Abstract
Plants have been an important source for discovery of anticancer compounds. With the current decline in the number of new molecular entities from the pharmaceutical industry, novel anticancer agents are being sought from traditional medicines; therefore the anticancer efficacy of many plants that are used in traditional medicine is yet to be verified. The objective of the study was to evaluate the cytotoxic potential of ethanolic leaf gall extract of Terminalia chebula are evaluated against buffalo rat liver 3A, MCF-7 (Human mammary gland adenocarcinoma) and A-549 (Human lung cancer) cell lines. The cytotoxic effect of the ethanolic extract was evaluated by MTT assay. The extract was potent and effective in inducing cytotoxic effects in all the cell lines with an IC50 value of 305.18 ± 1.7 μg/mL, 643.13 ± 4.2 μg/mL, and 208.16 ± 3.7 μ/mL, respectively. The extract was more effective against A549 cell lines when compared to others. The presences of phenolics, triterpenoids, and flavonoids were identified in the extract. The extract showed total phenolic and flavonoid content of 478 ± 2.2 mg of gallic acid equivalent/g d.w and 538 ± 1.4 mg of quercetinequivalent/g d.w, respectively. This higher content of total phenolics and flavonoids found in the ethanolic extract was directly associated to higher cytotoxicity activity.
Conclusion:
The ethanolic leaf gall extract of T. chebula showed effective cytotoxic activities; which might be attributed to the phenolics/flavonoids present in higher concentration. Future work will be interesting to know the chemical composition of the extract and also better understand the mechanism of action of the constituents present in the extract to develop it as drug for therapeutic application.
SUMMARY
The present investigation establishes the anticancer activities of T. chebula leaf gall extracts on BRL3A, MCF-7, and A-549 cells. Presumably, these activities could be attributed in part to the phenolics/flavanoids features of the extract that has been demonstrated to act as cytotoxic agents. The experimental evidence obtained in the laboratory model could provide a rationale for the traditional use of plant as a source of easily available effective anticancer agents to the people, particularly in developing countries.
Keywords: Alternative medicine, drugs, indian medicinal plants, inhibition, traditional medicine
INTRODUCTION
Plants and plant-based herbal preparations have been used to treat ailments since prehistoric times, and thus the treatment of various diseases with plant-based medicines has remained an integral part of many cultures across the globe. Side effects of several allopathic drugs and development of resistance to currently used drugs have led to increased emphasis on the use of plant materials as a source of medicines for a wide variety of human ailments. The World Health Organization estimates that 4 billion people (i.e., 80% of the World's population) use herbal medicines in some aspects of primary healthcare, and there is a growing tendency to “Go Natural.”;[1,2] It is well established that plants have always been a useful source, for the occurrence of anticancer compounds.[3,4,5] Approximately 60% of the currently used anticancer chemotherapeutic drugs (vinblastin, vincristine) are derived from plant resource.[6,7] Although most of the plants used in the traditional medicine have been identified and their applications are well-documented, the anticancer efficacy of many plants is yet to be verified.
Terminalia chebula (Gaertn.) Retz. (Combretaceae), is an important medicinal plant native to tropical regions of Southern Asia viz., India, Nepal, China, Sri Lanka, Malaysia, Vietnam. It is commonly known as black myrobalan and haritaki, which is amply referred to as “King of medicines” as it has been the component of many formulations for the treatment of various diseases in all the streams of Indian system of medicines such as Ayurveda, Siddha, Unani, and Homeopathy.[8,9] It consists of gall-like excrescences formed by plant insect Dixothrips onerosus (Thysanoptera) on the leaves, petioles and branches.[10] These galls are commonly known as Karkatshringi, which is an important Ayurvedic drug used in preparations such as the dasamularista, cyavanaprasa, and shringyadi churna and used in the treatment of diseases such as swasa (asthma), yakshma (tuberculosis), ajeerna (indigestion), hydroga (heart diseases), jwara (fevers) and yakrt roga (liver disorders) to mention a few.[8,9] Karkatshringi also finds usage in the treatment of children's ear infections, suppress hemorrhage from gums, and also used to suppress bleeding from nose.[11] Hakims considered galls useful in pulmonary infections, diarrhea, and vomiting.[12] Although the accepted source of Karkatasringi is the galls of Rhus succedanea L., however Pistacia integerrima and T. chebula are also generally used in the preparations.[9,13] Gall extracts of T. chebula have been found to possess antioxidant, antiinflammatory, antibacterial, antityrosinase, and antiaging activities.[14,15,16,17,18,19] In an earlier study it was found that the ethanolic leaf gall extract T. chebula possessed potent anthelmintic activities.[20] Henceforth, in the present study, the cytotoxic potential of ethanolic leaf gall extract of T. chebula is evaluated to exemplify its further potential use and develop it as an anticancer agent.
MATERIALS AND METHODS
Buffalo rat liver 3A (BRL3A) cell line, MCF-7 (Human mammary gland adenocarcinoma) and A-549 (Human lung cancer cell line) cell lines were procured from the National Centre for Cell Sciences, Pune, India. Fetal bovine serum (FBS), penicillin, streptomycin and amphotericin B were purchased from SRL, India. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT, No M5655) purchased from Sigma (St Louis, MO, USA). All the chemical reagents and solvents of analytical grade were purchased from SRL Chemicals, India.
Plant material
The gall induced leaves of T. chebula were purchased from local market of Bengaluru, India. The plant materials were authenticated by Dr. S. Sundara Rajan, and the voucher specimen (JU-RUV-52) were deposited at the Research Centre of Vrikshayurveda, Jain University, Bengaluru. The galls were cleaned with distilled water, dried and crushed into fine powder using an electric grinder.
Preparation of extract
The coarsely powdered gall materials were extracted with ethanol solvent in Soxhlet apparatus for 24 h. The extracts were evaporated to dryness under reduced pressure using a Rotavapor (BuchiFlawil, Switzerland), and a portion of the residue was used for the assay.
Phytochemical analysis
Qualitative analysis
The preliminary qualitative phytochemical analyses of carbohydrates, saponins, alkaloids, flavonoids, fixed oils and fats, phenolic and tannins, glycosides, phytosterols and triterpenoids in the ethanolic extracts were carried out using the standard methods as described.[2,21,22,23,24]
Quantitative analysis
Determination of total phenolic content
The total phenolics content in the ethanolic leaf gall extract of T. chebula was determined using Folin - Ciocalteau reagent method, employing gallic acid as standard.[25] Briefly, 200 ml ethanol extract (2 mg/ml) was made up to 3 ml with distilled water, then mixed thoroughly with 0.5 ml of Folin - Ciocalteu reagent. After mixing for 3 min, 2 ml of 20% (w/v) sodium carbonate was added and allowed to stand for a further 60 min in the dark. The absorbance of the reaction mixtures was measured at 650 nm, and the results were expressed as mg of gallic acid equivalent (GAE)/g of dry weight.
Determination of total flavonoid content
Total flavonoid content in the ethanolic leaf gall extract of T. chebula was determined using the aluminum chloride colorimetric method as described by Chang et al.[26] In brief, 50 μl of ethanol extract (2 mg/ml) was made up to 1 ml with methanol then mixed with 4 ml of distilled water and subsequently with 0.3 ml of 5% NaNO2 solution. After 5 min of incubation, 0.3 ml of 10% AlCl3 solution was added and then allowed to stand for 6 min, followed by adding 2 ml of 1 M NaOH solution to the mixture. Then water was added to the mixture to bring the final volume to 10 ml and the mixture was allowed to stand for 15 min. The absorbance was measured at 510 nm. Total flavonoid content was calculated as quercetin from a calibration curve. The calibration curve was prepared by preparing quercetin solutions at concentrations 12.5–100 mg/ml in methanol. The result was expressed as mg quercetin equivalent (QUE)/g of dry weight.
In vitro cytotoxicity assay (MTT assay)
BRL3A, MCF-7 and A-549 cell lines that were procured were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% inactivated FBS, penicillin (100 IU/mL), streptomycin (100 μg/mL) and amphotericin B (5 μg/mL) in a humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with trypsin phosphate versene glucose solution (0.2% trypsin, 0.02% ethylenediaminetetraacetic acid, 0.05% glucose in phosphate-buffered saline [PBS]). The stock cultures were grown in 25 cm3 culture flasks, and all experiments were carried out in 96 microtiter plates (Tarsons India Pvt. Ltd., Kolkata, India). All these cell lines were cultured, and cytotoxicity test were carried out using MTT assay.[27,28] The trypsinized 70–80% confluent cell lines (BRL3A, MCF-7, and A-549) of 1 × 105 cells/well were seeded in a 96 well plate and incubate for 24 h at 37°C, and varying concentrations (0–1000 μg/ml) of ethanolic leaf gall extract of T. chebula are added and incubated at 72 h. After 72 h, the drug solutions in the wells were discarded and 50 μL of MTT in PBS was added to each well. The plates were gently shaken and incubated for 3 h at 37°C in 5% CO2 atmosphere. The supernatant was removed and 100 μL of propanol was added, and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 540 nm. The percentage growth inhibition was calculated using the following formula and concentration of test drug needed to inhibit cell growth by 50% (CTC50) values is generated from the dose-response curves for each cell line.
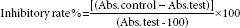
Statistical analysis
The experiments were carried out in triplicate, and results are given as the mean ± standard deviation. The data in all the experiments were analyzed (Microsoft Excel 2007) for statistical significance using Student's t-test, and differences were considered significant at P < 0.05.
RESULTS AND DISCUSSION
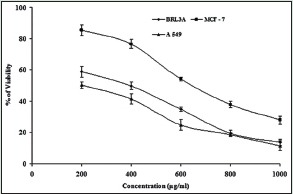
In the discovery of potent drugs for cancer treatment, plants have always been a potential source as lead molecules.[6,7] MTT is a simple, reliable technique, which measures cell viability and can be used for screening anti-proliferative agents.[27,28] MTT assay is a spectrophotometric analysis, which uses (3-4,5-dimethyl-thiazol-2-yl]-2,5-diphenyl tetrazolium bromide), known as MTT, a yellow color, water soluble compound. The MTT enters the cells through the plasma membrane and in contact with superoxide produced by the mitochondrial activity, is oxidized to MTT-formazan, a slat purplish color, which is insoluble in water. Then the oxidation of MTT is proportional to the mitochondrial activity and therefore to cell viability.[28] When the ethanol leaf gall extract of T. chebula was screened for their cytotoxic (anticancer) effect against BRL3A cell line, MCF-7 (Human mammary gland adenocarcinoma) and A-549 (Human lung cancer cell line) cell lines, it was found that the extract was potent and effective in inducing cytotoxic effect at increasing concentration of the extract tested [Figure 1]. The extract showed cytotoxic effect in BRL3A, MCF-7, and A-549 cells with an IC50 value of 305.18 ± 1.7 μg/mL, 643.13 ± 4.2 μg/mL, and 208.16 ± 3.7 μg/mL, respectively [Table 1]; indicating that the extract was more effective against A549 cell lines when compared to others. The preliminary phytochemical analysis shows the presence of flavonoids, steroids, triterpenes and alkaloids in the ethanolic gall extract of T. chebula [Table 2]. Further, the quantitative estimation of total phenolic and flavonoid content indicated the presence of phenolics and flavonoids up to 478 ± 2.2 mg of GAE/g d.w and 538 ± 1.4 mg of QUE/g d.w, respectively [Table 3]. This significantly high presence of phenolics and flavanoids in the extract[20] might be responsible for the cytotoxic effects on BRL3A, MCF7, and A549 cancer cell lines. This is in agreement with the earlier work of Lee et al.[29] and Saleem et al.,[30] who have shown that Terminalia species containing phenolic compounds that are rich in the hydroxy group are believed to be responsible for the cytotoxic activity. In our study, the ethanol extract of the gall of T. chebula is also found to be rich in phenolics (478 ± 2.2 mg/g). Most of the phenolic compounds in plants such as gallic acid, caffeic acid, flavonoids, and its derivatives are known to exhibit various pharmacological actions like antioxidant, free radical scavenging, pro-oxidant toxicity, and apoptosis.[31,32] The cytotoxic effects of the phenolic compound against various cell lines were shown to be higher than the gallic acid used.[29] However, some studies have shown that most of the phenolic compounds have same cytotoxicity effect as gallic acid on cancer cell lines.[33,34] Further, it has been demonstrated that the phenolic compounds in polar extracts are shown to have inhibition of proliferation and induce cell death by apoptosis and necrosis.[30] The results of this study show that the phenolic and flavonoid content which is higher in the ethanolic extract of gall extracts of T. chebula had significant cytotoxic potential, thus could act as lead for the development of anticancer agents These observations justify the ethnobotanical approach for the search of novel bioactive compounds for therapeutic application.
Figure 1.
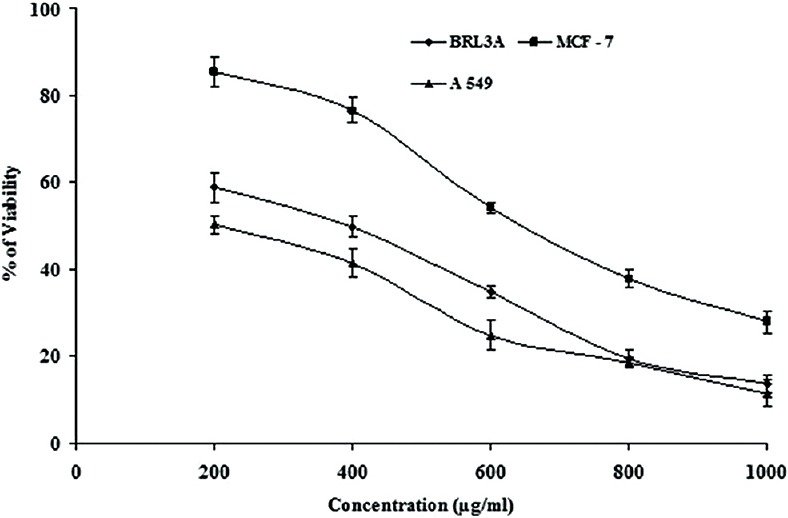
Cytotoxic activity of ethanolic gall extracts of Terminalia chebula on buffalo rat liver 3A, MCF-7, and A 549 cell lines. Extracts were incubated with 105 viable cells at concentrations ranging from 0 to 1000 μg/ml for 48 h. Cell viability was determined by the MTT method
Table 1.
IC50 values of ethanolic leaf gall extract of Terminalia chebula

Table 2.
Preliminary phytochemical analysis of ethanolic leaf gall extract of Terminalia chebula
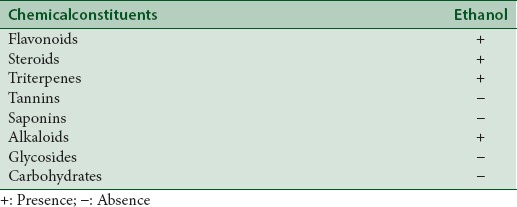
Table 3.
Total phenolic and flavonoid content of ethanolic leaf gall extract of Terminalia chebula

CONCLUSION
The result of this study establishes the anticancer activities of T. chebula leaf gall extracts, whereby it exerted potent cytotoxic effect on BRL3A, MCF-7, and A-549 cells. The potential anticancer activities exhibited by leaf gall extracts of T. chebula may be due to the rich presence of phytoconstituents such as the phenolics/flavanoids that have been demonstrated to act as cytotoxic agents. The experimental evidence obtained in the laboratory model could provide a rationale for the traditional use of plant as a source of easily available effective anticancer agents to the people, particularly in developing countries. Further work will be interesting to know the chemical composition and also better understand the mechanism of action of the constituents of the extract exerting anticancer activities for developing it as a drug for therapeutic application.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Bhadrapura Lakkappa Dhananjaya
Dr. Bhadrapura Lakkappa Dhananjaya, has been working in the field of Drug discovery from diverse source from the past 12 years and has published over 70 International papers.
Acknowledgements
We acknowledge Dr. R. Chenraj Jain, President, Jain University Trust., Dr. N Sundararajan, Vice Chancellor, Jain University and Prof. K.S. Shantamani, Chief Mentor, Jain University, Bengaluru for their kind support and encouragement. DBL thank Jain University for the constant support and encouragement given toward research progress.
REFERENCES
- 1.Petrovska BB. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eshwarappa RS, Iyer S, Subaramaihha SR, Richard SA, Dhananjaya BL. Antioxidant activities of Ficus glomerata (moraceae) leaf gall extracts. Pharmacognosy Res. 2015;7:114–20. doi: 10.4103/0974-8490.147225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stankovic MS, Curcic MG, Zizic JB, Topuzovic MD, Solujic SR, Markovic SD. Teucrium plant species as natural sources of novel anticancer compounds: Antiproliferative, proapoptotic and antioxidant properties. Int J Mol Sci. 2011;12:4190–205. doi: 10.3390/ijms12074190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: A global perspective. Pharmacol Ther. 2003;99:1–13. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 5.Aydemir EA, Simsek E, Imir N, Göktürk RS, Yesilada E, Fiskin K. Cytotoxic and apoptotic effects of Ebenus boissieri Barbey on human lung cancer cell line A549. Pharmacogn Mag. 2015;11(Suppl 1):S37–45. doi: 10.4103/0973-1296.157679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–9. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Tan G, Gyllenhaal C, Soejarto DD. Biodiversity as a source of anticancer drugs. Curr Drug Targets. 2006;7:265–77. doi: 10.2174/138945006776054942. [DOI] [PubMed] [Google Scholar]
- 8.Singh G, Kumar P. Extraction, gas chromatography-mass spectrometry analysis and screening of fruits of Terminalia chebula Retz. For its antimicrobial potential. Pharmacognosy Res. 2013;5:162–8. doi: 10.4103/0974-8490.112421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.1st ed. Delhi: Government of India; 1978. Anonymous. The Ayurvedic Formulary of India. Part; pp. 1–324. [Google Scholar]
- 10.Santha TR, Shetty JK, Yoganarasimhan SN, Sudha R. Farmacognostical studies on the South Indian market sample of karkatasringi (kadukkaipoo) – Terminalia chebul (gaertn. Leaf gall) Anc Sci Life. 1991;11:16–22. [PMC free article] [PubMed] [Google Scholar]
- 11.Sukh D. Ethanotherapeutics and modern drug development. The potential of ayurveda. Curr Sci. 1997;73:909–28. [Google Scholar]
- 12.Nadkarni KM. 3rd ed. India: Popular Prakashan; 1976. Indian Materia Medica; pp. 1062–3. [Google Scholar]
- 13.Eshwarappa RS, Lakshmikantha RY, Subaramaihha SR, Subbaiah SG, Surendranath AR, Dhananjaya BL. Antioxidant activity of insect gall extracts of Pistacia integerrima. Acta Sci Pol Technol Aliment. 2015;14:367–74. doi: 10.17306/J.AFS.2015.4.36. [DOI] [PubMed] [Google Scholar]
- 14.Vonshak A, Barazani O, Sathiyamoorthy P, Shalev R, Vardy D, Golan-Goldhirsh A. Screening South Indian medicinal plants for antifungal activity against cutaneous pathogens. Phytother Res. 2003;17:1123–5. doi: 10.1002/ptr.1399. [DOI] [PubMed] [Google Scholar]
- 15.Manosroi A, Jantrawut P, Akazawa H, Akihisa T, Manosroi J. Biological activities of phenolic compounds isolated from galls of Terminalia chebula Retz. (Combretaceae) Nat Prod Res. 2010;24:1915–26. doi: 10.1080/14786419.2010.488631. [DOI] [PubMed] [Google Scholar]
- 16.Upadhye AS, Rajopadhye AA. Pharmacognostic and phytochemical evaluation of leaf galls of kakadshringi used in Indian system of medicine. J Sci Ind Res. 2010;69:700–4. [Google Scholar]
- 17.Manosroi A, Jantrawut P, Akihisa T, Manosroi W, Manosroi J. In vitro and in vivo skin anti-aging evaluation of gel containing niosomes loaded with a semi-purified fraction containing gallic acid from Terminalia chebula galls. Pharm Biol. 2011;49:1190–203. doi: 10.3109/13880209.2011.576347. [DOI] [PubMed] [Google Scholar]
- 18.Shankara BE, Ramachandra YL, Sundara Rajan S, Preetham J, Sujan Ganapathy PS. In vitro antibacterial activity of Terminalia chebula leaf gall extracts against some human pathogenic strains. Int Cur Pharm J. 2012;1:217–20. [Google Scholar]
- 19.Manosroi A, Jantrawut P, Ogihara E, Yamamoto A, Fukatsu M, Yasukawa K, et al. Biological activities of phenolic compounds and triterpenoids from the galls of Terminalia chebula. Chem Biodivers. 2013;10:1448–63. doi: 10.1002/cbdv.201300149. [DOI] [PubMed] [Google Scholar]
- 20.Ravi Shankara BE, Ramachandra YL, Sundara Rajan S, Richard SA, Dhananjaya BL. Evaluating the anthelmintic potential of leaf gall extracts of Terminelia chebula (Gaertn.) Retz (Combretaceae) J Young Pharm. 2014;6:1–4. [Google Scholar]
- 21.Harborne JJ. 2nd ed. New York: Chapman and Hall; 1984. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; p. 85. [Google Scholar]
- 22.Trease GE, Evans WC. 13th ed. Delhi: ELBS Publication; 1989. Pharmacognosy; p. 171. [Google Scholar]
- 23.Kokate CK, Purohit AP, Gokhale SB. 23rd ed. Pune: Nirali Prakashan; 1998. Pharmacognosy; pp. 106–14. [Google Scholar]
- 24.Khandelwal KR. 13th ed. Pune: Nirali Prakashan; 2005. Practical Pharmacognosy: Techniques and Experiments; pp. 149–56. [Google Scholar]
- 25.Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37:153–61. [Google Scholar]
- 26.Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Edmondson J, Armstrong LS, Martinez AO. A rapid and simple MTT-based spectrophotometric assay for determining drug sensitivity in monolayer cultures. J Tissue Cult Methods. 1988;11:15–7. [Google Scholar]
- 29.Lee SH, Ryu SY, Sang U, Lee CO, No ZK, Kim SK, et al. Hydrolyzable tannins and related compound having cyotoxic activity from the fruits of Terminalia chebula. Arch Pharm Res. 1995;18:118–20. [Google Scholar]
- 30.Saleem A, Husheem M, Härkönen P, Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula Retz. Fruit J Ethnopharmacol. 2002;81:327–36. doi: 10.1016/s0378-8741(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 31.Cai Q, Rahn RO, Zhang R. Dietary flavonoids, quercetin, luteolin and genistein, reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Lett. 1997;119:99–107. doi: 10.1016/s0304-3835(97)00261-9. [DOI] [PubMed] [Google Scholar]
- 32.Sergediene E, Jönsson K, Szymusiak H, Tyrakowska B, Rietjens IM, Cenas N. Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: Description of quantitative structure-activity relationships. FEBS Lett. 1999;462:392–6. doi: 10.1016/s0014-5793(99)01561-6. [DOI] [PubMed] [Google Scholar]
- 33.Manosroi A, Jantrawut P, Sainakham M, Manosroi W, Manosroi J. Anticancer activities of the extract from Longkong (Lansium domesticum) young fruits. Pharm Biol. 2012;50:1397–407. doi: 10.3109/13880209.2012.682116. [DOI] [PubMed] [Google Scholar]
- 34.Wong SK, Lim YY, Abdullah NR, Nordin FJ. Antiproliferative and phytochemical analyses of leaf extracts of ten Apocynaceae species. Pharmacognosy Res. 2011;3:100–6. doi: 10.4103/0974-8490.81957. [DOI] [PMC free article] [PubMed] [Google Scholar]


