Abstract
Andrographis paniculata Nees is an important medicinal plant found in the tropical regions of the world, which has been traditionally used in Indian and Chinese medicinal systems. It is also used as medicinal food. A. paniculata is found to exhibit anti-inflammatory activities; however, its inhibitory potential on inflammatory Group IIA phospholipases A2 (PLA2) and its associated inflammatory reactions are not clearly understood. The aim of the present study is to evaluate the inhibitory/neutralizing potential of ethanolic extract of A. paniculata on the isolated inflammatory PLA2 (VRV-PL-VIIIa) from Daboii rusellii pulchella (belonging to Group IIA inflammatory secretory PLA2 [sPLA2]) and its associated edema-induced activities in Swiss albino mice. A. paniculata extract dose dependently inhibited the Group IIA sPLA2 enzymatic activity with an IC50 value of 10.3 ± 0.5 μg/ml. Further, the extract dose dependently inhibited the edema formation, when co-injected with enzyme indicating that a strong correlation exists between lipolytic and pro-inflammatory activities of the enzyme. In conclusion, results of this study shows that the ethanolic extract of A. paniculata effectively inhibits Group IIA sPLA2 and its associated inflammatory activities, which substantiate its anti-inflammatory properties. The results of the present study warranted further studies to develop bioactive compound (s) in ethanolic extract of A. paniculata as potent therapeutic agent (s) for inflammatory diseases.
SUMMARY
This study emphasis the anti-inflammatory effect of A. paniculata by inhibiting the inflammatory Group IIA sPLA2 and its associated inflammatory activities such as edema. It was found that there is a strong correlation between lipolytic activity and pro-inflammatory activity inhibition. Therefore, the study suggests that the extract processes potent anti-inflammatory agents, which could be developed as a potential therapeutic agent against inflammatory and related diseases.
Keywords: Disease, drug, inflammation, inhibition, neutralization, plants
INTRODUCTION
Secretory phospholipases A2 (sPLA2s) are known to regulate the arachidonic acid pathway by which pro-inflammatory mediators of inflammation are released.[1] In snake venoms, two groups of sPLA2s (GI and GII) have been identified. Group I includes the snake venom PLA2 (svPLA2s) from Elapinae and Hydrophiinae venoms with 115–120 amino acid residues and these svPLA2s are homologous to mammalian pancreatic GIB sPLA2. Group II comprises the svPLA2s from Crotalinae and Viperidae venoms with 120–125 amino acid residues and homologous to mammalian nonpancreatic Group IIA sPLA2.[2] These groups of svPLA2s are known to exhibit a wide variety of physiological and pathological effects that includes induction of inflammatory reactions.[3] Several studies have demonstrated that sPLA2 inhibitors are efficient suppressors of inflammatory processes and thus help in the management of inflammatory diseases.[1,4,5] Due to the central role of sPLA2s in the inflammatory process and considering the drawbacks and severe side effects exhibited by present anti-inflammatory therapeutic agents, which includes the nonsteroidal anti-inflammatory drugs that inhibit either lipoxygenase or cyclooxygenase (COX)-½ enzymes,[6] there is renewed pharmacological interest in search of potent and specific sPLA2 inhibitors from diverse sources.[1,5] In this context, many plant extracts and its constituents are reported for their anti-inflammatory activity through the inhibition of sPLA2s.[1,5,7] However, effective and specific inhibitors of sPLA2 are still not available to date.
Andrographis paniculata Nees (Acanthaceae), commonly known as king of bitters, is a widely distributed medicinal herb in tropical Asian countries including India. It has been used as medicinal herb in traditional medicinal systems of India, China, Thailand, and Europe.[8] As evident from Indian Pharmacopoeia, A. paniculata is prominently known in 26 ayurvedic formulations; while in traditional Chinese medicine, due to its important “cold property,” it is used to release body heat in fever.[9] This species is therapeutically well explored and effectively used for the treatment of asthma, gonorrhea, piles, dysentery and dyspepsia, influenza, gastric complaints, diarrhea, pharyngotonsillitis, fever, myocardial ischemia, common cold, diabetes, respiratory tract infections, and jaundice among others.[8,9] Further, it used as a medicinal food to treat various disorders.[8,9] It is reported to possess anti-microbial, hypotensive, anti-hyperglycemic, oxygen radical scavenging, anti-atherosclerotic, anti-malarial, anti-HIV, anti-platelet aggregation, hepatic lipid per-oxidation protective, hepatoprotective, choleretic, and anticancer properties.[8,9,10] In addition, the plant extract has also been reported to exhibit anti-typhoid, anti-fungal, anti-fertility, anti-nematicidal, anti-hepatitis, anti-hypoglycemic, antiulcerogenic, anti-snake venom, anti-thrombogenic, and anti-inflammatory properties.[8,9,10] In an earlier study, it was observed that the stem chloroform extract of A. paniculata is effective against carrageenan-induced edema showing statistically significant anti-inflammatory activity.[11] In this study, investigations were carried to evaluate the modulatory effect of whole plant ethanolic extract of A. paniculata on Group IIA sPLA2 (i.e., purified VRV-PL-VIIIa PLA2 enzyme from Russell's Viper venom), to further substantiate its anti-inflammatory properties.
MATERIALS AND METHODS
Materials
Venom of Vipera russelli (Russell's viper) was purchased from Irula Co-operative Society Ltd., Chennai, India. Ursolic acid was purchased from Sigma Chemicals, USA. All other reagents and chemicals used were of all analytical grades purchased from Sisco Research Laboratories, Bangalore, India.
Collection of plant and extraction of crude extract
A. paniculata plant was collected from nearby areas of Thanjavur District, Tamil Nadu, India. The plant was authenticated at the Centre for Research in Indian Systems of Medicine (CRISM), SASTRA University (SU), Thanjavur, Tamil Nadu, where a voucher specimen (SASTRA/CRISM/PL/182) was deposited. The whole plant collected of free of disease and injury was washed several times with distilled water and shade dried. The whole plant was crushed into fine powder by using electric grinder. The powdered plant (40 g dry weight) was extracted using 95% ethanol (400 ml) in soxhlet extractor solvent and the extract was concentrated using rotary vacuum evaporator and the residue obtained (dark brown was dried, weighed, and was preserved in an airtight glass container. Further, until use, it was kept inside the refrigerator at 4°C.
Animals
Swiss Wister albino mice weighing about 20–25 g were obtained from the central animal house facility. The animal experiments protocol of this study were carried out after reviewing the protocols by the Animal Ethical Committee of the University and have been approved by the SU-Institutional Animal Care and Use Committee-(IAEC No. SU/IAEC/PROTOCOL No. 14/2013). Animal care and handling were conducted in compliance with the national and international regulations for animal research.
Isolation of Group IIA (VRV-PL-VIIIa) secretary phospholipase A2
sPLA2 belonging to Group IIA-VRV-PL-VIIIa from the venom of Daboii russelli pulchella (Southern region) was purified up to homogeneity as described previously by the method of Kasturi and Gowda,[12] and as modified by Srinivasan.[13] The enzyme was further used for evaluating the anti-inflammatory potential of ethanolic extract of A. paniculata. The protein concentration was estimated according to the method of Lowry et al.[14] using bovine serum albumin as protein standard.
Inhibition of phospholipase A2 activity by Andrographis paniculata ethanolic extract
The PLA2 assay was carried out according to the method as described by Bhat and Gowda.[15] Phosphatidylcholine was diluted with petroleum ether (60–80°C) to get a concentration of 1000 nmoles/50 ml. The reaction mixture containing VRV-PL-VIIIa (3 μg) was made up to 680 ml with water. To the reaction mixture, 200 μl of ether, 100 μl of Tris – HCl buffer (0.05 M, pH 7.5), and 20 μl of CaCl2 (500 mM) were added. The total reaction mixture was incubated at 37°C for 60 min. After incubation, 0.5 ml of doles mixture (Isopropanol: Pet ether: 1NH2SO4, 40:10:1) was added, mixed, and centrifuged at 1000 rpm for 3 min. To the organic phase, 0.5 ml of CHCl3: Pet ether (1:5) was added, mixed, and centrifuged at 1000 rpm for 3 min. To the upper phase cobalt reagent 1.35 vol. of triethanolamine made up to 10 ml with solution A [6 g of CO (NO3)2.-6H2O + 0.8 ml glacial acetic acid] and 7 ml of solution B [saturated Na2SO4]) were added, mixed, and centrifuged at 1000 rpm for 3 min. The upper organic phase was carefully transferred and 0.75 ml of α-nitroso-β-naphthol reagent (0.4% α-nitroso-β-naphthol in 96% ethanol) was added. The intensity of the orange color is directly proportional to the amount of cobalt present. After 30 min, 2 ml of ethanol was added to dilute the contents and absorbance was read at 540 nm. The amount of free fatty acid released was estimated using standard linolenic acid curve. The enzyme activity was expressed as nmoles of fatty acid released/min/mg of protein.
For inhibition studies, VRV-PL-VIIIa (3 μg) was preincubated with or without different concentrations of ethanolic extract of A. paniculata (0–25 μg/ml) at 37°C for 15 min. Appropriate controls were carried and further experiments were carried out as described above. The inhibition is expressed as percentage, considering the activity of venom alone as 100%. IC50 values were calculated using GraphPad version 5.0.
Neutralization of edema-inducing activity of Andrographis paniculata ethanolic extract
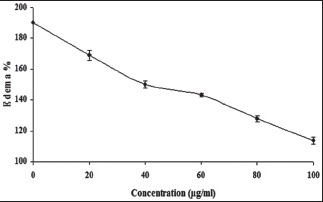
To examine the neutralization effect of ethanolic extract of A. paniculata on the VRV-PL-VIIIa induced edema, the procedure as modified by Vishwanath et al.[16] was followed. Here, the minimum edematic dose (MED) which is defined as the amount of protein concentration required to cause an edema ratio of 120% is used. Mice weighing between 20 and 25 g were used and distributed to seven groups each containing three mice. VRV-PL-VIIIa causing MED at 5 μg was preincubated without or with different concentrations of the extract (0–100 μg/ml) in a total volume of 20 μl saline and injected. Group – I was with VRV-P-VIIIa (5 μg) alone; Group-II with VRV-PL-VIIIa + 20 μg/ml of extract; Group-III with VRV-PL-VIIIa + 40 μg/ml of extract; Group-IV with VRV-PL-VIIIa + 60 μg/ml of extract; Group-V with VRV-PL-VIIIa + 80 μg/ml of extract; Group-VI with VRV-PL-VIIIa + 100 μg/ml of extract; and Group-VII with VRV-PL-VIIIa + ursolic acid. The reaction mixtures were injected into intra plantar surface of right hind footpad of mice and the left footpad that received saline (20 μl) served as control. After 45 min, the mice were sacrificed by giving anesthesia (Pentobarbitone, 30 mg/kg, i.p.) and both hind limbs were removed at the ankle joint and weighed individually. The increase in weight due to edema is expressed as the ratio of the weight of edematous limb to the weight of normal (sham injected) limb ×100. Minimum edema dose is defined as the microgram of protein causing an edema ratio of 120%. Injecting a fixed dose protein into mice footpads and sacrificing them at regular intervals of time obtained time course curve of edema-inducing activity. Edema ratio was calculated and expressed as percent.
Statistical analysis
The IC50 values were calculated using GraphPad version 5.0. Inhibition percentages were calculated from the difference between inhibitor-treated group and control animals, which received the vehicle. Student's t-test for comparisons of unpaired data was used for statistical evaluation.
RESULTS AND DISCUSSION
It is observed that effective inhibitors of sPLA2 are known to suppress the inflammation and its associated processes.[1] Although A. paniculata is found to exhibit anti-inflammatory activities, its inhibitory potential on inflammatory Group IIA phospholipases A2 and its associated inflammatory reactions are not clearly understood. In these line of studies, when analyzing the anti-inflammatory potential of A. paniculata for inhibiting inflammatory PLA2 belonging to Group IIA, i.e., VRV-PL-VIIIa, it was observed that the ethanolic extract of A. paniculata inhibited the enzymatic activity in a concentration dependent manner and the extent of inhibition was >95% at 25 μg/ml [Figure 1], with an IC50 value of 10.3 ± 0.5 μg/ml [Table 1]. Most of the inflammatory sPLA2s are known to induce edema when injected into mouse footpad as demonstrated before.[16] It is also observed that there are many inflammatory mediators which participate in the production of edema in a variety of inflammatory conditions.[17] Among others, histamine, prostaglandins, kinins, and leukotrienes could be implicated in resulting edema due to the action of PLA2s as in the case of snake venoms.[18,19] The edema induced by snake venom PLA2s belonging to Group IIA, follows the classical two phases, which is characterized by a rapid initial first phase produced by mediators such as histamine and serotonin, and a delayed second phase mediated by prostaglandins.[18,19] Several sPLA2 inhibitors are demonstrated to exhibit the concomitant inhibition of both enzyme activity and edema-inducing activity.[1,20] From our earlier studies, based on the dose-response edema responsive reaction of VRV-PL-VIIIa, a challenge dose of 5 μg was selected. This dose was selected because its effective inflammatory result without damaging the overall physical integrity of the animal and helping in the determination of the anti-inflammatory activity of the drugs.[20] It was observed that when the ethanolic extract of A. paniculata was co-injected with VRV-PL-VIIIa (5 μg), the extract significantly reduced the edema formation in a dose-dependent manner [Figure 2]. In addition, A. paniculata extract at the tested dose alone did not induce edema when injected into mice footpads. Further, it was observed that ursolic acid which was used as standard when co-injected with VRV-PL-VIIIa, significantly reduced the edema formation (results not shown). Since the extract significantly inhibits edema formation, it is most likely that the compounds present in these extracts are directly interacting with PLA2s activity. The study shows that the extract acts on the first and second phases of the inflammatory response, as reported by others.[18,19] A. paniculata has been reported to inhibit carrageenan-induced edema showing statistically significant anti-inflammatory activity.[11] Andrographolide, which is one of the therapeutically important constituents of A. paniculata, is known to significantly inhibit carrageenan, kaolin- and nystatin-induced paw edema.[21] Further, it is reported that dehydro andrographolide, andrographolide, and neo andrographolide exhibited strong anti-inflammatory activity by inhibiting COX-1 expression in ionophore A23187-induced human platelets and lipopolysaccharide (LPS) stimulated COX-2 activity in human blood. In addition, andrographolide is known to modulate the level of LPS-induced tumor necrosis factor-α, interleukin (IL)-6, IL-1 β, and IL-10 secretion in human blood.[21,22] It was also shown that A. paniculata extract neutralized the inflammatory and lethal effects induced by Indian Red Scorpion.[23] Our study further confirms the anti-inflammatory potential of A. paniculata by inhibiting Group IIA sPLA2.
Figure 1.
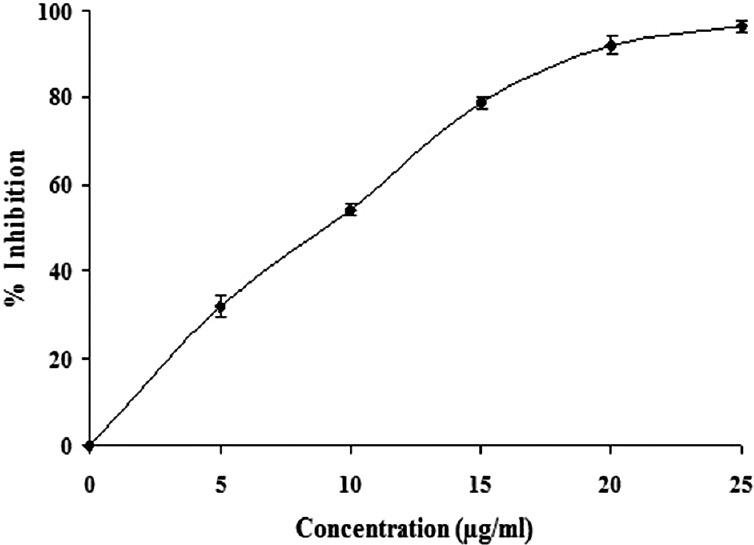
Dose-dependent inhibition of secretory phospholipase A2 (VRV-PLa-VIIIa) activities by ethanolic extract of Andrographis paniculata. Briefly, phosphatidylcholine corresponding to 1000 nmoles/ml was made up to 680 μl with VRV-PL-VIIIa (3 μg), with or without ethanolic extract of Andrographis paniculata at various concentrations (0.25 μg/ml) and was incubated with other reaction mixture at 37°C for 60 min and color developed was read at 540 nm. The results show ± standard error of the mean for n = 3
Table 1.
Specific activity of VRV-PL-VIIIa and IC50 values

Figure 2.
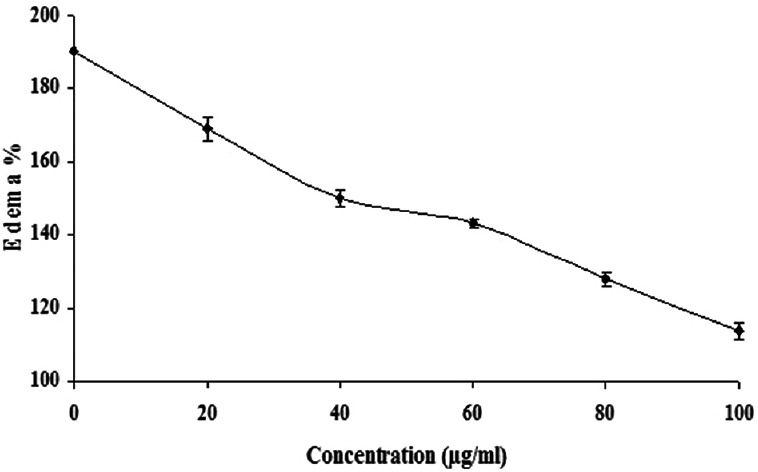
Dose-dependent neutralization of edema-inducing activity of secretory phospholipase A2 (VRV-PL-VIIIa) activities by ethanolic extract of Andrographis paniculata. The reaction mixture 30 μl containing VRV-PL-VIIIa (5 μg) was incubated for 30 min with increasing concentration of ethanolic extract of Andrographis paniculata (100 μg/ml) of Mangifera indica. Saline (20 μl) injected into the mouse foot-pad served as control. Data represent ± standard error of the mean for n = 3
Although at this point the mechanism of action of the extract is unclear and based on the finding that no visible change was detected in the electrophoretic pattern of VRV-PL-VIIIa when incubated with extracts (data not shown), it excludes the proteolytic degradation as a potential mechanism,[20] and the most likely mechanism for anti-inflammatory activities by this extract could be due to the direct binding of the constituents of the extract with sPLA2s active site, as it was observed that there was concernment inhibition of enzymatic and edematigenic activity of VRV-PL-V. The ethanolic extract of A. paniculata inhibiting both in vitro PLA2 enzymatic activity and in vivo edema-inducing activity of VRV-PL-V suggests a strong correlation between lipolytic activity and pro-inflammatory activity inhibition. It can be viewed that A. paniculata ethanolic extract, which contains potent anti-inflammatory molecules, can be developed for topical application as an effective anti-inflammatory formulation.
CONCLUSION
The ethanolic extract of A. paniculata effectively inhibited the inflammatory Group IIA sPLA2 and its associated inflammatory activities such as edema. It was found that there is a strong correlation between lipolytic activity and pro-inflammatory activity inhibition. Therefore, the study suggests that the extract processes potent anti-inflammatory agents, which could be developed as a potential therapeutic agent against inflammatory and related diseases. This study also substantiates their anti-inflammatory properties by inhibiting the Group IIA sPLA2. Further, in-depth studies on compounds present in the ethanolic extract of A. paniculata and the mechanism responsible for their anti-inflammatory activity will be investigated to develop them as a new class of anti-inflammatory agents for therapeutic applications.
Financial support and sponsorship
N. S. Yarla and D. G. Rao are thankful to University Grants Commission.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Bhadrapura Lakkappa Dhananjaya
Dr. Bhadrapura Lakkappa Dhananjaya, has been working in the field of Drug discovery from diverse source from the past 12 years and has published over 70 International papers.
Acknowledgments
Dr. Dhananjaya Bhadrapura Lakkappa (DBL) thanks Jain University for the constant encouragement given to progress in research. DBL and V. Kishore thank SU. DBL acknowledge the Department of Science and Technology, Government of India, for providing the grant INT/SLP/P-007/2012, dated 15th May, 2014. DBL also acknowledge SU for providing the T. R. Rajagopalan Fund. F. Zameer acknowledge University Grants Commission (UGC) for C. V. Raman Fellowship postdoctoral Fellowship 2014–15, Prof. Dr. K. V. Prabhakara, Principal, SBRR Mahajana First Grade College and the Management for their support and inspiration. N. S. Yarla and D. G. Rao are thankful to the UGC (F. No. 42-643/2013) for financial support.
REFERENCES
- 1.Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS. PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants – A new role as anti-inflammatory molecule. Curr Top Med Chem. 2007;7:765–77. doi: 10.2174/156802607780487623. [DOI] [PubMed] [Google Scholar]
- 2.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl 50):S237–42. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doley R, Zhou X, Kini RM. Snake venom phospholipase A2 enzymes. In: Mackessy SP, editor. Handbook of Venoms and Toxins of Reptiles. Boca Raton, FL, USA: CRC Press; 2010. pp. 173–205. [Google Scholar]
- 4.Meyer MC, Rastogi P, Beckett CS, McHowat J. Phospholipase A2 inhibitors as potential anti-inflammatory agents. Curr Pharm Des. 2005;11:1301–12. doi: 10.2174/1381612053507521. [DOI] [PubMed] [Google Scholar]
- 5.Narendra Sharath Chandra JN, Ponnappa KC, Sadashiva CT, Priya BS, Nanda BL, Gowda TV, et al. Chemistry and structural evaluation of different phospholipase A2 inhibitors in arachidonic acid pathway mediated inflammation and snake venom toxicity. Curr Top Med Chem. 2007;7:787–800. doi: 10.2174/156802607780487678. [DOI] [PubMed] [Google Scholar]
- 6.Vane JR, Botting RM. Anti-inflammatory drugs and their mechanism of action. Inflamm Res. 1998;47(Suppl 2):S78–87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 7.Springer DM. An update on inhibitors of human 14 kDa type II s-PLA2 in development. Curr Pharm Des. 2001;7:181–98. doi: 10.2174/1381612013398275. [DOI] [PubMed] [Google Scholar]
- 8.Akbar S. Andrographis paniculata: A review of pharmacological activities and clinical effects. Altern Med Rev. 2011;16:66–77. [PubMed] [Google Scholar]
- 9.Benoy GK, Aimesh DK, Aninda M, Priyanka DK, Sandip H. An overview on Andrographis paniculata (burn F.) nees. Int J Res Ayurveda Pharm. 2012;3:756–60. [Google Scholar]
- 10.Niranjan A, Tewari SK, Lehri SK. Biological activities of Kalmegh (Andrographis paniculata Nees) and its active principles – A review. Indian J Nat Prod Resour. 2010;1:125–35. [Google Scholar]
- 11.Radhika P, Rajendra YP, Sastry BS, Lakshmi KR. Anti-inflammatory activity of chloroform extract of Andrographis paniculata Nees Stem. Res J Biotechnol. 2009;4:35–8. [Google Scholar]
- 12.Kasturi S, Gowda TV. Purification and characterization of a major phospholipase A2 from Russell's viper (Vipera russelli) venom. Toxicon. 1989;27:229–37. doi: 10.1016/0041-0101(89)90136-0. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan S. University of Mysore; 2004. Mechanism of Action of Snake Venom Toxic Phospholipases. Thesis. [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 15.Bhat MK, Gowda TV. Purification and characterization of a myotoxic phospholipase A2 from Indian cobra (Naja naja naja) venom. Toxicon. 1989;27:861–73. doi: 10.1016/0041-0101(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 16.Vishwanath BS, Kini RM, Gowda TV. Characterization of three edema-inducing phospholipase A2 enzymes from habu (Trimeresurus flavoviridis) venom and their interaction with the alkaloid aristolochic acid. Toxicon. 1987;25:501–15. doi: 10.1016/0041-0101(87)90286-8. [DOI] [PubMed] [Google Scholar]
- 17.Posadas I, Terencio MC, Guillén I, Ferrandiz ML, Coloma J, Payá M, et al. Co-regulation between cyclo-oxygenase-2 and inducible nitric oxide synthase expression in the time-course of murine inflammation. Arch Pharmacol. 2000;361:98–106. doi: 10.1007/s002109900150. [DOI] [PubMed] [Google Scholar]
- 18.Chaves F, Barboza M, Gutiérrez JM. Pharmacological study of edema induced by venom of the snake Bothrops asper (terciopelo) in mice. Toxicon. 1995;33:31–9. doi: 10.1016/0041-0101(94)00135-u. [DOI] [PubMed] [Google Scholar]
- 19.Badilla B, Chaves F, Mora G, Poveda LJ. Edema induced by Bothrops asper (Squamata: Viperidae) snake venom and its inhibition by Costa Rican plant extracts. Rev Biol Trop. 2006;54:245–52. doi: 10.15517/rbt.v54i2.13865. [DOI] [PubMed] [Google Scholar]
- 20.Dhananjaya BL, Sudarshan S. Inhibition of secretary PLA2 – VRV-PL-VIIIa of Russell's viper venom by standard aqueous stem bark extract of Mangifera indica L. Trop Biomed. 2015;32:24–35. [PubMed] [Google Scholar]
- 21.Liu J, Wang ZT, Ji LL. In vivo and in vitro anti-inflammatory activities of neoandrographolide. Am J Chin Med. 2007;35:317–28. doi: 10.1142/S0192415X07004849. [DOI] [PubMed] [Google Scholar]
- 22.Parichatikanond W, Suthisisang C, Dhepakson P, Herunsalee A. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm.f.) Nees and their effects on gene expression. Int Immunopharmacol. 2010;10:1361–73. doi: 10.1016/j.intimp.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Brahmane RI, Pathak SS, Wanmali VV, Salwe KJ, Premendran SJ, Shinde BB. Partial in vitro and in vivo red scorpion venom neutralization activity of Andrographis paniculata. Pharmacognosy Res. 2011;3:44–8. doi: 10.4103/0974-8490.79115. [DOI] [PMC free article] [PubMed] [Google Scholar]


