Abstract
Using yeast two-hybrid screens, we have defined an interaction group of six Tra proteins encoded by the F plasmid and required by F+ cells to elaborate F pili. The six proteins are TraH, TraF, TraW, TraU, TrbI, and TrbB. Except for TrbI, these proteins were all identified as hallmarks of F-like type IV secretion systems (TFSSs), with no homologues among TFSS genes of P-type or I-type systems (T. Lawley, W. Klimke, M. Gubbins, and L. Frost, FEMS Microbiol. Lett. 224:1-15, 2003). Also with the exception of TrbI, which is an inner membrane protein, the remaining proteins are or are predicted to be periplasmic. TrbI consists of one membrane-spanning segment near its N terminus and an 88-residue, hydrophilic domain that extends into the periplasm. Hence, the proteins of this group probably form a periplasmic cluster in Escherichia coli. The interaction network identifies TraH as the most highly connected node, with two-hybrid links to TrbI, TraU, and TraF. As measured by transcriptional activation of lacZ, the TrbI-TraH interaction in Saccharomyces cerevisiae requires the TraH amino acid segment from residues 193 to 225. The TraU and TraF interactions are localized to C-terminal segments of TraH (amino acids 315 to 458 for TraF and amino acids 341 to 458 for TraU). The TrbI-TraH interaction with full-length (less the signal peptide) TraH is weak but increases 40-fold with N-terminal TraH deletions; the first 50 amino acids appear to be critical for inhibiting TrbI binding in yeast. Previous studies by others have shown that, with the exception of trbB mutations, which do not affect the elaboration or function of F pili under laboratory conditions, a mutation in any of the other genes in this interaction group alters the number or length distribution of F pili. We propose a model whereby one function of the TraH interaction group is to control F-pilus extension and retraction.
Type IV secretion systems (TFSSs) comprise a broadly distributed group of molecular machines that function to secrete macromolecules from gram-negative bacteria into other bacterial or eukaryotic cells (3, 4). These systems are responsible for the pathogenic effects of some bacterial species (4) and contribute to the dissemination of antibiotic resistance genes to pathogens of humans and domestic animals (37). Several classes of TFSSs are distinguishable by sequence comparisons (3, 6, 7, 26), and these systems may have arisen more than once during bacterial evolution.
TFSSs typically mediate DNA transfer (conjugation) (5, 14, 21). This activity has been broadly divided into two experimentally separable stages. The first is the establishment of secure cell-cell contacts, and the second is DNA transfer per se. These two stages are linked by coupling proteins, which are membrane or membrane-associated complexes responsible for substrate recognition and presentation to a channel or pore complex that spans the cell envelope (4, 27, 35). Individual functions associated with the first stage are designated Mpf (mating pair formation) functions and the corresponding proteins are Mpf proteins; proteins and functions associated with the second stage are designated Dtr (donor transfer replication). Most TFSS genes fall into the Mpf class (14, 17). However, these functional assignments are often based on mutant phenotypes. For proteins that function at more than one stage, null mutations would identify only the earliest stage. For this reason, the Mpf designation does not preclude a function at later stages of conjugal DNA transfer.
One feature common to all conjugal DNA transfer systems of gram-negative bacteria is the presence of conjugative pili (21, 32). These surface filaments function in the earliest stages of conjugation, when donor and recipient cells make initial contacts that eventually lead to DNA transfer. Insofar as they have been examined, conjugative pili are repeats of one quantitatively predominant subunit (12, 32). These subunits and the corresponding filaments are designated according to the conjugal DNA transfer system of which they are a part, e.g., F pili(n), RP4 pili(n), T pili(n), etc. Notwithstanding their apparent structural simplicity, the formation of conjugative pili requires numerous Mpf proteins. For the 25-gene tra system borne by the F plasmid, a mutation in any of 16 genes abolishes the formation of extended F pili or alters F-pilus length or number distribution (14).
After initial contacts, F pili retract (8, 29), such that DNA transfer occurs primarily, if not exclusively, between cells that are firmly joined at their surfaces (11, 25, 33). Retraction also occurs when filamentous DNA bacteriophages bind to the F-pilus tip (22). It is unclear how widely distributed retraction is among TFSSs other than the F-like group.
Type IV Mpf systems typically include core components that are recognizable by sequence similarities among classes (3, 26). Other components, however, appear to be class specific (26). The TFSSs encoded by F and the F-like R factors include several such components. Five of the 16 F-plasmid-encoded Tra proteins required for the formation of F pili or for normal F-pilus number and length distributions appear not to have homologues in TFSSs outside of the F and F-like families (26). These are TraF, TraH, TraW, TrbC, and TraU. There are no data regarding physical interactions among these proteins, but genetic data suggest that they have a common function(s). A mutation in traF, traH, traW, or trbC abolished the ability of F+ cells to form extended F pili that were visible by electron microscopy (14). However, TraF, TraH, and TraW mutants retained significant sensitivity to filamentous bacteriophages that bind to the F-pilus tip (1), suggesting that these Tra proteins are required for F-pilus extension. (The trbC mutant could not be tested.) Mutations in traU also reduced the number of F pili per cell and the mean F-pilus length, but not as drastically as mutations in the other genes (31). (Such mutations reduced DNA donor activity more than expected from the reduction in F-pili, suggesting that TraU affects multiple stages of conjugation [31].)
Here we show by yeast two-hybrid analyses that TraH, TraF, TraW, TraU, and TrbB are components of the same Tra protein interaction group. An additional member of this group is TrbI. While trbB mutations had no effect on F-pilus functions in otherwise tra+ cells (24), trbI mutants were reported to elaborate unusually long F pili (28). The properties of mutants with mutations in individual components of this interaction group suggest a role for the group in regulating F-pilus retraction and extension.
MATERIALS AND METHODS
Strains and plasmids.
Saccharomyces cerevisiae strain Y190 (MATa gal4 gal80 his3 trp1-901 ade2-101 ura3-52 leu2-3,112 Cyhr GAL1::lacZ@URA3 GAL1::HIS3@LYS2) and plasmids pACTII and pAS1CYH2 were originally obtained from Steven Elledge, Baylor University College of Medicine (10). Bait and prey libraries derived from plasmid pTG801 (16) and constructed in plasmids pAS1CYH2 and pACTII, respectively, were described previously (18). Specific bait plasmids were constructed by PCR amplification of tra segments from JCFL0 (F′ lac+ tra+) (traH and traF) or pTG801 (traU, traW, and trbI), as described by Harris et al. (19). The primers used for each such construct are shown in Table 1. PCR products were purified from agarose gels by centrifugation of frozen (liquid N2) gel segments (10 min at 13,000 × g) through siliconized glass wool, digested with NcoI and BamHI, and cloned into pAS1CYH2 digested with the same two enzymes. All constructs were tested alone for transcriptional activation; only the traF construct gave low and variable levels of activation under some conditions (see Results).
TABLE 1.
Primers used to amplify tra sequences of bait plasmids
| Bait construct | Primersa (5′ primer, 3′ primer) | tra ntb |
|---|---|---|
| pAS1traH | GCAGCGTCcatGGATGTGAACAGCG, CGTCCAGGaTCCCATAAAGGCG | 8090-8115, 19486-19466 |
| pAS1traF | CCGGCGccatgGGAAAGATGCAGGC, CATAAATCAggATCCGCGATTAAAAATTGG | 15127-15150, 15843-15815 |
| pAS1traW | GGAACCATGggATGCCGGG, CAGCCACAGgaTgCGCTTCAT | 10726-10742, 11381-11361 |
| pAS1traU | CAGAGGAGGcAtGgAAATGAAGC, GCTTCATCATggAtCCTCACAGGA | 11345-11374, 12368-12346 |
| pAS1trbl | TACAGGAGccaTgGGCATGAGTTC, GCCCCCGGATCcTCATGGTTC | 10335-10356, 10747-10727 |
Lowercase letters indicate mutations that were introduced to aid cloning.
tra sequence coordinates were obtained from reference 15.
pAS1CYH2 traH deletions were isolated from a pAS1CYH2tra fragment library (18) by colony lift hybridization. Radioactive traH DNA was obtained by random primer labeling (13) with [α-32P]CTP. DNA was obtained by PCR amplification from JCFL0 with the forward primer 5′ GCAAGAATGATGCCACG (tra nucleotides [nt] 18024 to 18040) and the reverse primer 3′ TCATTCACAGCGTGCTG (tra nt 19410 to 19374). (Numbering of the tra nucleotides was from reference 15.) The pAS1CYH2tra library was plated at 60,000 CFU/plate. Of 360,000 colonies screened, 85 potential positive colonies were isolated and sequenced. Several of these were selected based on the extent of the deletions and, where necessary, were frame shifted to correspond to the GAL4 sequence of pAS1CYH2 by a fill-in reaction catalyzed by the Klenow fragment of DNA polymerase I or by mung bean nuclease. In all cases, a polypeptide of the appropriate size could be detected in yeast extracts analyzed by Western blotting with antibodies against the hemagglutinin (HA) epitope included in the GAL4 segment of pAS1CYH2 (18).
Media and growth conditions.
Yeast extract-peptone-dextrose and synthetic complete dropout media were described previously (23). Yeast strains were routinely grown at 30°C with aeration, and growth was monitored by total cell counts in a hemocytometer or by measuring the optical density at 600 nm. For interaction screens, yeast colonies appearing within 5 days at 30°C on Leu− His− plates containing 40 mM 3-aminotriazole (added to reduce HIS3 [imidazole glycerol phosphate dehydratase] activity) were considered to be His+, and those yielding a blue color upon colony lifting within 18 h at 41°C were considered to be LacZ+.
Escherichia coli was routinely cultured in Luria-Bertani medium at 37°C with aeration. Growth was monitored by measuring the optical density at 600 nm. Ampicillin was added, when necessary, at 100 μg/ml.
Methods.
Yeast transformations were carried out by the lithium acetate-polyethylene glycol method, as described previously (23); transformation frequencies with the pACTtra library were generally 104 to 105 Leu+ Trp+ colonies/μg of DNA. Plasmid DNAs were prepared from Zymolyase-treated yeast cells (23) and introduced into E. coli by electroporation. Otherwise, E. coli cells were transformed by the CaCl2 method.
Beta-galactosidase activities in yeast were measured with chlorophenyl-red-β-d-galactopyranoside (CPRG; Boehringer-Mannheim) essentially as described previously (10). Cells in 5 ml of culture at an optical density at 600 nm of 0.6 to 0.8 were collected by sedimentation, suspended in 1 ml of H buffer (10), permeabilized with sodium dodecyl sulfate and CHCl3, and assayed. Chlorophenyl-red released by hydrolysis was measured as the absorbance at 574 nm.
Western blot analyses of yeast proteins were performed as described previously (18).
RESULTS
TraH and TraF interact in yeast two-hybrid assay.
TraH is one of several periplasmic Tra proteins that are required for the elaboration of F pili (14). We used traH as bait in a two-hybrid screen of a library of tra fragments derived from plasmid pTG801 which includes all of the F plasmid tra genes required to elaborate functional F pili and only those genes, with a few exceptions (16). The traH gene comprises 458 codons, with a predicted 24-amino-acid leader peptide (15). The bait plasmid included traH codons 24 to 458. Of 250,000 Leu+ Trp+ transformants containing both the bait and prey plasmids, 45 were His+ LacZ+ as well, indicating transcriptional activation. Forty-four of these were sequenced, of which 40 contained traF segments (Table 2). The remaining prey plasmids contained traC, traB, or traL. The traC and traB inserts were in frame with respect to the upstream GAL4 sequence, whereas the traL insert was not.
TABLE 2.
TraH-TraF interaction by yeast two-hybrid screen
| tra bait | No. of codons (full gene) | No. of Leu+ Trp+ transformants | No. of His+ LacZ+ transformants | tra gene (no. of sequences/total sequences) |
|---|---|---|---|---|
| traH24-458 | 458 | 254,000 | 45 | traF (40/44) |
| traF19-247 | 247 | 156,400 | 860 | traH (12/40) |
| traC (4/40) | ||||
| traU (3/40) | ||||
| traW (2/40) | ||||
| traN (2/40) |
TraF is itself a periplasmic protein of 247 amino acids with a predicted 19-amino-acid leader peptide (15, 38). The 5′ termini of the traF fragments contained in the 40 prey plasmids were all within the segment comprising traF codons R64 to W88. The segment R64-W88 might border a region required for protein folding in yeast or might be amino acids that are directly required for the TraF-TraH interaction.
We next used a traF bait, comprising traF codons 19 to 247, to screen the tra fragment library. We performed two separate screens, which combined yielded His+ LacZ+ transformants at a frequency of 5.5 × 10−3, or 30-fold higher than that in the traH screen (Table 2). Only two tra genes were identified in both screens, among 40 total sequences. Twelve contained traH segments, all in frame, and four contained traC sequences, with three in frame. Several other tra genes were identified in one screen or the other, but not both, along with reverse tra and unidentified, possibly vector, sequences (Table 2).
The high fraction of questionable positive results in the screen TraF is unusual in our experience. While we have no definitive explanation, we observed unusually variable levels of transcriptional activation in yeast cells containing both the traF bait and random prey plasmids. In our screens, we selected those with higher levels, estimated from the intensity of the blue color in colony lifts for β-galactosidase activity, but we could easily have chosen a significant number of false-positive transformants. For this reason, we are inclined for the time being to credit only the traH isolates, both because they still constituted nearly a third of the total sequences and because the traH screen identified exclusively traF preys. Also note that TraC is a cytoplasmic, peripheral membrane protein in tra+ cells (34), whereas TraF is periplasmic (38). These different cellular locations make it improbable, though certainly not impossible, that the TraC interaction is functionally significant.
In contrast to the results with traF preys isolated with the traH bait, the 5′ end points of the 12 traH preys were distributed throughout the central half of the coding region, from F135 to T326. These results suggest that a segment of TraH between T326 and the C terminus of the molecule, L458, is sufficient for TraF binding in yeast (Fig. 1). In a reciprocal experiment, we found that yeast cells with a bait plasmid containing traH encoding amino acids 259 to 458 (traH259-458 and a prey plasmid containing traF75-247 were His+ and LacZ+ (data not shown), again suggesting that a C-terminal segment of TraH contains a TraF binding site.
FIG. 1.
Interaction map of TraH. Segments required for two-hybrid interactions between TraH (458 amino acids) and other Tra proteins were derived from sequence analyses of prey plasmids and β-galactosidase assays. The TraH leader peptide (LP; amino acids 1 to 24) is also shown. TrbIi, segment required for inhibition of interaction with TrbI in yeast.
Interactions involving TrbI.
An additional interaction involving TraH was detected with bait plasmids lacking 5′-terminal traH codons beyond the 24 codons encoding the TraH signal peptide. We identified these deletions by colony hybridization with the bait plasmid library and by DNA sequence analysis, as described in Materials and Methods. Surprisingly, when these deletions were used in small-scale screens of the tra fragment prey library, the predominant prey in His+ LacZ+ yeast transformants was TrbI, a 128-amino-acid inner membrane protein in E. coli (28). A larger-scale screen with one of these deletions, traH199-458, yielded 16 trbI bait plasmids of 18 that were sequenced (Table 3). The overall frequency of positive transformants was fivefold higher in this screen than in the traH19-458 screen (Table 2), perhaps explaining why no traF preys were identified among the 18 that were sequenced from the traH199-458 screen. Most of the trbI segments identified in this screen contained the entire gene, which at 128 codons is smaller than the mean fragment length of 1 kb for the prey library (18). Four segments of the 16, however, began at codons N32, V35, I39, and R41. All of these are within or, in the case of R41, immediately adjacent to a putative membrane-spanning segment (codons W18 to V40 [15]). Hence, the interaction domain of TrbI for TraH is within the segment from R41 to the C-terminal P128. This region is relatively hydrophilic and extends into the periplasm (34).
TABLE 3.
TrbI-TraH interaction by yeast two-hybrid screen
| tra bait | No. of codons (full gene) | No. of Leu+ Trp+ transformants | No. of His+ LacZ+ transformants | tra gene (no. of sequences/total sequences) |
|---|---|---|---|---|
| traH199-458 | 458 | 277,000 | 237 | trbl (16/18) |
| trbl1-128 | 128 | 85,000 | 31 | traH (5/31) |
| trbl (25/31) |
To explain why no trbI preys were identified among the 44 that were sequenced from the traH24-458 screen (Table 2), we considered the possibility that an N-terminal segment of TraH inhibits the TrbI-TraH interaction in yeast. To test this, we measured β-galactosidase activity in yeast strains containing different traH bait plasmids and the same trbI5-128 prey. This experiment confirmed that β-galactosidase activity increased about 40-fold when an N-terminal segment of TraH, between A21 and L137, was deleted from TraH (Fig. 2). A further deletion of TraH to G193 had no effect on the activity, which remained at a relatively high level, but deletion to A225 or further essentially abolished activity. These results indicate that the TraH segment from G193 to A225 is required for the binding of TrbI in the yeast two-hybrid assay and that this binding is inhibited by an N-terminal segment of TraH. Whether or not this also occurs in E. coli remains to be determined. The putative TrbI binding segment of TraH is predicted to be very hydrophilic, with 12 charged and 8 polar amino acids (Fig. 2). It is therefore, in all likelihood, solvent exposed and hence available for protein-protein interactions.
FIG. 2.
TrbI interaction domain and inhibitory segment of TraH. Transcriptional activation (LacZ activity) in cells containing a trbI5-128 prey and the indicated traH bait plasmids shows the inhibitory effect of the N-terminal segment D24-L137 and the requirement for the segment G193-A225. The figure also indicates the five traH preys isolated with a trbI bait and the predicted amino acid sequence of the hydrophilic segment containing the TrbI binding site. LP, leader peptide.
We also performed a two-hybrid screen with a trbI1-128 bait plasmid. We sequenced each of the 31 His+ LacZ+ transformants (Table 3) that we obtained. Twenty-five of these carried trbI, indicating that TrbI segments interact in yeast. None of the trbI segments of these prey plasmids lacked more than the first 10 codons. We infer that the TrbI-TrbI interaction domain lies close to the N terminus of the molecule and is conceivably the hydrophobic segment beginning at codon W18 and ending at codon V40 (15). Thus, TrbI interacts with other TrbI monomers through an N-terminal putative membrane-spanning segment (34) and with TraH through its periplasmic domain.
Five of the 31 preys contained traH (Table 3). Three of these contained the same segment, encompassing codons 74 through 233. One encompassed codons 76 to 225 and one encompassed codons 74 to 230. Two features of these segments are consistent with previous results. First, all five lacked N-terminal codons. Given that codons 1 to 24 encode the TraH signal peptide, the data suggest that TraH amino acids 25 to 75 are sufficient to inhibit TraH binding to TrbI in yeast (Fig. 1). Second, all five traH isolates included the region encoding G193 to A225, which is suggested to contain the binding site for TrbI (Fig. 1). We were unable to detect any β-galactosidase activity at all (<0.05 U) in yeast cells containing the trbI5-128 prey and a bait plasmid with a 3′-terminal traH deletion that still encoded an intact N-terminal domain (traH15-330) (Fig. 2). In a LacZ colony lift assay, this segment as well as that comprising TraH amino acids 253 to 458 still interacted with TraF (data not shown), suggesting that these segments were not merely misfolded in yeast.
The differences in activity with different traH bait constructs cannot be attributed to different GAL4-TraH fusion protein levels. As measured by Western blotting against the HA epitope common to all of the fusion proteins, the levels of inactive fusion proteins (TraH225-458 and TraH15-330) (Fig. 2) were no less than those of active fusion proteins (TraH193-458 and TraH199-458) (Fig. 3). Moreover, it seems unlikely that the different transcriptional activation levels of cells with GAL4-TraH199-458, which was active in TrbI binding (Table 3), and cells with GAL4-TraH225-458, which was inactive (Fig. 2), can be explained by effects of the 26-amino-acid difference between the two fusion proteins on folding, nuclear transport, or other factors that are not directly related to transcriptional activation.
FIG. 3.

Levels of GAL4-TraH fusion proteins in yeast. Yeast cells with segments of traH fused to the GAL4 domain of pAS1CYH2 were assayed for fusion protein levels by Western blotting with antibodies against the HA epitope of the GAL4 polypeptide segment (18). The range of numbers under each lane denotes the TraH amino acids contained in the corresponding fusion. Full-length TraH is 458 amino acids long (13).
Interactions involving TraU and TraW.
We performed additional two-hybrid screens to define new Tra protein interaction groups. Unexpectedly, two of these screens expanded the TraH/TraF/TrbI group. The first utilized traU48-330 as bait (15, 31). Positives were obtained at a frequency of 5.6 × 10−4 (Table 4). Of those that were sequenced, 67% carried in-frame traH segments; no other tra gene was represented more than once. The 5′ termini of the traH segments were all between codons T265 and V343 (of 458 codons), indicating that TraU interacts with a C-terminal segment of TraH, as does TraF (Fig. 1).
TABLE 4.
TraU and TraW interactions by yeast two-hybrid screen
| tra bait | No. of codons (full gene) | No. of Leu+ Trp+ transformants | No. of His+ LacZ+ transformants | tra gene (no. of sequences/total sequences) |
|---|---|---|---|---|
| traU48-330 | 330 | 169,000 | 95 | traH (39/58) |
| traW1-210 | 210 | 236,000 | 121 | traU (16/44) |
| trbB (22/44) |
We also performed a screen with a bait plasmid containing traW1-210 (15, 28). This screen yielded positive transformants at a frequency of 5.1 × 10−4 (Table 4). Of those prey plasmids that were sequenced, 86% carried either of two tra genes, trbB and traU, at similar frequencies (Table 4). While trbB insertion alleles had no effect on F-pilus-related functions under laboratory conditions (24), the trbB gene products encoded by the F and R100 plasmids are 90% identical (1), suggesting that there is some evolutionary pressure for conservation. The data showing that the F trbB gene product is part of a Tra protein interaction group also suggest a function for TrbB, albeit one either not required in routine assays for conjugal DNA transfer or donor-specific bacteriophage sensitivity or for which another Tra protein is redundant (24, 26).
DISCUSSION
The data presented here establish a new interaction group among F-plasmid Tra proteins required for the elaboration of F pili. This group consists of six proteins: they are TraH, TraF, TrbI, TraU, TraW, and TrbB. Except for TrbI, the other proteins of this group are or are predicted to be periplasmic (14). TrbI is an inner membrane protein but has a large periplasmic domain (34). Hence, interactions among the six proteins are consistent with their cellular localization.
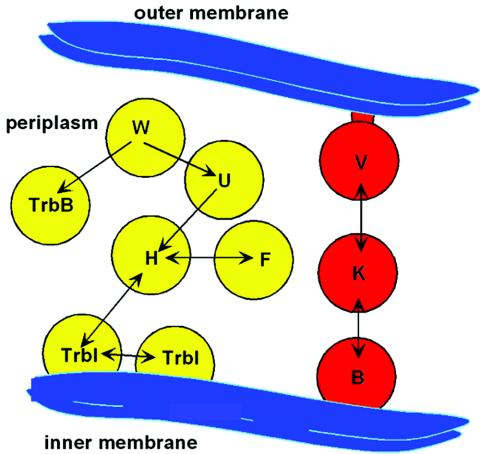
The two-hybrid data identify TraH as the most highly connected member of the group, with two-hybrid links to TrbI, TraF, and TraU (Fig. 4). At 434 amino acids, periplasmic TraH is also the largest protein in this interaction group. These data suggest a central role for TraH in the group’s function(s).
FIG. 4.
Protein interactions of the TraV/TraK/TraB and TraH/TraF/TraW/TraU/TrbI/TrbB interaction groups. Arrows connect proteins that were shown to interact by yeast two-hybrid assays; arrowheads point to the prey. The interaction map was overlaid on the intracellular location of each protein in E. coli, as described previously (14). The TraV/TraK/TraB data are from reference 19; other data are from the present communication.
Of the six Tra proteins in the TraH interaction group, five (TraH, TraF, TraW, TraU, and TrbB) were reported by Lawley et al. (26) to be characteristic of F-like TFSSs, insofar as sequence database searches returned no homologues among P-type or I-type TFSSs (26). Conversely, using virB query sequences, Cao and Saier found no homologues among F-plasmid tra genes for virB6-virB9 or virB11 (3). They did find tra homologues of virB2-5 and virB10, but none were among the genes found by Lawley et al. to be characteristic of F-like tra systems (3, 26). Cao and Saier attributed this to incomplete sequencing of the F tra region (3), but perhaps a more plausible hypothesis is that gram-negative TFSSs, at least in part, arose more than once during bacterial evolution.
A mutation in traH, traF, traW, traU, or trbI altered the F-pilus length or number distribution, suggesting that there are functional relationships among the corresponding Tra proteins. (The numbers of F pili per cell and F-pilus length distribution could amount to the same thing, since factors leading to shorter F pili might favor filaments that are too short to be visible by electron microscopy. This would be scored as a reduction in the number of F pili per cell [2, 9].) The effects of mutations in traH, traF, and traW were similar (reference 14 and references therein). Each abolished the formation of F pili that were visible by electron microscopy of negatively stained cells. None affected the amount of membrane F pilin (30). All reduced DNA donor activity by several orders of magnitude and all were resistant to donor-specific bacteriophages by a plaque assay (14). However, by a more sensitive transduction assay, traH, traF, and traW mutants retained significant levels (1 to 100%) of sensitivity to a filamentous DNA bacteriophage that binds to the F-pilus tip (1). In comparison, an amber mutation in traA or a mutation in any of several other tra genes reduced phage sensitivity by at least 6 orders of magnitude, to an undetectable level (1). The simplest interpretation of these data is that traH, traF, and traW mutants are defective in F-pilus extension, such that mutant cells have unusually short filaments that are still able to bind bacteriophage at their tips. Amber and insertion mutations in traU also had the effect of reducing both the number and length distributions of F pili, though not as drastically (31), whereas trbI mutants were reported to have unusually long F pili (28).
In addition to evolutionary and functional data, the data presented here add a third line of evidence linking TraH, TraF, TraW, and TraU: all four proteins are components of a yeast two-hybrid interaction group, along with TrbI. Three independent lines of evidence thus converge on the hypothesis that these proteins function together in F+ strains of E. coli and that the function of the group is either unique to F-like TFSSs, or if common to other TFSS classes, arose independently in F-like systems. One possibility for the main function of the TraH group arose from conclusions reached by Sowa et al. (36); to account for the difficulty they encountered in exhausting the pool of membrane F pilin, they proposed that F-pilus extension and retraction alternate stochastically. The Tra protein interaction group defined here could function as a switch to regulate F-pilus extension and retraction cycles. TraW, TraF, TraH, and to a lesser degree, TraU would be pro-extension components, since a mutation in any of these genes leads to the formation of unusually short F pili and/or fewer F pili per cell (14, 28, 31). (TrbB and TraF have been suggested to have overlapping functions [26], which might explain why trbB mutations had no effect in a traF+ background [24].) TrbI would be pro-retraction, insofar as trbI mutants have been reported to elaborate unusually long F pili (28). Given that two-hybrid data cannot reveal interaction dynamics, this model will have to be tested in E. coli, in which the complete F-pilus assembly system can be analyzed (16).
A summary of this and our previous work on Tra protein interactions related to F-pilus formation is shown in Fig. 4. The TraB/TraK/TraV interaction group (19) consists of proteins with homologues, or at least obvious functional equivalents, among other TFSSs (20, 26). The TraH interaction group described here consists of Tra proteins that are characteristic of F and F-like systems (1, 26). How these interaction groups function in F-pilus formation and perhaps other stages of conjugal DNA transfer and especially whether the TraH and TraV/TraK/TraB groups interact with each other are topics of immediate interest.
Acknowledgments
We are indebted to Veronica Hombs for excellent technical assistance.
This work was supported by National Science Foundation grants MCB-9900533 and MCB-0212365 and by funds provided by the Oklahoma Medical Research Foundation. P.M.S. acknowledges support from the Marjorie Nichlos Chair in Medical Research.
REFERENCES
- 1.Anthony, K., W. Klimke, J. Manchak, and L. Frost. 1999. Comparison of proteins involved in pilus synthesis and mating pair stabilization from the related plasmids F and R100-1: insights into the mechanism of conjugation. J. Bacteriol. 181:5149-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biebricher, C., and E.-M. Duker. 1984. F and type I piliation of Escherichia coli. J. Gen. Microbiol. 130:951-957. [DOI] [PubMed] [Google Scholar]
- 3.Cao, T., and M. Saier. 2001. Conjugal type IV macromolecular transfer systems of gram-negative bacteria: organismal distribution, structural constraints and evolutionary conclusions. Microbiology 147:3201-3214. [DOI] [PubMed] [Google Scholar]
- 4.Cascales, E., and P. Christie. 2003. The versatile bacterial type IV secretion systems. Nat. Rev. Microbiol. 1:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie, P. 1997. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in Eubacteria. J. Bacteriol. 179:3085-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie, P., and J. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covacci, A., J. Telford, G. Del Giudice, J. Parsonnet, and R. Rappuoli. 1999. Helicobacter pylori virulence and genetic geography. Science 284:1328-1333. [DOI] [PubMed] [Google Scholar]
- 8.Curtiss, R., III. 1969. Bacterial conjugation. Annu. Rev. Microbiol. 23:69-127. [DOI] [PubMed] [Google Scholar]
- 9.Curtiss, R., III, L. Caro, D. Allison, and D. Stallions. 1969. Early stages of conjugation in Escherichia coli. J. Bacteriol. 100:1091-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durfee, T., K. Becherer, P.-L. Chen, S.-L. Yeh, Y. Yang, A. Kilburn, W.-H. Lee, and S. Elledge. 1993. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 7:555-569. [DOI] [PubMed] [Google Scholar]
- 11.Durrenberger, M., W. Villiger, and T. H. Bachi. 1991. Conjugational junctions: morphology of specific contacts in conjugating Escherichia coli bacteria. J. Struct. Biol. 107:146-156. [DOI] [PubMed] [Google Scholar]
- 12.Eisenbrandt, R., M. Kalkum, E. Lai, R. Lurz, C. Kado, and E. Lanka. 1999. Conjugative pili of IncP plasmids, and the Ti plasmid T pilus are composed of cyclic subunits. J. Biol. Chem. 274:22548-22555. [DOI] [PubMed] [Google Scholar]
- 13.Feinberg, A., and B. Vogelstein. 1983. A technique for radiolabeling DNA restriction fragments to high specific activity. Anal. Biochem. 132:6-13. [DOI] [PubMed] [Google Scholar]
- 14.Firth, N., K. Ippen-Ihler, and R. Skurray. 1996. Structure and function of the F factor and mechanism of conjugation, p. 2377-2401. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 15.Frost, L., K. Ippen-Ihler, and R. Skurray. 1994. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol. Rev. 58:162-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman, T., and P. Silverman. 1989. Structure and function of conjugative pili: inducible synthesis of functional F pili by Escherichia coli K-12 containing a lac-tra operon fusion. J. Bacteriol. 171:650-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haase, J., R. Lurz, A. Grahn, D. Bamford, and E. Lanka. 1995. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J. Bacteriol. 177:4779-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, R., A. Sholl, M. Conrad, M. Dresser, and P. Silverman. 1999. Interaction between the F plasmid TraA (F-pilin) and TraQ proteins. Mol. Microbiol. 34:780-791. [DOI] [PubMed] [Google Scholar]
- 19.Harris, R., V. Hombs, and P. Silverman. 2001. Evidence that F-plasmid proteins TraV, TraK, and TraB assemble into an envelope-spanning structure in Escherichia coli. Mol. Microbiol. 42:757-766. [DOI] [PubMed] [Google Scholar]
- 20.Harris, R. L., and P. M. Silverman. 2002. Role of internal cysteines in the function, localization, and reactivity of the TraV outer membrane lipoprotein encoded by the F plasmid. J. Bacteriol. 184:3126-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ippen-Ihler, K., and S. Maneewannakul. 1991. Conjugation among enteric bacteria: mating systems dependent on expression of pili, p. 35-69. In M. Dworkin (ed.), Microbial cell-cell interactions. ASM Press, Washington, D.C.
- 22.Jacobson, A. 1972. Role of F pili in the penetration of bacteriophage f1. J. Virol. 10:835-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Kathir, P., and K. Ippen-Ihler. 1991. Construction and characterization of derivatives carrying insertion mutations in F plasmid transfer region genes trbA, artA, traQ, and trbB. Plasmid 26:40-54. [DOI] [PubMed] [Google Scholar]
- 25.Lawley, T., G. Gordon, A. Wright, and D. Taylor. 2002. Bacterial conjugative transfer: visualization of successful mating pairs and plasmid establishment in live Escherichia coli. Mol. Microbiol. 44:947-956. [DOI] [PubMed] [Google Scholar]
- 26.Lawley, T., W. Klimke, M. Gubbins, and L. Frost. 2003. F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 224:1-15. [DOI] [PubMed] [Google Scholar]
- 27.Llosa, M., F. Gomis-Ruth, M. Coll, and F. de la Cruz. 2002. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 28.Maneewannakul, S., K. Maneewannakul, and K. Ippen-Ihler. 1992. Characterization, localization, and sequence of F transfer region products: the pilus assembly gene product TraW and a new product, TrbI. J. Bacteriol. 174:5567-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marvin, D., and B. Hohn. 1969. Filamentous bacterial viruses. Bacteriol. Rev. 33:172-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore, D., B. Sowa, and K. Ippen-Ihler. 1981. The effect of tra mutations on the F-pilin polypeptide. Mol. Gen. Genet. 184:260-264. [DOI] [PubMed] [Google Scholar]
- 31.Moore, D., K. Maneewannakul, S. Maneewannakul, J. Wu, K. Ippen-Ihler, and D. Bradley. 1990. Characterization of the F-plasmid transfer gene traU. J. Bacteriol. 172:4263-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paranchych, W., and L. Frost. 1988. The physiology and biochemistry of pili. Adv. Microb. Physiol. 29:53-114. [DOI] [PubMed] [Google Scholar]
- 33.Samuels, A., E. Lanka, and J. Davies. 2000. Conjugative junctions in RP4-mediated mating of Escherichia coli. J. Bacteriol. 182:2709-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schandel, K., M. Muller, and R. Webster. 1992. Localization of TraC, a protein involved in assembly of the F conjugative pilus. J. Bacteriol. 174:3800-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroder, G., S. Krause, E. Zechner, B. Traxler, H.-J. Yeo, R. Lurz, G. Waksman, and E. Lanka. 2002. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J. Bacteriol. 184:2767-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowa, B., D. Moore, and K. Ippen-Ihler. 1983. Physiology of F-pilin synthesis and utilization. J. Bacteriol. 153:962-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teuber, M. 1999. Spread of antibiotic resistance with food-borne pathogens. Cell Mol. Life Sci. 56:755-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, J., P. Kathir, and K. Ippen-Ihler. 1988. The product of the F plasmid transfer operon gene, traF, is a periplasmic protein. J . Bacteriol. 170:3633-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]