QUE)/g of dry weight.
Brine shrimp lethality bioassay (cytotoxicity)
The cytotoxicity test of leaf gall extracts (petroleum ether, chloroform, ethanol, and aqueous) of T. chebula was evaluated using the standard procedure as described by Meyer et al.,[17] McLaughlin,[18] and Logarto Parra et al.[19] The test was conducted by using brine shrimp (A. salina). Brine shrimp eggs (A. salina Leach) were hatched in a hatching chamber, filled with fresh sea water. Plant extracts are tested at a concentration of 10, 100 and 1000 μg/ml in the multi-welled culture plates containing brine and 15 shrimps in each replicate. They are then incubated at a temperature of 25°C for 24 h. Potassium dichromate was used as reference standard. Survivors were counted after 24 h, and the percentage death at each concentration was determined.[17,18,19,20,21] The LC50 value at 95% confidence interval was determined from the count using the statistical method of probit analysis.[19,20,21]
Statistical analysis
Statistical analysis was performed using SPSS (Windows version 10.0.1; SPSS Inc., Chicago, IL, USA) using a one-way Student's t-test; P < 0.05 was considered as statistically significant, when comparing with relevant controls. All results refer to mean ± standard deviation.
RESULTS AND DISCUSSION
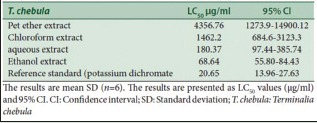
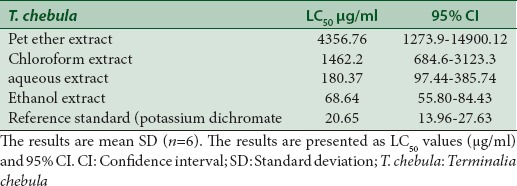
The galls of T. chebula have been used in many ayurvedic formulations in the name of Karkatasringi for treating various diseases.[1,2,13] These studies have revealed its potential to provide novel drug candidates for various diseases, however its toxic effect if any have not been evaluated until date. Henceforth, the galls of T. chebula was analysed for its cytotoxicity by brine shrimp assay. The brine shrimp lethality assay represents a rapid, inexpensive and simple bioassay for testing plant extracts bioactivity, which in most cases correlates reasonably well with cytotoxic properties. Brine shrimp assay results are presented in Table 1, which shows that the petroleum ether extract was virtually nontoxic on the shrimps, and exhibited very low toxicity with LC50 value of 4356.76 μg/ml. Furthermore, the chloroform extract exhibited very low toxicity, giving LC50 value of 1462.2 μg/ml. On the other hand, the ethanol extract was very toxic to brine shrimps with LC50 value of 68.64 μg/ml. The aqueous extract exhibited the moderate toxicity with an LC50 of 180.37 μg/ml. Further, it was found that the degree of lethality was found to be directly proportional to the concentration of the extract used.
Table 1.
Brine shrimp lethality test of Terminalia chebula leaf gall extracts
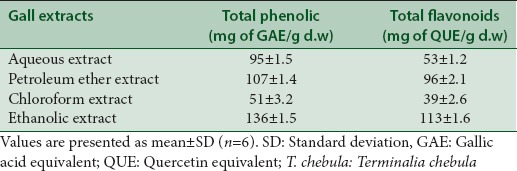
In the preliminary phytochemical studies, the qualitative presence of phenolics, flavonoids, triterpenes, saponins, glycosides, phytosterols, reducing sugars were identified in the extracts [Table 2]. The total amount of phenolic and flavonoid content of extracts of leaf galls of T. chebula is presented in [Table 3]. The results obtained indicate that in comparison with all the exact, the ethanol extract had the highest total phenolic and flavonoid content of 136 ± 1.5 mg of GAE/g d.w and 113 ± 1.6 mg of QUE/g d.w, respectively. It was observed that there was a correlation between the total phenolics and flavonoids content in the extract and toxicity exhibited by different extracts [Table 3], thus the higher toxicity exhibited by ethanolic extract of T. chebula might be related to the significantly high polyphenolic and flavonoids content. The leaf gall of T. chebula are reported to be very rich in tannins, triterpenoids, flavonoids, essential oils, and others phenolic constituents.[13] The results also demonstrates that the ethanol extract possessed significant activity in releasing most of the secondary metabolites from leave galls of T. chebula. This may be due to the fact that phenolic and flavonoid compounds are often extracted in higher amounts by using polar solvents such as aqueous methanol/ethanol.[13,22,23] It is reported that differences in the polarity of the extracting solvents could result in a wide variation in the polyphenolic and flavonoid contents of the extract.[13,22,23] Some of the phyto-constituents present in the gall extracts such as alkaloids, tannins, and phenols may be accountable to have this significant cytotoxic activity. The significant lethality of different extracts to brine shrimp is an indicative of the presence of potent cytotoxic components which warrants further investigation.
Table 2.
Preliminary phytochemical analysis of leaf gall extracts of Terminalia chebula
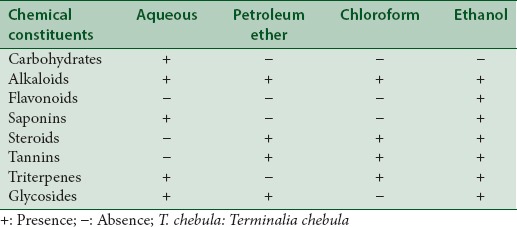
Table 3.
Total phenolic and total flavonoid content of Terminalia chebula leaf gall extracts
CONCLUSION
The results of this study shows the toxicity effect of different extracts of galls of T. chebulla, which would serve as an index for formulation of drugs for treatment of various diseases. Presumably, these activities could be attributed in part to the polyphenolic features of the extract, as there was a strong correlation of higher toxic effect with that of high total phenolic and flavonoid content in the ethanolic leaf gall extracts of T. chebula. Our study shows that although the brine shrimp lethality assay is rather inadequate regarding the elucidation of the mechanism of action, it is very useful to assess the bioactivity of the plant extracts. In the course of our studies, the brine shrimp lethality assay actually has proven to be a convenient system for monitoring biological activities of the plants that are used in the Indian traditional medicine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge Dr. R. Chenraj Jain, President, Jain University Trust., Dr. N Sundararajan, Vice Chancellor, Jain University and Prof. K.S. Shantamani, Chief Mentor, Jain University, Bangalore for their kind support and encouragement and for providing facilities for carrying out this work. DBL thank Jain University for the constant encouragement provided to proceed in research activities.
REFERENCES
- 1.Vol. 8. New Delhi: CSIR; 1969. Anonymous. The Wealth of India; p. 121. [Google Scholar]
- 2.1st ed. Delhi: Government of India; 1978. Anonymous. The Ayurvedic Formulary of India. Part. I; pp. 1–324. [Google Scholar]
- 3.Santha TR, Shetty JK, Yoganarasimhan SN, Sudha R. Farmacognostical studies on the South Indian market sample of karkatasringi (kadukkaipoo) – Terminalia chebul (Gaertn.Leaf gall) Anc Sci Life. 1991;11:16–22. [PMC free article] [PubMed] [Google Scholar]
- 4.Sukh D. Ethano therapeutics and modern drug development. The potential of Ayurveda. Curr Sci. 1997;73:909–28. [Google Scholar]
- 5.Nadkarni KM. 3rd ed. India: Popular Prakashan; 1976. Indian Materia Medica; pp. 1062–3. [Google Scholar]
- 6.New Delhi: Government of India; 1978b. Anonymous. The Siddha Formulary of India. [Google Scholar]
- 7.Vonshak A, Barazani O, Sathiyamoorthy P, Shalev R, Vardy D, Golan-Goldhirsh A. Screening South Indian medicinal plants for antifungal activity against cutaneous pathogens. Phytother Res. 2003;17:1123–5. doi: 10.1002/ptr.1399. [DOI] [PubMed] [Google Scholar]
- 8.Manosroi A, Jantrawut P, Akazawa H, Akihisa T, Manosroi J. Biological activities of phenolic compounds isolated from galls of Terminalia chebula Retz. (Combretaceae) Nat Prod Res. 2010;24:1915–26. doi: 10.1080/14786419.2010.488631. [DOI] [PubMed] [Google Scholar]
- 9.Upadhye AS, Rajopadhye AA. Pharmacognostic and phytochemical evaluation of leaf galls of Kakadshringi used in Indian system of medicine. J Sci Ind Res. 2010;69:700–4. [Google Scholar]
- 10.Manosroi A, Jantrawut P, Akihisa T, Manosroi W, Manosroi J. In vitro and in vivo skin anti-aging evaluation of gel containing niosomes loaded with a semi-purified fraction containing gallic acid from Terminalia chebula galls. Pharm Biol. 2011;49:1190–203. doi: 10.3109/13880209.2011.576347. [DOI] [PubMed] [Google Scholar]
- 11.Shankara BE, Ramachandra YL, Sundara Rajan S, Preetham J, Sujan Ganapathy PS. In vitro anti-bacterial activity of Terminalia chebula leaf gall extracts against some human pathogenic strains. Int Curr Pharm J. 2012;1:217–20. [Google Scholar]
- 12.Manosroi A, Jantrawut P, Ogihara E, Yamamoto A, Fukatsu M, Yasukawa K, et al. Biological activities of phenolic compounds and triterpenoids from the galls of Terminalia chebula. Chem Biodivers. 2013;10:1448–63. doi: 10.1002/cbdv.201300149. [DOI] [PubMed] [Google Scholar]
- 13.Ravi Shankara BE, Ramachandra YL, Sundara Rajan S, Richard SA, Dhananjaya BL. Evaluating the anthelmintic potential of leaf gall extracts of Terminelia chebula (Gaertn.) Retz. (Combretaceae) J Young Pharm. 2014;6:1–4. [Google Scholar]
- 14.Carballo JL, Hernández-Inda ZL, Pérez P, García-Grávalos MD. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002;2:17. doi: 10.1186/1472-6750-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37:153–61. [Google Scholar]
- 16.Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 17.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 18.McLaughlin JL. Assays for bioactivity. In: Hostettmann K, editor. Methods in Plant Biochemistry. Vol. 6. London: Academic Press; 1991. pp. 1–33. [Google Scholar]
- 19.Logarto Parra A, Silva Yhebra R, Guerra Sardiñas I, Iglesias Buela L. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8:395–400. doi: 10.1078/0944-7113-00044. [DOI] [PubMed] [Google Scholar]
- 20.Finney DJ. 3rd ed. Cambridge: Cambridge University Press; 1971. Probit Analysis. [Google Scholar]
- 21.Sauders L, Fleming R. 2nd ed. London: The Pharmaceutical Press; 1971. Mathematics Statistics for Use in the Biological and Pharmaceutical Sciences; pp. 225–86. [Google Scholar]
- 22.Eshwarappa RS, Iyer S, Subaramaihha SR, Richard SA, Dhananjaya BL. Antioxidant activities of Ficus glomerata (Moraceae) leaf gall extracts. Pharmacognosy Res. 2015;7:114–20. doi: 10.4103/0974-8490.147225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshwarappa RS, Ramachandra YL, Subaramaihha SR, Subbaiah SG, Austin RS, Dhananjaya BL. Antioxidant activities of leaf galls extracts of Terminalia chebula (Gaertn.) Retz. (Combretaceae) Acta Sci Pol Technol Aliment. 2015;14:97–105. doi: 10.17306/J.AFS.2015.2.11. [DOI] [PubMed] [Google Scholar]


