Abstract
Objectives
To identify studies of existing instruments available for clinicians to record overall patient preferences and priorities for care, suitable for use in routine primary care practice in patients with multimorbidity. To examine the data for all identified tools with respect to validity, acceptability and effect on health outcomes.
Design
Systematic Review.
Data sources
MEDLINE, EMBASE and Cochrane databases, each with a predefined search strategy.
Eligibility criteria
Citations were included if they reported a tool used to record patient priorities or preferences for treatment, and quantitative or qualitative results following administration of the tool.
Results
Our search identified 189 potential studies of which 6 original studies and 2 discussion papers were included after screening for relevance. 5 of 6 studies (83%) were of cross-sectional design and of moderate quality. All studies reported on the usability of a tool in order to elicit patient preferences. No studies reported on changes to patient-specific healthcare outcomes as a consequence of recording preferences and priorities. 1 of 6 studies reported on eliciting patient preference in the context of multimorbidity. No studies incorporated patient preferences into an electronic medical record.
Conclusions
Given the importance of eliciting patient priorities and preferences in providing patient-centred care in the context of multimorbidity and polypharmacy, we found surprisingly few relevant tools. Some aspects of the tools used for single-disease contexts may also be useful in the context of multimorbidity. There is an urgent need to develop ways to make patient priorities explicitly visible in the clinical record and medical decision-making and to test the effect on patient-relevant outcomes.
Keywords: PRIMARY CARE, GERIATRIC MEDICINE, REHABILITATION MEDICINE
Strengths and limitations of this study.
Descriptions of good medical care for people with multimorbidity commonly include terms such as patient centered care and person focused care.
Identifying patient priorities and preferences for care is important in providing patient-centred care and person focused care over time in the context of polypharmacy and multimorbidity.
It is therefore surprising that our systematic review identified only one tool for recording priorities or preferences in clinical primary care that was suitable for use in patients with multimorbidity.
Our results highlight an urgent need to develop ways to make patient priorities explicitly visible in clinical records, and in medical decision-making, and to test their effect on patient-relevant outcomes.
The lack of studies assessing the effect on patient relevant health outcomes or adverse effects limited any ability to assess the impact of using these tools.
Introduction
In an ageing population, the pattern of illness has shifted such that multimorbidity is the norm. Clinicians face substantial challenges in dealing well with chronic multimorbidity. It is recognised that the existing single-disease approach to healthcare, health policy and measures of quality of care makes polypharmacy inevitable in this situation, and adverse drug effects add to the burden of morbidity and mortality.1 The burden of this complex care may also exceed the patient's ability to cope, and the effects on compliance may reduce the effectiveness of the treatments most likely to benefit the patient.2 3
The system of healthcare is beginning to make adjustments around the move from single disease-centred care to an approach that focuses on multimorbidity and values person-focused care over time, recognising that an improvement in health status is not an end in itself but the means to fulfilment and possibility in the life of the patient.1 4 Patient-centred care can be confused with satisfaction; however, it involves multiple domains: exploring both the disease and the illness experience, understanding the whole person, finding common ground, incorporating prevention and health promotion, enhancing the patient–doctor relationship, and being realistic.5 This is not ‘giving the patients what they want’ (preferences) as might be implied by patient satisfaction but does involve taking account of individual preferences (what patients do and do not want) as well as their priorities (relative importance) in decisions about care. Patient-centred care can occur in a single consultation; however, person-focused care adds the additional dimension of care over time, which in the context of multimorbidity is both essential and requires priority setting as well as preferences for care.
The incorporation of patients' priorities and preferences for treatment in their care is then an important aspect of operationalising this shift in rhetoric, to minimise the burden of care and the harms of overtreatment by prioritising interventions most important to the patient. Incorporating patient priorities and preferences into their healthcare can improve desirable proximal outcomes related to communication such as the patient feeling heard, understood, respected and engaged in their care, which in themselves can mitigate the negative effects of being ill and can assist clinicians in decision-making.6–11 This can improve medical and physiological outcomes, as well as result in decreased anxiety, greater confidence in and adherence to treatment plans, increased satisfaction with care and higher levels of trust in healthcare providers.11–13
The best informant regarding individual patient preferences and values is the patient.8 14 It is important that clinicians know and integrate these patient priorities and preferences with other information, such as diagnoses, available treatments and patient status indicators.10 It is also important that these priorities and preferences are visible in integrating care between multiple treatment providers, and visible so that measures of care and health policy and service delivery models can value person-focused care, not just disease focused decision-making.
The issue that then arises is the best method of determining and recording patient preferences and priorities, and the effect this has on outcomes of relevance to the patient.
In order to accurately elicit patient priorities and preferences for care, clinicians must often translate confusing and convoluted medical information into a form that their patients can understand; the way in which information is presented can affect how patients interpret it and the decisions they choose to make using it.15 To do this, various decision aids have been designed to support patients in making choices about the healthcare they receive. The most basic aids use layman's terms to provide information about available options—including, where reasonable, the option of taking no action, and the associated outcomes.16 There are excellent decision aids being developed to try to support patient understanding of the existing research on risks and benefits of single treatments and diagnostic tests in this way, as well as standards for evaluating these decision aids.17 However, dealing with multimorbidity, for example, in reducing harmful polypharmacy, requires an understanding of patients' priorities and preferences for care across multiple illnesses. Clinicians acknowledge that they struggle with this process, and that it is a barrier to addressing polypharmacy.18 19
Noting that there may be a difference between the kind of tool that might be developed for research purposes and one for use in clinical practice, we carried out a systematic review to answer the following question: In adults with multimorbidity, what tools exist that are suitable for recording priorities and preferences in clinical primary care, and what impact do these have on patient relevant outcomes?
We noted whether any tools had been incorporated into routine electronic or clinical records, planning a subgroup analysis if possible.
Methods
We followed the MOOSE and PRISMA guidelines for meta-analyses and systematic reviews of observational studies in designing and reporting this study.20
Selection and inclusion criteria: We searched EMBASE, MEDLINE and the Cochrane Library to identify English language abstracts published up to January 2015. Search terms and strategy were determined by the investigators with advice from a librarian (box 1). We also used the Google search engine to identify articles in the grey literature, and manually searched reference lists of included and excluded articles for other articles of potential relevance.
Box 1. Search terms.
1 Recording AND Patient
2 Patient Preferenc$ OR Patient Goal$ OR Patient Choice$ OR patient priorit$
3 Patient AND Preferenc$
4 Tool$ OR Scale$
5 Record OR Document OR Account OR Incorporate$
Subssearch:
7 EMR OR Electronic OR Technology OR Digital
Abstracts were retrieved and screened, and articles that were determined to be of potential relevance were retained. These articles were screened in detail and those meeting the inclusion criteria were retained. Each abstract screened and each potentially relevant article retrieved was reviewed independently by two authors (by DM and either GS or VB). We did not restrict our search to particular types of patients or settings. However, we included only papers reporting tools suitable for use in primary care settings, and for use in patients with multimorbidity.
Abstracts were included if they satisfied the following criteria: (1) development, validation or evaluation of a tool, or a trial incorporating priorities or preferences that (2) evaluated making patient priorities or preference for care visible, defined as the process of asking about, determining and recording patient priorities or preferences. While the objective was to find tools used in multimorbidity, disease or treatment-specific tools were reviewed for any aspects that might be suitable for use in multimorbidity. We did not restrict by study design but quantitative or qualitative results had to be available.
Exclusions: We excluded tools used to elicit group norms. Decision support type tools were also excluded where they were primarily aimed at summarising research literature but did not provide for explicit recording of patient preferences and priorities for care. Abstracts only and overviews or reviews were excluded in this study. A subgroup assessment was planned to assess any of these tools that had been tested in an e-Health setting (ie, had been incorporated into patient electronic medical records (EMR)).
Data from included articles were extracted using a standardised form describing the following study characteristics: year, target population, location, sample size, validity testing, name of tool, study design, outcome measure, incorporation into EMR, clinical utility, patient relevance of outcome, knowledge gaps, key conclusions and study setting. We assessed for duplicate citations and tools.
Quality measure
We did not perform a meta-analysis as the studies we retrieved were heterogeneous, and all were observational designs. Therefore we present here a narrative summary and review of the studies retrieved. The quality of included studies was assessed using the National Institutes of Health Quality Assessment tool for Observational Cohort and Cross Sectional Studies (http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort). Reporting of studies was assessed using an adapted version of the STROBE statement, which is a checklist of items that should be addressed in articles reporting on three main study designs: cohort, case–control and cross-sectional.21 This is included in the online supplementary material.
bmjopen-2015-010903supp.pdf (332.3KB, pdf)
Results
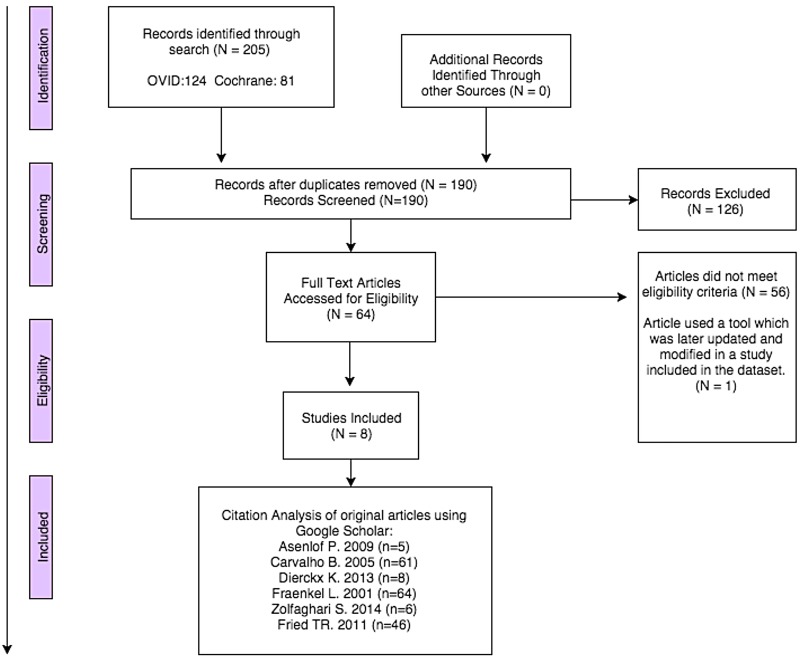
Our search retrieved 190 citations of which 64 were potentially relevant abstracts. Figure 1 shows a flow chart of the search, identification and screening results.
Figure 1.
Flow chart of search, identification and screening of studies for inclusion.
There was little disagreement between the two reviewers doing the screening; one reviewer included two additional papers, one of which was a background review and the other was a tool for extracting patient clinical indicators. Of these 64 abstracts screened, 8 articles fit the inclusion criteria, including 1 article that was a later updated publication of data on the same tool described in another article. These eight articles were included in the data set and included six original articles22–27 and two discussion papers.10 28 These discussion papers were not systematic reviews but described important patient preference-related concepts that were felt to have possible relevance, one illustrating the use of semantic structure mapping for EMRs10 and the other highlighting theoretical and practical considerations in eliciting preferred priorities for care using four case studies.28 Aspects of these papers are included in the discussion section and online supplementary materials. The six original articles and their characteristics are summarised in table 1. Excluded studies and reasons for exclusion are listed in the online supplementary material.
Table 1.
Characteristics of included studies regarding study design, patient preference tool, outcome measures and relevance to multimorbidity
| Study ID (reference) | Type of study | Location | Target population and sample size | Setting of study | Name and type of tool used to elicit patient priorities and preferences | Outcome measured | Patient relevance of outcome | Validity testing | Incorporation into electronic medical records | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Asenlof (2009) | Prospective cohort | Uppsala, Sweden | Patients with subacute or chronic (>4weeks) musculoskeletal pain (n=54) | Primary care | Patient Goal Priority Questionnaire (PGPQ) and Patient Goal Priority List (PGPL) (Questionnaire) |
Patient behavioural goals in the context of physical therapy. PGPL differs from PGPQ in that PGPL is a questionnaire that does not place restrictions on activities and is administered with the help of the physical therapist. | Not relevant, while the tool used was for measuring patient priority; it was used as a decision aid as opposed to a tool which was directly tied to patient health outcomes. | 52% raw percentage of agreement of the PGPQ questionnaire on two occasions. 14% raw percentage agreement between the PGPQ and PGPL questionnaires. |
No | None |
| Carvalho, et al23 | Cross-sectional | California, USA | Pregnant women attending a prenatal class (n=82) | Outpatient | Pre-CS survey (Questionnaire) | Patient's preference in regard to potential outcomes in a caesarean delivery, measured by both a ranking and relative value score methodology. | Not relevant; the tool was a measure of patient preference for anaesthesia but was not directly tied to patient health outcomes before or after surgery. | Ranking and relative outcome scores for each outcome were positively correlated (R2=0.6–0.8 for 9 outcome variables, P<0.001). | No | None |
| Dierckx, et al24 | Cross-sectional | Ghent, Belgium | Patients >18 years attending physical therapy (n=237) | Outpatient | Control Preference Scale (CPS) (Questionnaire) | To document the patient’s preference in the degree of control they wish to have in their level of care. Also measured agreement of perceived patient level of control between the therapist and the patient. The OPTION tool was used to measure the degree to which patient involvement occurred during the decision-making process. | Not relevant; qualitative study model which did not measure the effectiveness of SDM vs no SDM but rather was looking at agreement between therapist and patient perception of level of involvement in care. | The CPS questionnaire was based on a Dutch version of the instrument which was not validity tested. | No | None |
| Fraenkel, et al25 | Cross-sectional | Connecticut, USA | Women with diagnosed systemic lupus erythematosus (n=103) | Primary care | Adaptive Conjoint Analysis (ACA) (Questionnaire) | Ask patients to choose between competing products, stimulating the way the patient would actually make choices faced with a task. Based on three assumptions: (1) Each treatment option can be broken down into attributes which are defined by a number of levels (eg, Risk of infection on medication A, B, and C are respectively 10%, 20% and 30%. (2) Respondents have unique values for each attribute level. (3) Utilities and values can be combined across attributes (eg, if the sum of the attributes for medication A is more than for medication B, then the patient should prefer medication A) | Not relevant; the goal of the study was to assess the effectiveness of adaptive conjoint analysis and also to see patient-related preferences on cytotoxic medications. The study did not measure changes in health outcome as a direct consequence of applying adaptive conjoint analysis. | Study did not explicitly measure the validity of the tool but instead reported good ‘face validity’. | No | None |
| Zolfaghari et al (2014) | Cross-sectional | Mannheim, Germany | Patients with atrial fibrillation on oral anti-coagulant therapy (n=180) | Primary care | Likert scale (Questionnaire) | The study looked to objectively identify the patient's preferences for the use of a vitamin K antagonist compared to direct oral anticoagulants. | Not a patient-relevant outcome measure, the tool was a measure of patient preference for anticoagulant therapy but did not measure direct changes to health outcome as a consequence of introducing the tool. | The reliability of the items measured in the questionnaire was tested and had a reliability of the seven items with a probability of 98%. | No | None;, did not present outcome results |
| Fried, et al26 | Cross-sectional | Connecticut, USA | Older patients (>65) with multiple chronic conditions (n=357) | Community dwelling | Priority (Questionnaire) | Measured patient priorities regarding the following outcome measures (Tool domains): “Keeping you alive maintaining independence pain management symptom management” | Not relevant, the study was validating health outcome prioritisation as a tool for decision-making. Study did not evaluate the effectiveness of such a tool on improving health outcomes but did use a tool relevant in the context of multimorbidity | Percentage agreement amount 35 participants who completed the tool regarding the most and least important outcome ranged from 85% to 100%. | No | None |
The study participant size ranged from 54 to 357. We found that four22 25–27 of the six original research studies were conducted in a primary care or community setting, while the remaining two were conducted in a tertiary care setting.23 24 One22 of these six studies used a prospective cohort design and five were cross-sectional.23–27 Four of six studies related to a single disease-specific context22 23 25 27 and the other two were relevant to primary care patients' preferences and priorities for care in the context of multimorbidity.24 26 Four22 23 26 27 of six articles explicitly recorded any type of validity testing of the tool used. No articles provided patient relevant outcome measures and none reported on potential adverse outcomes of using the tool.
Population characteristics in included studies
As outlined in table 1, the geographic range of studies was wide: three were carried out in the USA, one in Germany, one in Belgium and one in Sweden. Among the four studies that focused on eliciting patient preference in a disease or condition-specific context, patient preference was explored in the following groups:
Women attending prenatal class preparing for a caesarean section delivery;
Patients with subacute or chronic musculoskeletal pain;
Women diagnosed with lupus erythematosus;
Patients with atrial fibrillation on anticoagulant therapy.
Mode for recording priorities and preferences
Three of six studies explicitly or indirectly used a priority ranking scale to elicit patient preference for either personal treatment goals or most valued treatment outcomes.22 23 26 Two studies24 27 used a Likert scale to evaluate responses to patient preference specific questions: one had a focus on patient preference for control in decision-making and the other used a Likert scale to compare patient preference for two different treatment types. Finally, adaptive conjoint analysis (ACA) was used in one of six studies.25 The internal validity of the ACA tool was not formally measured in this study. All tools were delivered in the form of surveys which were converted to an interview script format for discussion with patients. A detailed description of these four studies is included in the online supplementary material.
Quality of included studies
Table 2 describes the quality of included studies. All studies were of cross-sectional design and of low–moderate quality. Five of six studies demonstrated some degree of selection bias. Four of six studies considered and identified potential biases either in the methods or discussion section but did not take further steps to adjust for or address the potential bias described. Furthermore, four of six studies did not clearly describe their study design or the study design described did not match the methods actually used. Finally, three of six studies used tools that were translated into a second language without description of validation in that language.22 24 27
Table 2.
Quality of included studies (NIH Quality Assessment Tool)
| Criteria | Asenlof (2009) | Carvalho, et al25 | Dierckx, et al24 | Fraenkel, et al25 | Zolfaghari, et al (2014) | Fried, et al26 |
|---|---|---|---|---|---|---|
| Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the study population clearly specified and defined? | Yes | No; a source of recruiting participants was discussed but there were no clear inclusion/exclusion criteria provided. | Yes | Yes | Yes | Yes |
| Was the participation rate of eligible persons at least 50%? | Yes | Yes | No; initial recruitment of physical therapists had a ‘very low participation rate’ according to the study. Of the 125 physiotherapists who were invited to participate, 10% agreed. There was a 90.5% participation rate among patients recruited. |
Participation rate not mentioned. | Participation rate was not included in the study. | Yes |
| Were all the participants selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes | Yes | Yes | Yes; study recruited based on another study’s population whose inclusion/exclusion criteria were explicit. |
| Was a sample size justification, power description, or variance and effect estimates provided? | Yes; describes a pragmatic approach. Sample size was dependent on a recruitment within the predetermined time limit set by the study organisers. | No | No | Yes; sample size was based on the number needed to stabilise conjoint results. | No justification for the sample size was included in the study. | No justification for the sample size was included in the study. |
| For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the time frame sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | Yes | NA (Cross-Sectional Study) | Yes | NA | NA | NA |
| For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (eg, categories of exposure, or exposure measured as a continuous variable)? | NA | NA (Cross-Sectional Study) | NA (Tools administered once) | NA | NA | NA |
| Were the exposure measures (independent variables) clearly defined, valid, reliable and implemented consistently across all study participants? | No; all participants were not included in each analysis and measurements. | Yes | Yes | Yes | Yes | Yes |
| Was the exposure(s) assessed more than once over time? | Yes | No | No | No | No | Tool was applied twice to check test–retest validity |
| Were the outcome measures (dependent variables) clearly defined, valid, reliable and implemented consistently across all study participants? | No; while the outcome measures were clearly defined, they did not get implemented consistently across all study participants as not all participants completed each type of questionnaire. | Yes | Yes | Yes | Yes | Yes |
| Were the outcome assessors blinded to the exposure status of participants? | Yes | NA | No mention of blinding | NA | No mention of blinding | No mention of blinding |
| Was loss to follow-up after baseline 20% or less? | No; only 32 patients in the sample completed the survey at all measurement points. | No; while 82% completed the tool initially, only 23% completed it again post-caesarean section | NA | NA | NA | NA |
| Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes; the study addresses parity, social education and previous surgeries in the results section and identifies no statistically significant effects on current preferences. | Yes; the study identified no significant difference in agreement of tools based on age, sex, level of education and several other demographic/personal characteristics of clients | Yes; the study assessed for differences in outcome based on patient demographics or characteristics. | Study identified differences in patient characteristics, but these differences were not analysed when interpreting results. | Yes |
NA, not applicable.
We found no studies that examined the effect on health outcomes either from a patient or medical perspective.
Health outcome prioritisation as a tool for decision-making
Most (3 of 6) studies used some kind of priority ranking scale for eliciting patient perspectives.22 25 26 Of these three studies, we found one26 that was relevant to patient priorities or preferences in the context of multimorbidity and included universal outcomes that were of relevance to patients. This was a cross-sectional study among seniors in the USA.26 Sixty-nine per cent of participants had >4 chronic conditions and 49% had >4 prescribed medications. A tool for determining patient's priorities for care was administered in the form of a face-to-face interview in which participants were instructed to evaluate the following universal health outcomes: ‘staying alive, maintaining independence, reducing/eliminating pain and reducing/eliminating other symptoms (eg, dizziness, fatigue or shortness of breath).’ Participants were subsequently asked to provide a rank in order of their priorities by ranking these different outcomes along a single visual analogue scale from 0 to 100, with higher scores indicating that the outcome was more important to them.
The tool used was a modified version of a tool described in a previous article by the same authors. Test–retest validity in the earlier study was low, so the tool was modified on the basis of the results to try to improve this, by changing the script describing use of the tool to better communicate the concept of ‘trade off’. The test–retest validity of the modified tool was significantly higher (85–100% vs 45–100%). Per cent agreement for the second iteration of the tool regarding the most and least important outcomes ranged from 85% to 100%. Agreement across all four ranks was higher for maintaining independence and staying alive (83% and 77%) than for reducing pain and reducing other symptoms (46% and 51%).
Adverse effects of measuring patient preferences or priorities
No included studies reported on adverse effects of using patient preference elicitation tools.
Conclusion
In shared decision-making, decision aids provide summaries of the research evidence for incorporation into clinical decision-making and there is evidence supporting their use. Despite this, a discussion paper10 concluded that decision aids, while useful, are stand-alone systems that occasionally are offered to patients but currently rarely used by clinicians as a part of routine practice.
Incorporating patient priorities and preferences for care into clinical decision-making is another equally important domain of evidence-based medicine. Despite the value ascribed to taking account of patient priorities and preferences for care in decision-making for treatments in the context of multimorbidity, we found few explicit tools available to support this process. Most studies focused on capturing preferences, two on capturing priorities, and no available studies measured any effect on health outcomes of importance for patients. Overall, we found that most tools developed with the purpose of incorporating patient preferences or priorities in primary care are single disease or context-specific with little direct utility in multimorbidity and managing polypharmacy. With the exception of two studies, patient preference incorporation as a concept was interpreted as the elicitation of patient experiences and priorities in order to make appropriate healthcare service delivery decisions or choices for patient groups. The exceptions included one study that explored patient preference from the perspective of ‘locus of control’ for decision-making and viewed patient preference elicitation as a concept driven by the agreement between healthcare provider and patient on the preferred level of involvement in care decision-making.24 The results of the study indicated a low level of shared decision-making in clinical practice and reinforced previous studies which showed that the clinician's perception of patient preference when determined implicitly may differ substantially from the patient’s actual preferences.29 30 In this study, in 64% of cases the patient preferred to have more active involvement in decision-making than the physical therapist had perceived they would like.24
Two studies24 26 looked at eliciting patient priorities or preferences in multimorbidity and one of these had good test–retest validity.26
Overall, it appears that while SDM and inclusion of patient priorities and preferences in patient care have been embraced in theory, high-quality research is lacking in the development of clinically applicable tools for eliciting patient priorities and preferences in multimorbidity settings. No study measured direct health outcomes as a consequence of introducing tools to help elicit and record patient preference. We found no studies that had included tools as part of EMR. There were two relevant discussion papers: one reviewed dimensions for consideration when incorporating patient preferences into electronic medical records. Another reviewed potential methods used for preference elicitation and the underlying patient preference concepts that may be helpful in developing and testing such tools. These are discussed in the online supplementary material.
Healthcare providers sometimes avoid advanced care planning discussions and the elicitation of patient preferences out of fear of causing distress.28 A weakness of all studies was the absence of explicit elicitation or measurement of adverse effects of using tools eliciting patient priorities and preferences. It cannot be assumed that a tool for eliciting patient preferences and priorities has only positive or neutral effects. This is hinted at in a study by Nakagowa al31 on advanced care planning, where 1 in 20 participants indicated that they were unwilling to discuss preferences for care, perhaps suggesting seeking that this kind of information has the potential for making patients feel uncomfortable.
Though we searched the grey literature beyond published articles, and manually searched reference lists, it is possible that other tools have been developed as part of routine service delivery and that we potentially omitted relevant articles as well as unpublished tools. This area is relatively new in published research, and our search strategy relied on the keywords we chose for the search and also on the authors of studies assigning these keywords. We also focused on tools that were suitable for use in multimorbidity, though we looked at tools from other settings that might have relevance for use in multimorbidity. The lack of outcomes data meant that a meta-analysis was not possible to determine the extent to which any approach improves patient-important outcomes.
In order to address the challenges of multimorbidity well, including dealing with polypharmacy, the rhetoric of incorporating patient priorities and preferences into care must be transformed into action. The existing literature indicates that many physicians feel uncomfortable starting these conversations and, as outlined, implicit clinician and patient views of patient priorities and preferences may not be concordant. A controlled trial in older adults suggested that eliciting patient preferences for care and providing this to clinicians changes the clinician's care priorities to be more consistent to patient preferences.11
The results of this review highlight the important work still required to systematically develop tools to support this core domain of primary care, and to make patient priorities and preferences explicitly visible in the clinical record and medical decision-making. At present, a look at any EMR will show a wealth of data on individual diseases, but no systematic way or place to record patient priorities or preferences. However, evidence-based clinical decision-making requires that clinicians give equal weight to research evidence, patient context, priorities and preferences in supporting decision-making, and test the effect on patient-relevant outcomes. Without routine recording of patient priorities and preferences, it is unlikely that this will be transmitted in discussions between clinicians and, also importantly, valued in measures of quality of care or in policy.
Excellent work is being performed to create decision aids to inform patient about specific treatments and decisions; however, multimorbidity care, if it is to avoid the problems of polypharmacy, is going to require prioritisation of treatments based both on the evidence and on patient preferences. We found little literature addressing priority ranking. The paper by Fried et al26 provide a model that illustrates the novel approach required. It is important that such tools are rigorously evaluated for validity, feasibility in clinical practice, patient relevance and effect on improving health outcomes in randomised trials if they are to deliver on their promise. End of life care literature indicates that preferences for care may be stable over time. However, it can be envisaged that priorities may change in the light of new problems.32 33 The Fried study reinforces this: broad priorities remained similar while there was some movement in priorities related to specific symptoms. To prioritise treatments most important to the patient requires a horizontal assessment across treatments and diseases that takes account of an individual patient's universal priorities and trade-offs as well as the expression of their particular pattern of illness and the significance of particular symptoms. Work is needed with patients to gain their perspectives to understand how a tool for use in routine primary care might be structured. We would envisage that such a tool would be adjusted over time, reviewed when major clinical reviews are carried out, for example, a yearly or two-yearly medication review and transferred as a routine into referral letters to other clinicians to avoid the patient repeating their story, or a lack of acknowledgement of these priorities where care is shared between providers in different areas, or at transitions of care.
Looking at the current situation, T.S. Eliot might well ask, “where is the patient we have lost in diseases?”34
Acknowledgments
Thanks to A Drake for helpful comments on the manuscript and J Parascandalo for help with preparing the manuscript and formatting of tables and figures.
Footnotes
Twitter: Follow Cathy Risdon at @risdonc
Contributors: DM conceived the review and was responsible for the overall design. DM, GS and VB designed the detailed search strategy. GS and VB extracted the data. GS VB and DM screened and analysed the data. DM, GS, VB and CR interpreted the analysis, wrote the paper and contributed to revisions.
Funding: VB was a summer student at the Department of Family Medicine, McMaster University, and was funded by a MacWork student bursary and the David Braley Nancy Gordon Chair in Family Medicine. Jenna Parascandalo is funded by the David Braley Nancy Gordon Chair in Family Medicine. DM's work is supported by the David Braley Nancy Gordon Chair in Family Medicine.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data are provided in the paper and supplementary files.
References
- 1.Mangin D, Heath I, Jamoulle M. Beyond diagnosis: responding to the comorbidity challenge. BMJ 2012;344:e3526 10.1136/bmj.e3526 [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. Micronutrient deficiencies. Battling iron deficiency anaemia. World Health Organisation, 2003. http://www.who.int/nut/ida.htm (updated 9 Apr 2003; cited 25 July 2003). [Google Scholar]
- 3.Vervloet M, Linn AJ, van Weert JCM et al. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc 2012;19:696–704. 10.1136/amiajnl-2011-000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starfield B. Primary care: balancing health needs, services, and technology. New York: Oxford University Press, 1998. [Google Scholar]
- 5.Stewart M, Brown JB, Weston WW et al. Patient-centred medicine: transforming the clinical method. United Kingdom: Radcliffe Medical Press, 2003. [Google Scholar]
- 6.Arora NK, Weaver KE, Clayman ML et al. Physicians’ decision-making style and psychosocial outcomes among cancer survivors. Patient Educ Couns 2009;77:404–12. 10.1016/j.pec.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laine C, Davidoff F. Patient-centered medicine. A professional evolution. JAMA 1996;275:152–6. [PubMed] [Google Scholar]
- 8.Little P, Everitt H, Williamson I et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ 2001;323:908–11. 10.1136/bmj.323.7318.908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Street RL Jr, Makoul G, Arora NK et al. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Couns 2009;74:295–301. 10.1016/j.pec.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 10.Ruland CM, Bakken S. Representing patient preference-related concepts for inclusion in electronic health records. J Biomed Inform 2001;34:415–22. 10.1006/jbin.2002.1035 [DOI] [PubMed] [Google Scholar]
- 11.Ruland CM. Improving patient outcomes by including patient preferences in nursing care. Proc AMIA Symp 1998:448–52. [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez KL, Appelt CJ, Switzer GE et al. Veterans’ decision-making preferences and perceived involvement in care for chronic heart failure. Heart Lung 2008;37:440–8. 10.1016/j.hrtlng.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Stewart M, Brown J, Weston W et al. Patient-centred medicine: transforming the clinical method. In: Patient Centred Care. Stewart M, Brown J, Freeman T, edrs. UK: Radcliff Medical Press Ltd, 2003:361. [Google Scholar]
- 14.Casper GR, Brennan PF. Improving the quality of patient care: the role of patient preferences in the clinical record. Proceedings of the Annual Symposium on Computer Application in Medical Care; 1993:8–11. [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ 2002;324:827–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elwyn G, O'Connor A, Stacey D et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ 2006;333:417 10.1136/bmj.38926.629329.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph-Williams N, Newcombe R, Politi M et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process. Med Decis Making 2013;34:699–710. 10.1177/0272989X13501721 [DOI] [PubMed] [Google Scholar]
- 18.Scott IA, Gray LC, Martin JH et al. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med 2012;125:529–37.e4. 10.1016/j.amjmed.2011.09.021 [DOI] [PubMed] [Google Scholar]
- 19.Nixon M, Vendelø MT. General practitioners' decisions about discontinuation of medication: an explorative study. J Health Organ Manag. 2016;30(4). [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asenlöf P, Siljebäck K. The patient goal priority questionnaire is moderately reproducible in people with persistent musculoskeletal pain. Phys Ther 2009;89:1226–34. 10.2522/ptj.20090030 [DOI] [PubMed] [Google Scholar]
- 23.Carvalho B, Cohen SE, Lipman SS et al. Patient preferences for anesthesia outcomes associated with cesarean delivery. Anesth Analg 2005;101:1182–7, table of contents. [DOI] [PubMed] [Google Scholar]
- 24.Dierckx K, Deveugele M, Roosen P et al. Implementation of shared decision making in physical therapy: observed level of involvement and patient preference. Phys Ther 2013;93:1321–30. 10.2522/ptj.20120286 [DOI] [PubMed] [Google Scholar]
- 25.Fraenkel L, Bodardus S, Wittnik DR. Understanding patient preferences for the treatment of lupus nephritis with adaptive conjoint analysis. Med Care 2001;39:1203–16. 10.1097/00005650-200111000-00007 [DOI] [PubMed] [Google Scholar]
- 26.Fried TR, Tinetti M, Agostini J et al. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ Couns 2011;83:278–82. 10.1016/j.pec.2010.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zolfaghari S, Harenberg J, Froelich L et al. Development of a tool to identify patients’ preference for vitamin K antagonist or direct oral anticoagulant therapy. Semin Thromb Hemost 2014;40:121–8. 10.1055/s-0033-1361940 [DOI] [PubMed] [Google Scholar]
- 28.Reynolds J, Croft S. Applying the preferred priorities for care document in practice. Nurs Stand 2011;25:35–42. 10.7748/ns2011.05.25.36.35.c8515 [DOI] [PubMed] [Google Scholar]
- 29.Sonntag U, Wiesner J, Fahrenkrog S et al. Motivational interviewing and shared decision making in primary care. Patient Educ Couns 2012;87:62–6. 10.1016/j.pec.2011.07.026 [DOI] [PubMed] [Google Scholar]
- 30.Elwyn G, Hutchings H, Edwards A et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect 2005;8:34–42. 10.1111/j.1369-7625.2004.00311.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa S, Clark EM, Cobbs EL et al. Promoting advance care planning documentation for veterans through an innovative electronic medical record template. J Am Geriatr Soc 2014;62:1811–13. 10.1111/jgs.13008 [DOI] [PubMed] [Google Scholar]
- 32.Everhart MA, Pearlman RA. Stability of patient preferences regarding life-sustaining treatments. Chest 1990;97:159–64. 10.1378/chest.97.1.159 [DOI] [PubMed] [Google Scholar]
- 33.Pruchno RA, Rovine MJ, Cartwright F et al. Stability and change in patient preferences and spouse substituted judgments regarding dialysis continuation. J Gerontol B Psychol Sci Soc Sci 2008;63:S81–91. 10.1093/geronb/63.2.S81 [DOI] [PubMed] [Google Scholar]
- 34.Adapted from Eliot TS. In: Choruses from the Rock. UK: Faber & Faber Ltd, 1934.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2015-010903supp.pdf (332.3KB, pdf)