Abstract
Background
Hair loss is caused by a variety of hair growth disorders, each with its own pathogenetic mechanism.
Methods
This review is based on pertinent articles retrieved by a selective search in PubMed, on the current German and European guidelines, and on the authors’ clinical and scientific experience.
Results
Excessive daily hair loss (effluvium) may be physiological, as in the postpartum state, or pathological, due for example to thyroid disturbances, drug effects, iron deficiency, or syphilis. Androgenetic alopecia generally manifests itself in women as diffuse thinning of the hair over the top of the scalp, and in men as receding temporal hairlines and loss of hair in the region of the whorl on the back of the head. Alopecia areata is patchy hair loss arising over a short time and involving the scalp, eyebrows, beard, or entire body. The hair loss of alopecia areata is reversible in principle but hard to treat. Folliculitis decalvans is a form of alopecia with scarring, characterized by inflamed papules, pustules, and crusts at the edges of the lesions. Lichen planopilaris generally presents with small patches of baldness, peripilar erythema, and round areas of skin scaling. Kossard’s frontal fibrosing alopecia is characterized by a receding hairline and loss of eyebrows.
Conclusion
Hair loss is a symptom, not a diagnosis. The pathogenesis of the alopecias involves a range of genetic, endocrine, immune, and inflammatory processes, each of which calls for its own form of treatment.
Hair is of both physiological and psychological importance. It protects against the sun’s ultraviolet rays and serves a biological signaling function. In the Western world at least, long and full women’s hair is considered beautiful and a sign of youth, while thick pigmented men’s hair signifies youth and vitality (1). It is thus hardly surprising that people with excessive hair loss often seek medical help.
Learning objectives
Reading this article should enable readers to
understand the normal physiology and pathophysiology of hair growth,
know the most common and cosmetically most disturbing types of increased hair loss and alopecia, and
carry out the indicated treatments in collaboration with a dermatologist.
Anatomy and physiology of hair growth
Healthy men and women generally have 80 000 to 120 000 vital terminal hairs on the scalp. Hair is composed of keratin and is produced in the hair follicles. All hair follicles go through repeated cycles of growth and rest (2).
During the growth (anagen) phase, which is 2–6 years long, a hair grows at a rate of about 0.3 mm per day, or 1 cm per month. The maximum attainable hair length depends on the duration of the anagen phase. A brief transitional (catagen) phase follows, and then a rest (telogen) phase lasting 2–4 months, after which the hair falls out (2).
Normally, the ca. 100 000 hairs on a person’s head grow independently of one another. Intrinsic or extrinsic factors can synchronize the hair follicles by inducing a premature transition from the anagen to the telogen phase, leading to noticeable hair loss 2–4 months later (2). These factors include hormones, growth factors, drugs, and the seasons (2, 3).
Hair growth cycle.
Each of the ca. 100 000 hairs on the head independently goes through a growth cycle consisting of three phases: anagen (3–6 years), catagen (1–2 weeks), and telogen (2–4 months).
History-taking in a patient with hair loss
A complaint of “hair loss” may refer to either of two things: an increased amount of hair falling out daily (effluvium) or visible hairlessness (alopecia). Up to 100 hairs normally fall out every day (3). It is important to ask patients about the drugs they are taking. While “hair loss” is listed as a possible side effect on practically all package inserts, only a few drugs are truly relevant (e1). For example, hair loss 2–4 months after the administration of multiple heparin injections is not at all rare (e2). Women should be asked about gynecological factors such as the initiation or discontinuation of hormonal contraceptive drugs. Transient postpartum effluvium is normal: the stress of delivery, and the hormonal changes afterward, cause many hair follicles to undergo a transition from the anagen to the telogen phase, so that hair loss is seen 2–4 months later (2).
Highly toxic factors such as chemotherapeutic drugs can induce severe follicular damage, causing hairs to break off in their follicles within one to three weeks. As a consolation, patients can be told that this process synchronizes the growth phases of the follicles, so that the hair, once it has grown back, is often thicker than before. Structural changes can occur in which originally straight hair regrows as curly hair, or vice versa (4).
Nomenclature.
Increased daily hair loss is called “effluvium”; visible hairlessness is called “alopecia.”
Physical examination
Inspection of the scalp (capillitium) reveals whether there is a visible reduction of the amount of hair (alopecia) and, if so, in what pattern. Any inflammatory redness or scaling should be noted, as psoriasis and eczema can cause effluvium (e3). Dermatoscopic examination of the scalp is helpful as well (5).
A clinical hair-pull test is supplemented by a tricho(rhizo)gram, in which 20–50 hairs are epilated with a rubber-shod artery clamp and then analyzed under a microscope. The differently formed roots in each of the growth phases can then be counted. A percentage of hairs in the telogen phase that exceeds 20% indicates increased hair shedding (6, e4). A non-invasive phototrichogram can also yield an estimate of the anagen-to-telogen ratio (e5) but cannot reveal root anomalies such as dystrophic hairs.
Laboratory tests for diffuse effluvium
In patients with effluvium of unknown cause, laboratory testing should be performed to exclude, in particular, the following:
Iron deficiency (ferritin) (7)
Thyroid dysfunction (TSH, T3, T4)
Stage II syphilis (TPPA test).
Syphilis is very rarely detected, but omitting to take the relevant history or to perform the TPPA test in a patient with hair loss can have serious consequences for the patient, and for the physician as well, if neurosyphilis should later develop.
Androgenetic alopecia
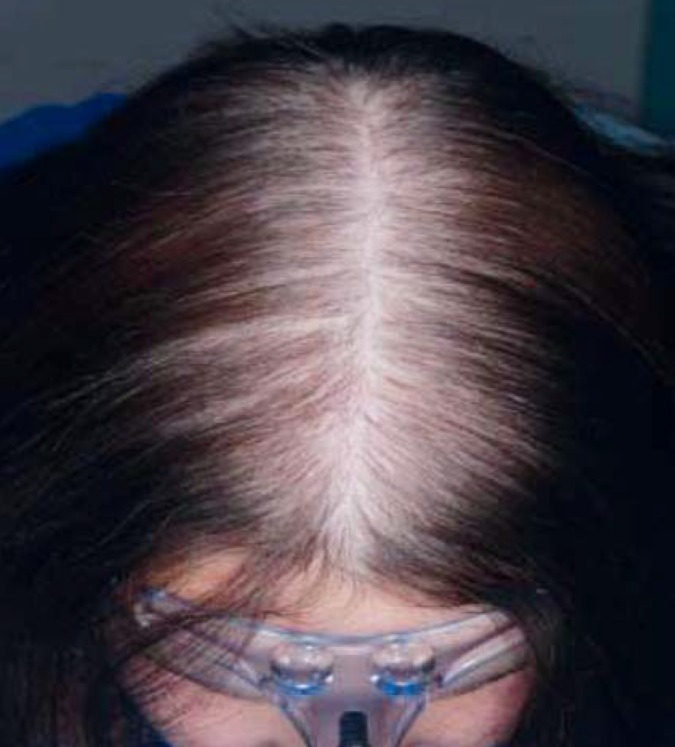
Androgenetic alopecia is the most common type of hair loss, affecting up to 70% of men and 40% of women (8). Histological examination reveals diminished size of terminal hair follicles in genetically predisposed areas of the scalp, shortening of hair growth phases, and decreased thickness of hair shafts (8). The pattern of hair loss is characteristic: in men, receding temporal hairlines and hair loss in the region of the whorl at the back of the head (Norwood–Hamilton type); in women, diffuse midline thinning on the top of the scalp (Ludwig type) (Figure 1).
Figure 1.
Androgenetic alopecia, female type, Ludwig grade I
Trichogram.
A trichogram yields an estimate of the percentages of actively growing (anagen) hairs and resting (telogen) hairs: the normal values are >80% and <20%, respectively.
Androgenetic alopecia in men is ascribed to genetic variants of the androgen receptor (8, 9). Dihydrotestosterone (DHT), generated from testosterone through the activity of the enzyme 5a-reductase, plays a key role (10). There is no association of male-pattern baldness and androgen levels in the blood; rather, the condition is thought to reflect a genetically variable sensitivity of hair follicles in the affected areas to normal levels of circulating androgen. Multiple genes are apparently involved (9). The gene for the androgen receptor lies on the X chromosome; thus, a man’s tendency to develop androgenetic alopecia in later life can be inherited in the maternal line (e6).
The data on androgenetic alopecia in women are sparse, but here, too, there is clear evidence of a genetic predisposition (e7).
The appropriate diagnostic evaluation of androgenetic alopecia, including a diagnostic algorithm, was presented in a European consensus statement in 2011 (11), with the following main conclusions:
Men with a typical balding pattern need no further laboratory evaluation. The diagnosis can be made on clinical grounds alone.
Women should undergo further laboratory testing depending on the history and physical examination. A gynecologic-endocrinological evaluation is indicated if there is evidence of hormone dysregulation (acne, hirsutism).
Androgenetic alopecia.
Androgenetic alopecia, the most common type of alopecia, is caused by a genetic predisposition and reflects the sensitivity of hair follicles to circulating androgens.
Treatment
The evidence-based European guideline for the diagnostic evaluation and treatment of androgenetic alopecia recently became available (12). The main goal is to stop hair loss and, if possible, reverse the shrinking of hair follicles, in order to promote the resumption of hair growth (12). The success or failure of treatment should be objectively documented with standardized photographic documentation and, optionally, a phototrichogram.
Two drugs are now recognized as effective against androgenetic alopecia: topical minoxidil solution (for women and men) and finasteride tablets, 1 mg (for men only).
Topical treatment with minoxidil.
The topical application of minoxidil is an effective treatment for androgenetic alopecia in both men and women.
Topical treatment with minoxidil
Minoxidil, a calcium channel opener, has been approved as a 2% solution for women (13, 14) and a 5% solution or foam for men (15) and can be bought in a pharmacy without a prescription in most parts of the world. A 5% minoxidil foam to be used once daily by women would simplify the treatment and is expected to be approved in Germany in 2016. Minoxidil solution is the only topically applied drug against androgenetic alopecia whose efficacy has been documented by high-level (level 1) evidence (12). It was found, in large-scale studies, to stop hair loss in 80–90% of persons treated; hair became visibly thicker in about 50% (13– 15). Its side effects include redness and scaling of the scalp; rarely, contact dermatitis; and hypertrichosis in women—mostly at the temples.
Systemic treatment with finasteride
Finasteride, a type II inhibitor of 5a-reductase, was approved in Germany in 1999 for use by men aged 18–41 at a dose of 1 mg po qd (10). Level 1 evidence documents its efficacy against androgenetic alopecia (12).
Systemic treatment with finasteride.
Finasteride 1 mg po qd can be given to men for the systemic treatment of androgenetic alopecia. Antiandrogens (EL 4) can be helpful for women with hyperandrogenemia.
Like minoxidil solution, finasteride at a dose of 1 mg per day stops hair loss in 80–90% of persons treated and visibly thickens hair in about 50% (10, 12). It is relatively well tolerated, with a mildly elevated rate of reversible loss of libido and erectile dysfunction (appr. 2%) as well as a mildly elevated incidence of gynecomastia (10). The notion, which appears in some Internet fora, that finasteride might increase the risk of prostate cancer, breast cancer, or infertility has made patient education about this drug increasingly difficult but is not supported by valid scientific evidence (16, e8).
Finasteride is not approved for use by women, as it can cause developmental genital defects in male fetuses (10) and is ineffective against androgenetic alopecia in postmenopausal women (17).
Dutasteride, a dual (type I and type II) inhibitor of 5a-reductase, is currently approved in Germany only for the treatment of benign prostatic hyperplasia. Therefore, we advise against its off-label use to treat alopecia (18).
Topical or systemic treatment with hormones
There is as yet inadequate evidence for the topical use of natural estrogens, progesterone, or antiandrogens to treat androgenetic alopecia in women who do not have hormonal dysregulation (Table) (12).
Table. Treatment options for androgenetic alopecia and the evidence supporting them*.
| Treatment | Evidencelevel | Efficacy in preventing progression | Efficacy in improving manifestations | Safety | Practicality for the patient | Practicalityfor the physician |
|---|---|---|---|---|---|---|
| Finasteride (men) | 1 | +++ | ++ | +++ | ++++ | ++ |
| Minoxidil 5% (men) | 1 | +++ | ++ | ++++ | +/++ | +++ |
| Minoxidil 2% (women) | 1 | +++ | ++ | ++++ | + | +++ |
| Oral hormones (for women) - with hyperandrogenism - with normal hormonal status | 3 | + +/– | + +/– | + + | +++ +++ | ++ ++ |
| Hair transplantation (men und women) |
4 | – | +++ (men) ++ (women) |
++ | + procedure +++ long-term effect |
+ |
| Various other treatments (aloe vera, aminexil, ginkgo, food supplements, etc.) |
insufficient evidence |
inadequately studied in clinical trials |
+/– | unknown | + | + |
*Adapted from (12), Blumeyer et al.: J Dtsch Dermatol Ges 2011; for a complete list of all randomized trials, cf. “Guideline Androgenetic Alopecia” at the Internet address
www.euroderm.org/edf/index.php/edf-guidelines/;globalratingfrom-to ++++
Nor is there adequate evidence to support the use of systemic antiandrogens in women with androgenetic alopecia whose menstrual cycles are normal, other than a proof-of-principle study in which these hormones were given at very high doses (19). In women who do have hormonal dysregulation, androgenetic alopecia can be treated with antiandrogens such as cyproterone acetate, chlormadinone acetate, or dienogest (level 3 evidence) (Table) (19).
Surgical treatment
Autologous hair transplantation is a supplementary treatment for advanced androgenetic alopecia. Hair is removed from the occipital (androgen-insensitive) scalp and transplanted into the affected areas. Hair follicle transplantation can thicken the hair not only in men with androgenetic alopecia, but also in women (level 4 evidence). A further measure—the easiest of all, and one that affected persons can carry out for themselves—is the comb-over, i.e., appropriate hair styling to cover up areas of androgenetic alopecia. If restyling cannot achieve the desired effect, a toupee or wig can be worn.
Androgenetic alopecia in women.
There is as yet inadequate evidence for the topical use of natural estrogens, progesterone, or antiandrogens to treat androgenetic alopecia in women.
Alopecia areata
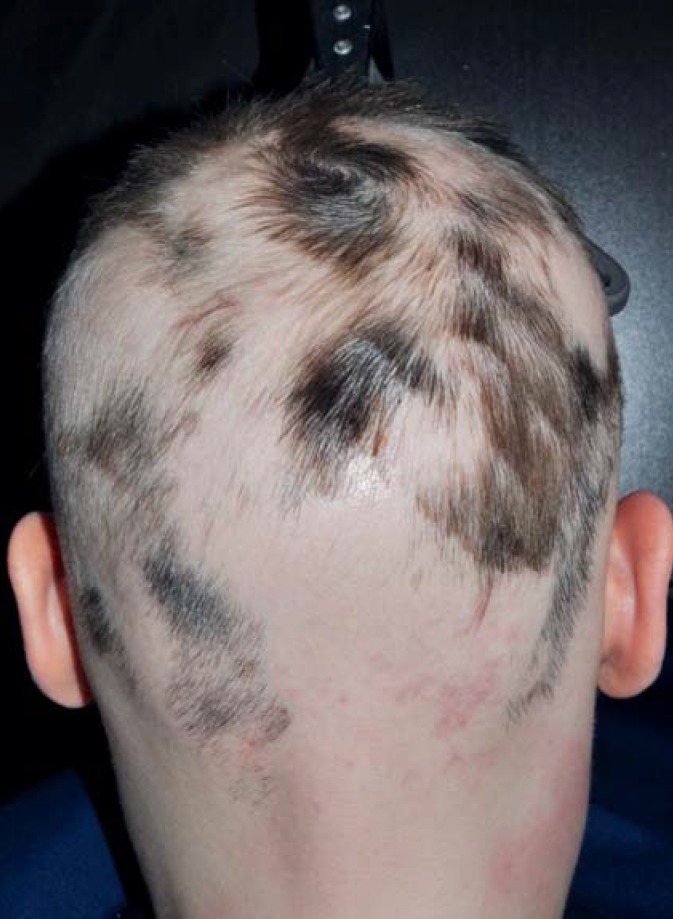
Alopecia areata (“baldness in a circle”) often arises suddenly; it usually affects a round patch of scalp at first, then spreads in a centrifugal or multilocular pattern (Figure 2). This disorder has a genetic component (e9). Alopecia areata affecting the entire scalp is called alopecia areata totalis; that affecting the entire body is called alopecia areata universalis. Acute alopecia areata begins with marked, diffuse hair loss (20). Alopecia areata has a lifelong incidence of 1–2% and is the third most common type of hair loss after androgenetic and diffuse alopecia (31).
Figure 2.
Alopecia areata: sharply demarcated hairless areas
This condition is associated with other inflammatory and autoimmune diseases including atopic eczema, Hashimoto’s thyroiditis, Graves’ disease, and vitiligo.
The following factors imply a worse prognosis (20):
Onset in childhood (21)
Extensive involvement and long duration
Ophiasis type (nuchal involvement)
Nail involvement (pitting; sandpaper nails)
Atopic dermatitis and autoimmune disease
Positive family history.
The condition is thought to be due to an autoimmune reaction, because scalp biopsies from patients with alopecia areata have revealed dense infiltration of lymphocytes and other immune cells in the deepest part of the hair follicles (bulb and dermal hair papilla). The hair follicles are reversibly damaged, mainly by cytotoxic T lymphocytes and cytokines (interferon-?, interleukin-2, and interleukin-15 receptor ß); in consequence, the hair falls out (22).
It remains unclear why and when alopecia areata arises, why the hair a few centimeters away from an area of alopecia grows normally, and how spontaneous remission comes about. Emotional and physical stress are thought to precipitate alopecia areata, but this has not been scientifically confirmed. The condition does not seem to be explicable as the product of an infection or a toxic environmental influence.
Alopecia areata.
Alopecia areata is an autoimmune disease in which T lymphocytes and other immune cells reversibly “paralyze” the hair follicles.
Alopecia areata takes a highly variable course, manifesting itself clinically in any of the following ways:
A single small focus that resolves spontaneously
Multiple simultaneously present areas of alopecia, including some with regrowth of hair, and others newly arising
Multiple large foci, often confluent, that can persist for years
Total hair loss that persists for decades.
One-third of patients have a spontaneous remission within six months of the initial manifestation; 50–80% are asymptomatic after one year (23).
Treatment
The treatment of alopecia areata depends on the severity of involvement (23). If the disorder is mild and does not cause the patient very much distress, waiting for a spontaneous remission is a sensible option. Treatment with zinc as a putative immune modulator generally has no side effects and is therefore suitable for use in children (e10). Topical corticosteroids can be applied for several weeks without risk, but their efficacy against alopecia areata has not been established (20). A clear benefit was seen in a double-blind trial of a high-dose steroid foam with an intrapatient control (right vs. left side of the head) (24).
Alternatively, alopecia areata can be treated by the intralesional injection of triamcinolone crystals. This can be tried if the patient has only a few, stable foci of baldness. In rare cases, systemic corticosteroids are helpful (25).
Spontaneous remission of alopecia areata.
If the disorder is mild and does not cause the patient very much distress, waiting for a spontaneous remission is a sensible option.
The most effective treatment (level 2 evidence) is topical immune therapy with diphenylcyclopropenone or squaric acid dibutylester (23). The mechanism of action is competitive inhibition of the responsible T lymphocytes by the induction of type IV allergy to whichever of these two substances is used; both of them are obligate chemical allergens not normally present in the environment. Once an allergic dermatitis has been induced in this way, the hair may grow back in 3–6 months. The response rate varies from 30 to 80% depending on the baseline findings, i.e., the total surface area of the lesions and the length of time that they have been present (20).
Various other therapeutic approaches will not be discussed here for lack of space and scientific evidence, e.g.: stimulation with dithranol, psoralen-UVA turban therapy, 308 nm excimer laser, methotrexate/prednisolone and sulfasalazine (20, 23, 25).
Modern biologic agents, e.g., TNF-a antagonists, are surprisingly ineffective and can even induce alopecia areata; thus, they are not recommended (22, 23). It is hoped that Janus kinase inhibitors (mainly for topical use) will be found to have a positive effect (e11).
Effective treatment for alopecia areata.
The most effective treatment (level 2 evidence) is topical immune therapy with diphenylcyclopropenone or squaric acid dibutylester.
Scarring and atrophizing alopecias
The scarring (cicatricial) and atrophizing alopecias are a heterogeneous group of diseases that destroy hair follicles irreversibly (26). They include the following:
Folliculitis decalvans
Folliculitis et perifolliculitis capitis abscedens et suffodiens
Chronic discoid lupus erythematosus
Lichen planus follicularis (lichen planopilaris)
Postmenopausal frontal fibrosing alopecia (Kossard)
Brocq’s pseudopelade.
Most of these types of alopecia are easily recognizable from their clinical appearance, although they may be hard to distinguish from one another in early stages. If necessary, a biopsy taken from the edge of the lesion where there is still hair can help establish the diagnosis (e12).
Folliculitis decalvans
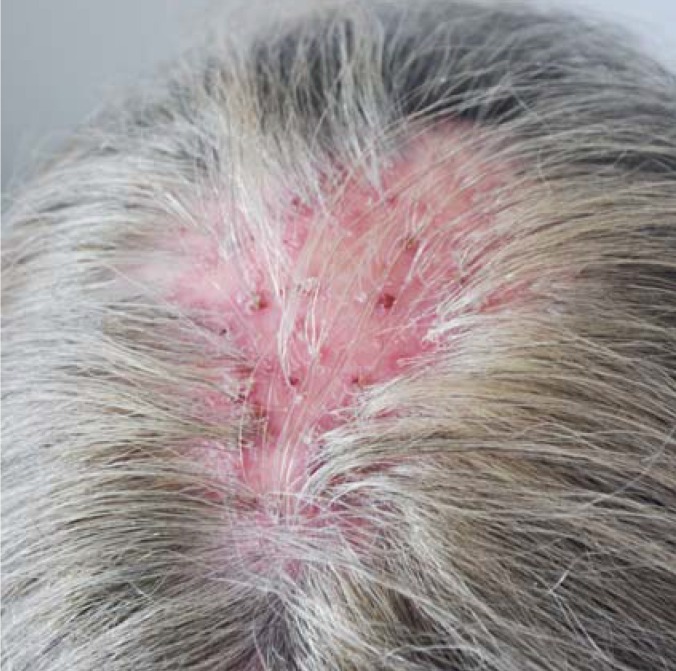
Folliculitis decalvans, which affects both men and women, is one of the most difficult scalp diseases to treat (26, 27). It manifests itself as intense granulocytic inflammation that destroys both the hair follicles and the skin of the scalp. Staphylococcal organisms and an excessive inflammatory response are involved in the pathogenesis of this disease. Inspection reveals scarred and atrophic areas with a red, inflamed margin (Figure 3). Hair tufts, consisting of 5–20 hairs, are often found at the edges of lesions; they are the portal of entry for staphylococci and thus play a role in the progression of the inflammation (27).
Figure 3.
Folliculitis decalvans: scarring alopecia with inflammatory papules, pustules, crusts, and tufted hairs
The treatment of folliculitis decalvans is long and difficult. Basic treatment consists of daily antimicrobial shampooing to lessen the load of staphylococci. Systemic antibiotic treatment with clarithromycin or doxycycline for 4–8 weeks often leads to improvement, but recurrences are common.
The most effective antibiotic treatment, a combination of clindamycin 300 mg po bid with rifampicin 300 mg po bid for 6 to 12 weeks, renders nearly all patients asymptomatic, sometimes for many months (27, 28). About half, however, have recurrent disease requiring further treatment. In a small fraction of patients (1 in 17), gastrointestinal side effects necessitate the discontinuation of treatment (28).
Dapsone 50 mg po bid for several months or years can likewise keep the inflammatory activity under control (e13, e14).
To prevent recurrences, all tufted hair follicles should be surgically removed from the scalp. Once the disease process has been arrested, larger areas of scarring can be surgically reduced.
Folliculitis decalvans.
Folliculitis decalvans is a chronic, destructive, scarring alopecia. Its pathogenesis is not entirely clear; staphylococcal organisms and an excessive inflammatory response in the scalp are involved.
Folliculitis et perifolliculitis capitis abscedens et suffodiens
This disease affects men almost exclusively. It manifests itself initially with hemispherical, lividly inflamed, hairless lesions on the hair-bearing scalp that are soft and fluctuant to palpation. The bloody exudate that can be aspirated from them is generally microbiologically sterile. In the extreme case, the entire scalp is undermined by confluent inflammatory exudate (29).
If there are only a few fluctuating nodules, the initial treatment can consist of fluid aspiration followed by the injection of a triamcinolone crystal suspension (10 mg/mL) through the same needle. Marked inflammation can be treated with a combination of systemic glucocorticoids (e.g., methylprednisolone, 1 mg/kg body weight) and isotretinoin (0.5 mg/kg BW) or else with a combination of dapsone and isotretinoin (30). This being a rare disease, there are no guidelines on how to treat it, and the therapeutic recommendations given here are only the authors’ expert opinions (level 5 evidence).
Lichen planus follicularis (lichen planopilaris)
In lichen planus of the scalp, a dense collection of T lymphocytes appears under the epidermal and follicular basal membrane zone (31).
The pathogenesis of this condition is thought to involve a misdirected cellular immune response to an unknown antigen in the basal membrane zone. The T lymphocytes destroy the follicular stem cells in the bulge area of the hair follicle, leading to irreversible hair loss (e14).
The characteristic clinical finding is that of small patches of alopecia with peripheral follicular hyperkeratosis. The hairs at the edge of an affected area seem to possess a tight-fitting white collar surrounded by perifollicular erythema (Figure 4).
Figure 4.
Lichen planus follicularis: atrophizing alopecia with peripilar erythema and collar-like scaling
Lichen planus of the scalp is usually asymptomatic and is often present for years before it is noticed. Further lichen planus lesions are only rarely seen elsewhere on the patient’s body. Lassueur–Graham-Little–Piccardi syndrome consists of lichen planus follicularis that involves the scalp and the body and is accompanied by dystrophic changes of the fingernails and toenails (31).
Preventing recurrence of folliculitis decalvans.
To prevent recurrences, all tufted hair follicles should be surgically removed from the scalp. Once the disease process has been arrested, larger areas of scarring can be surgically reduced.
To arrest or at least slow the gradual inflammatory destruction of the hair follicles, we prefer to use topically applied foam preparations of class III or IV corticosteroids, which have a favorable side-effect profile (31).
Systemic corticosteroids are not used to treat this condition because of the serious adverse effects of their long-term use. The use of the retinoid acitretin is limited by the cutaneous and mucosal dryness that arises in more than 80% of patients who take it, as well as other side effects. The best-tolerated systemic treatment seems to be with hydroxychloroquine 200 mg po bid (level 4 evidence) (31).
Frontal fibrosing alopecia of Kossard
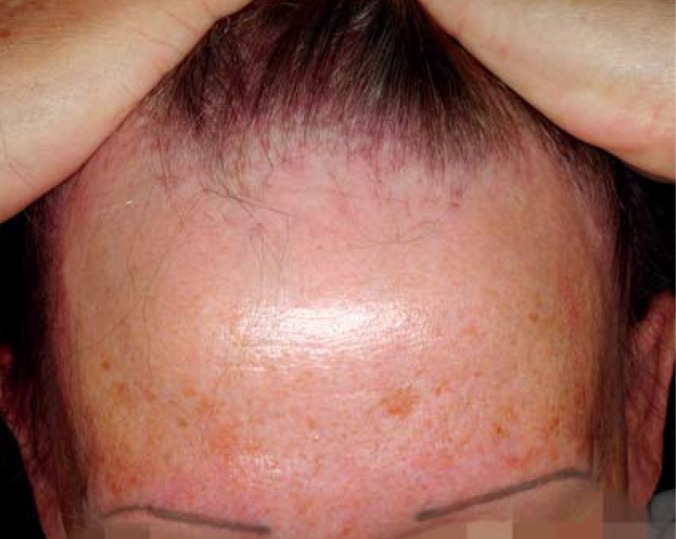
In 1994, the Australian physician Steven Kossard described a disease that he called “postmenopausal frontal fibrosing alopecia” (32). This condition, considered a variant of lichen planopilaris, arises nearly exclusively in elderly women; it does, however, affect a small number of premenopausal women and even men (33), and we therefore omit the word “postmenopausal” from its name. The pattern of hair loss resembles at first glance that of androgenetic alopecia in men (Figure 5).
Figure 5.
Frontal fibrosing alopecia of Kossard with perifollicular erythema in a 66-year-old woman. Total eyebrow loss, covered up in this case with permanent make-up
Perifollicular erythema and hyperkeratoses can often be seen at the affected hairline. The condition is often restricted to the frontal region, although there are also temporal, occipital, and even centripetal distribution patterns. The eyebrows are nearly always thinned or completely lost.
Asymptomatic loss of hair follicles can be seen on the body as well (33). The treatment is analogous to that of lichen planus (33– 36).
Lichen planus follicularis.
In the autoimmune disease lichen planus the hair follicles are destroyed by T lymphocytes, yielding an irreversible, atrophizing alopecia usually consisting of small bald patches on the scalp.
Frontal fibrosing alopecia.
The pattern of hair loss resembles that of androgenetic alopecia in men. Perifollicular erythema and hyperkeratoses can often be seen at the hairline. The condition is not always restricted to the frontal region.
Further information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
This CME unit can be accessed until 21 August 2016, and earlier CME units until the dates indicated:
“Alcohol Dependence and Harmful Use of Alcohol” (Issue 17/2016) until 24 July 2016,
“Acute Lumbar Back Pain” (Issue 13/2016) until 26 June 2016,
“Evidence-Based Hernia Treatment in Adults” (Issue 9/2016) until 29 May 2016.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the most appropriate answer.
Question 1
What is the name of the resting phase that lasts two to four months before a hair falls out?
catagen
telogen
anagen phase
anaphase
miophase
Question 2
How long is the growth phase of a hair follicle on the scalp?
about 48 hours
about 6 months
2–6 years
12–18 years
a lifetime
Question 3
In effluvium of unknown cause, which of the following should be ruled out by laboratory testing?
vitamin C deficiency
vitamin B deficiency
iron deficiency
zinc deficiency
calcium deficiency
Question 4
What is the most common type of hair loss?
frontal fibrosing alopecia
lichen planus follicularis
folliculitis decalvans
alopecia areata
androgenetic alopecia
Question 5
A 23-year-old male student complains of marked hair loss over a period of four weeks, and this is confirmed by a trichogram. Which of the following tests must not be omitted in the further work-up?
blood sugar profile over the course of the day
serum zinc level
urinary protein
syphilis test
ECG
Question 6
Which of the following factors implies a worse prognosis for alopecia areata?
onset in adulthood
nail involvement (pitting; sandpaper nails)
female sex
location on the top of the head
limited involvement
Question 7
What is a possible side effect of minoxidil treatment in women with androgenetic alopecia?
irregular menstrual cycles
acne
loss of libido
hypertrichosis
infertility
Question 8
What measure can lessen the likelihood of recurrent folliculitis decalvans?
use of a shampoo that contains caffeine
weekly scalp baths in basic salt solution
at least 6 months of treatment with TNF- α antagonists after all the affected areas have healed
surgical removal of all tufted hair follicles from the scalp
UVB irradiation of the affected scalp areas
Question 9
Which of the following types of alopecia is reversible?
lichen planopilaris
alopecia areata
Brocq’s pseudopelade
chronic discoid lupus erythematosus
folliculitis decalvans
Question 10
In what type of alopecia do staphylococci play an important pathogenetic role?
lichen planopilaris
alopecia areata
frontal fibrosing alopecia of Kossard
chronic discoid lupus erythematosus
folliculitis decalvans
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Wolff has received lecture honoraria from the MSD Sharp & Dohme, Johnson & Johnson, Pierre Fabre, Grünenthal, and Bayer companies.
Dr. Fischer has received lecture honoraria from, and has served as a paid consultant for, the following companies: MSD Sharp & Dohme, Johnson & Johnson, Galderma, Pierre Fabre, ASATONA, ISDIN, Dr. Kurt Wolff, HairDreams, and Bayer.
Prof. Blume-Peytavi has received lecture honoraria from, and has served as a paid consultant for, the following companies: Almirall, Johnson & Johnson, Galderma, Pierre Fabre, Procter & Gamble, and Bayer.
References
- 1.Cash T. The psychosocial consequences of androgenetic alopecia: a review of the research literature. Br J Dermatol. 1999;141:398–405. doi: 10.1046/j.1365-2133.1999.03030.x. [DOI] [PubMed] [Google Scholar]
- 2.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 3.Courtois M, Loussouarn G, Hourseau S, Grollier JF. Periodicity in the growth and shedding of hair. Br J Dermatol. 1996;134:47–54. [PubMed] [Google Scholar]
- 4.Lindner J, Hillmann K, Blume-Peytavi U, et al. Hair shaft abnormalities after chemotherapy and tamoxifen therapy in patients with breast cancer evaluated by optical coherence tomography. Br J Dermatol. 2012;167:1272–1278. doi: 10.1111/j.1365-2133.2012.11180.x. [DOI] [PubMed] [Google Scholar]
- 5.Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 6.Whiting DA. Chronic telogen effluvium: increased scalp hair shedding in middle-aged women. J Am Acad Dermatol. 1996;35:899–906. doi: 10.1016/s0190-9622(96)90113-9. [DOI] [PubMed] [Google Scholar]
- 7.Kantor J, Kessler LJ, Brooks DG, Cotsarelis G. Decreased serum ferritin is associated with alopecia in women. J Invest Dermatol. 2003;121:985–988. doi: 10.1046/j.1523-1747.2003.12540.x. [DOI] [PubMed] [Google Scholar]
- 8.Blume-Peytavi U, Blumeyer A, Tosti A, et al. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol. 2011;164:5–15. doi: 10.1111/j.1365-2133.2010.10011.x. [DOI] [PubMed] [Google Scholar]
- 9.Heilmann S, Brockschmidt FF, Hillmer AM, et al. Evidence for a polygenic contribution to androgenetic alopecia. Br J Dermatol. 2013;169:927–930. doi: 10.1111/bjd.12443. [DOI] [PubMed] [Google Scholar]
- 10.The Finasteride Male Pattern Hair Loss Study Group. Long-term (5-year) multinational experience with finasteride 1 mg in the treatment of men with androgenetic alopecia. Eur J Dermatol. 2002;12:38–49. [PubMed] [Google Scholar]
- 11.Blume-Peytavi U, Vogt A. Current standards in the diagnostics and therapy of hair diseases—hair consultation. J Dtsch Dermatol Ges. 2011;9:394–410. doi: 10.1111/j.1610-0387.2011.07609.x. [DOI] [PubMed] [Google Scholar]
- 12.Blumeyer A, Tosti A, Messenger A, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(Suppl 6):1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 13.Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541–553. doi: 10.1016/j.jaad.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 14.van Zuuren EJ, Fedorowicz Z, Carter B. Evidence-based treatments for female pattern hair loss: a summary of a Cochrane systematic review. Br J Dermatol. 2012;167:995–1010. doi: 10.1111/j.1365-2133.2012.11166.x. [DOI] [PubMed] [Google Scholar]
- 15.Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47:377–385. doi: 10.1067/mjd.2002.124088. [DOI] [PubMed] [Google Scholar]
- 16.Mella JM, Perret MC, Manzotti M, Catalano HN, Guyatt G. Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141–1150. doi: 10.1001/archdermatol.2010.256. [DOI] [PubMed] [Google Scholar]
- 17.Price V, Roberts JL, Hordinsky M. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43:768–776. doi: 10.1067/mjd.2000.107953. [DOI] [PubMed] [Google Scholar]
- 18.Gubelin Harcha W, Barboza Martinez J, Tsai TF, et al. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014;70:489–498. doi: 10.1016/j.jaad.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466–473. doi: 10.1111/j.1365-2133.2005.06218.x. [DOI] [PubMed] [Google Scholar]
- 20.Freyschmidt-Paul P, Happle R, Hoffmann R. Alopecia areata Klinik, Pathogenese und rationale Therapie einer T-Zell-vermittelten Autoimmunerkrankung. Hautarzt. 2003;54:713–722. doi: 10.1007/s00105-003-0560-z. [DOI] [PubMed] [Google Scholar]
- 21.Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438–441. doi: 10.1016/j.jaad.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515–1525. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 23.Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166:916–926. doi: 10.1111/j.1365-2133.2012.10955.x. [DOI] [PubMed] [Google Scholar]
- 24.Tosti A, Iorizzo M, Botta GL, Milani M. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243–1247. doi: 10.1111/j.1468-3083.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- 25.Fischer TW. Alopezien - Diagnostisches und therapeutisches Management. Akt Dermatol. 2008;34:209–225. [Google Scholar]
- 26.Trüeb RM. Vernarbende Alopezien. Hautarzt. 2013;64:810–819. doi: 10.1007/s00105-013-2577-2. [DOI] [PubMed] [Google Scholar]
- 27.Powell JJ, Dawber RPR, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140:328–333. doi: 10.1046/j.1365-2133.1999.02675.x. [DOI] [PubMed] [Google Scholar]
- 28.Stockmeier M, Kunte C, Feldmann KA, Messer G, Wolff H. Folliculitis decalvans - Behandlung mit einer systemischen Rifampicin-Clindamycin-Kombinationstherapie bei 17 Patienten. Akt Dermatol. 2001;27:361–363. [Google Scholar]
- 29.Bolz S, Jappe U, Hartschuh W. Successful treatment of perifolliculitis capitis abscedens et suffodiens with combined isotretinoin and dapsone. J Dtsch Dermatol Ges. 2008;6:44–47. doi: 10.1111/j.1610-0387.2007.06399.x. [DOI] [PubMed] [Google Scholar]
- 30.Scerri L, Williams HC, Allen BR. Dissecting cellulitis of the scalp: response to isotretinoin. Br J Dermatol. 1996;134:1105–1108. [PubMed] [Google Scholar]
- 31.Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723–732. doi: 10.1056/NEJMcp1103641. [DOI] [PubMed] [Google Scholar]
- 32.Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770–774. [PubMed] [Google Scholar]
- 33.Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670–678. doi: 10.1016/j.jaad.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Manousaridis I, Manousaridis K, Peitsch WK, Schneider SW. Individualisierte Behandlung und Therapiewahl bei Lichen planus: ein schrittweiser Ansatz. J Dtsch Dermatol Ges. 2013:981–991. doi: 10.1111/ddg.12141. [DOI] [PubMed] [Google Scholar]
- 35.Racz E, Gho C, Moorman PW, Noordhoek Hegt V, Neumann HA. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461–1470. doi: 10.1111/jdv.12139. [DOI] [PubMed] [Google Scholar]
- 36.Harries MJ, Messenger A. Treatment of frontal fibrosing alopecia and lichen planopilaris. J Eur Acad Dermatol Venereol. 2014;28:1404–1405. doi: 10.1111/jdv.12362. [DOI] [PubMed] [Google Scholar]
- e1.Sinclair R. Diffuse hair loss. Int J Dermatol. 1999;38(Suppl 1):8–18. doi: 10.1046/j.1365-4362.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- e2.Apsner R, Horl WH, Sunder-Plassmann G. Dalteparin-induced alopecia in hemodialysis patients: reversal by regional citrate anticoagulation. Blood. 2001;97:2914–2915. doi: 10.1182/blood.v97.9.2914. [DOI] [PubMed] [Google Scholar]
- e3.Wilsmann-Theis D, Bieber T. Psoriasis und Ekzeme am Capillitum. Hautarzt. 2014;65:1043–1049. doi: 10.1007/s00105-014-3542-4. [DOI] [PubMed] [Google Scholar]
- e4.Trüeb RM. Systematisches Vorgehen bei Frauen mit Haaarausfall. JDDG. 2010;8:284–298. [Google Scholar]
- e5.Hoffmann R. TrichoScan, a GCP-validated tool to measure hair growth. J Eur Acad Dermatol Venereol. 2008;22:132–134. doi: 10.1111/j.1468-3083.2007.02470.x. [DOI] [PubMed] [Google Scholar]
- e6.Hillmer AM, Brockschmidt FF, Hanneken S, et al. Susceptibility variants for male-pattern baldness on chromosome 20p11. Nat Genet. 2008;40:1279–1281. doi: 10.1038/ng.228. [DOI] [PubMed] [Google Scholar]
- e7.Redler S, Dobson K, Drichel D, et al. Investigation of six novel susceptibility loci for male androgenetic alopecia in women with female pattern hair loss. J Dermatol Sci. 2013;72:186–188. doi: 10.1016/j.jdermsci.2013.06.012. [DOI] [PubMed] [Google Scholar]
- e8.Yim E, Nole KL, Tosti A. 5-alpha-Reductase inhibitors in androgenetic alopecia. Curr Opin Endocrinol Diabetes Obes. 2014;21:493–498. doi: 10.1097/MED.0000000000000112. [DOI] [PubMed] [Google Scholar]
- e9.Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6 doi: 10.1038/ncomms6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Peter C, Hoting E. Therapie der Alopecia areata mit Zinksulfat - eine placebokontrollierte Studie an 307 Patienten. Z Hautkr. 1996;71:175–189. [Google Scholar]
- e11.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Headington JT. Transverse microscopic anatomy of the human scalp. Arch Dermatol. 1984;120:449–456. [PubMed] [Google Scholar]
- e13.Kunte C, Loeser C, Wolff H. Folliculitis spinulosa decalvans: successful therapy with dapsone. J Am Acad Dermatol. 1998;39:891–893. doi: 10.1016/s0190-9622(98)70374-3. [DOI] [PubMed] [Google Scholar]
- e14.Tan E, Martinka M, Ball N, Shapiro J. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25–32. doi: 10.1016/j.jaad.2003.04.001. [DOI] [PubMed] [Google Scholar]