Abstract
Triple-negative breast cancers, which lack estrogen receptor, progesterone receptor, and HER2/neu overexpression, account for approximately 15% of breast cancers, but occur more commonly in African Americans. The poor survival outcomes seen with triple-negative breast cancers patients are, in part, due to a lack of therapeutic targets. Epidermal growth factor receptor (EGFR) is overexpressed in 50% of triple-negative breast cancers, but EGFR inhibitors have not been effective in patients with metastatic breast cancers. However, mTOR inhibition has been shown to reverse resistance to EGFR inhibitors. We examined the combination effects of mTOR inhibition with EGFR inhibition in triple-negative breast cancer in vitro and in vivo. The combination of EGFR inhibition by using lapatinib and mTOR inhibition with rapamycin resulted in significantly greater cytotoxicity than the single agents alone and these effects were synergistic in vitro. The combination of rapamycin and lapatinib significantly decreased growth of triple-negative breast cancers in vivo compared with either agent alone. EGFR inhibition abrogated the expression of rapamycin-induced activated Akt in triple-negative breast cancer cells in vitro. The combination of EGFR and mTOR inhibition resulted in increased apoptosis in some, but not all, triple-negative cell lines, and these apoptotic effects correlated with a decrease in activated eukaryotic translation initiation factor (eIF4E). These results suggest that mTOR inhibitors could sensitize a subset of triple-negative breast cancers to EGFR inhibitors. Given the paucity of effective targeted agents in triple-negative breast cancers, these results warrant further evaluation.
Introduction
Triple-negative breast cancers, which lack expression of estrogen receptor, progesterone receptor, and HER2/neu (HER2), account for approximately 15% of all diagnosed breast cancers (1). We and others have noted a 2-fold higher incidence of triple-negative breast cancers in African-American patients compared with their Caucasian counterparts, regardless of age at diagnosis (2, 3). In African-American patients ages less than 40 years, triple-negative breast cancers account for 50% of all diagnosed breast cancer cases (3). Triple-negative breast cancers are commonly high grade and run an aggressive course, with a significant risk of developing metastases in the 5 years following diagnosis (4). Survival for patients with triple-negative breast cancers is consequently poor, especially in African-American patients (3). The poor survival associated with triple-negative breast cancers is, at least in part, due to a lack of effective targeted agents, which have positively impacted outcomes for patients with other subtypes of breast cancers (5, 6).
Genetic and immunohistochemical analyses show that 50% of basal-like breast cancers, which account for approximately three fourth of triple-negative breast cancers, express epidermal growth factor receptor (EGFR; ref. 7) and that EGFR expression has been associated with poor prognosis (8). However, the use of tyrosine kinase inhibitors directed toward EGFR in patients with unselected metastatic breast cancers produced little efficacy (9, 10). More recently, the use of single-agent cetuximab (an EGFR monoclonal antibody) in metastatic triple-negative breast cancers patients resulted in a response rate of only 6% and a clinical benefit rate of 20% (11). The addition of chemotherapy to cetuximab marginally increased the response rate to 17% (11). Given these disappointing results, it seems that EGFR inhibition alone will not prove to be an effective therapeutic approach for patients with triple-negative breast cancers.
The mTOR inhibitors, temsirolimus and everolimus, are currently approved for the treatment of metastatic renal carcinoma. The use of single-agent mTOR inhibitors in patients with unselected metastatic breast cancers has not shown encouraging results (12). The suboptimal outcomes obtained from the use of single-agent mTOR inhibitors, such as rapamycin and its analogues (or rapalogues), in the treatment of metastatic solid tumors is thought to be partly due to an increase in phosphorylated Akt levels following exposure to these rapalogues (13). mTOR inhibitor-induced Akt activation can be abrogated by the inhibition of upstream growth factors such as insulin-like growth factor I receptors (13, 14). Given the fact that many triple-negative breast tumors express EGFR (1), another upstream regulator in the phosphoinositide 3-kinase (PI3K)/Akt pathway, we postulated that mTOR inhibitors would sensitize triple-negative breast cancer cells to upstream inhibitors of the EGFR family. In support of this hypothesis, everolimus has been shown to reverse resistance to trastuzumab in patients with trastuzumab-resistant HER2-positive metastatic breast cancers (15). The approach of targeting mTOR and EGFR concurrently has been preclinically evaluated previously in breast and other cancers (13, 16) but has not been specifically evaluated in triple-negative breast cancers in vitro and in vivo.
On the basis of these data, we assessed the effects of coinhibition of mTOR (using rapamycin) and EGFR (using lapatinib; Fig. 1A) in triple-negative breast cancer cell lines and nude mice models. Our results show that cotargeting mTOR and EGFR was synergistic in decreasing cell survival and resulted in increased apoptosis in some but not all triple-negative breast cancer cell. Interestingly, the apoptotic effects noted were associated with changes in the expression of activated elF4E following combined treatment with EGFR and mTOR inhibitors. Furthermore, combined EGFR and mTOR inhibition downregulated rapamycin-induced activation of Akt in vitro. These findings suggest that mTOR inhibition could improve the efficacy of EGFR inhibition in some triple-negative breast cancers, and that the combination of an mTOR inhibitor with an EGFR inhibitor could warrant further evaluation in patients with triple-negative breast cancers.
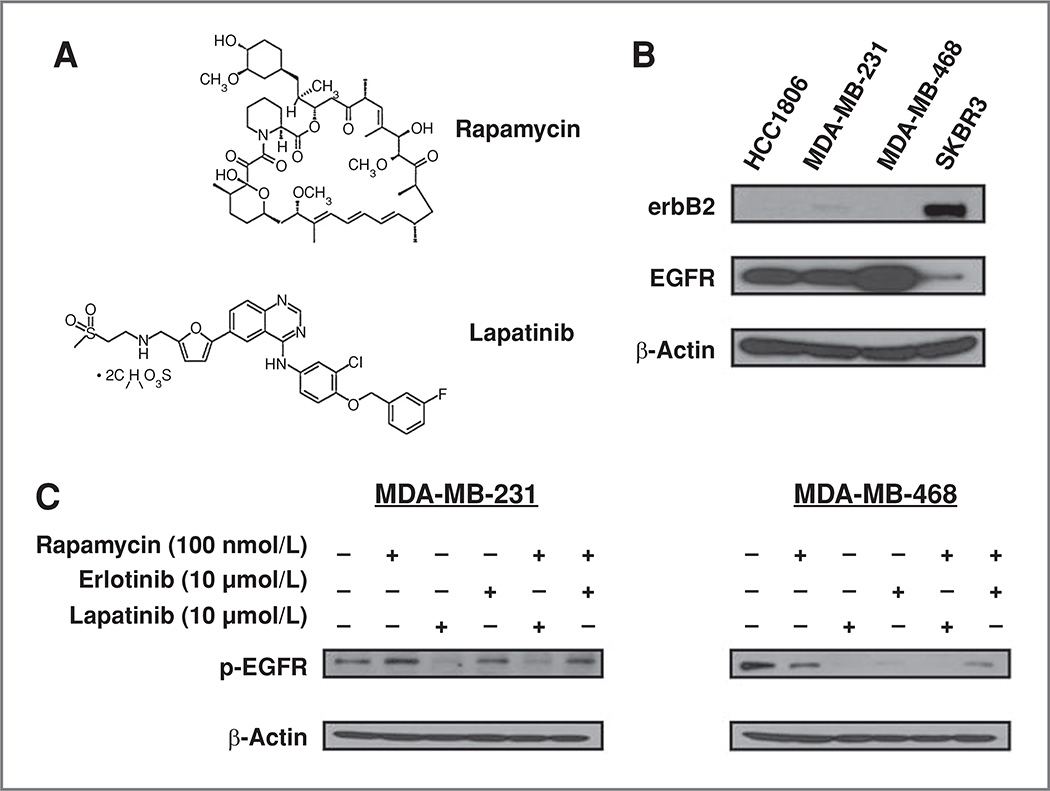
Figure 1.
Rapamycin plus lapatinib combined treatments reduce phosphorylation of EGFR in human triple-negative breast cancer cell lines. A, chemical structures of rapamycin and lapatinib (used with permission from LC Laboratories). B, basal levels of erbB2 and EGFR expression among HCC1806, MDA-MB-231, MDA-MB-468, and SKBR3 breast cancer cell lines were compared by Western blotting. C, MDA-MB-231 and MDA-MB-468 were treated with rapamycin (100 nmol/L), lapatinib (10 µmol/L), erlotinib (10 µmol/L), rapamycin plus lapatinib, or rapamycin plus erlotinib for 3 hours. Cells were then harvested and whole cell protein lysates were collected and analyzed by SDS-PAGE and Western blotting. Phosphorylation of EGFR (p-EGFR) was detected and compared among all treatment conditions in both cells lines. Representative blots of 3 independent experiments are shown. β-Actin was used as a loading control.
Materials and Methods
Cell lines, antibodies, and reagents
The MDA-MB-231 breast cancer cell line was purchased from American Type Culture Collection. The breast cancer cell lines HCC1806 and MDA-MB-468 and lung cancer cell line A549 were generously provided by Drs. Sean Kimbro, Paula Vertino, and Wei Zhou, respectively, at the Winship Cancer Institute, Emory University. These cell lines were not authenticated. MDA-MB-231, HCC1806, and A549 cells were routinely maintained in RPMI 1640 medium supplemented with 5% FBS. MDA-MB-468 cells were maintained in Dulbecco’s Modified of Eagle’s Medium supplemented with 5% FBS. The antibodies against p-Akt (S473), pS6 (S235/236), p-p44/42 MAPK (p-ERK, Thr202/Tyr204), and caspase-3 were obtained from Cell Signaling Technology, Inc. Antibody against p-EGFR (Tyr1173) was obtained from Santa Cruz Biotechnology, Inc. The antibody against p-eIF4E (pS209) was from Epitomics, Inc. Rapamycin was purchased from LC Laboratories. Erlotinib (Tarceva; Genetech) and lapatinib (Tykerb; GlaxoSmithKline) are commercially available. For in vivo studies, lapatinib was dissolved in 1% Tween-80 (Sigma) and rapamycin was dissolved in 2% ethyl alcohol. Protease inhibitor cocktail was purchased from Sigma.
Cell survival assay
MDA-MB-231 and MDA-MB-468 cells were seeded at a density of 5,000 cells/well in 96-well plates. They were grown overnight before treatment with various concentrations of rapamycin (0–100 nmol/L) and lapatinib (0–20 µmol/L) alone and in combination for 72 hours. Cell viability was assessed by the sulforhodamine B (SRB) assay following procedures described previously (17).
Combination index assay
Combination index (CI) equations allow for the quantitative measurement of dose–effect relationships of single drugs and their combinations to determine synergistic drug interactions (18). The synergistic interactions between rapamycin and lapatinib were analyzed by CalcuSyn software (Biosoft), which is based on the Chou and Talalay method (18, 19). We further tested synergy of these drugs at several combinations by using the method of Laska and colleagues (20). MDA-MB-231 and MDA-MB-468 were seeded in 96-well plates at a density of 5,000 cells per well overnight before drug treatment. After incubation with 1:2 serial dilutions of the drug based on their IC50 for rapamycin alone, lapatinib alone, or rapamycin in combination with lapatinib, the cells were subject to SRB assays (17). Data from SRB assays were expressed as fraction of cells with growth affected (comparison of drug-treated cells versus untreated ones). CI was calculated by CalcuSyn software. CI > 1 indicates antagonism, CI = 1 indicates additivity, and CI < 1 indicates synergism.
Western blotting
Breast cancer cell lines MDA-MB-231, HCC1806, and MDA-MB-468 and lung cancer cell line A549 were harvested and lysed in lysis buffer containing protease inhibitors (Sigma). Twenty micrograms of whole cell protein lysate were separated by SDS-PAGE followed by Western blot analysis with antibodies following procedures described in manufacturer’s instruction. The signals were detected with enhanced chemiluminescence reagents (GE-Amersham), exposed on Hyblot CL autoradiography films (Denville Scientific), and developed by Konica SRX-101A medical film processor (Konica Medical & Graphic Corporation).
Apoptosis assay
Apoptotic MDA-MB468 and MDA-MB-231 cells were determined by using Annexin V–phycoerythrin (PE) and 7-amino-actinomycin D (7-AAD; BD Biosciences). Cells were treated with rapamycin (25 nmol/L) alone, lapatinib (5 µmol/L) alone, and in combination for 72 hours. Both floating and adherent cells were collected and labeled followed by fluorescence-activated cell sorting (FACS) analysis. Student’s t test was used to evaluate P values.
In vivo xenograft tumor model
The animal protocol was approved by Emory University Institutional Animal Care and Use Committee. Female nude mice (athymic, nude-foxnl nu; Harlan) ages 4 to 5 weeks were injected with 5 × 106 MDA-MB-231 or MDA-MB-468 cells into the mammary fat pad and were randomized into 4 groups and treated as follows: for the mice inoculated with MDA-MB-231, there are vehicle [1% Tween-80, per os (orally) 5 days a week and 2% ethyl alcohol, intraperitoneally (i.p.) twice a week, n = 6], rapamycin (3 mg/kg, i.p. twice a week, n = 10), lapatinib (75 mg/kg, orally 5 days a week, n = 10), and the combination of rapamycin (3 mg/kg, i.p. twice a week) and lapatinib (75 mg/kg, orally 5 days a week) treatment group (n = 12). For the study with MDA-MB-468, both treatment and dosage are the same as MDA-MB-231. But the sample size is 10 mice in each of the 4 groups. For both studies, treatment was started 1 week after the cells were injected. Mouse weight and tumor sizes were measured twice weekly. Tumor volume was calculated by using the equation, V (mm3) = largest diameter × smallest diameter2/2. The mice were sacrificed following treatment. Tumors were harvested, weighed, and snap frozen or placed in formalin for immunohistochemistry studies.
Immunohistochemistry
Serial sections of 4-µm thick tumor tissues were cut from the formalin-fixed, paraffin-embedded tissue blocks. Antigen retrieval was done in 1× EDTA buffer (pH 8.0), using the LabVision PTmodule. The immunohistochemistry assay was carried out by using DAKO LSAB 2 kit in a DAKO Autostainer (DakoCytomation). The endogenous peroxidase was blocked with 3% hydrogen peroxide followed by incubation with primary antibodies for cleaved caspase-3 (1:500 dilution) and Ki67 (1:500 dilution; Epitomics) for 30 minutes at room temperature. The tissues were then incubated with biotinylated secondary antibody (DakoCytomation) for 30 minutes followed by enzyme labeling with freshly prepared horseradish peroxidase–labeled streptavidin (DakoCytomation). The developing chromogen DAB+ solution (DakoCytomation) was added for 2 minutes and then the sections were lightly counterstained (1:6 dilution) with hematoxylin in dH2O (Richard-Allan Scientific). The negative control consisted of nonimmune mouse or rabbit IgG. Digital images were captured by the Aperio ScanScope XT slide scanner (Aperio Technologies). Three images of different tissue sections were scored on the basis of intensity level (0, negative; 1, weak; 2, moderate; and 3, strong) multiplied by percentage of area staining. Student’s t test was used to calculate the P.
Statistical analysis
Synergy of rapamycin and lapatinib was tested at the dose combinations listed in Table 1, using the method of Laska and colleagues (20). For each dose combination (r*, l*), we tested the null hypothesis that e(r*, l*) ≤ e(r* + l*/m, 0) or e(r*, l*) ≤ e(0, mr* + l*) versus the alternative hypothesis that e(r*, l*) > e(r* + l*/m, 0) and e(r*, l*) > e(0, mr* + l*), where e(r*, l*) denotes the fraction affected at (r*, l*). Here, we took m = 0.2 because each dose combination (r*, l*) in Table 1 is the midpoint of the line joining the points (r* + l*/m, 0) and (0, mr* + l*) and the fractions affected at these points are available from the experiments. Laska and colleagues (20) showed that if the null hypothesis H0 is rejected in favor of the alternative hypothesis H1, then the combination is synergistic at (r*, l*). The hypotheses were tested by two 2-sample t tests, using 4 replicates at each point and the higher of the 2 P values were reported in Table 1. Repeated measure ANOVA was used to compare the mean tumor volumes between the 4 different groups. Bonferroni correction to the P values was adopted when comparing pairs of treatment groups.
Table 1.
P values for testing synergy at selected dose combinations
| Combination rapamycin (nmol/L), lapatinib (µmol/L) |
(50, 10) | (25, 5) | (12.5, 2.5) | (6.25, 1.25) | (3.125, 0.625) | (1.56, 0.3125) | (0.78, 0.156) |
|---|---|---|---|---|---|---|---|
| MDA-MB-231 | 0.996 | <0.0001 | 0.006 | <0.0001 | 0.007 | 0.005 | 0.003 |
| MDA-MB-468 | 0.999 | 0.002 | 0.007 | 0.005 | 0.003 | 0.045 | 0.426 |
NOTE: Two-sample t test was used to evaluate the synergistic effects at 7 different dose-combinations. The P values are summarized in the table.
Results
Combination of mTOR and EGFR inhibition is synergistic in triple-negative breast cancer cells
EGFR and erbB2 expression levels were determined by Western blotting in MDA-MB-231, MDA-MB-468, and HCC1806 triple-negative cell lines (Fig. 1B). ErbB2 expression levels were undetectable in MDA-MB-468 and HCC1806 whereas MDA-MB-231 had extremely low expression of erbB2 compared with erbB2-positive SKBR3 cell lines. MDA-MB-468 showed strong expression of EGFR, compared with MDA-MB-231 and HCC1806, which expressed moderate levels of EGFR. Given the differences in EGFR expression levels and response to lapatinib, we chose MDA-MB-231 and MDA-MB-468 cell lines for further evaluation.
We next compared the combined effects of rapamycin with erlotinib or lapatinib on activated EGFR protein levels in both MDA-MB-231 and MBA-MD-468 cells. Lapatinib alone and in combination with rapamycin decreased expression of activated EGFR more effectively in both cell lines, compared with erlotinib alone or in combination with rapamycin (Fig. 1C). On the basis of these findings, we selected lapatinib for subsequent experiments. The fact that the triple-negative cells express no or extremely low levels of erB2 suggests that the growth inhibitory effects of lapatinib are mediated through EGFR and not erbB2.
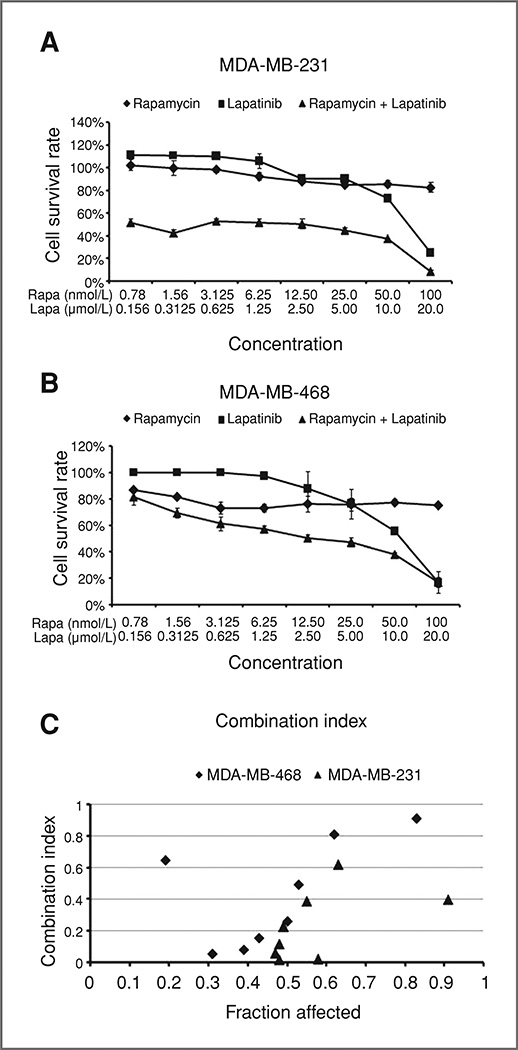
To determine the sensitivity of triple-negative cells to mTOR inhibition in combination with EGFR inhibition, MDA-MB-231 and MDA-MB-468 triple-negative breast cancer cell lines were treated with rapamycin (0.78–100 nmol/L), lapatinib (0.156–20 µmol/L), or both agents in combination for 72 hours. Twofold serial dilutions were conducted for both drugs. As shown in Figure 2A and B, rapamycin alone had very limited cytotoxic effect and cell survival rate remained between 85% to 100% for MDA-MB-231 and 75% to 90% for MDA-MB-468 after a 72-hour treatment. Lapatinib alone produced a gradual dose-dependent growth inhibition in both cell lines. The inhibitory effect of combined treatment with rapamycin and lapatinib in 2 triple-negative breast cancer cell lines was determined by using a CI and formal statistical tests. Table 1 gives the P values for testing the synergy between rapamycin and lapatinib at each of 7 dose combinations and for each cell line. Using Bonferroni correction for multiple testing, we conclude that for the MDA-MB-231 cell line, the combination is synergistic at (25, 5), (12.5, 2.5), (6.25, 1.25), (3.125, 0.625), (1.56, 0.3125), and (0.78, 0.156). There is no evidence of synergy at (50, 10). For the MDA-MB-468 line, the combination is synergistic at (25, 5), (12.5, 2.5), (6.25, 1.25), and (3.125, 0.625). There is no evidence of synergy at (50, 10), (1.56, 0.3125), and (0.78, 0.156). Synergy at these dose combinations is also confirmed by the CI value in Figure 2C. We also note that the software CalcuSyn truncated cell survival fractions over 100% to values just below 100%. Also, the combined erlotinib and rapamycin treatments have been verified with CI to synergistically reduce cell growth in MDA-MB-468 triple-negative cells (data not shown). These experiments indicate a synergistic interaction between rapamycin and lapatinib in suppressing growth of triple-negative breast cancer cells.
Figure 2.
Rapamycin combined with lapatinib synergistically inhibits survival of triple-negative breast cancer cells. MDA-MB-231 (A) and MDA-MB-468 (B) were treated with 2-fold serial dilution of rapamycin alone (0.78–100 nmol/L), lapatinib alone (0.156–20 µmol/L), and the agents in combination for 72 hours and cell survival was analyzed by SRB assays. C, CI was calculated via CalcuSyn software. Lapatinib plus rapamycin combined data of cell growth inhibition rate (1 − survival rate) from SRB assay was expressed as fraction of affected cells. CI > 1 indicates antagonism, CI = 1 indicates additivity, and CI < 1 indicates synergism. The assay was prepared in quadruplicates. The error bars represent the SE of replicates. Rapa, rapamycin; Lapa, lapatinib.
Effects of combined mTOR inhibition and EGFR inhibition on downstream signaling pathways
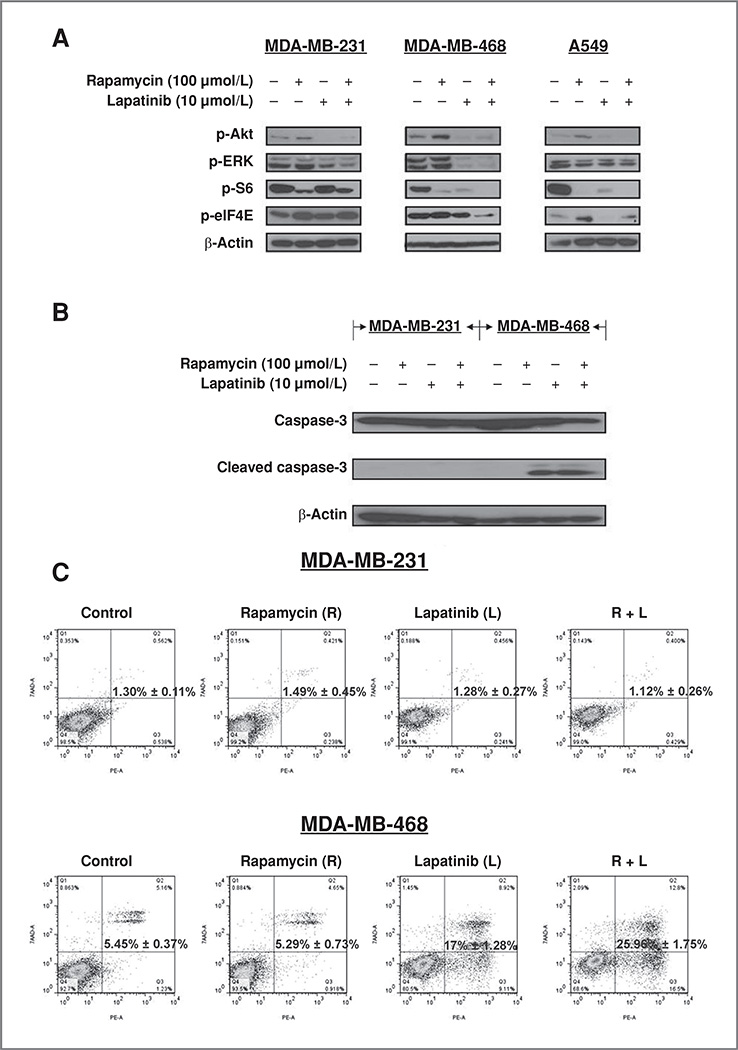
mTOR inhibition may promote pharmacologic mechanisms of resistance in cancer cells via feedback activation of the PI3K/Akt/mTOR and RAF/MEK/ERK signaling pathways (21). Rapamycin and a number of clinically available mTOR inhibitors activate Akt while inhibiting mTOR and downstream signaling (13, 14), and the precise mechanism of rapamycin-induced Akt activation remains unknown. This aberrant Akt upregulation may partly explain the modest clinical responses elicited by these single agents in the treatment of many solid tumors, including breast cancer (12, 22). We explored the possibility that rapamycin-induced Akt activation could be repressed with combined EGFR and mTOR inhibition in triple-negative breast cancer cells. Cells were treated with vehicle, rapamycin alone, lapatinib alone, or with rapamycin in combination with lapatinib. Lapatinib decreased the expression of p-Akt and p-ERK in both triple-negative breast cancer cell lines (Fig. 3A). As expected, rapamycin increased the expression of p-Akt while abolishing mTOR signaling, as evidenced by decreased expression of the downstream target pS6 (Fig. 3A). Interestingly, rapamycin also increased the expression of upstream p-EGFR in MDA-MB-231 cells (Fig. 1B), possibly because of a similar feedback loop that resulted in increased Akt activation. In all cell lines, the combination of inhibitors decreased rapamycin-induced activation of p-Akt, and reduced p-ERK and pS6 expression levels (Fig. 3A).
Figure 3.
Effects of lapatinib and rapamycin on signaling and apoptosis in triple-negative breast cancer cells. A, triple-negative breast cancer cell lines MDA-MB-231 and MDA-MB-468, and lung cancer cell line A549 were treated with vehicle, rapamycin (100 nmol/L), lapatinib (10 µmol/L), and rapamycin combined with lapatinib (same doses) for 3 hours. Protein lysates from each treatment group were analyzed for the expression of p-Akt, p-ERK, pS6, p-eIF4E, and β-actin. B, MDA-MB-231 and MDA-MB-468 cells were treated with vehicle, rapamycin, lapatinib, or rapamycin combined with lapatinib at the above concentrations for 48 hours followed by SDS-PAGE and Western blotting analysis of caspase-3 activity. C, MDA-MB-231 and MDA-MB468 cells were treated with rapamycin alone, lapatinib alone, and the 2 drugs in combination for 72 hours. Cells were then stained with Annexin V–PE and 7-AAD and analyzed by FACSorting analysis. The group of 4 graphs shown for each cell line is a one-time experiment representative of the 4 repeated ones. The bold number indicated in the middle of the graph is the mean ± SE of 4 replicates. Student's t test was used to calculate P value.
Besides activating p-Akt, mTOR inhibition has been shown to increase phosphorylated eIF4E levels, a protein that plays a key role in cell proliferation and apoptotic resistance (23–25). Activated eIF4E attenuates apoptosis (23, 24), and EGFR inhibition suppresses rapamycin-induced p-eIF4E in lung cancer cell lines (25). We observed that eIF4E activity differed in the 2 triple-negative breast cancer cell lines following combined EGFR and mTOR inhibition. We found that p-eIF4E expression was decreased following rapamycin plus lapatinib treatment in MDA-MB-468cells (Fig. 3A). In contrast, dual inhibition of mTOR and EGFR in MDA-MB-231 breast cancer cells increased the expression of activated p-eIF4E, similar to what has been described for A549 non–small cell lung cancer cells (19). Expression of p-eIF4E activity is closely associated with apoptotic resistance (17, 18).
Given the fact that the combination of lapatinib and rapamycin decreased p-elF4E levels in MDA-MB-468 cells, but not in MDA-MB-231 cells, we evaluated whether apoptosis was stimulated in response to the combination therapy. As expected from our data with p-elF4E, rapamycin combined with lapatinib induced cleavage of caspase-3 in MDA-MB-468 cells but not in MDA-MB-231 cells (Fig. 3B). These data suggest that the failure to induce apoptosis in MDA-MB-231 treated with combined EGFR and mTOR inhibitors may be due to acquired apoptotic resistance via activated eIF4E.
To quantitatively determine whether the combination of mTOR and EGFR inhibition increased apoptosis, MDA-MB-231 and MDA-MB468 cells were treated with rapamycin alone, lapatinib alone, or the combination of rapamycin and lapatinib for 72 hours. The percentage of apoptotic cell death was measured with Annexin V–PE and 7-AAD staining followed by FACS analysis (Fig. 3C). We noted a significant increase in apoptotic MDA-MB-468 cells treated with the combination of rapamycin and lapatinib (25.96%), compared with rapamycin (5.29%) and lapatinib (17.0%) alone (lapatinib versus combination P < 0.01). There was no increase in apoptosis in MDA-MB-231 cells treated with either single agent alone or rapamycin and lapatinib in combination (P = 0.54). Interestingly, the fact that both cell lines had less apoptotic dead cells than the growth-inhibited cells detected by SRB assay indicated that other inhibition mechanisms, such as cytostasis, could also play roles in the observed inhibitory effects induced by the combined treatment.
EGFR and mTOR inhibition suppresses growth of triple-negative breast tumor xenografts
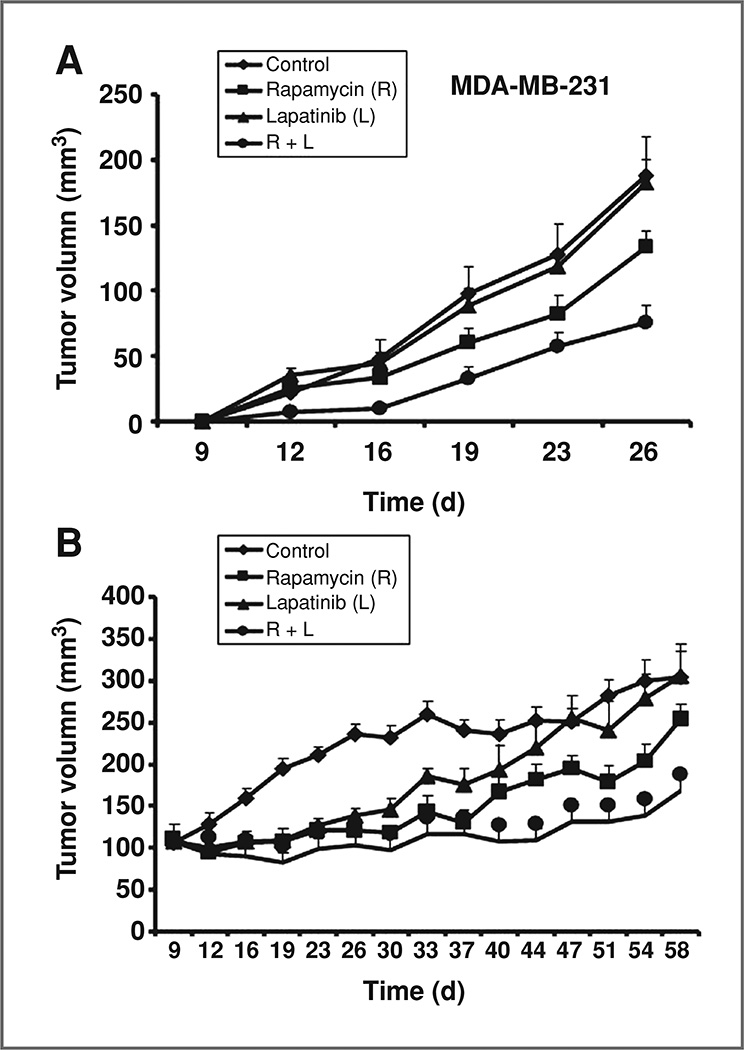
To confirm our in vitro results, triple-negative breast cancer cells MDA-MB-231 and MDA-MB-468 were injected into the mammary fat pads of athymic mice in 2 independent experiments. Mice were randomized into 4 treatment groups and administered rapamycin, lapatinib, combination of rapamycin and lapatinib, or vehicle. Tumor diameters were measured bidimensionally twice weekly. At the end of the overall treatments, neither lapatinib (mean tumor size = 183 mm3, P = 0.87) nor rapamycin (mean tumor size = 133 mm3, P = 0.098) alone significantly decreased the volume of MDA-MB-231 tumors compared with vehicle-treated animals (mean tumor size = 188 mm3). In contrast, the combination of lapatinib and rapamycin (mean tumor size = 76 mm3) significantly inhibited MDA-MB-231 tumor progression (60%), compared with lapatinib alone (4%, P < 0.0001), rapamycin alone (29%, P = 0.0096), and vehicle-treated animals (P = 0.0005; Fig. 4A). All P values were adjusted by Bonferroni correction because we are comparing 5 pairs of groups.
Figure 4.
Inhibitory effects of EGFR plus mTOR inhibitors on triple-negative breast cancers in vivo. Nude mice were injected with 5 × 106 MDA-MB-231 cells or 5 × 106 MDA-MB-468 cells. Animals were treated with vehicle (1% Tween-80, orally and 2% ethyl alcohol, i.p., n = 6/10), rapamycin alone (3 mg/kg, i.p., n = 10/10), lapatinib alone (75 mg/kg, orally, n = 10/10), and their combination (same doses and route, n = 12/10). Rapamycin was given twice a week and lapatinib was given 5 days a week. Mice injected with MDA-MB-231 cells (A) were treated for a total of 3 weeks, and mice injected with MDA-MB-468 cells (B) were treated for a total of 7 weeks. Tumor volumes of MDA-MB-231 and MDA-MB-468 xenografts were measured twice weekly. Tumor sizes were compared among treatment groups. Vertical bars on the tumor growth curve chart indicate the SE.
Initially, MDA-MB-468 xenograft tumor growth was inhibited to a similar degree by each single-agent and combined treatments. However, at the end of continued treatment, the combination of rapamycin and lapatinib significantly limited tumor growth (45%, mean tumor size = 168 mm3), compared with the vehicle-treated group (mean tumor size = 305 mm3, P < 0.0001), whereas the rapamycin was only able to inhibit the tumor growth by 16% (mean tumor size = 254 mm3, P < 0.0001) and the lapatinib alone had no inhibition effect at the end (mean tumor size = 306 mm3, P = 0.03; Fig. 4B). All P values were adjusted by Bonferroni correction.
There was no significant difference in mouse weight between the treatment groups in either experiment, suggesting that the combination of an EGFR and mTOR inhibitor does not significantly increase toxicity (data not shown). In summary, in triple-negative xenografts breast cancer models, lapatinib significantly enhances the effect of rapamycin on tumor progression.
Effects of EGFR and mTOR inhibition on apoptosis and proliferation in vivo
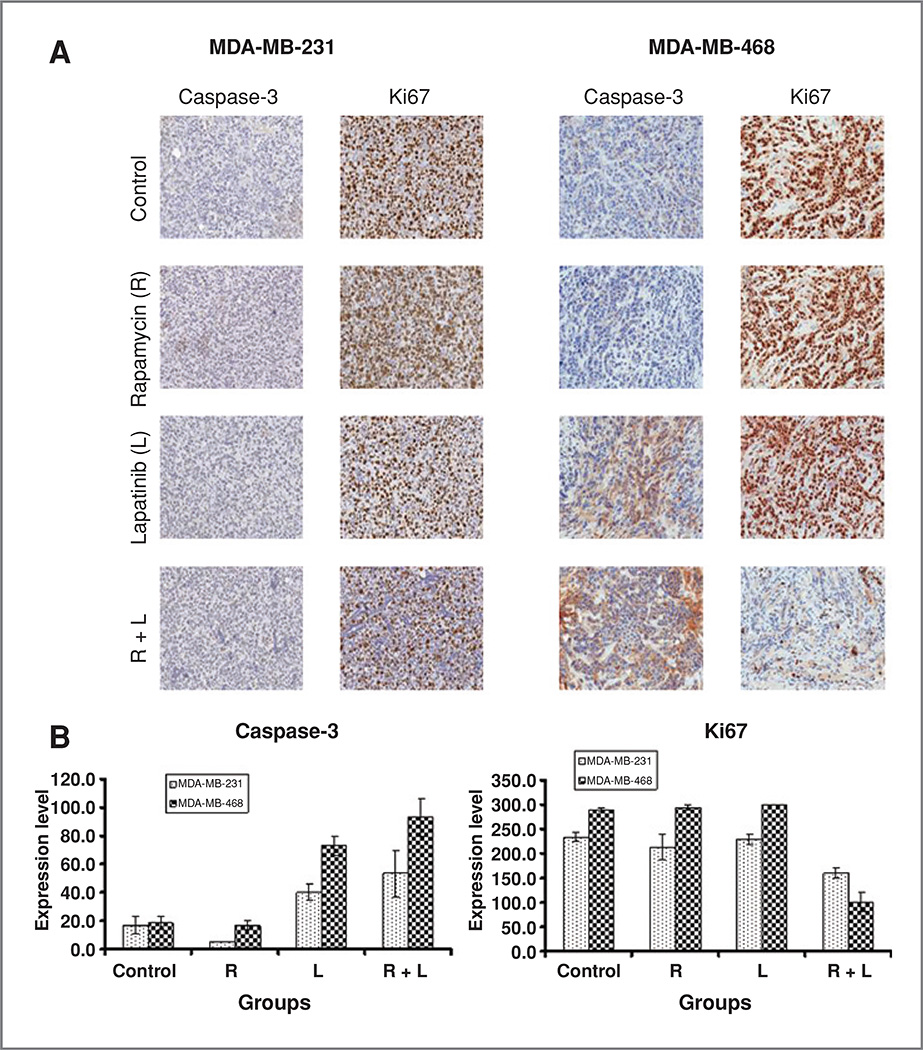
To evaluate the apoptotic effects of combined and single-agent treatments on both MDA-MB-231 and MDA-MB-468 tumors, we examined caspase-3 activity by immunohistochemistry (Fig. 5A and B). Basal levels of caspase-3 were low in both MDA-MB-231 and MDA-MB-468 tumor tissues and remained low in rapamycin-treated tumor tissues. The combination of lapatinib and rapamycin resulted in a greater increase in caspase-3 expression in MDA-MB-468 tumors (P < 0.01). Even though we saw a trend in increased caspase-3 induced by combination treatment, there was no statistical difference in expression level in MDA-MB-231–treated groups (P = 0.1). Interestingly, caspase-3 expression was increased in MDA-MB-468 (P < 0.01) xenografts exposed to lapatinib alone, despite the fact that single-agent lapatinib was less effective in inhibiting tumor growth than the combination of lapatinib and rapamycin in vivo (Fig. 5B). These results suggest that apoptosis may play only a partial role in the tumor inhibitory effects noted with combined EGFR and mTOR inhibition.
Figure 5.
Effects of combined mTOR/EGFR inhibition on proliferation and apoptosis in vivo. A, representative images of cleaved caspase-3 and Ki67 immunostaining for formalin-fixed, paraffin-embedded tumor tissues in MDA-MB-231 and MDA-MB-468 formed tumors. B, comparison of caspase-3 and Ki67 expression level among control and treatment groups for MDA-MB-231 and MDA-MB-468 cells. Overall intensity level (ranging from 0 to 300, intensity score times percentage of area staining) is used to evaluate the protein expression level. Error bars represent SE of scores. Student's t test was used to calculate P values. R, rapamycin; L, lapatinib.
As tumor development is regulated by both decreased apoptosis and increased proliferation, we assessed the expression of Ki67 (Fig. 5A and B). Ki67 expression was markedly decreased in MDA-MB-468 tumors (P < 0.001) and MDA-MB-231 tumors (P < 0.01) treated with the combination of rapamycin and lapatinib, indicating that the combination therapy decreased tumor proliferation. In contrast, neither rapamycin nor lapatinib alone was able to affect Ki67 expression in both triple-negative tumors (Fig. 5B).
Discussion
The outcome for metastatic hormone receptor–positive and HER2-positive cancers has improved over the past 15 years with the availability of targeted therapies, such as tamoxifen, aromatase inhibitors, and trastuzumab (26, 27). The median survival time for patients with metastatic hormone receptor–positive breast cancers is approximately 4 years, whereas the median survival for patients with HER2-positive metastatic breast cancers treated with trastuzumab-based chemotherapy approaches 3 years (26, 27). Triple-negative breast cancers pose a significant therapeutic problem because of a lack of targeted therapies, and the median survival for patients with metastatic ER-negative breast cancers is less than 12 months (26). Chemotherapy remains the mainstay of therapy for metastatic triple-negative breast cancers, but resistance is common and can develop rapidly (11). Response rates have been shown to be lower and time to progression shorter in patients with triple-negative breast cancers treated with single or combination chemotherapy compared with any of the other subtypes (28), suggesting that triple-negative breast cancers are intrinsically more chemoresistant than other breast cancer subtypes. Antiangiogenic approaches, when added to chemotherapy, seem promising for patients with metastatic triple-negative breast cancers (29). The use of the PARP inhibitor, iniparib, has been shown to prolong progression free and overall survival in patients with triple-negative metastatic breast cancers, compared with chemotherapy alone (30) in the phase 2 setting, though these results were not confirmed in a larger phase 3 clinical trial. Therefore, there is a critical need for new therapeutic approaches in triple-negative breast cancers.
EGFR is upregulated and overexpressed in a significant percentage of triple-negative or basal-like cancers (1). However, previous trials in which patients with unselected metastatic breast cancers were treated with tyrosine kinase inhibitors targeting EGFR produced disappointing results, with response rates of 2% and time to progression of less than 2 months (9, 10). Response rates to single-agent cetuximab are only 6%, and increased to 17% with the addition of carboplatin, in patients with triple-negative metastatic breast cancer (11). Notably, in many patients, cancers progressed so rapidly that they were taken off the study before their first staging assessment. The addition of cetuximab increased response rates from 30% to 49% in patients with triple-negative breast cancers but prolonged time to progression by only 1 week (31). On the basis of these trials, EGFR inhibitors alone or in combination with chemotherapy do not seem to be particularly effective in triple-negative breast cancers.
mTOR inhibitors activate the Akt pathway, possibly through a feedback mechanism (13). Treatment of breast and lung cancer cells lines with mTOR inhibitors increases expression of activated Akt (16, 32). This paradoxical activation of Akt is believed to be one possible resistance mechanism to mTOR inhibitors by cancer cells and may explain the disappointing results to date reported with these agents when used as single therapies (12). However, recent clinical data suggest that mTOR inhibition can sensitize resistant HER2-positive breast cancers to the EGFR inhibitor, trastuzumab (15).
Therefore, we postulated that mTOR inhibition could sensitize triple-negative breast cancer cells to EGFR inhibitors. We noted that the combination of rapamycin with lapatinib was synergistic in inhibiting triple-negative breast cancer cell survival. We showed that the combination of mTOR and EGFR inhibition increased apoptosis in some triple-negative cell lines compared with either treatment given alone. Combined rapamycin and lapatinib also resulted in significant suppression of triple-negative breast cancers in vivo compared with either agent alone. Lapatinib did not inhibit growth of triple-negative breast cancers in vivo, which is in keeping with clinical trials, where minimal efficacy is noted in HER2-negative metastatic breast cancers (33). Consistent with our in vitro results, the combination of lapatinib and rapamycin induced moderate apoptosis in MDA-MB-468 tumors, whereas induction of apoptosis was not significant in MDA-MB-231 tumors. Combination therapy markedly decreased the expression of Ki67 in MDA-MB-468 tumors and MDA-MB-231 tumors. These results suggest that the inhibitory effects of combined mTOR and EGFR inhibition on the growth of MDA-MB-468 tumors are due to effects on both apoptosis and cell proliferation. In contrast, the growth inhibition noted in MDA-MB-231 tumors treated with combined therapy is probably due to reduced cellular proliferation. Taking our in vitro and in vivo data together, a subset of triple-negative breast cancer seem to be more susceptible to concurrent inhibition of EGFR and mTOR. Lapatinib decreased the increase in activated Akt observed with the use of mTOR inhibitors in vitro. This finding potentially explains the synergistic effects we noted with combination therapy in vitro, though we were unable to show this conclusively in vivo (data not shown).
In summary, mTOR inhibitors in combination with EGFR inhibitors result in synergistic effects in triple-negative breast cancer models in vitro and suppressed triple-negative tumor growth in vivo, which warrant further evaluation. Given the lack of targeted agents and the rapid onset of chemoresistance in metastatic triple-negative breast cancers, there is a critical need for novel approaches to target these tumors. The mTOR inhibitor, everolimus has been combined with erlotinib in a phase 1 clinical trial, showing varying degrees of toxicity (34), but overall confirming the validity of this approach. Given the very poor outcomes for patients with metastatic triple-negative breast cancers, we believe the combination of an mTOR inhibitor and an EGFR inhibitor warrants clinical investigation, and we are currently accruing to a trial evaluating the combination of ever-olimus and lapatinib patients with minimally pretreated metastatic triple-negative breast cancers.
Acknowledgments
Grant Support
This study was supported by Wilbur and Hilda Glenn Foundation and Georgia Cancer Coalition (both to R. O’Regan).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta GA. Breast Cancer Res Treat. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 4.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke M, Collins R, Davies C, Godwin J, Gray R, Peto R, et al. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 8.Hudson R, Laska M, Berger T, Heye B, Schopohl J, Danek A. Olfactory function in patients with hypogonadotropic hypogonadism: an all-or-none phenomenon? Chem Senses. 1994;19:57–69. doi: 10.1093/chemse/19.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Dickler MN, Cobleigh MA, Miller KD, Klein PM, Winer EP. Efficacy and safety of erlotinib in patients with locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2009;115:115–121. doi: 10.1007/s10549-008-0055-9. [DOI] [PubMed] [Google Scholar]
- 10.Baselga J, Albanell J, Ruiz A, Lluch A, Gascon P, Guillem V, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–5333. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 11.Carey LA, Rugo HS, Marcom PK, Irvin W, Ferraro M, Burrows E, et al. On behalf of the Translational Breast Cancer Research Consortium TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer. J Clin Oncol. 2008;26:1009. [Google Scholar]
- 12.Hahn OM, Ma CX, Lin L, Hou D, Sattar H, Olopade FO, et al. A phase II trial of a mammalian target of rapamycin inhibitor, temsirolimus, in patients with metastatic breast cancer. SABCS. 2008:407. [Google Scholar]
- 13.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 14.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Regan R, Andre F, Campone M, Naughton M, Manlius C, Pylvaenaeinen I, et al. RAD001 (everolimus) in combination with weekly paclitaxel and trastuzumab in patients with HER-2-overexpressing metastatic breast cancer with prior resistance to trastuzumab: a multicenter phase I clinical trial. SABCS. 2008:3119. [Google Scholar]
- 16.Buck E, Eyzaguirre A, Brown E, Petti F, McCormack S, Haley JD, et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5:2676–2684. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR, et al. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123:1005–1014. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 19.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 20.Laska EM, Meisner M, Siegel C. Simple designs and model-free tests for synergy. Biometrics. 1994;50:834–841. [PubMed] [Google Scholar]
- 21.Lagravere MO, Fang Y, Carey J, Toogood RW, Packota GV, Major PW. Density conversion factor determined using a cone-beam computed tomography unit NewTom QR-DVT 9000. Dentomaxillofac Radiol. 2006;35:407–409. doi: 10.1259/dmfr/55276404. [DOI] [PubMed] [Google Scholar]
- 22.Copenhaver MM, Johnson BT, Lee IC, Harman JJ, Carey MP. Behavioral HIV risk reduction among people who inject drugs: meta-analytic evidence of efficacy. J Subst Abuse Treat. 2006;31:163–171. doi: 10.1016/j.jsat.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- 24.Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21:3232–3237. doi: 10.1101/gad.1604407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Hawk N, Yue P, Kauh J, Ramalingam SS, Fu H, et al. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors’ anticancer efficacy. Cancer Biol Ther. 2008;7:1952–1958. doi: 10.4161/cbt.7.12.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andre F, Slimane K, Bachelot T, Dunant A, Namer M, Barrelier A, et al. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol. 2004;22:3302–3308. doi: 10.1200/JCO.2004.08.095. [DOI] [PubMed] [Google Scholar]
- 27.Dawood SS, Kristine B, Hortobagyi GN, Giordano SH. Prognosis of women with stage IV breast cancer by HER2 status and trastuzumab treatment: an institutional based review. J Clin Oncol. 2008;26:1018. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rugo HS, Thomas ES, Lee RK, Fein LE, Peck R, Verrill M, et al. Combination therapy with the novel epothilone B analog, ixabepilone, plus capecitabine has efficacy in ER/PR/HER2-negative breast cancer resistant to anthracyclines and taxanes. SABCS. 2007 [Google Scholar]
- 29.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 30.Bottorff JL, Kalaw C, Johnson JL, Stewart M, Greaves L, Carey J. Couple dynamics during women's tobacco reduction in pregnancy and postpartum. Nicotine Tob Res. 2006;8:499–509. doi: 10.1080/14622200600789551. [DOI] [PubMed] [Google Scholar]
- 31.O’Shaughnessy J, Weckstein DJ, Vukelja SJ, McIntyre K, Krekow L, Holmes FA, et al. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer. SABCS. 2007:308. [Google Scholar]
- 32.Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–7418. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26:5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadimitrakopoulou V, Blumenschein GR, Leighl NB, Bennouna J, Soria JC, Burris HA, et al. A phase 1/2 study investigating the combination of RAD001 (R) (everolimus) and erlotinib (E) as 2nd and 3rd line therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) previously treated with chemotherapy (C): phase 1 results. J Clin Oncol. 2008;26:8051. [Google Scholar]